Abstract
Why do more intelligent people live healthier and longer lives? One possibility is that intelligence tests assess health of the brain, but psychological science has lacked technology to evaluate this hypothesis. Digital retinal imaging, a new non-invasive method to visualize microcirculation in the eye, may reflect vascular conditions in the brain. We studied the association between retinal vessel caliber and neuropsychological functioning in the representative Dunedin birth cohort. Wider venular caliber was associated with poorer neuropsychological functioning at midlife, independent of potentially confounding factors. This association was not limited to any specific test domain, and extended to informant-reports of cognitive difficulties in everyday life. Moreover, wider venular caliber was associated with lower childhood IQ tested 25 years earlier. The finding indicates that retinal venular caliber may be an indicator of neuropsychological health years before dementing diseases’ onset, and suggests digital retinal imaging as an investigative tool for psychological science.
Young people with lower intelligence test scores tend to be in poorer health as adults and die earlier (Whalley & Deary, 2001). The emerging field of cognitive epidemiology is now focused on uncovering the mechanisms linking early-life intelligence to later illness and early death (Gottfredson & Deary, 2004). The obvious explanations for the intelligence-illness association have not fully explained it; for example, the association is not simply an artifact of low socioeconomic status (SES), and poor health behaviors alone do not account for the link (Gottfredson, 2004; Jokela, Batty, Deary, Gale, & Kivimaki, 2009). One intriguing hypothesis is that intelligence is a marker for system integrity; that is, of a healthy, well-maintained body and brain (Deary, 2010; Deary, Weiss, & Batty, 2010). However, psychological science is limited in the technology to identify indicators of system integrity. Here we borrow insights from a surprising quarter (the discipline of ophthalmology) and show that retinal vessel caliber, which provides a window on microcirculation of the brain, may index lifelong neuropsychological health.
The vascular network of the brain contributes to neuropsychological ability by supplying oxygen and nutrients through a dense network of blood vessels (Paulson, 2002). A possible window into the health of the brain’s vascular network is provided by the closely related retinal blood vessels of the eye. Retinal and cerebral small vessels share similar embryological origin, structural and physiological features (Patton et al., 2005). Thus, assessing retinal vasculature may provide a useful noninvasive method to visualize the state of microcirculation in vivo, and to investigate the relationship between cerebral vascular state and neuropsychological health.
Advances in fundus photography (photographing the interior surface of the eye) and retinal image analysis now allow for the accurate quantitative measurement of retinal vessel caliber in large population-based samples (Sun et al., 2009). Of particular interest is the caliber of the arterioles and venules (that is, the size of the internal space of the vessel). Arterioles are small branches of the arteries. They carry oxygen-rich blood away from the heart to the capillaries, where oxygen and nutrients from the blood diffuse into the surrounding tissue. Arterioles regulate blood flow through changes in caliber (vasodilation and vasoconstriction) and are the primary determinants of blood pressure. Venules, in contrast, carry blood from the capillaries to the veins and back to the heart. Recent studies suggest that retinal arterioles and venules may represent different vascular pathophysiological processes (Sun et al., 2009). In particular, retinal arteriolar narrowing may be an early sign of hypertensive retinopathy and a prognostic indicator of hypertension (Wong & Mitchell, 2004). In contrast, retinal venular widening is associated with obesity (Wang et al., 2006), inflammatory markers (de Jong et al., 2007), and smoking behavior (Kifley et al., 2007), and predicts the risk of stroke and coronary heart disease (Wong et al., 2001).
Additional studies have shown an association specifically between retinal venular widening and brain-related vascular events such as stroke, cerebral infarction, and cerebral hypoxia (de Jong et al., 2008; Doubal, Hokke, & Wardlaw, 2009; Ikram, de Jong, Bos, et al., 2006; Ikram, De Jong, Van Dijk, et al., 2006; Wong, Kamineni, et al., 2006). These associations support the hypothesis of a connection between retinal venular caliber and cerebrovascular function. Several studies have also linked retinal vascular abnormalities, including venular widening, with neuropsychological dysfunction, with a particular focus on impaired memory or dementia. However, most studies examined the elderly or midlife adults with diabetes (Ding et al., 2008). Neuropsychological impairment related to advanced age and diabetes may have different underlying mechanisms than neuropsychological dysfunction in the general population. Moreover, whether or not retinal vessel caliber is associated with neuropsychological status in younger people, prior to declines seen in late life, remains to be addressed.
The purpose of this study was to examine the association between retinal vessel caliber and neuropsychological functioning in a representative birth cohort. First, we tested the article’s central hypothesis that retinal vessel caliber (arteriolar and venular caliber) is associated with concurrent neuropsychological functioning in adulthood (age 38 years). Based on prior studies cited above, we expected a stronger association for venular caliber and neuropsychological tests scores. An important step in cognitive epidemiology is to make sure that the correlates of neuropsychological functioning are untangled from other influences on such functioning (Lubinski, 2009). Thus, we tested whether poor health, lifestyle factors or environmental factors could explain the association between retinal vessel caliber and neuropsychological functioning, as all of these factors have been found to predict both retinal vessel caliber and neuropsychological test scores (Hubbard et al., 1999; Klein, Klein, Knudtson, Wong, & Tsai, 2006; Nguyen et al., 2008; Plassman, Williams, Burke, Holsinger, & Benjamin, 2010; Wong, Islam, et al., 2006). Second, we tested whether retinal vessel caliber was associated with specific versus generalized neuropsychological impairment. Studies linking retinal vessel caliber to neuropsychological performance have assessed a narrow set of neuropsychological domains, raising questions about the breadth of intellectual deficits (Ding et al., 2008). Specific impairment might indicate brain pathology (such as stroke), whereas generalized impairment would be more consistent with the theoretical concept of brain/body system integrity. Third, we tested whether retinal vessel caliber was associated with third-party informant reports of cognitive problems to determine if retinal signs translate into functional problems in daily living. Fourth, we tested the hypothesis that the association between retinal vessel caliber and neuropsychological functioning begins early in life by examining whether retinal vessel caliber was associated with childhood IQ tested over 25 years earlier.
Methods
Participants
Participants are members of the Dunedin Multidisciplinary Health and Development Study, a longitudinal investigation of health and behavior in a complete birth cohort. Study members (N=1,037; 91% of eligible births; 52% male) were all individuals born between April 1972 and March 1973 in Dunedin, New Zealand, who were eligible for the longitudinal study based on residence in the province at age 3 and who participated in the first follow-up assessment at age 3. The cohort represents the full range of SES in the general population of New Zealand’s South Island and is primarily white. Assessments were carried out at birth and at ages 3, 5, 7, 9, 11, 13, 15, 18, 21, 26, 32, and, most recently, 38 years, when 96% of the 1,004 living study members underwent assessment in 2010–2012. At each assessment wave, each Study member is brought to the Dunedin Research Unit for a full day of interviews and examinations.
Neuropsychological functioning
Intelligence tests were administered in childhood at ages 7, 9, 11 and 13 years (scores were averaged over ages 7 to 13 years) and again in adulthood at age 38 years. We report results from the Wechsler Intelligence Scale for Children-Revised (WISC-R) (Wechsler, 1974) and the Wechsler Adult Intelligence Scale-IV (WAIS-IV) (Wechsler, 2008). These tests comprise a series of subtests that yield indexes standardized to population norms with M=100 and SD=15. At age 38, additional neuropsychological tests were administered, including the Trail-Making, Wechsler Mental Control, Wechsler Verbal Paired Associates, Rey Auditory Verbal Learning (total and delayed recall), Grooved Pegboard, One-legged Balance, Grip Strength tests and 3 tasks from the Cambridge Neuropsychological Test Automated Battery (CANTAB) (Sahakian & Owen, 1992). Table S1 provides further details. Tests were administered in the morning in counterbalanced order.
Informant reports of Study members’ neuropsychological functioning were obtained at age 38 years. Study members nominated people "who knew them well." These informants were mailed questionnaires and asked to complete a checklist, including whether the study member had problems with attention and memory over the past year. The informant-reported cognitive problems scale consisted of seven items: “is easily distracted, gets sidetracked easily”, “can’t concentrate, mind wanders”, “tunes out instead of focusing”, “has difficulty organizing tasks that have many steps”, “has problems with memory”, “misplaces wallet, keys, eyeglasses, paperwork”, and “forgets to do errands, return calls, pay bills” (internal consistency reliability=.875). Informant reports of cognitive problems were significantly correlated with Study members’ IQ scores (r=−.22, p<.001).
Additional variables
Physical examinations were conducted at age 38 and Study members provided blood samples (venepunctures always between 4:15–4:45 pm). Pregnant women were excluded from analyses.
High-sensitivity C-Reactive Protein (hsCRP, mg/L) was measured on a Hitachi 917 analyzer (Roche Diagnostics, GmbH, D-68298, Mannheim, Germany) using a particle-enhanced immunoturbidimetric assay. The CDC/AHA definition of high cardiovascular risk (hsCRP >3 mg/L) was adopted to identify our risk group (Ridker, Wilson, & Grundy, 2004). 20.4% of the cohort members were defined as having high hsCRP levels.
Diabetes was defined using the American Diabetes Association cut points; 5.7– 6.5% glycated hemoglobin (HbA1c) levels for pre-diabetes, and 6.5% or higher HbA1c levels for diabetes (Seino et al., 2010). 18.2% were defined as pre-diabetic or diabetic.
High blood pressure was assessed according to standard protocols (Perloff et al., 1993) using a Hawksley Random-zero Sphygmomanometer with a constant deflation valve. High blood pressure was defined as systolic BP >140 mm Hg or diastolic BP >90 mm Hg (Chobanian et al., 2003). 12.5% were defined as having high blood pressure.
Obesity. Height was measured to the nearest millimeter using a portable Harpenden Stadiometer (Holtain, Crymych, UK). Weight was recorded to the nearest 0.1kg using a Tanita calibrated scale. BMI was computed as weight (kg)/height (m2). Obesity was defined as BMI≥30 (National Institutes of Health, 1998). 23.7% were defined as obese.
Current smoking. 22.6% were current smokers.
Low adult SES. Study members’ occupation was coded to a 6-point scale for contemporary occupations in New Zealand (1= unskilled laborer, 6= professional) based on education and income associated with that occupation in the New Zealand census. The SES of homemakers and those not working was estimated from their education. Low adult SES was defined as 1 or 2 on the SES scale. 30.2% were defined as low adult SES.
Assessment of retinal vessel caliber
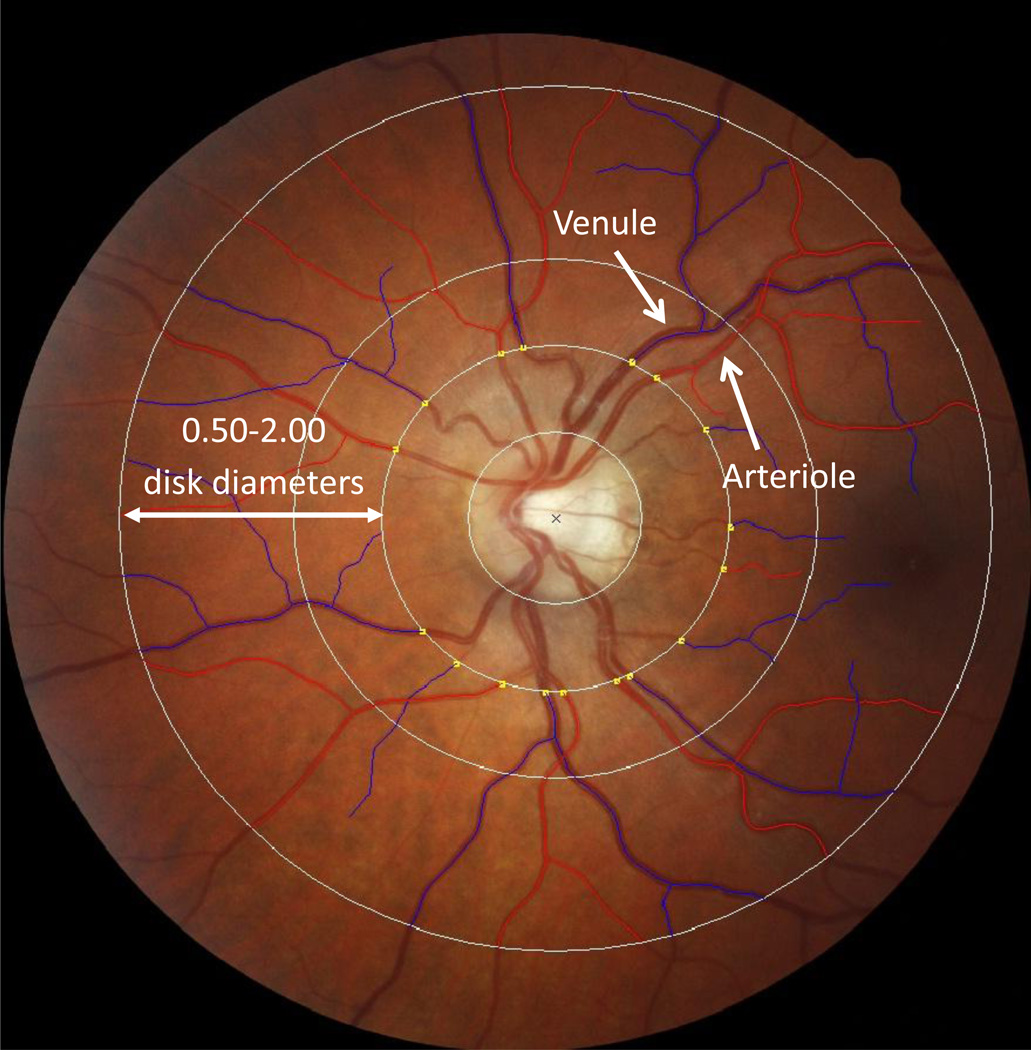
Digital fundus photographs were taken at the Research Unit after 5 minutes of dark adaptation. The same camera (Canon NMR-45 with a 20D SLR backing, Japan) was used for all photographs, thereby preventing artifactual variation from different cameras. Both the left and right eyes were photographed and we report analyses of the average of the two eyes. Retinal photographs were graded at the Singapore Eye Research Institute, National University of Singapore, using semi-automated computer software (Singapore I Vessel Assessment [SIVA], software version 3.0). Trained graders, masked to participant characteristics, used the SIVA program to measure the retinal vessel diameters according to a standardized protocol with high inter-grader reliability (Cheung et al., 2011). Diameter (or caliber) denotes the size of the lumen, which is the internal space of the vessel. Measurements were made for arterioles and venules where they passed through a region located 0.50–2.00 disk diameters from the optic disk margin (Cheung et al., 2011) (Figure 1). Vessel calibers were based on the six largest arterioles and venules passing though this region and were summarized as central retinal artery equivalent (CRAE) and central retinal vein equivalent (CRVE) using the revised Knudtson-Parr-Hubbard formula (Cheung et al., 2010; Knudtson et al., 2003). Of 938 study members with retinal images, only 7 could not be graded because the images were either too dark or not centered on the optic disk. An additional 9 study members were excluded from analyses due to pregnancy. This left 922 study members with retinal vessel data. Arteriolar and venular calibers were normally distributed within our population-representative cohort. The mean arteriolar caliber among the 922 study members was 137.33 measuring units (SD=10.86, Median=137.30, Range=105.66, 179.47), and the mean venular caliber was 196.20 measuring units (SD=14.83, Median = 195.51, Range=141.07, 245.68).
Figure 1.
Measurements were made for the 6 largest arterioles (red) and venules (blue) passing through 0.50–2.00 disk diameters from the optic disk margin.
Statistical analysis
Linear regression was used to test the hypotheses that retinal vessel caliber was related to (a) neuropsychological test performance at 38 years of age (beginning with the IQ, and then progressing to other neuropsychological scores), (b) informant ratings of cognitive impairment at age 38 years, and (c) IQ performance in childhood (averaged across ages 7, 9, 11 and 13 years). Individuals with wider arterioles were more likely to have wider venules (r=.67, p<.001); following recommended procedures, we included both artieriolar and venular caliber in all analyses to control for the confounding effects of the fellow vessel (Liew et al., 2007; Sun et al., 2009). To rule out the possibility that the association between retinal vessel caliber and IQ was a spurious artifact of current poor health, lifestyle factors or socio-environmental factors, we re-analyzed the association between retinal vessel caliber and IQ controlling for these potential confounding conditions. Table S2 shows that high levels of hsCRP, diabetes, high blood pressure, obesity, cigarette smoking, and low SES were significantly correlated with retinal vessel caliber- replicating previous medical and epidemiological studies-- as well as with IQ. Sex was included as a covariate in all analyses. Results are presented with and without adjustment for the covariance between arteriolar and venular retinal caliber.
Results
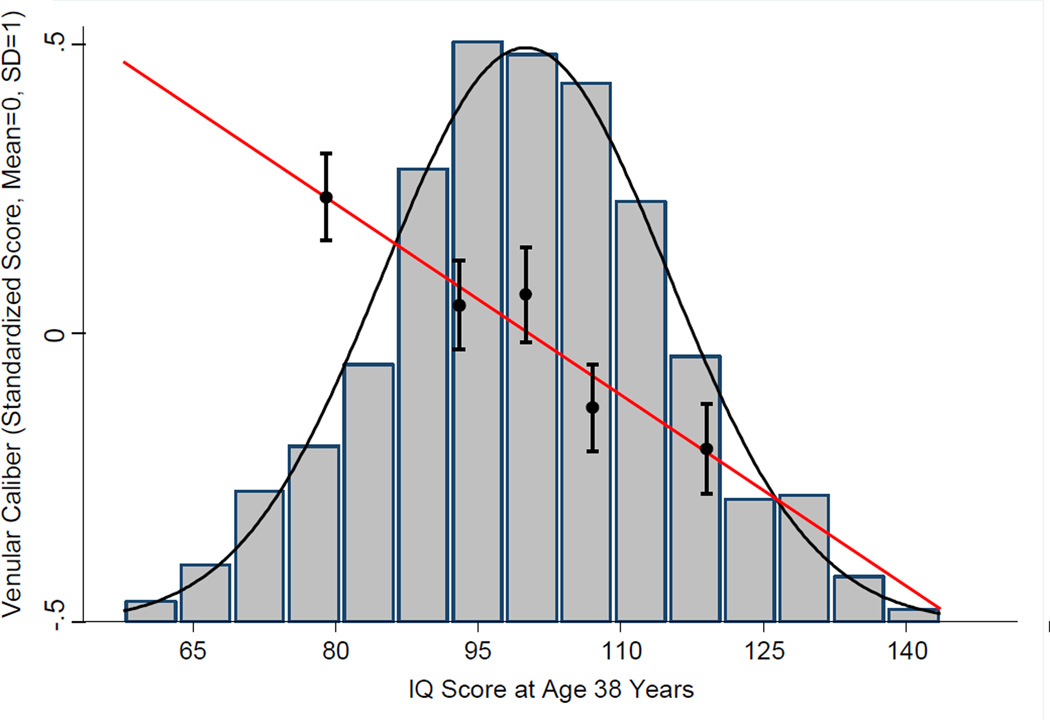
Wider venular caliber was associated with lower IQ scores at age 38 years (β= − .155, 95% CI: −.220, −.091, p< .001), even after adjusting for arteriole caliber (β= −.194, 95% CI: −.282, −.108, p<.001) (Figure 2). Similar associations between wider venular caliber and lower IQ scores were observed among both males (β=−.212, 95% CI: −.350, − .092, p= .001) and females (β=−.177, 95% CI: −.288, −.054, p= .004).
Figure 2.
The association between retinal venular caliber and IQ. The histogram depicts the distribution of IQ scores at age 38 years. The scatter plot and regression line show the association between venular caliber and IQ score (Y-axis). For illustrative purposes, plotted points show the mean venular caliber for cohort members in each of the quintile groups of the IQ distribution (mean quintiles: 79.7, 92.7, 100.7, 107.7, and 119.6). Error bars reflect standard errors. The negative slope of the regression line shows that cohort members with wider venular caliber had lower IQ scores at age 38 years.
Retinal arteriolar caliber was significantly associated with IQ scores (β= −.071, 95% CI: −.137, −.006, p=.032), but not after adjusting for venular caliber (β= .060, 95% CI: −.027, .148, p= .178), nor when adjusted for venular caliber and analyzed separately among males (β=.074, 95% CI: −.053, .208, p= .245) and females (β=.046, 95% CI: −.073, .162, p=.455). Given the null association with arteriolar caliber, all further analyses were performed on venular caliber only.
We ruled out several explanations for the observed association between wider venular caliber and adult IQ, namely that this association could be an artifact of: (a) current poor health (high hsCRP, diabetes, high blood pressure), (b) lifestyle (smoking, obesity), or (c) socio-environmental risk factors (low adult SES). Table 1 shows that excluding study members with each of these conditions from analysis did not alter the initial finding. Further, a multivariate regression analysis, controlling for all of the above confounding factors and arteriolar caliber simultaneously, showed that wider venular caliber remained significantly associated with lower IQ scores (β= −.107, 95% CI: −.195, − .022, p=.014).
Table 1.
The association between retinal venular caliber and IQ at age 38 years after excluding cases for potential confounders (poor health factors, lifestyle factors or environmental factors). The standardized regression coefficients (β) reflect change in neuropsychological test performance as a function of a 1-SD unit change in retinal venular caliber.
| IQ at Age 38 Years Model 1 |
IQ at Age 38 Years Model 2 |
|||||
|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | |
| Total Cohort (n=916)* | −.155 | −.219, −.091 | < .001 | −.194 | −.282, −.108 | < .001 |
| Excluding cases of (n= total number of study members after exclusion): | ||||||
| High hsCRP (n=708) | −.161 | −.234, −.088 | < .001 | −.233 | −.334, −.131 | < .001 |
| Pre-diabtetes or Diabetes (n=718) | −.174 | −.238, −.099 | < .001 | −.221 | −.309, −.118 | < .001 |
| High blood pressure (n=800) | −.155 | −.222, −.086 | < .001 | −.178 | −.272, −.083 | < .001 |
| Obesity (n=699) | −.186 | −.267, −.117 | < .001 | −.204 | −.313, −.107 | < .001 |
| Smokers (n=709) | −.124 | −.198, −.051 | .001 | −.192 | −.292, −.094 | < .001 |
| Low Adult SES (n=638) | −.175 | −.249, −.097 | < .001 | −.210 | −.309, −.107 | < .001 |
Note: Model 1: control for sex. Model 2: Model 1 + control for arteriolar caliber.
Out of the n=922 with retinal imaging information, 6 Study members did not have neuropsychological tests scores leaving an effective group size of n=916.
hsCRP = high sensitive C reactive protein; SES = Socio-economic status.
Table 2 shows that wider venular caliber was associated with lower scores on tests of verbal comprehension, perceptual reasoning, working memory, processing speed, executive function, memory, and motor functions. This pattern emphasizes general rather than specific neuropsychological deficits associated with wider venular caliber.
Table 2.
The association between venular caliber and neuropsychological test performance at age 38 years. The standardized regression coefficients (β) reflect change in neuropsychological test performance as a function of a 1-SD unit change in retinal venular caliber.
| Neuropsychological Test | Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | ||
| WAIS-IV | Verbal Comprehension | −.141 | −.207, −.078 | <.001 | −.181 | −.269, −.095 | <.001 |
| Perceptual Reasoning | −.103 | −.168, −.039 | .002 | −.124 | −.211, −.037 | .005 | |
| Working Memory | −.096 | −.161, −.032 | .003 | −.147 | −.234, −.061 | .001 | |
| Processing Speed | −.124 | −.186, −.061 | <.001 | −.141 | −.224, −.055 | .001 | |
| Executive Function |
Trail Making Test B* | .098 | .034, .163 | .003 | .114 | .027, .201 | .010 |
| WMS-III: Mental Control | −.123 | −.186, −.060 | <.001 | −.096 | −.180, −.010 | .028 | |
| CANTAB Rapid Visual Information Processing: A Prime | −.075 | −.141, −.010 | .023 | −.126 | −.215, −.039 | .005 | |
| Memory Tests |
Rey Auditory Verbal Learning: Total Recall | −.086 | −.150, −.025 | .006 | −.113 | −.198, −.029 | .008 |
| Rey Auditory Verbal Learning: Delayed Recall | −.060 | −.122, .002 | .060 | −.087 | −.171, −.003 | .043 | |
| WMS-III: Paired Associates: Total Recall | −.048 | −.113, .017 | .145 | −.068 | −.155, .019 | .126 | |
| WMS-III: Paired Associates: Delayed Recall | .008 | −.057, .073 | .801 | .040 | −.048, .128 | .372 | |
| CANTAB Visual Paired Associates Learning: Total Errors* | .083 | .018, .144 | .012 | .080 | −.007, .164 | .072 | |
| Motor Tests |
Grooved Pegboard* | .103 | .040, .166 | .001 | .113 | .028, .198 | .009 |
| One-legged Balance | −.061 | −.126, .004 | .067 | −.152 | −.240, −.065 | .001 | |
| Grip Strength | .011 | −.033, .054 | .629 | .025 | −.034, .083 | .408 | |
| CANTAB Reaction Time: 5-Choice Reaction Time* | .011 | −.053, .075 | .739 | .007 | −.079, .094 | .872 | |
Note: Model 1: control for sex. Model 2: Model 1 + control for arteriolar caliber.
Higher scores indicate slower performance or more errors, and thus worse functioning.
WAIS-IV= Wechsler Adult Intelligence Scale-IV; WMS-III = Wechsler Memory Scale-III; CANTAB = Cambridge Neuropsychological Test Automated Battery.
Moreover, informants reported observing more cognitive problems (β=.133, 95% CI: .067, .194, p< .001) among cohort members with wider venular caliber, even after controlling for arteriolar caliber (β=.130, 95% CI: .042, .213, p=.004).
Finally, we tested the hypothesis that the association between wider venular caliber and IQ begins early in life by examining participants’ childhood IQ scores. Wider venular caliber at age 38 was associated with lower childhood IQ (β= −.117, 95% CI: − .171, −.049, p< .001), even after controlling for arteriolar caliber (β=−.152, 95% CI: −.226, −.061, p= .001).
Discussion
Wider venular caliber was associated with worse neuropsychological functioning in a population-based cohort of adults as they approach midlife. The present study extends current knowledge in several ways. First, wider venular caliber (but not arteriolar caliber) assessed at age 38 years was correlated with poorer neuropsychological functioning, years before onset and diagnosis of age-related diseases. Second, the association between wider venular caliber and neuropsychological performance was not limited to any specific neuropsychological domain, and could not be explained by poor health, lifestyle factors or environmental factors that might affect both the condition of the retinal vessel and neuropsychological performance. Third, third-party informant reports of cognitive problems were associated with wider venular caliber, suggesting that retinal venular caliber also can predict cognitive problems in everyday life among relatively young adults. Finally, poorer neuropsychological functioning in childhood antedated wider venular caliber in adulthood.
Previous studies have documented association between wider retinal venules and dementia (Ikram, Ong, Cheung, & Wong, 2012; Sun et al., 2009). However, to date, it has not been clear whether vessel caliber is related to neuropsychological status prior to declines seen later in life or to those declines associated with illness and disease. To us it seems unremarkable that venular caliber in the eye is abnormal in elderly individuals who have documented vascular disease, yet rather more remarkable that venular caliber in the eye is related, however modestly, to mental test scores of individuals in their thirties, and even to IQ scores assessed in childhood. Taken together, these findings suggest that the developmental processes linking retinal vessel abnormalities to neuropsychological functioning begin at much younger ages than previously assumed in studies of retinal vasculature and memory loss in the elderly. It appears that digital retinal imaging may serve as a tool for testing the theory that general intelligence represents brain/body system integrity across the life course.
The pathophysiological mechanism that links wider venular caliber with poorer neuropsychological functioning is not well understood. It is possible that low oxygen perfusion in the brain results in damaged cerebral (and retinal vessel) microvasculature, having a negative impact on cognitive functioning (Qaum et al., 2001). This is consistent with studies showing that venular caliber signs are associated with low cerebral oxygen supply, progression of cerebral small vessel disease and stroke, chronic ischemia and cerebral atrophy (de Jong et al., 2008; Doubal et al., 2009; Ikram, de Jong, Bos, et al., 2006; Ikram, De Jong, Van Dijk, et al., 2006; Wong, Kamineni, et al., 2006). As arterioles are the main source for supplying the tissue with oxygen and nutrients, it may seem surprising that only venules were linked to IQ. Previous studies have emphasized the specific link between venules and neuropsychological functioning. In contrast, the lack of association with arterioles is not entirely understood. One potential explanation for this discrepancy may lie with the underlying structural differences between arterioles and venules. Arterioles have higher relative proportions of connective tissue and smooth muscle than venules; thus, arterioles are less elastic and may not be as good an indicator of brain health as venules. Whatever the process, our finding that IQ was lower during childhood among individuals who presented with wider venular caliber as adults suggests it is underway as early as childhood.
Our findings must be interpreted in the context of several limitations. First, as digital retinal imaging is a relatively new technology, we assessed it at one time point only: at age 38 years. Thus, we could not estimate change in retinal vessel caliber from childhood to adulthood, or rule out whether factors associated with low childhood IQ caused change in retinal vessel caliber. However, we ruled out key mechanisms by which low premorbid IQ might affect retinal vasculature (high levels of hsCRP, diabetes, high blood pressure, obesity, cigarette smoking and low adult SES; Table 1). Second, although we ruled out these numerous possible explanations of the association between retinal venular caliber and neuropsychological impairment, it is possible that other unmeasured factors may explain the results. Third, we noticed that venular caliber was un-related to another measure that has been proposed to index brain/body integrity, “choice reaction time” (Deary & Der, 2005). In our cohort, choice reaction time was correlated with the IQ (r = .21, controlling for sex); thus, perhaps both reaction time and venular caliber can help to account for the IQ-health connection, albeit via complementary mechanisms. Fourth, the association between neuropsychological health and retinal venular caliber reflected a small effect size in the population (~r=.2). However, at the extremes, the effects were more marked: the highest and lowest fifths of the population on IQ differed by 0.5 SD units in the size of their venular caliber (Figure 2). Effect sizes for new research findings should be evaluated against other, well-established findings. The effect sizes we observed between IQ and venular caliber can be evaluated against correlations between IQ and other factors such as occupational status (~r=.4) and income (~r=.2) (Deary, 2012), birth weight (~r=.2) (Shenkin et al., 2001) and reaction time (~r=.3) (Deary, 2012). The modest effect size cautions against the use of retinal vessel caliber as a surrogate marker for general IQ, but digital retinal imaging may prove to be a useful tool as an indicator of brain/body system health. Finally, the present findings need to be confirmed in independent samples.
Retinal imaging may prove to be a valuable new tool for psychological science in studying development, aging, and health. It is noninvasive, can be administered to children and adults, measured repeatedly, and compared across different populations. Several new research directions suggest themselves. Developmental research is needed to establish when in the life course associations emerge between retinal vessel caliber and neuropsychological functioning. Neuroscience research is needed to combine retinal and neuroimaging tools to test associations between retinal caliber and brain structure (for example, gray matter volume) and function (changes in blood flow). Longitudinal research is needed to test if changes in retinal vasculature track with changes in neuropsychological functioning over time. Epidemiological research is needed to test whether retinal caliber may, in part, explain the associations between IQ and morbidity and mortality and whether this applies especially to specific illnesses and causes of death involving vascular pathology in the brain, a topic we hope to pursue as the Dunedin cohort ages. More knowledge about retinal vessels could inform prevention and intervention strategies aimed at increasing oxygenation of the brain and preventing agerelated worsening of neuropsychological problems (de Jong et al., 2008). Our initial findings support the hypothesis that retinal venular caliber may be an indicator of neuropsychological health throughout the life-course and its application need not be limited to the study of age-related diseases such as dementia. We think these findings open the door for digital retinal imaging to be used as an investigative tool for psychological science.
Supplementary Material
Acknowledgments
We thank the Dunedin Study members, their families, Unit research staff, and Study founder Phil Silva. The Dunedin Multidisciplinary Health and Development Research Unit is supported by the New Zealand Health Research Council. This research is supported by the National Institute on Aging (AG032282) and the UK Medical Research Council (MR/K00381X, G0601483). Additional support was provided by the National Institute of Child Health and Human Development (HD061298) and the Jacobs Foundation; MHM was supported by the National Institute on Drug Abuse (P30 DA023026). The study protocol was approved by the institutional ethical review boards of the participating universities. Study members gave informed consent before participating.
References
- Cheung CY, Hsu W, Lee ML, Wang JJ, Mitchell P, Lau QP, Wong TY. A new method to measure peripheral retinal vascular caliber over an extended area. Microcirculation. 2010;17(7):495–503. doi: 10.1111/j.1549-8719.2010.00048.x. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Tay WT, Mitchell P, Wang JJ, Hsu W, Lee ML, Wong TY. Quantitative and qualitative retinal microvascular characteristics and blood pressure. J Hypertens. 2011;29(7):1380–1391. doi: 10.1097/HJH.0b013e328347266c. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Wright JT. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- de Jong FJ, Ikram MK, Witteman JC, Hofman A, de Jong PT, Breteler MM. Retinal vessel diameters and the role of inflammation in cerebrovascular disease. Ann Neurol. 2007;61(5):491–495. doi: 10.1002/ana.21129. [DOI] [PubMed] [Google Scholar]
- de Jong FJ, Vernooij MW, Ikram MK, Ikram MA, Hofman A, Krestin GP, Breteler MM. Arteriolar oxygen saturation, cerebral blood flow, and retinal vessel diameters. The Rotterdam Study. Ophthalmology. 2008;115(5):887–892. doi: 10.1016/j.ophtha.2007.06.036. [DOI] [PubMed] [Google Scholar]
- Deary IJ. Cognitive epidemiology: its rise, its current issues, and its challenges. Personality and Individual Differences. 2010;49(4):337–343. [Google Scholar]
- Deary IJ. Intelligence. Annual Review of Psychology, Vol 63. 2012;63:453–482. doi: 10.1146/annurev-psych-120710-100353. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Der G. Reaction time explains IQ's association with death. Psychol Sci. 2005;16(1):64–69. doi: 10.1111/j.0956-7976.2005.00781.x. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Weiss A, Batty GD. Intelligence and personality as predictors of illness and death how researchers in differential psychology and chronic disease epidemiology are collaborating to understand and address health inequalities. Psychological Science in the Public Interest. 2010;11(2):53–79. doi: 10.1177/1529100610387081. [DOI] [PubMed] [Google Scholar]
- Ding J, Patton N, Deary IJ, Strachan MW, Fowkes FG, Mitchell RJ, Price JF. Retinal microvascular abnormalities and cognitive dysfunction: a systematic review. Br J Ophthalmol. 2008;92(8):1017–1025. doi: 10.1136/bjo.2008.141994. [DOI] [PubMed] [Google Scholar]
- Doubal FN, Hokke PE, Wardlaw JM. Retinal microvascular abnormalities and stroke: a systematic review. J Neurol Neurosurg Psychiatry. 2009;80(2):158–165. doi: 10.1136/jnnp.2008.153460. [DOI] [PubMed] [Google Scholar]
- Gottfredson LS. Intelligence: is it the epidemiologists' elusive" fundamental cause" of social class inequalities in health? Journal of personality and social psychology. 2004;86(1):174. doi: 10.1037/0022-3514.86.1.174. [DOI] [PubMed] [Google Scholar]
- Gottfredson LS, Deary IJ. Intelligence predicts health and longevity, but why? Current Directions in Psychological Science. 2004;13(1):1–4. [Google Scholar]
- Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, Cai J. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106(12):2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- Ikram MK, de Jong FJ, Bos MJ, Vingerling JR, Hofman A, Koudstaal PJ, Breteler MM. Retinal vessel diameters and risk of stroke: the Rotterdam Study. Neurology. 2006;66(9):1339–1343. doi: 10.1212/01.wnl.0000210533.24338.ea. [DOI] [PubMed] [Google Scholar]
- Ikram MK, De Jong FJ, Van Dijk EJ, Prins ND, Hofman A, Breteler MM, De Jong PT. Retinal vessel diameters and cerebral small vessel disease: the Rotterdam Scan Study. Brain. 2006;129(Pt 1):182–188. doi: 10.1093/brain/awh688. [DOI] [PubMed] [Google Scholar]
- Ikram MK, Ong YT, Cheung CY, Wong TY. Retinal vascular caliber measurements: clinical significance, current knowledge and future perspectives. Ophthalmologica. 2012 doi: 10.1159/000342158. [DOI] [PubMed] [Google Scholar]
- Jokela M, Batty GD, Deary IJ, Gale CR, Kivimaki M. Low childhood IQ and early adult mortality: the role of explanatory factors in the 1958 British birth cohort. Pediatrics. 2009;124(3):E380–E388. doi: 10.1542/peds.2009-0334. [DOI] [PubMed] [Google Scholar]
- Kifley A, Liew G, Wang JJ, Kaushik S, Smith W, Wong TY, Mitchell P. Long-term effects of smoking on retinal microvascular caliber. Am J Epidemiol. 2007;166(11):1288–1297. doi: 10.1093/aje/kwm255. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BEK, Knudtson MD, Wong TY, Tsai MY. Are inflammatory factors related to retinal vessel caliber?: the Beaver Dam Eye Study. Archives of ophthalmology. 2006;124(1):87. doi: 10.1001/archopht.124.1.87. [DOI] [PubMed] [Google Scholar]
- Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27(3):143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- Liew G, Sharrett AR, Kronmal R, Klein R, Wong TY, Mitchell P, Wang JJ. Measurement of retinal vascular caliber: issues and alternatives to using the arteriole to venule ratio. Invest Ophthalmol Vis Sci. 2007;48(1):52–57. doi: 10.1167/iovs.06-0672. [DOI] [PubMed] [Google Scholar]
- Lubinski D. Cognitive epidemiology: with emphasis on untangling cognitive ability and socioeconomic status. Intelligence. 2009;37(6):625–633. [Google Scholar]
- National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. National Institutes of Health; 1998. [PubMed] [Google Scholar]
- Nguyen TT, Wang JJ, Sharrett AR, Islam F, Klein R, Klein BEK, Wong TY. Relationship of retinal vascular caliber with diabetes and retinopathy. Diabetes Care. 2008;31(3):544. doi: 10.2337/dc07-1528. [DOI] [PubMed] [Google Scholar]
- Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005;206(4):319–348. doi: 10.1111/j.1469-7580.2005.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson OB. Blood-brain barrier, brain metabolism and cerebral blood flow. Eur Neuropsychopharmacol. 2002;12(6):495–501. doi: 10.1016/s0924-977x(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88(5 Pt 1):2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Williams JW, Jr, Burke JR, Holsinger T, Benjamin S. Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med. 2010;153(3):182–193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, Adamis AP. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42(10):2408–2413. [PubMed] [Google Scholar]
- Ridker PM, Wilson PW, Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109(23):2818–2825. doi: 10.1161/01.CIR.0000132467.45278.59. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Owen AM. Computerized assessment in neuropsychiatry using Cantab - discussion paper. Journal of the Royal Society of Medicine. 1992;85(7):399–402. [PMC free article] [PubMed] [Google Scholar]
- Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, Diagnostic CJDS. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Journal of Diabetes Investigation. 2010;1(5):212–228. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenkin SD, Starr JM, Pattie A, Rush MA, Whalley LJ, Deary IJ. Birth weight and cognitive function at age 11 years: the Scottish Mental Survey 1932. Archives of Disease in Childhood. 2001;85(3):189–195. doi: 10.1136/adc.85.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Wang JJ, Mackey DA, Wong TY. Retinal vascular caliber: systemic, environmental, and genetic associations. Surv Ophthalmol. 2009;54(1):74–95. doi: 10.1016/j.survophthal.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Taylor B, Wong TY, Chua B, Rochtchina E, Klein R, Mitchell P. Retinal vessel diameters and obesity: a population-based study in older persons. Obesity (Silver Spring) 2006;14(2):206–214. doi: 10.1038/oby.2006.27. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for children, revised. Psychological Corp; 1974. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. Fourth Edition. San Antonio, TX: Pearson Assessment; 2008. [Google Scholar]
- Whalley LJ, Deary IJ. Longitudinal cohort study of childhood IQ and survival up to age 76. British medical journal. 2001;322(7290):819–822. doi: 10.1136/bmj.322.7290.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, Shahar E. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multiethnic study of atherosclerosis (MESA) Invest Ophthalmol Vis Sci. 2006;47(6):2341–2350. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Kamineni A, Klein R, Sharrett AR, Klein BE, Siscovick DS, Duncan BB. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the cardiovascular health study. Arch Intern Med. 2006;166(21):2388–2394. doi: 10.1001/archinte.166.21.2388. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, Sharrett AR. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358(9288):1134–1140. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- Wong TY, Mitchell P. Hypertensive retinopathy. N Engl J Med. 2004;351(22):2310–2317. doi: 10.1056/NEJMra032865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.