Abstract
Recent evidence has linked mitochondrial dysfunction and DNA damage, increased oxidative stress in skeletal muscle, and insulin resistance (IR). The purpose of this study was to determine the role of the DNA repair enzyme, human 8-oxoguanine DNA glycosylase/apurinic/apyrimidinic lyase (hOGG1), on palmitate-induced mitochondrial dysfunction and IR in primary cultures of skeletal muscle derived from hind limb of ogg1−/− knockout mice and transgenic mice, which overexpress human (hOGG1) in mitochondria (transgenic [Tg]/MTS-hOGG1). Following exposure to palmitate, we evaluated mitochondrial DNA (mtDNA) damage, mitochondrial function, production of mitochondrial reactive oxygen species (mtROS), mitochondrial mass, JNK activation, insulin signaling pathways, and glucose uptake. Palmitate-induced mtDNA damage, mtROS, mitochondrial dysfunction, and activation of JNK were all diminished, whereas ATP levels, mitochondrial mass, insulin-stimulated phosphorylation of Akt (Ser 473), and insulin sensitivity were increased in primary myotubes isolated from Tg/MTS-hOGG1 mice compared to myotubes isolated from either knockout or wild-type mice. In addition, both basal and maximal respiratory rates during mitochondrial oxidation on pyruvate showed a variable response, with some animals displaying an increased respiration in muscle fibers isolated from the transgenic mice. Our results support the model that DNA repair enzyme OGG1 plays a pivotal role in repairing mtDNA damage, and consequently, in mtROS production and regulating downstream events leading to IR in skeletal muscle.
Insulin resistance (IR) in skeletal muscle is a critical step in the pathogenesis of type 2 diabetes (1) and metabolic syndrome (2). Diet enriched in saturated fat leads to obesity and, thus, to IR in skeletal muscle. IR represents a major risk factor for metabolic syndrome, type 2 diabetes, and cardiovascular disorders. Increasing evidence, accumulated over the last decade, indicates that mitochondrial dysfunction and oxidative stress are important components in these disease processes (3, 4), but the underlying mechanisms responsible for these events are still unknown. Because diet-induced obesity and its consequences have became a major problem in western society, with adverse consequences for public health and health care costs, it is an urgent priority to define the pathogenic mechanisms and identify pathways for prevention, reversal, and possible treatments of diet-induced obesity, metabolic syndrome, and their associated cardiovascular disorders. On a molecular level, the mechanisms of reactive oxygen species (ROS)-induced mitochondrial dysfunction remain unclear. Among the potential ROS targets is mitochondrial DNA (mtDNA), because mtDNA is highly specialized and encodes for proteins that are essential for energy metabolism. Until recently, the integrity of mtDNA and its repair mechanisms have received little attention in obesity and diabetes research. However, we have shown that damage to mtDNA, induced by the saturated free fatty acid palmitate, heightens mitochondrial ROS (mtROS) production, which is very critical for both mitochondrial dysfunction and insulin signaling pathways in L6 myotubes (5). Damage to mtDNA can occur in the form of base modifications, abasic sites, or various other types of lesions to the sugar-phosphate backbone (6, 7). 8-Oxoguanine (8-OxoG) is the most abundant oxidative lesion in nucleotide bases. Its presence can be used as an indicator of oxidative damage to DNA. If left unrepaired, 8-OxoG is highly mutagenic, resulting in G-C to T-A transversions (8). Because mammalian mtDNA encodes 13 polypeptides of the electron transport chain, 2 rRNAs and 22 tRNAs, alterations in mitochondrial transcription could change electron transport complexes to cause decreased ATP production and, also, lead to defective electron transfer, which would cause additional ROS production, thus, initiating a vicious cycle between mtDNA damage and ROS generation, because any damage to the respiratory chain may enhance ROS production and, therefore, heighten the oxidative stress to all other mitochondrial components, including mtDNA. We and others have reported that targeting the DNA repair protein human 8-OxoG glycosylase/apurinic/apyrimidinic (AP) lyase (hOGG1) to mitochondria augments mtDNA repair and enhances cellular survival and proliferation following oxidative stress (9–16). Furthermore, by targeting the hOGG1 into skeletal muscle mitochondria, we have revealed that the vicious cycle between mtDNA damage and mtROS production can be interrupted, which leads to improved insulin sensitivity in vitro (5). hOGG1 encodes two isoforms, hOGG1 1-α, which has a nuclear localization, whereas the 1-β isoform is targeted to mitochondrion (17). There are discrepancies in the results for the overexpression of hOGG1 in mitochondria both in vitro and in vivo, which possibly could depend on the isoform of the hOGG1 that was used. Several groups, including our own, have reported beneficial effects of mitochondrial expression of the hOGG1 1-α during oxidative stress (9–16). In contrast, the 1-β isoform did not have the same rescuing effect, but on the contrary resulted in increased mtDNA damage in both in vitro and in vivo experiments (18, 19). In the present work, we have used primary muscle cell (PMC) cultures derived from hind limb skeletal muscle isolated from 1) wild-type mice (WT), 2) mice lacking OGG1 (ogg1−/−, knockout [KO]), and 3) transgenic mice, which overexpress human OGG1 (hOGG1, 1-α isoform) only in mitochondria (transgenic [Tg]/mitochondrial targeting sequence [MTS]-hOGG1). Our results reveal that upon treatment with palmitate, PMC from Tg/MTS-hOGG1 accumulate significantly less mtDNA damage and thus produce less mtROS and have improved mitochondrial function and insulin sensitivity compared to PMC from WT and KO animals. By using PMC isolated from all three groups, we addressed two major issues: 1) we further defined the role of hOGG1 in repairing of mtDNA damage, and consequently in mtROS production and downstream events leading to IR; and 2) we confirmed that mtDNA damage is critical in triggering mitochondrial dysfunction, increased oxidative stress, and the development of IR. Whether similar mechanisms exist in the skeletal muscle tissue of Tg/KO animals in vivo is the focus of our future investigations.
Materials and Methods
Materials
DMEM and penicillin-streptomycin were from Invitrogen (Carlsbad, California), and fetal bovine serum (FBS) was from Hyclone (Logan, Utah). Palmitate, bovine serum albumin (BSA) (fatty acid-free), insulin (from bovine pancreas), penicillin/streptomycin were from Sigma (St Louis, Missouri). 2-Deoxy-d-[3H] glucose was purchased from PerkinElmer (Shelton, Colorado).
Genotyping of animals
The animals used for establishing the colonies of WT, KO, and Tg mice were inbred at the University of South Alabama Animal Care Facility and were previously described (20, 21). Determination of the genotype of newborn mice was done by PCR, using the sets of primers and PCR conditions as described previously (20, 21). All procedures used in this study were approved by the Institutional Animal Care and Use Committee of University at South Alabama and were in full compliance with the guidelines for the National Institutes of Health. Both sexes of mice were used in this study.
Isolation of cellular fractions and 8-OxoG activity assay
Mitochondrial and nuclear fractions from gastrocnemius skeletal muscle were isolated using the protocol for the MITO-ISO kit provided by Sigma. The 8-OxoG activity assay was performed, as previously described, with minor modifications (10, 12). Briefly, 20 μg of mitochondrial or nuclear fractions isolated from skeletal muscles from either KO, Tg, or WT mice were incubated with a radiolabeled duplex containing 8-OxoG for 3 hours at 37°C. The incised product was separated by denaturing PAGE; wet gels were autoradiographed at −70°C, and the resultant images were scanned from films. As a positive control, recombinant hOGG1 (New England Biolabs, Ipswich, Massachusetts) was used.
Mitochondrial respiration
To address the effects of OGG1 in mitochondrial respiration, lower hind limb skeletal muscle from adult animals (8-10 wk old) were isolated from all three groups of animals. Thin sections of approximately 25 mg each were obtained after careful dissection and suspended in sterile MiR06 buffer (MiR05 + 280 u/mL Catalase) (MiR05 contains [mM]: sucrose, 110; K-lactobionate, 60; EGTA, 0.5; MgCl2, 3; taurine, 20; KH2PO4, 10; HEPES, 20, adjusted to pH 7.1 with KOH at 37°C; and 1 g/L BSA essentially fatty acid free). Sections were transferred to individual chambers of an oxygraph-2k (Oroboros Instruments, Innsbruck, Austria) containing 2 mL MiR06 for high-resolution respirometry. After a 10-minute equilibration at 37°C, pyruvate (1 M) was added as an electron donor and basal oxygen flux was assessed. Specimens then were treated with trifluorocarbonylcyanide phenylhydrazone (3.4 mM) as an uncoupler to measure maximal respiration. Basal and maximal oxygen flux are reported in picomole per second per milligram wet tissue.
Preparation of primary muscle cells and treatment
Satellite cells were obtained from lower hind limb muscle from adult animals (8-10 wk old) using the modified protocol as described previously (22). The muscles were excised from the lower limbs of mice, thoroughly minced, and digested in 0.2% type II collagenase (Worthington Biochemicals, Lakewood, New Jersey) in Dulbecco's phosphate buffered saline buffer with calcium (Invitrogen) for 30 minutes at 37°C. After digestion, the cells were collected via a 5-minute centrifugation at 1800g. The collected cells were exposed to trypsin-EDTA (0.25% with 1 mM EDTA from Invitrogen) at 37°C for 10 minutes. The cells were diluted in 30 mL of culture medium containing 20% FBS, and 1% penicillin-streptomycin in DMEM and filtered through 40-μm cell strainers (BD Biosciences, San Jose, California). Cells were grown in 5% CO2 at 37°C. Fibroblasts adhere to uncoated culture dishes more readily than muscle cells (23), so fibroblast contamination was minimized by preplating the cells onto 100-mm tissue culture dishes for 3 to 4 hours at 37°C. The unattached cells were centrifuged at 1800g for 5 minutes, suspended in culture medium, plated onto dishes coated with 0.1% gelatin (Millipore, Temecula, California), and incubated at 37°C overnight. The attached cells were trypsinized, suspended in culture medium, and plated at 1 to 2 × 105 cells/mL in culture dishes, 6-well, or 24-well plates. After 1 to 2 days in culture, the culture medium was replaced with differentiation medium containing 2% FBS, 1% horse serum (Invitrogen), and antibiotics. Primary myotubes were used 4 to 5 days after induction of differentiation, when most cells were multinucleated. A stock concentration of palmitate was prepared as discussed previously (5, 24). Control cells were treated with drug diluent only (2% BSA) in the DMEM medium. A stock concentration of 10-N-nonyl acridine orange (NAO, Invitrogen) was dissolved in dimethylsulfoxide. Control cultures, not treated with NAO, received the same concentration of dimethylsulfoxide as in the compound treated cultures. In the Akt (Ser 473) phosphorylation experiments, PMC were incubated with palmitate for 16 hours, then serum starved for 2 hours, and incubated with 100 nM of insulin for 15 minutes.
Assay for mtDNA copy number and damage analysis
To evaluate mtDNA copy number, a slot blot analysis (25) was performed using the samples of DNA isolated and treated with EcoRI (5, 24). A measure of 0.5 to 2 μg DNA was denatured by 0.3 M NaOH, linked to a nylon membrane in a GS Gene Linker (Bio-Rad, Hercules, California), and probed with a mitochondrial (cytochrome c oxidase, I subunit) or nuclear (Immunoglobulin E) probe. Hybridization images were scanned and band intensities were determined, which allowed a direct comparison between the amount of mtDNA and nuclear DNA present at each sample. Relative values from band intensities were calculated by comparing each sample with the average of WT mice. To evaluate mtDNA damage, PMC were exposed to palmitate for 6 hours and quantitative alkaline Southern blots were performed (5, 24). Break frequency was determined using the Poisson expression (s = −ln P0, where s is the number of breaks per fragment and P0 is the fraction of fragments free of breaks) as previously described (5, 10, 24).
Measurement of ATP levels
Cells were grown in 24-well culture plates, differentiated, and treated with 0.5 or 0.75 mM palmitate for 24 hours. To determine the total cellular ATP level, an ATP bioluminescence assay kit (Roche, Mannheim, Germany) was used (5, 24, 26). Values are shown as a percentage of the corresponding untreated control.
Cell viability
The CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, Wisconsin), a colorimetric method for determining the number of viable cells by assessing mitochondrial function, was performed 24 hours after exposure to 0.5 or 0.75 mM palmitate, as previously described (5, 24, 26). The reagent contains a tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] and an electron-coupling reagent (phenazine methosulfate). MTS is bioreduced by the dehydrogenase enzymes found in metabolically active cells into a formazan product soluble in tissue culture medium. The quantity of formazan product measured by the amount of 490 nm absorbance is directly proportional to the number of living cells in culture. The reagent is added to culture wells and the cells are incubated for 2 hours. Optical density was read at 490 nm in a microplate reader. Data are displayed as a percentage of untreated controls.
Mitochondrial mass measuring
The fluorescent dye NAO, which specifically binds to cardiolipin independently of the mitochondrial membrane potential, was used to monitor mitochondrial mass (27). Cells were treated with the indicated concentration of palmitate for 24 hours and 20 μM NAO was added for the last 30 minutes of the treatment and incubated in the dark.
Mitochondrial ROS production
Primary myotubes were exposed to the indicated concentrations of palmitate for 24 hours and then analyzed for mtROS production. MitoSOX Red, a mitochondrial superoxide indicator for live cell imaging (Invitrogen), was used to analyze mitochondrial superoxide generation within myotube mitochondria as described (5, 24). Values are shown as a percentage of the corresponding untreated control.
Protein isolation and Western blot analysis
Proteins were extracted from frozen hind limb skeletal muscles from all three groups of animals by stirring in liquid nitrogen followed by incubation in cell lysis buffer (Cell Signaling, Beverly, Massachusetts) supplemented with 0.1 mg phenylmethylsulfonylfluoride and a 1/100 dilution of protease and phosphatase inhibitor cocktails (Sigma). The extracts were additionally homogenized using glass homogenizers. Samples were centrifuged for 10 minutes at 14 000g and the supernatants were used for Western blots. Protein extracts for total cellular fractions were obtained from one 100-mm dish of each cell type. Proteins were isolated in cell lysis buffer as described above for isolation of proteins from muscle. The cells were scraped to dislodge them from the bottom of the dishes and then passed 4 times through a 1-mL gauge syringe (Becton Dickinson, Franklin Lakes, New Jersey). Samples were centrifuged for 10 minutes at 14 000g and the supernatants were used for Western blots. Protein concentrations were determined using the Bio-Rad protein dye microassay. SDS-PAGE and transfer of separated proteins to nylon or polyvinyldifluoride membranes were performed as previously described (5, 24). The antibodies used were actin (Sigma); phospho-Akt (Ser 473), total Akt, phospho-SAP/JNK (T183/Y185), total SAP/JNK, insulin receptor substrate-1 (IRS-1), GAPDH (Cell Signaling); Glut4, MnSOD (Abcam); mitochondrial transcription factor (TFAM), peroxisome proliferator activator receptor-γ coactivator 1α (PGC-1α), lamin A (Santa Cruz Biotechnology, Santa Cruz, California). All antibodies to oxidative phosphorylation (OXPHOS) complexes: complex I, subunit NDUFA9; complex II, subunit 70-kDa FAD (Fp); complex IV, subunits 3 and 4; and complex V, subunit F1α were from Mitosciences (Eugene, Oregon). Complexes formed were detected with horseradish peroxidase–conjugated antimouse, antigoat, or antirabbit immunoglobulin G antibodies (Promega) using chemiluminescent reagents (Cell Signaling). Where indicated, the resultant band images were scanned and analyzed using Fujifilm Image Gauge Version 2.2 software (Fujifilm USA, Stamford, Connecticut).
2-Deoxy-d-[3H] glucose uptake
Primary skeletal myotubes were cultured in six-well plates and treated with medium containing palmitate or BSA as a control, as indicated, and 2-deoxyglucose uptake (2DG) was performed as previously described (5) for 5 minutes in HEPES-buffered saline containing 10 μM 2-deoxy-d-[3H]glucose (1 μCi/ml).
Statistical analysis
Data are expressed as means ± SE. Differences between the two groups were assessed using unpaired, two-tailed Student t test. Differences between more than two groups in normally distributed data were assessed using one-way ANOVA followed by Bonferroni analysis. The nonparametric Kruskal-Wallis test was used for the analysis of mitochondrial respiration data, which were not normally distributed. The choice between parametric and nonparametric tests was based on the Bartlett test for homogeneity of variances. Statistical significance was determined at the 0.05 level, and all tests were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, California).
Results
Analysis of animals
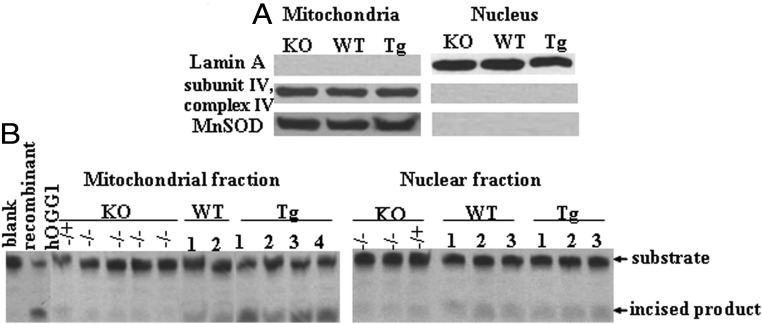
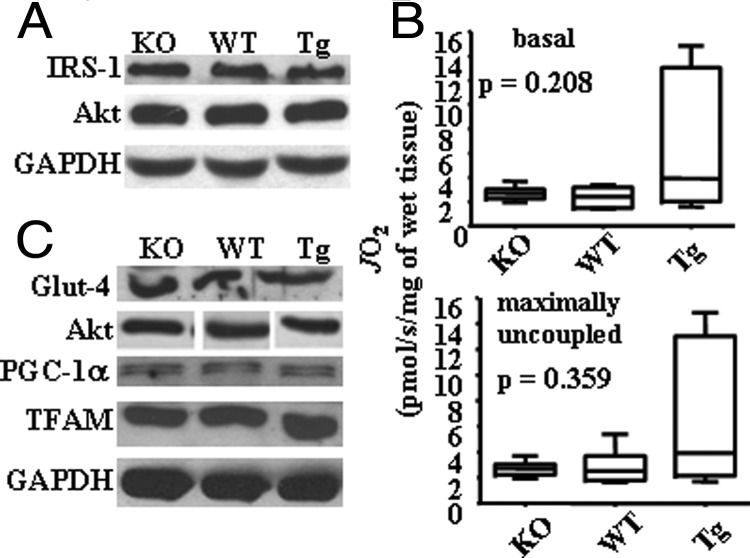
To analyze the impact of mtDNA damage during palmitate administration, we used three groups of mice with different repair capacity toward mtDNA damage: WT, ogg1−/− (KO), and Tg mice. In addition to the previously described analysis of KO and Tg animals (20, 21), we performed a further evaluation of OGG1 activity in mitochondria isolated from skeletal muscle from KO and Tg animals. To prove that hOGG1 targeting was confined exclusively to mitochondria in Tg mice and to exclude possible leakage expression in nucleus, OGG1 activity was also evaluated in nuclear fractions isolated from skeletal muscle. First, mitochondrial and nuclear fractions were isolated using a MITO-ISO kit and the purity of the preparation was checked by the enrichment of mitochondrial or nuclear specific proteins as determined by Western blot (Figure 1A). As shown in Figure 1B, OGG1 activity was elevated in skeletal muscle mitochondria of Tg mice, compared to mitochondria isolated from WT mice. On the other hand, no difference in OGG1 activity was observed in lanes containing nuclear fractions, thus confirming that hOGG1 is predominantly expressed in mitochondria without leakage expression in nucleus (Figure 1B). As expected, no incision activity against 8-OxoG was detected in mitochondrial or nuclear extracts from KO mice (Figure 1B). In addition, we evaluated the expression of some proteins of the insulin signaling pathway in skeletal muscles harvested from all groups of animals, as well as in PMC. For this, we isolated proteins from lower hind limb muscle dissected from all three groups and performed Western blot analysis using antibodies against IRS-1 and Akt proteins. As shown in Figure 2A, all three animal groups have similar levels of expression of the insulin signaling proteins analyzed. Also, to ensure that the OGG1 transgene over/underexpression did not affect the expression of proteins of insulin signaling and proteins involved in mitochondrial biogenesis, we analyzed the expression of the glucose transporter Glut4, Akt, and proteins involved in mitochondrial biogenesis in cultures of PMC. These latter include PGC-1α, a major protein implicated in mitochondrial biogenesis and TFAM, a mitochondrial replication factor. Consistent with the data obtained for in vivo analysis, there appeared to be no difference in expression of the various proteins in vitro in PMC isolated from all groups of mice (Figure 2C). In addition, we tested whether OGG1 has an effect on mitochondrial respiration of a carbohydrate-derived substrate (pyruvate) in muscle fibers isolated from lower hind limb of KO, WT, and Tg mice in the absence of any exogenous oxidative stress. As seen in Figure 2B, muscle fibers isolated from the Tg animals showed a distinct trend toward an increase of both basal and maximal respiratory rates during mitochondrial oxidation of pyruvate, although the difference was not found to be statistically significant, using a nonparametric Kruskall-Wallis test.
Figure 1.
OGG1 expression in skeletal muscle isolated from KO and Tg animals. A, Skeletal muscle mitochondrial and nuclear fractions were isolated from different genotype littermates of KO, WT, and Tg mice and analyzed by Western blot for the purity of fractions. Lamin A was used as a marker for nuclear proteins, and subunit IV of mitochondrial complex IV and MnSOD were used as markers for mitochondrial proteins. Equal loading was confirmed using Ponceau staining of the membrane. No detected nuclear contamination was present in the skeletal muscle mitochondria and no contamination of mitochondrial proteins was found in nuclear fraction. B, OGG1 activity in mitochondrial and nuclear fractions isolated from skeletal muscle of KO, WT, and Tg mice. Note the almost complete absence of the product in the KO and minimal product in WT animals and increased amount of incised product in mitochondrial fractions isolated from Tg mice.
Figure 2.
Analysis of KO and Tg animals, and PMC. Expression of various proteins in skeletal muscle (A) and in PMC (C) isolated from different genotypes (as indicated). B, Mitochondrial respiration in skeletal muscle fibers on pyruvate, basal (top) and maximally uncoupled (bottom) (n = 5). P values were assessed using the nonparametric Kruskal-Wallis test.
Mitochondrial overexpression of hOGG1 prevented palmitate-induced mtDNA damage and mitochondrial dysfunction in PMC
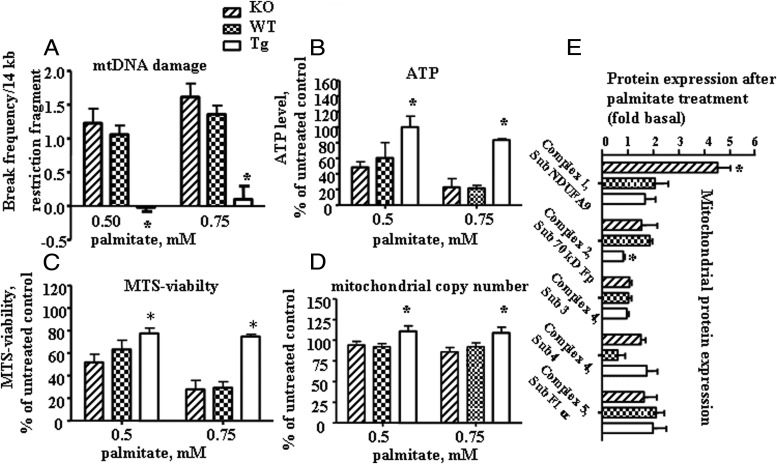
Before we proceeded to analyze primary muscle cultures, we evaluated the morphology of the PMC isolated from all three groups of animals. Myoblasts derived from all three groups of mice morphologically appeared very similar and, after 3 to 4 days in culture, they fused into large, multinucleated myotubes, which displayed spontaneous contractile activity (data not shown). To evaluate mtDNA damage, PMC cultures were exposed to 0.5 or 0.75 mM palmitate conjugated to 2% BSA for 6 hours. Control cells were incubated with diluent (2% BSA) only. Cells were lysed; total DNA was isolated, and quantitative Southern hybridizations were performed using an mtDNA-specific probe. The results revealed a significant decrease in palmitate-induced mtDNA damage in primary myotubes isolated from skeletal muscle of Tg mice compared with cells isolated from KO or WT mice (Figure 3A). mtDNA copy number normalized to WT animals was 0.95, 1, and 1.5 for PMC isolated from KO, WT, and Tg mice, respectively. For measuring ATP levels, PMC isolated from all groups of animals were grown in 24-well culture plates and treated with palmitate for 24 hours. Control cultures were exposed to diluent only for the same amount of time. As can be seen in Figure 3B, ATP levels were significantly higher in PMC isolated from Tg mice, as compared to cells isolated from KO or WT mice incubated with the same concentrations of palmitate. Also, mitochondrial metabolic function and mass were evaluated in cultures treated with 0.5 or 0.75 mM palmitate for 24 hours. PMC isolated from Tg mice showed significantly enhanced mitochondrial function (also named as an MTS viability; Figure 3C) and mass (Figure 3D) as compared to PMC isolated from either KO or WT animals. Interestingly, the increase in mitochondrial function/mass was not due to an increase in the basal level of the two major proteins involved in mitochondrial biogenesis, PGC-1α and TFAM (Figure 3E). We next evaluated the expression of mitochondrial proteins after 24 hours of treatment with 0.5 mM palmitate in the PMC from all three groups of animals. To our surprise, with the exception of complex 4, subunit 3, which is encoded by mtDNA, protein levels of other complexes, which were encoded by nuclear DNA (nDNA) (with the exceptions, which are discussed in the next sentence), were increased after 24 hours of palmitate treatment (Figure 3E). Interestingly, Tg mice show virtually no increase in complex 2 expression, and the WT animals show a similar trend for complex 4, subunit 4.
Figure 3.
Overexpression of hOGG1 in mitochondria from PMC isolated from Tg mice prevented palmitate-induced mtDNA damage and increased mitochondrial function. A, Break frequency/14-kb mtDNA fragment. ATP level (B), MTS-viability (C), and mitochondrial copy number (D) were increased in Tg as compared to both KO or WT cultures after exposure to indicated concentrations of palmitate. The average results ± SE are shown (n ≥ 3). *, P < .05 vs PMC from KO and WT mice treated with the same concentration of palmitate. E, Primary skeletal muscle myotubes were treated with 0.5 mM palmitate for 24 hours. Total cell lysates were isolated and expression of mitochondrial proteins was analyzed by Western blot with the indicated antibodies. The values from densitometry from at least four independent experiments were normalized to the level of actin and expressed as fold difference compared to the corresponding control (2% BSA) conditions (n ≥ 4). *, P < .05 vs other groups.
Effect of hOGG1 expression in mitochondria on mtROS production and JNK activation in primary muscle myotubes
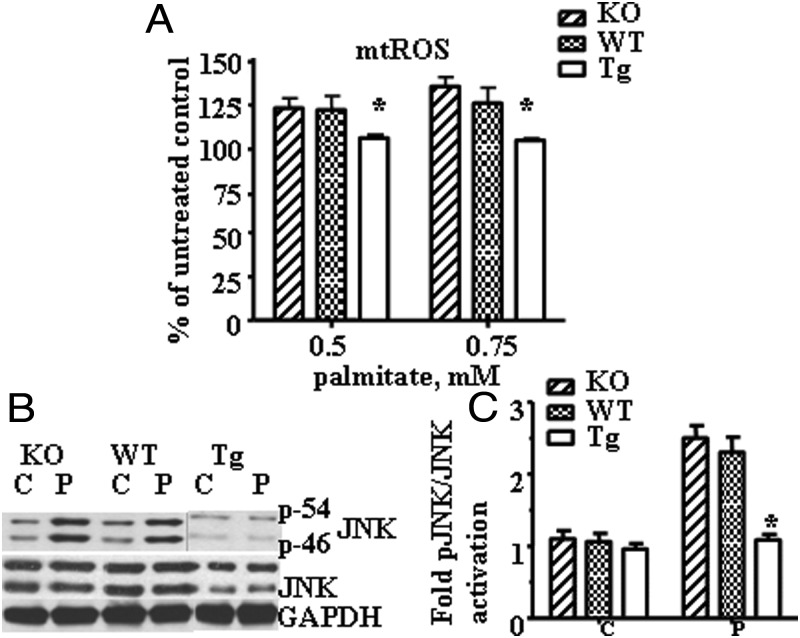
Next, we evaluated whether protection from palmitate-induced mtDNA damage by hOGG1 targeted to mitochondria would have an effect on mtROS generation in PMC after palmitate treatment. KO, WT, and Tg cultures were grown in 24-well culture plates and treated with palmitate (0.5 or 0.75 mM) for 24 hours, and mtROS generation was measured as described in Materials and Methods. As shown in Figure 4A, palmitate treatment significantly increased mitochondrial superoxide generation in both KO and WT PMC, whereas there was no increase in mtROS generation in Tg PMC. In addition, we compared the phosphorylation/activation level of JNK in PMC isolated from all three groups of mice following incubation with 0.5 mM palmitate. As shown in Figure 4, B and C, Tg PMC showed reduced palmitate-induced JNK phosphorylation.
Figure 4.
Overexpression of hOGG1 in mitochondria from PMC isolated from Tg mice prevented palmitate-induced mtROS generation and activation of JNK kinase. A, Mitochondrial superoxide production in primary myotubes isolated from KO, WT, and Tg animals and treated with the indicated concentrations of palmitate for 24 hours. Cells were analyzed in a fluorescent plate reader, and the increase in ROS production was calculated as a percentage increase compared to control. The means ± SE are shown (n ≥ 3). *, P < .05 vs PMC isolated from KO and WT mice treated with the same concentration of palmitate. B, Primary skeletal muscle myotubes were exposed to control medium (C, 2% BSA) or medium containing 0.5 mM palmitate (P). Total cell lysates were isolated and analyzed by Western blot with the indicated antibodies. The figure shows a representative experiment, which has been repeated 3 times. Equal loading was confirmed using Ponceau staining of the membrane. C, The values from densitometry from three (pJNK) independent experiments were normalized to the level of total JNK and expressed as fold of difference normalized to control data (2% BSA) ± SE. *, P < .05 vs all other groups.
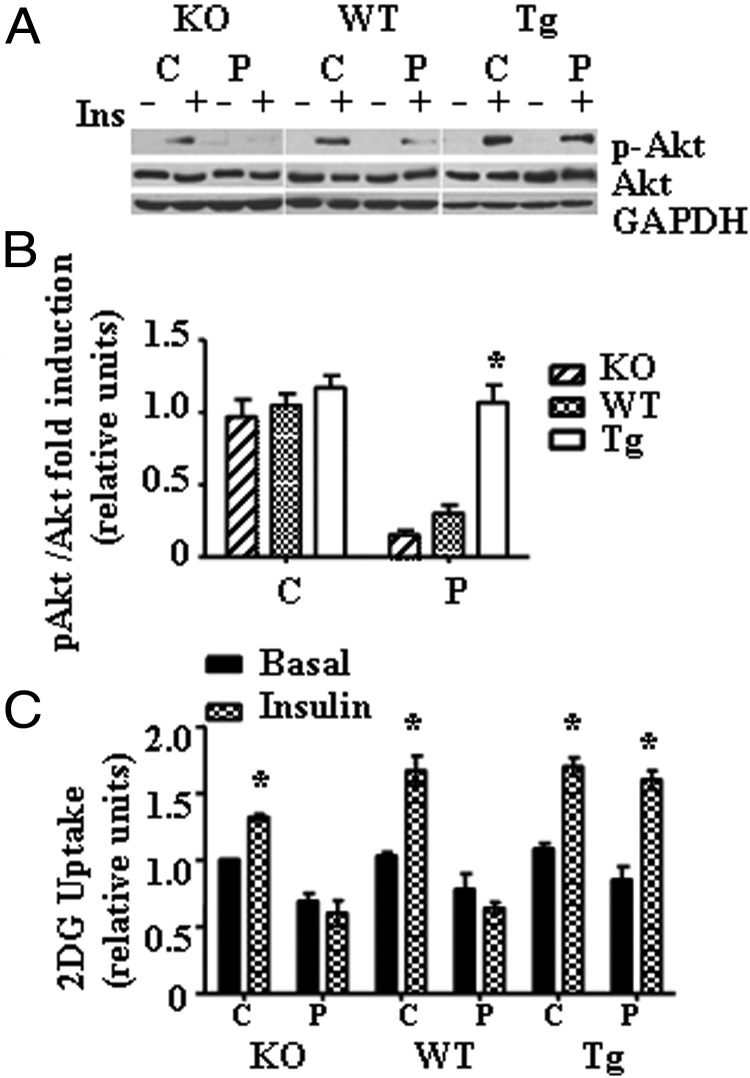
Palmitate-induced inhibition of insulin signaling was prevented and insulin-stimulated glucose uptake was increased in PMC from Tg mice
We have evaluated phosphorylated Akt levels in total protein fractions of PMC isolated from all three groups of mice treated with palmitate. PMC were treated with 0.5 mM palmitate for 16 hours, then serum starved for 2 hours, and incubated in the presence or absence of 100 nM insulin for 15 minutes. Representative blots for phosphorylated Akt (Ser 473) and Akt protein expression levels in total protein fractions are shown in Figure 5A. As shown in Figure 5, A and B, mitochondrial expression of hOGG1 prevented palmitate-induced inhibition of insulin-stimulated phosphorylation of Akt (Ser 473). Next, we evaluated the effect of mitochondrial over/underexpression of hOGG1 on palmitate-induced inhibition of 2DG uptake in PMC. Interestingly, there was a slight decrease in insulin-stimulated 2DG uptake in KO control cultures (treated with 2% BSA) compared to both WT and Tg PMC (Figure 5C). In correlation with decreased mtDNA damage and mtROS production, insulin-stimulated 2DG uptake was significantly greater in Tg PMC compared to both KO and WT cultures after treatment with 0.5 mM palmitate (Figure 5C), thus confirming that Tg PMC were more insulin sensitive following palmitate treatment. Differences in Akt phosphorylation/insulin signaling and insulin sensitivity were not due to changes in Akt or Glut4 expression, as we found no differences in Akt or Glut4 protein expression (Figure 2C).
Figure 5.
Targeting of hOGG1 to mitochondria of PMC from Tg mice prevented against palmitate-mediated inhibition of insulin-signaling and IR. A, Akt (Ser 473) phosphorylation in primary myotubes isolated from KO, WT, and Tg mice. PMC were exposed to control medium (C, 2% BSA) or to medium containing 0.5 mM palmitate (P) for 16 hours and then serum starved and incubated in the presence or absence of 100 nM insulin for 15 minutes as described in Materials and Methods. Total cell lysates were isolated and analyzed by Western blot analysis with the indicated antibodies. B, The values from densitometry from at least three (p-Akt) independent experiments were normalized to the level of total Akt and expressed as fold of difference after addition of insulin normalized to the control (2% BSA) plus insulin data. The mean results ± SE are shown. *, P < .05 vs both KO and WT transduced cells treated with palmitate. C, Primary myotubes cultures were treated with 2% BSA (C, 2% BSA) or 2% BSA plus 0.5 mM palmitate (P) for 16 hours. Next, cells were incubated for 20 minutes in the absence or presence of insulin and then for 5 minutes with 2DG and uptake was measured. Values were normalized to the PMC from KO mice control basal data and are the means ± SE (n ≥ 3). *, P < .05 vs respective basal, unpaired two-tailed Student t test.
Discussion
This study was designed to identify the role of hOGG1 and mtDNA damage in the molecular mechanisms leading to both mitochondrial dysfunction and IR in conditions resembling in vivo. To accomplish this, we used primary cultured skeletal muscle cells, because they have been found to be an excellent model for the study of the effects of hormones, drugs, and other factors on muscle membrane transport systems and metabolism (28–30). The pattern of development of several membrane proteins in primary mouse skeletal muscle cells has been shown to closely resemble that observed in vivo. This is in contrast to various immortalized skeletal muscle cell lines that are deficient in many of these membrane properties. Using an in vitro model of PMC derived from three groups of mice (KO, WT, and Tg), a few novel findings were obtained. Surprisingly, we have not found a major increase in palmitate-induced mtDNA damage and mitochondrial dysfunction and mtROS production in PMC derived from KO animals as compared to WT or Tg cultures. Second, our data indicate that treatment with palmitate increased protein levels of some nuclear-encoded subunits of mitochondrial OXPHOS complexes in PMC isolated from all three groups of mice. Third, we provide new additional evidence that mitochondrial OGG1 is an enzyme that is involved in the regulation of mitochondrial function and thus insulin sensitivity.
Although associated with aging and aging-associated diseases, until recently, the role of DNA repair enzyme, OGG1 in metabolic syndrome, and obesity-associated disorders has been largely underestimated. A few previous reports indicated that type 2 diabetes may be associated with increased oxidative DNA damage and decreased efficacy of DNA repair and a polymorphism in OGG1 (31, 32). Furthermore, more recent studies have shown a positive correlation between oxidative DNA damage and obesity in humans with type 2 diabetes (33). The first in vivo investigation that showed a critical role for DNA repair enzyme in the underlying molecular mechanisms for these diseases was the study of a DNA repair-deficient mouse model, which lacked the oxidative DNA base damage repair enzyme NEIL1 (neil1−/− mouse). These animals exhibit many of the defining hallmarks of metabolic syndrome, including severe obesity, fatty liver disease, dyslipidemia, and IR (34). Regarding OGG1 enzyme, a previous study (35), which was designed to investigate tumor induction in ogg1−/− mice, showed that male ogg1−/− mice were found to have weight gains nearly identical to neil1−/− mice (34). Also, more recent study has shown that ogg1−/− mice have increased adiposity and hepatic steatosis following exposure to a high-fat diet (HFD) (36). Also, OGG1 deficiency altered cellular substrate metabolism, favoring a fat-sparing phenotype, that results in increased susceptibility to obesity and increased insulin resistance in ogg1−/− mice (36). It is important to note that the ogg1−/− animals in our study (20) and the ogg1−/− mice mentioned previously (35, 36) were obtained by two independent groups. In addition, recently we have reported that HFD significantly increased protein content for both mitochondrial and nuclear OGG1 in skeletal muscle, probably indicating a compensatory effect for the increased oxidative stress. Interestingly, although we have found increased mtDNA damage in liver, levels of either nuclear or mitochondrial OGG1 were not significantly influenced by a HFD in liver (37).
Consistent with the previous data obtained for analysis of nuclear fractions (20), our data showed that mitochondrial or nuclear extracts isolated from the skeletal muscle from KO mice do not excise 8-OxoG. There was no effect of genotype on the ability of myoblasts to differentiate in vitro, because PMC developed from KO animals were not morphologically distinct from either WT or Tg cultures and they retain their ability to differentiate into myotubes. Although we have found a slight increase in palmitate-induced mtDNA damage, mtROS production, and activation of JNK in PMC derived from KO animals as compared to WT cultures, to our surprise, this increase was not as great as expected. First, we cannot rule out the compensatory effect of some alternative mtDNA glycosylase/AP lyase involved in the repair of palmitate-induced mtDNA damage. Because ogg1−/− animals are only moderately prone to cancer and exhibited no marked age-associated pathological changes (20, 38), despite 20-fold increase in 8-OxoG accumulation in mtDNA (39), existence of alternative pathways has been discussed previously (40). The second possible explanation is that PMC with substantial mtDNA damage and subsequent increased mtROS production get eliminated through a mechanism that has been described before and suggests that in the absence of OGG1, a surveillance system is activated to remove cells with extreme DNA damage and subsequent ROS production from ogg1−/− cultures (41). Furthermore, analysis of mitochondrial protein expression after palmitate treatment clearly indicates the existence of different responses of the different genotypes in the protein levels of various subunits of mitochondrial complexes. Interestingly, PMC isolated from KO mice showed the distinctive trend for the augmented levels of nDNA encoded subunits after palmitate treatment, probably indicating some compensatory effect for the loss of OGG1 and possible consequent changes in mitochondrial proteins on increased palmitate-induced oxidative stress. On the contrary, after this treatment time, the level of mtDNA encoded subunit remained unchanged in all three groups. In addition, as we already indicated in the Results, Tg mice show virtually no increase in complex 2 expression, and the WT animals show a similar trend for complex 4, subunit 4. Obviously, further studies that will involve more prolonged treatment time and additional antibodies to both nDNA and mtDNA encoded subunits of OXPHOS complexes are required for a better understanding of the influence of mtDNA damage on the protein levels of OXPHOS complexes. Furthermore, we believe that future experiments on OGG1 Tg/KO animals in vivo will shed additional light on the relationships of mtDNA damage, OXPHOS function, and development of IR. On the other hand, we observed a trend toward increased mitochondrial oxidation of pyruvate (both basal and maximally uncoupled) in muscle fibers isolated from Tg animals, even though the difference was not found to be statistically significant by nonparametric Kruskall-Wallis test. Additional experiments are underway to characterize the effect of OGG1 genotype further on mitochondrial respiration and function in muscle fibers. Also, an increase in both mitochondrial respiration in vivo and ATP level in vitro in skeletal muscle isolated from Tg mice compared to WT could be accounted for by an increase in the amount of mitochondria rather than to improvement of mitochondrial function.
Furthermore, as expected, PMC isolated from Tg mice showed a significant decrease in mtDNA damage and mtROS generation after treatment with palmitate. Therefore, these cultures were protected from palmitate-induced mitochondrial dysfunction, activation of JNK, consequent decrease in insulin-stimulated Akt (Ser 473) phosphorylation, and glucose uptake as compared to PMC isolated from both KO and WT skeletal muscle. Impairment of insulin-induced Akt phosphorylation by saturated fatty acids is often accounted for by enhancement of IRS-1 serine/threonine phosphorylation, which triggers its degradation by various ser/threonine kinases, including JNK (42, 43). The results obtained in the current study are in agreement with our previous data obtained in L6 myotubes (5), which showed that targeting of hOGG1 to mitochondria decreased mtROS and thus prevented activation of JNK, leading to improvement of palmitate-mediated inhibition of insulin-induced 1) tyrosine phosphorylation of IRS-1; 2) Akt (Ser 473) phosphorylation, and thus to overall increased insulin-stimulated translocation of GLUT4 to the plasma membrane and the consequent restoration of insulin-stimulated glucose uptake in L6 myotubes, which had been inhibited by palmitate. We believe the same mechanisms are involved in the protective effect of mitochondrial hOGG1 in PMC isolated from Tg mice.
These data disagree with a previous study that showed that overexpression of hOGG1 in mitochondria in vivo increased the abundance of free radicals and major deletions in mtDNA and led to obesity and hepatosteatosis in mice (19). As has previously been discussed (19), we believe that the different phenotypes are caused by using different isoforms of hOGG1, 1-α in our study vs 1-β isoform in the previous study (19). Further studies are required to determine whether similar mechanisms exist in different tissues in vivo.
Collectively, our data have two important implications. First, in the conditions close to in vivo, we identify mitochondrial OGG1 as an enzyme that can improve both mitochondrial function and insulin sensitivity and, therefore, could act as a therapeutic target for treating skeletal muscle IR caused by lipid excess. Second, because the role of mtROS in developing lipid-induced IR in skeletal muscle is still controversial, with some studies favoring this hypothesis (3, 4, 44) and others proving otherwise (45), our data provide additional evidence that saturated fat-induced mtDNA damage and consequent mitochondrial oxidative stress triggers skeletal muscle mitochondrial dysfunction and IR.
Acknowledgments
We are grateful to Dr Jonathan Scammell (Department of Comparative Medicine, University of South Alabama) for assistance in the animals' breeding and Luanne Galanopoulos (Department of Cell Biology and Neuroscience, University of South Alabama) for help in the preparation the manuscript.
This work was supported by National Institutes of Health Grants DK073808 (L.I.R.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AP
- apurinic/apyrimidinic
- 2DG
- 2-deoxy-d-glucose
- FBS
- fetal bovine serum
- HFD
- high-fat diet
- hOGG1
- human 8-oxoguanine (8-OxoG) glycosylase/AP lyase
- IR
- insulin resistance
- IRS-1
- insulin receptor substrate-1
- KO
- knockout
- mtDNA
- mitochondrial DNA
- mtROS
- mitochondrial ROS
- MTS
- mitochondrial targeting sequence
- NAO
- 10-N-nonyl acridine orange
- nDNA
- nuclear DNA
- OGG1
- 8-oxoguanine DNA glycosylase/AP lyase
- OXPHOS
- oxidative phosphorylation
- 8-OxoG
- 8-oxoguanine
- PGC-1α
- peroxisome proliferator activator receptor-γ coactivator 1α
- PMC
- primary muscle cells
- ROS
- reactive oxygen species
- TFAM
- mitochondrial transcription factor
- Tg
- transgenic
- WT
- wild type.
References
- 1. DeFronzo RA. Lilly lecture 1987. The triumvirate: β-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37:667-687 [DOI] [PubMed] [Google Scholar]
- 2. Petersen KF, Dufour S, Savage DB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:12587-12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonnard C, Durand A, Peyrol S, et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest. 2008;118:789-800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson EJ, Lustig ME, Boyle KE, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573-581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yuzefovych LV, Solodushko VA, Wilson GL, Rachek LI. Protection from palmitate-induced mitochondrial DNA damage prevents from mitochondrial oxidative stress, mitochondrial dysfunction, apoptosis, and impaired insulin signaling in rat L6 skeletal muscle cells. Endocrinology. 2012;153:92-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gutteridge JM, Halliwell B. Iron toxicity and oxygen radicals. Baillieres Clin Haematol. 1989;2:195-256 [DOI] [PubMed] [Google Scholar]
- 7. Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996;313 (Pt 1):17-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shibutani S, Takeshita M, Grollman AP. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991;349:431-434 [DOI] [PubMed] [Google Scholar]
- 9. Dobson AW, Xu Y, Kelley MR, LeDoux SP, Wilson GL. Enhanced mitochondrial DNA repair and cellular survival after oxidative stress by targeting the human 8-oxoguanine glycosylase repair enzyme to mitochondria. J Biol Chem. 2000;275:37518-37523 [DOI] [PubMed] [Google Scholar]
- 10. Rachek LI, Grishko VI, Musiyenko SI, Kelley MR, LeDoux SP, Wilson GL. Conditional targeting of the DNA repair enzyme hOGG1 into mitochondria. J Biol Chem. 2002;277:44932-44937 [DOI] [PubMed] [Google Scholar]
- 11. Rachek LI, Grishko VI, Ledoux SP, Wilson GL. Role of nitric oxide-induced mtDNA damage in mitochondrial dysfunction and apoptosis. Free Radic Biol Med. 2006;40:754-762 [DOI] [PubMed] [Google Scholar]
- 12. Rachek LI, Thornley NP, Grishko VI, LeDoux SP, Wilson GL. Protection of INS-1 cells from free fatty acid-induced apoptosis by targeting hOGG1 to mitochondria. Diabetes. 2006;55:1022-1028 [DOI] [PubMed] [Google Scholar]
- 13. Ruchko M, Gorodnya O, LeDoux SP, Alexeyev MF, Al-Mehdi AB, Gillespie MN. Mitochondrial DNA damage triggers mitochondrial dysfunction and apoptosis in oxidant-challenged lung endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L530–L535 [DOI] [PubMed] [Google Scholar]
- 14. Ricci C, Pastukh V, Leonard J, et al. Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis. Am J Physiol Cell Physiol. 2008;294:C413–C422 [DOI] [PubMed] [Google Scholar]
- 15. Ruchko MV, Gorodnya OM, Zuleta A, Pastukh VM, Gillespie MN. The DNA glycosylase Ogg1 defends against oxidant-induced mtDNA damage and apoptosis in pulmonary artery endothelial cells. Free Radic Biol Med. 2011;50:1107-1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Panduri V, Liu G, Surapureddi S, et al. Role of mitochondrial hOGG1 and aconitase in oxidant-induced lung epithelial cell apoptosis. Free Radic Biol Med. 2009;47:750-759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boiteux S, Radicella JP. Base excision repair of 8-hydroxyguanine protects DNA from endogenous oxidative stress. Biochimie. 1999;81:59-67 [DOI] [PubMed] [Google Scholar]
- 18. Zhang H, Mizumachi T, Carcel-Trullols J, et al. Targeting human 8-oxoguanine DNA glycosylase (hOGG1) to mitochondria enhances cisplatin cytotoxicity in hepatoma cells. Carcinogenesis. 2007;28:1629-1637 [DOI] [PubMed] [Google Scholar]
- 19. Zhang H, Xie C, Spencer HJ, et al. Obesity and hepatosteatosis in mice with enhanced oxidative DNA damage processing in mitochondria. Am J Pathol. 2008;178:1715-1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klungland A, Rosewell I, Hollenbach S, et al. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc Natl Acad Sci USA. 1999;96:13300-13305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang W, Esbensen Y, Kunke D, et al. Mitochondrial DNA damage level determines neural stem cell differentiation fate. J Neurosci. 2011;31:9746-9751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu S, Borst DE, Horowits R. Expression and alternative splicing of N-RAP during mouse skeletal muscle development. Cell Motil Cytoskeleton. 2008;65:945-954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sanger JW, Chowrashi P, Shaner NC, et al. Myofibrillogenesis in skeletal muscle cells. Clin Orthop Relat Res. 2002;S153–S62 [DOI] [PubMed] [Google Scholar]
- 24. Yuzefovych L, Wilson G, Rachek L. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. Am J Physiol Endocrinol Metab. 2010;299:E1096–E1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Driggers WJ, Holmquist GP, LeDoux SP, Wilson GL. Mapping frequencies of endogenous oxidative damage and the kinetic response to oxidative stress in a region of rat mtDNA. Nucleic Acids Res. 1997;25:4362-4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rachek LI, Musiyenko SI, LeDoux SP, Wilson GL. Palmitate induced mitochondrial deoxyribonucleic acid damage and apoptosis in l6 rat skeletal muscle cells. Endocrinology. 2007;148:293-299 [DOI] [PubMed] [Google Scholar]
- 27. Kaewsuya P, Danielson ND, Ekhterae D. Fluorescent determination of cardiolipin using 10-N-nonyl acridine orange. Anal Bioanal Chem. 2007;387:2775-2782 [DOI] [PubMed] [Google Scholar]
- 28. Brodie C. Regulation by thyroid hormones of glucose transport in cultured rat myotubes. J Neurochem. 1990;55:186-191 [DOI] [PubMed] [Google Scholar]
- 29. Sampson SR, Brodie C, Alboim SV. Role of protein kinase C in insulin activation of the Na-K pump in cultured skeletal muscle. Am J Physiol. 1994;266:C751–C758 [DOI] [PubMed] [Google Scholar]
- 30. Shefi-Friedman L, Wertheimer E, Shen S, Bak A, Accili D, Sampson SR. Increased IGFR activity and glucose transport in cultured skeletal muscle from insulin receptor null mice. Am J Physiol Endocrinol Metab. 2001;281:E16–E24 [DOI] [PubMed] [Google Scholar]
- 31. Thameem F, Puppala S, Lehman DM, et al. The Ser(326)Cys polymorphism of 8-oxoguanine glycosylase 1 (OGG1) is associated with type 2 diabetes in Mexican Americans. Hum Hered. 2010;70:97-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gonul N, Kadioglu E, Kocabas NA, Ozkaya M, Karakaya AE, Karahalil B. The role of GSTM1, GSTT1, GSTP1, and OGG1 polymorphisms in type 2 diabetes mellitus risk: a case-control study in a Turkish population. Gene. 2012;505:121-127 [DOI] [PubMed] [Google Scholar]
- 33. Al-Aubaidy HA, Jelinek HF. Oxidative DNA damage and obesity in type 2 diabetes mellitus. Eur J Endocrinol. 2011;164:899-904 [DOI] [PubMed] [Google Scholar]
- 34. Vartanian V, Lowell B, Minko IG, et al. The metabolic syndrome resulting from a knockout of the NEIL1 DNA glycosylase. Proc Natl Acad Sci USA. 2006;103:1864-1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arai T, Kelly VP, Minowa O, Noda T, Nishimura S. The study using wild-type and Ogg1 knockout mice exposed to potassium bromate shows no tumor induction despite an extensive accumulation of 8-hydroxyguanine in kidney DNA. Toxicology. 2006;221:179-186 [DOI] [PubMed] [Google Scholar]
- 36. Sampath H, Vartanian V, Rollins MR, Sakumi K, Nakabeppu Y, Lloyd RS. 8-Oxoguanine DNA glycosylase (OGG1) deficiency increases susceptibility to obesity and metabolic dysfunction. PLoS One. 2012;7:e51697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yuzefovych LV, Musiyenko SI, Wilson GL, Rachek LI. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One. 2013;8:e54059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sakumi K, Tominaga Y, Furuichi M, et al. Ogg1 knockout-associated lung tumorigenesis and its suppression by Mth1 gene disruption. Cancer Res. 2003;63:902-905 [PubMed] [Google Scholar]
- 39. de Souza-Pinto NC, Eide L, Hogue BA, et al. Repair of 8-oxodeoxyguanosine lesions in mitochondrial dna depends on the oxoguanine DNA glycosylase (OGG1) gene and 8-oxoguanine accumulates in the mitochondrial DNA of OGG1-defective mice. Cancer Res. 2001;61:5378-5381 [PubMed] [Google Scholar]
- 40. Stuart JA, Bourque BM, de Souza-Pinto NC, Bohr VA. No evidence of mitochondrial respiratory dysfunction in OGG1-null mice deficient in removal of 8-oxodeoxyguanine from mitochondrial DNA. Free Radic Biol Med. 2005;38:737-745 [DOI] [PubMed] [Google Scholar]
- 41. Bacsi A, Chodaczek G, Hazra TK, Konkel D, Boldogh I. Increased ROS generation in subsets of OGG1 knockout fibroblast cells. Mech Ageing Dev. 2007;128:637-649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Han MS, Lim YM, Quan W, et al. Lysophosphatidylcholine as an effector of fatty acid-induced insulin resistance. J Lipid Res. 2011;52:1234-1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gao Z, Zhang X, Zuberi A, et al. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3–L1 adipocytes. Mol Endocrinol. 2004;18:2024-2034 [DOI] [PubMed] [Google Scholar]
- 44. Hoehn KL, Salmon AB, Hohnen-Behrens C, et al. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci USA. 2009;106:17787-17792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Paglialunga S, van Bree B, Bosma M, et al. Targeting of mitochondrial reactive oxygen species production does not avert lipid-induced insulin resistance in muscle tissue from mice. Diabetologia. 2012;55:2759-2768 [DOI] [PubMed] [Google Scholar]