Abstract
Vagal afferent nerve fibers transmit gastrointestinal satiation signals to the brain via synapses in the nucleus of the solitary tract (NTS). Despite their pivotal role in energy homeostasis, little is known about the cellular mechanisms enabling fleeting synaptic events at vagal sensory endings to sustain behavioral changes lasting minutes to hours. Previous reports suggest that the reduction of food intake by the satiation peptide, cholecystokinin (CCK), requires activation of N-methyl-D-aspartate-type glutamate receptors (NMDAR) in the NTS, with subsequent phosphorylation of ERK1/2 (pERK1/2) in NTS vagal afferent terminals. The synaptic vesicle protein synapsin I is phosphorylated by pERK1/2 at serines 62 and 67. This pERK1/2-catalyzed phosphorylation increases synaptic strength by increasing the readily releasable pool of the neurotransmitter. Conversely, dephosphorylation of serines 62 and 67 by calcineurin reduces the size of the readily releasable transmitter pool. Hence, the balance of synapsin I phosphorylation and dephosphorylation can modulate synaptic strength. We postulated that CCK-evoked activation of vagal afferent NMDARs results in pERK1/2-catalyzed phosphorylation of synapsin I in vagal afferent terminals, leading to the suppression of food intake. We found that CCK injection increased the phosphorylation of synapsin I in the NTS and that this increase is abolished after surgical or chemical ablation of vagal afferent fibers. Furthermore, fourth ventricle injection of an NMDAR antagonist or the mitogen-activated ERK kinase inhibitor blocked CCK-induced synapsin I phosphorylation, indicating that synapsin phosphorylation in vagal afferent terminals depends on NMDAR activation and ERK1/2 phosphorylation. Finally, hindbrain inhibition of calcineurin enhanced and prolonged synapsin I phosphorylation and potentiated reduction of food intake by CCK. Our findings are consistent with a mechanism in which NMDAR-dependent phosphorylation of ERK1/2 modulates satiation signals via synapsin I phosphorylation in vagal afferent endings.
Gastrointestinal signals that inform the brain of the quantity and quality of food being consumed during ongoing meals are important controllers of food intake. These signals contribute to the process of satiation, which results in meal termination. The nucleus of the solitary tract (NTS) in the caudal brain stem is the site at which the vagal sensory nerve fibers that transmit gastrointestinal (GI) satiation signals make their central synapses (1–3). In addition to receiving vagal afferent inputs, the NTS receives afferent inputs from the hypothalamus and other areas that potentially modulate incoming GI signals according to metabolic and endocrine conditions (4, 5). Hence, the NTS is in a pivotal position to contribute to energy homeostasis. Little is known, however, about the cellular mechanisms that enable fleeting synaptic events in the NTS to be translated into behaviors over minutes to hours.
Glutamate is the principal excitatory neurotransmitter released by vagal afferents (6), and antagonism of hindbrain N-methyl-D-aspartate-type glutamate receptors (NMDARs) delays satiation and increases food intake by increasing meal size (7, 8). Although NMDARs are expressed by both intrinsic NTS neurons (9) and vagal afferent terminals in the NTS (10), previously published results support a role for vagal afferent NMDARs in the control of food intake. For example, subnodose vagotomy, which leaves central vagal terminals intact, does not attenuate the feeding effects of NTS NMDAR antagonist injection (11). However, after degeneration of central vagal afferent terminals, NTS injection of an NMDAR antagonist no longer increases food intake (11). Although these findings do not eliminate the participation of NMDARs on intrinsic NTS neurons, they do support an important role for NMDARs on vagal afferent endings in the control of food intake.
We previously reported that the phosphorylation of ERK 1/2 (pERK1/2) in vagal afferent endings is necessary for the reduction of food intake by the archetypical satiation peptide, cholecystokinin (CCK) and that hindbrain administration of an NMDAR antagonist prevents both CCK-induced increase of pERK1/2 in vagal afferent terminals and reduction of food intake (12). The mechanism by which NMDAR-mediated ERK1/2 phosphorylation in vagal afferent terminals contributes to CCK-induced reduction of food intake is not known. However, it is well documented that pERK1/2 phosphorylates the synaptic vesicle-associated protein, synapsin I (13, 14), and that pERK1/2-catalyzed synapsin I phosphorylation increases the pool of transmitter readily available for release (15–17). We hypothesized that CCK-evoked activation of vagal afferents results in NMDAR-dependent, pERK1/2-catalyzed phosphorylation of synapsin I (pSyn) in vagal afferent endings in the NTS. Synapsin I phosphorylation by pERK1/2 would be expected to enhance transmitter release and thereby contribute to the ability of CCK-induced vagal afferent activation to reduce food intake. Here we report that CCK triggers an increase in synapsin I phosphorylation in NTS vagal afferent endings. Increased synapsin I phosphorylation is mediated by type 1 CCK receptor (CCK1R) activation and requires NMDAR activation and the phosphorylation of ERK1/2. In addition, hindbrain inhibition of synapsin I dephosphorylation potentiates the reduction of food intake by CCK. Our findings are consistent with a mechanism in which NMDAR-dependent phosphorylation of ERK1/2 modulates satiation signals via synapsin I phosphorylation in vagal afferent endings.
Materials and Methods
Animals and housing
Male adult Sprague Dawley rats (280–300 g; Simonsen Laboratories, Gilroy, California) were individually housed in suspended wire mesh cages in a vivarium with 12-hour light, 12-hour dark cycle and controlled temperature and humidity. Rats had ad libitum access to water and pelleted rodent diet (Teklad, Kent, Washington) except during overnight fasts and food intake experiments, as described below. All animal housing and experiments reported here were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals under a protocol approved by the Washington State University Institutional Animal Care and Use Committee.
Drugs and antibodies
D-3-(2-carboxypiperazine-4-yl)-1-propenyl-1-phosphonic acid (D-CPP-ene), mitogen-activated ERK kinase (MEK) inhibitor U0126, and cyclosporin A (CsA) were purchased from Tocris (Ellisville, Missouri). Sulfated cholecystokinin octapeptide (CCK-8) was from Peptides International (Louisville, Kentucky). Cell Signaling Technology (Beverly, Massachusetts) supplied the primary antibody for pERK1/2 (phospho-p44/42 MAPK Thr202/Tyr 204, lot 38). The antibody for pSyn (phospho-synapsin I Ser62/67, lot cvp409y) was obtained from Acris (San Diego, California). Isolectin B4 (IB4) was purchased from Vector Laboratories (Burlingame, California). Capsaicin and devazepide were obtained from Sigma-Aldrich Inc (St Louis, Missouri).
Fourth ventricular cannulations
Rats were anesthetized with a ketamine (50 mg/kg), xylazine (25 mg/kg), and acepromazine (2 mg/kg) mixture, placed in a stereotaxic instrument, and implanted with 26-gauge stainless steel guide cannulas (McMaster-Carr, Santa Fe Springs, California) aimed for the fourth ventricle (2.0 mm anterior to occipital suture, on midline, 6.6 mm ventral from dura). Cannulas were cemented to the skull using stainless steel screws and methacrylate (Orthojet, Spokane, Washington). Rats were allowed 2 weeks postsurgical recovery.
Synapsin I phosphorylation time course
Twenty-four rats were handled for a week and habituated to the ip injection with mock saline injections. On the test day, overnight-fasted (16 hours) rats were injected with sterile saline or 2 μg/kg CCK dissolved in saline (1 mL/kg, ip). Separate groups of rats were removed from the colony room and were rapidly anesthetized with isoflurane anesthetic (Vedco, St Joseph, Missouri) 2.5, 5, 10, 15, and 30 minutes after injection and then perfused intracardially with 0.1 M phosphate buffer NaCl followed by 4% paraformaldehyde (Electron Microscopy, Hatfield, Pennsylvania) in 0.1 M phosphate buffer (pH 7.4). Immediately after perfusion, brains were collected, postfixed in the same fixative for 2 hours, and cryoprotected in 0.1 M phosphate buffer containing 25% sucrose overnight at 4°C. Thirty-micrometer coronal cryostat hindbrain sections were collected for immunohistochemical detection of pSyn. Hindbrain sections were incubated for 36 hours at room temperature in rabbit anti-pSyn (1:400) antiserum. Then sections were washed and incubated in Alexa 555-conjugated goat antirabbit (1:600; Invitrogen, Carlsbad, California) for 3 hours. The stained sections were then mounted on slides and coverslipped with ProLong Gold (Invitrogen) prior to microscope examination.
Phosphorylated synapsin I immunoreactivity was quantified by measuring the mean fluorescence intensity sampled from the medial NTS and area postrema (AP) in 1 section at each of 4 different brain levels, corresponding to 14.1, 13.8, 13.6, and 13.3 mm caudal to bregma, according to the stereotaxic atlas of Paxinos and Watson (18). Mean fluorescence intensity was sampled on both sides of the hindbrain in each section containing a given region of interest for each rat. These values were normalized to the background intensity, which was sampled from the noncellular area of the hypoglossal nucleus. The data are presented as the average fluorescence intensity for each brain across the 4 rostrocaudal levels listed above. The fluorescent intensity was calculated by applying the following formula: pSyn fluorescent intensity = (100 × pSyn intensity / background intensity) − 100.
Immunohistochemical detection of phosphoproteins and double-labeling procedures
Overnight-fasted rats were given an ip injection of saline (n = 3) or 2 μg/kg CCK (n = 4). Fifteen minutes after ip injection, rats were rapidly anesthetized with isoflurane and perfused. Hindbrain tissue was then collected and prepared for immunostaining. For pERK1/2 and pSyn double-labeling, hindbrain sections were incubated for 36 hours at room temperature in mouse anti-pERK1/2 (1:400) and rabbit anti-pSyn (1:400) antisera. Then sections were washed and incubated in Alexa 488-conjugated goat antimouse (1:600; Invitrogen) and Alexa 555-conjugated goat antirabbit (1:600) secondaries for 3 hours. For pSyn and IB4 double labeling, hindbrain sections were incubated in biotintylated IB4 (1:400) and rabbit anti-pSyn (1:400) antisera with subsequent incubation in Alexa 555-conjugated goat antirabbit (1:600) and Alexa 488-conjugated streptavidin (1:600; Invitrogen).
Effect of MEK inhibitor on CCK-induced synapsin I phosphorylation
A group of overnight-fasted rats (total n = 15) were separated into the following treatment groups: vehicle/NaCl (n = 4), vehicle/CCK (n = 4), u0126/CCK (n = 4), and U0126/NaCl (n = 3). Fourth ventricle injections of vehicle [50% dimethylsulfoxide (DMSO) dissolved in saline] or U0126 (4 μg dissolved in vehicle solution) in volumes of 3 μL were injected over a 2-minute period. Forty-five minutes after the fourth ventricle injections, rats received an ip injection of either CCK (2 μg/kg) or saline. Fifteen minutes after the ip injection, animals were anesthetized, perfused intracardially, and prepared for immunohistochemical detection of pSyn, as described above. Hindbrain pSyn immunofluorescent intensity was quantified as previously described.
Unilateral nodose ganglion removal
The left cervical vagus nerve was exposed and visualized. Once exposed, the vagus nerve was cut at the caudal end of the nodose, thereby allowing lifting of the nodose stump to retract, visualize, and cut the vagus rostral to the nodose and remove the entire ganglion. After a 2-week recovery time, rats were fasted overnight and received an ip injection of saline (n = 4) or 2 μg/kg CCK (n = 4). Fifteen minutes after the ip injection, the rats were quickly anesthetized, perfused, and brains harvested for immunohistochemical detection of pSyn. Quantification of pSyn immunofluorescence intensity was done as previously described, except the fluorescence intensity sampled from each side of the hindbrain are reported as separate values to compare the amount of pSyn immunoreactivity contralateral or ipsilateral to the side of the nodose ganglion removal.
Capsaicin treatment
Systemic treatment with capsaicin was used to destroy small unmyelinated primary afferent neurons, including those in the vagi, as previously described (19). A total capsaicin dose (125 mg/kg) was administered ip as a series of 3 injections (25, 50, and 50 mg/kg) at an injection volume of 1 mL/kg (n = 8). All 3 injections were made within a 24-hour period (0, 6, and 24 hours, respectively). An additional group of rats were injected with the vehicle solution (n = 8; 10% ethanol and 10% Tween-80 in 0.9% NaCl) at the same volumes and schedule as above. The eye wipe response to mild corneal stimulation, a response mediated by the capsaicin-sensitive trigeminal innervation of the cornea, was tested to screen the effectiveness of capsaicin treatment. The test involved the application of a drop of 1% NH4OH to 1 eye. All of our vehicle-treated rats immediately wiped the stimulated eye. None of the capsaicin-treated rats exhibited any eye wipe response to the test, confirming that our capsaicin treatment successfully destroyed afferent C fibers.
After a 2-week recovery period, capsaicin- and vehicle-treated groups were fasted overnight and injected ip with CCK (2 μg/kg) or 0.9% NaCl to achieve the following treatment conditions: vehicle/NaCl (n = 4), vehicle/CCK (n = 4), capsaicin/NaCl (n = 4), and capsaicin/CCK (n = 4). Fifteen minutes after the ip injection, animals were anesthetized and perfused, and brain tissue was prepared for immunohistochemical detection and quantification of pSyn.
Effect of CCK1R antagonist on CCK-induced synapsin I phosphorylation
Overnight-fasted rats (total n = 16) were divided into the following treatment conditions: vehicle/NaCl (n = 4), vehicle/CCK (n = 4), devazepide/NaCl (n = 4), and devazepide/CCK (n = 4). Devazepide (300 μg/kg) or vehicle (50% DMSO in saline) was administered ip 10 minutes prior to the ip injection of CCK (2 μg/kg) or saline. Fifteen minutes after the final ip injection, animals were anesthetized, perfused, and prepared for immunohistochemical detection of pSyn. Hindbrain pSyn fluorescent intensity was quantified as previously described.
Effect of hindbrain injection of NMDAR antagonist on CCK-induced synapsin I phosphorylation
A group of rats (total n = 20) was divided into subgroups of 4 rats each. After an overnight fast, each subgroup received 1 of the following treatments: NaCl/NaCl (n = 3), NaCl/CCK (n = 6), D-CPP-ene/CCK (n = 6), and D-CPP-ene/NaCl (n = 5). Fourth ventricle injections of saline or D-CPP-ene (40 ng dissolved in saline) in volumes of 3 μL over a 2-minute period. Fourth ventricle injections were followed immediately by an ip injection of either CCK (2 μg/kg) or saline. Fifteen minutes after the ip injection, animals were rapidly anesthetized with isofluorane, perfused, and prepared for immunohistochemical detection of pSyn. Hindbrain pSyn immunofluorescent intensity was quantified as described above.
Effect of calcineurin inhibitor on CCK-induced suppression of food intake and synapsin I phosphorylation
Twenty rats were cannulated and adapted as above. Following an overnight fast, rats were separated into the following treatment groups: vehicle/NaCl (n = 4), CsA/NaCl (n = 4), vehicle/CCK 15 minutes (n = 4), CsA/CCK 15 minutes (n = 4), and CsA/CCK 30 minutes (n = 4). Fourth ventricle injections of vehicle (DMSO) or CsA (12 μg dissolved in vehicle solution) were made in volumes of 3 μL. Forty-five minutes after fourth ventricle injections, rats received an ip injection of either CCK (2 μg/kg) or saline. All treatment groups were killed 15 minutes after the ip injection, with the exception of a second CsA/CCK group that was killed 30 minutes after the ip injection to determine whether CsA treatment prolonged CCK-induced synapsin I phosphorylation. Tissue was harvested and prepared for immunohistochemical detection of pSyn and pERK1/2. NTS pSyn immunofluorescent intensity was quantified as previously described, and counts of pERK1/2-labeled cell bodies were made according to our previously described protocol (12).
A separate group (n = 16) of cannulated animals was used to examine the effect of CsA on CCK-evoked suppression of food intake. Rats were adapted to computerized meal-monitoring boxes. The experimental procedure involved the removal of food, but not water, 1 hour prior to lights out the day prior to the feeding measures. Food was returned between 9:00 and 10:00 am the next day. On experimental days, after the overnight fast, each rat received a 3-μL fourth ventricle injection of either vehicle or CsA (12 μg). Forty-five minutes after the fourth ventricle injection and immediately prior to return of food, each animal received an ip injection of either CCK (2 μg/kg) or saline. Food intake was monitored remotely. Meals were defined as periods of food intake with 0.3 g or more of food eaten and not interrupted by more than 10 minutes. Postmeal intervals were defined as the interval between the end of a meal and the start of the subsequent meal. Each rat received all 4 combinations of injections, which were carried out in a counterbalanced order with at least 78 hours between injections. Meal size and postmeal intervals were calculated for each animal under each condition and data analyzed using repeated-measures ANOVA.
Data and statistical analyses
Appropriate 1- and 2-way ANOVAs were used to analyze data, followed by a Holm-Sidac post hoc analysis. For analysis of behavioral data, the repeated factor was treatment condition. The confidence limit for statistical significance was set at P < .05. However, wherever actual confidence limits were substantially less than .05, those P values are provided. Results are presented as means ± SEM.
Results
CCK stimulates synapsin I phosphorylation in the hindbrain
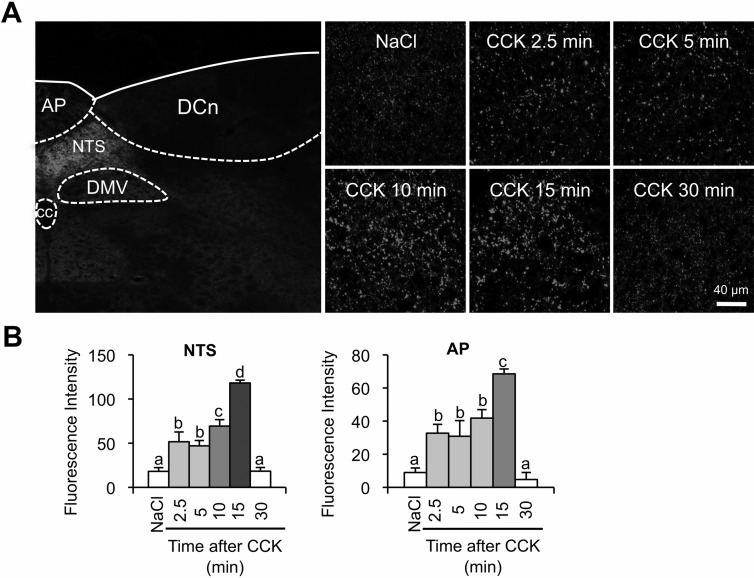
Immunohistochemistry of hindbrain sections using an antibody specific for synapsin I phosphorylated at the pERK1/2-specific sites, Ser 62 and Ser 67, revealed a significant increase in phosphorylated synapsin I after systemic administration of CCK. The increase in synapsin I phosphorylation after the CCK injection was limited to the dorsal vagal complex (Figure 1A), which is comprised of the NTS, dorsal vagal motor nucleus, and the AP, all of which receive direct innervation by vagal afferent terminals. We observed no increase of synapsin I phosphorylation in the surrounding hindbrain structures. Compared with saline control injection, CCK increased pSyn immunoreactivity in the AP and NTS as early as 2.5 minutes after injection (Figure 1B). Synapsin I phosphorylation peaked at 15 minutes after CCK injection, and by 30 minutes after CCK, there was no difference in pSyn immunoreactivity compared with saline control (Figure 1B).
Figure 1.
Time course for the development of CCK-induced phosphorylated synapsin I (pSer62/67) (pSyn) immunofluorescence in the rat hindbrain. A, The image on the left demonstrates the specificity of pSyn immunoreactivity to dorsal vagal structures in the dorsomedial hindbrain after CCK (2 μg/kg) injection. The images on the right are high-magnification images (×40) illustrating the time course of increased pSyn in the NTS after CCK (2 μg/kg) administration. B, CCK-induced increase in pSyn expressed as the average fluorescence intensity of pSyn immunoreactivity sampled from the medial NTS and AP across 4 rostrocaudal levels of the hindbrain and normalized to background intensity. Data are means ± SEM. Means sharing a common letter do not differ significantly, cc, central canal; DMV, dorsal motor nucleus of the vagus; DCn, dorsal column nuclei.
CCK-triggered synapsin I phosphorylation in the NTS requires pERK1/2
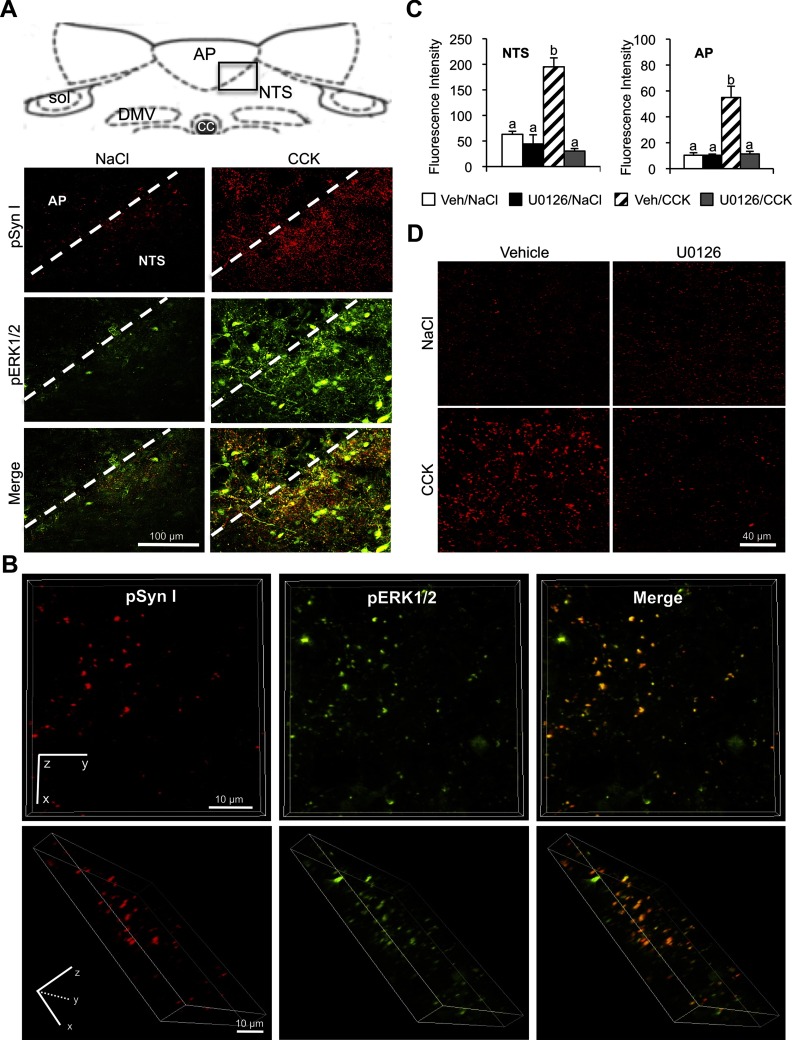
Synapsin I is selectively phosphorylated at serines 62 and 67 by pERK1/2 (13). The time course for CCK-induced increase of pSyn in the NTS and AP is congruent with previously published time course for CCK-induced pERK1/2 in these structures (12, 20). Images collected at ×40 magnification revealed an overlap of pERK1/2 and pSyn immunoreactivities in the NTS and AP after CCK injection (Figure 2A), suggesting that pERK1/2 and pSyn are colocalized. Optical sections collected at ×100 magnification definitively demonstrated colocalization of pERK1/2 and pSyn immunoreactivity in varicosity-like structures within the NTS (Figure 2B).
Figure 2.
Relation of CCK-induced synapsin I phosphorylation to ERK1/2 phosphorylation. A, Dual-label immunofluorescence images (×40 magnification) illustrating anatomical overlap between pERK1/2 and pSyn immunoreactivity in the neuropil of the AP and NTS after CCK (2 μg/kg) treatment (right column) but not after control saline injection (left column). The boxed region in the diagram of the dorsal hindbrain indicates the area included in the fluorescent images below. DMV, dorsal motor nucleus of the vagus; NTS, nucleus of the solitary tract; sol, solitary tract. B, Three-dimensional images rendered from high-magnification (×100) optical sections (24 sections 0.5 μm apart for a total thickness of 12 μm) showing colocalization of pERK1/2 and pSyn in the NTS. The top row is a view from the z-plane and the bottom row is a rotated view of the same rendering. C, Average fluorescence intensity of pSyn in NTS and AP of rats treated with fourth ventricle u0126 (4 μg) or vehicle prior to an ip injection of CCK (2 μg/kg) or saline. Data are means ± SEM. Means sharing a common letter are not differ significantly. D, Representative images of dorsal hindbrain sections stained to reveal pSyn immunoreactivity in NTS of rats treated with a fourth ventricle injection of the MEK inhibitor, u0126 (4 μg) or vehicle prior to an ip injection of CCK (2 μg/kg) or saline. Column labels indicate fourth ventricle treatment and row labels indicate the ip treatment condition.
Phosphorylation of ERK1/2 is mediated by upstream activation of MEK. To confirm that CCK-induced synapsin I phosphorylation in the NTS and AP is pERK1/2-dependent, we injected a MEK inhibitor (U0126) into the fourth ventricle prior to ip CCK injection. Pretreatment with U0126 significantly decreased CCK-induced pSyn in the NTS (P < .01) and AP (P < .01) compared with fourth ventricle vehicle injection (Figure 2, C and D).
CCK-induced synapsin I phosphorylation in vagal afferent endings is abolished by lesion of vagal C-type afferent fibers
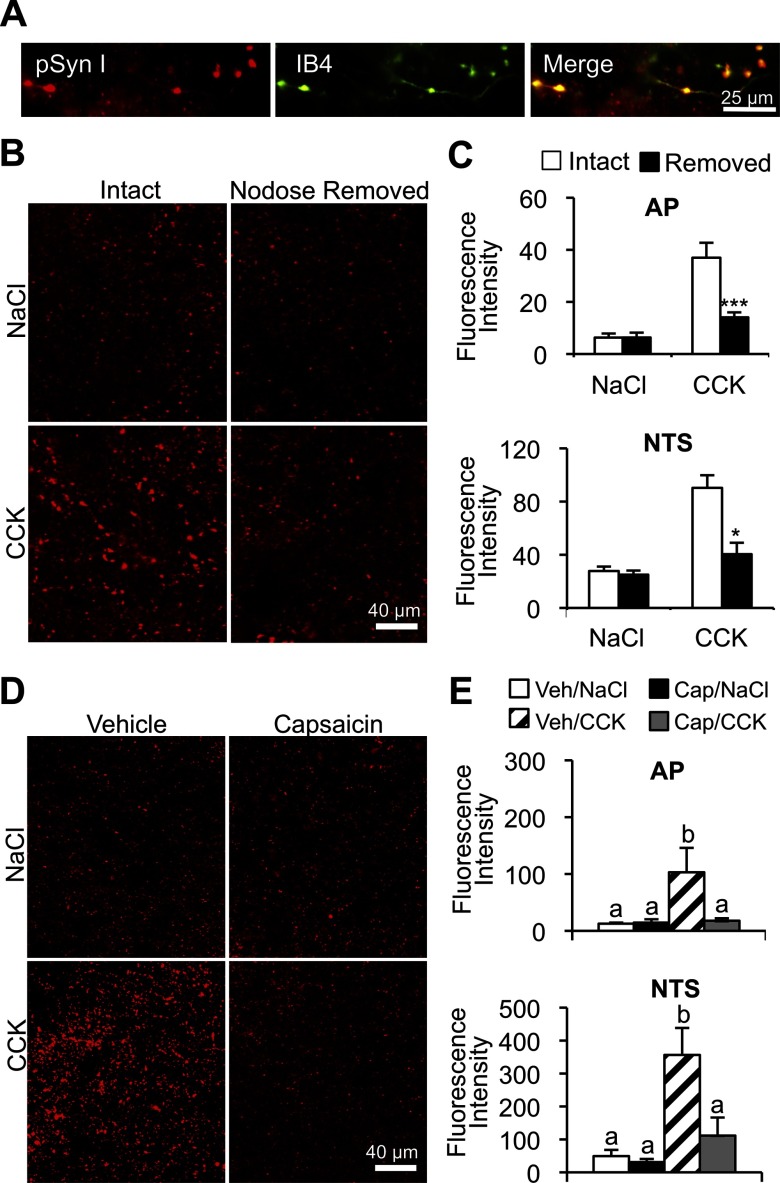
We previously reported that CCK-induced ERK1/2 phosphorylation is localized in vagal afferent endings in the NTS and AP (12). To determine whether CCK-induced synapsin I phosphorylation is localized in vagal afferent endings, 60% of which are unmyelinated C type (21), hindbrain tissue was stained for pSyn and IB4 binding. IB4 does not bind to intrinsic NTS neurons or fibers but has a high affinity for unmyelinated C-type vagal afferent endings in the NTS (22, 23). Using z-stacks of high-magnification (×100) optical sections, pSyn was definitively colocalized with IB4-bound vagal afferent fibers and terminals in the NTS (Figure 3A). We also observed pSyn and IB4 colocalization in the AP, which also receives direct vagal afferent innervation (24, 25). To further establish that CCK triggers synapsin I phosphorylation in vagal afferent endings, the nodose ganglion was unilaterally removed. Removal of the nodose ganglion results in the degeneration of vagal afferent fibers and terminals in the hindbrain (11). We found that CCK-induced synapsin I phosphorylation was reduced in the NTS (P < .05) and AP (P < .001) ipsilateral to nodosectomy compared with the contralateral side, innervated by an intact nodose ganglion (Figure 3, B and C).
Figure 3.
Localization of CCK-induced pSyn in vagal afferent C-type endings. A, Magnification image (×100) of multilabel fluorescence hindbrain images stained to reveal pSyn (red) and IB4 binding (green). Far right image depicts overlap of the 2 labels. B and C, Representative images of CCK-induced pSyn immunoreactivity in NTS after the unilateral nodose removal. Column labels indicate NTS ipsilateral (nodose removed) or contralateral (intact) to nodose ganglion removal. Row labels indicate treatment conditions ip CCK or NaCl. Data are means ± SEM. *, P < .05; ***, P < .001. D, Representative images depicting CCK-induced pSyn immunoreactivity in the NTS of rats pretreated with capsaicin or vehicle. Row labels and column labels indicate experimental conditions (2 μg/kg CCK or saline administered ip). E, Average fluorescence intensity of pSyn in the NTS and AP of capsaicin- and vehicle-treated rats 15 minutes after ip injection of CCK or NaCl. Data are means ± SEM. Means sharing a common letter do not differ significantly.
Although CCK is known to activate both A- and C-type vagal afferent neurons (26, 27), selective destruction of vagal C fibers with capsaicin abolishes reduction of food intake by CCK (19). Furthermore, capsaicin lesion of vagal C fibers alone is sufficient to abolish CCK-induced pERK1/2 in NTS vagal afferent endings (12). Similarly, we found that capsaicin treatment abolished CCK-induced increases of pSyn immunoreactivity in the AP (P < .05) and NTS (P < .05) (Figure 3, D and E).
Blockade of CCK1Rs prevents CCK-induced synapsin I phosphorylation in hindbrain vagal afferent endings
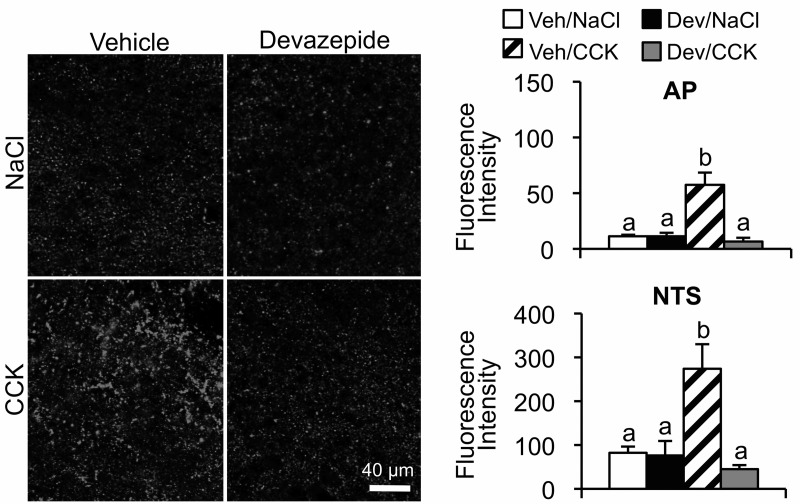
Previous reports indicate that reduction of food intake by exogenous or endogenously released CCK is mediated by GI vagal afferents (2, 28–30). Electrophysiological experiments indicated that vagal afferent CCK1R sensitivity is localized primarily in afferents that innervate the stomach and upper small intestine (21). Therefore, we examined CCK-induced synapsin I phosphorylation in the hindbrain after an ip injection of a CCK1R antagonist. We found that CCK-induced increase in pSyn immunoreactivity in the AP and NTS after ip CCK was reduced (P < .01) by the ip injection of devazepide, a CCK1R antagonist (Figure 4).
Figure 4.
Attenuation of CCK-induced NTS and AP pSyn immunoreactivity by CCK1R antagonist. Left panel shows representative fluorescence images of NTS from rats that received ip CCK (2 μg/kg) or NaCl 10 minutes after ip injection of the CCK1R antagonist, devazepide (300 μg/kg) or vehicle. Right panel is the quantification of pSyn immunoreactivity in AP and NTS expressed as the average fluorescence intensity. Data are means ± SEM. Means sharing a common letter do not differ significantly.
CCK-induced synapsin I phosphorylation requires activation of hindbrain NMDARs
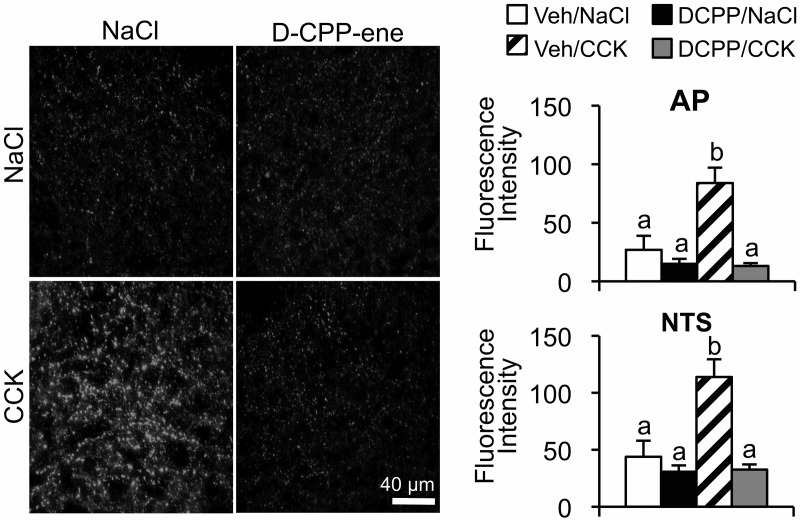
CCK-induced ERK1/2 phosphorylation in vagal afferent endings requires activation of hindbrain NMDARs (12). Because NMDAR activation is upstream of ERK1/2 phosphorylation, we hypothesized that NMDAR activation is necessary for CCK-induced, pERK1/2-catalyzed synapsin I phosphorylation. To determine whether hindbrain NMDAR activation is necessary for CCK-induced synapsin I phosphorylation, a competitive NMDAR antagonist (d-CPP-ene) was administered via the fourth ventricle prior to an ip injection of CCK (Figure 5). We found that pretreatment with d-CPP-ene significantly reduced CCK-induced pSyn immunoreactivity in the AP and NTS (P < .01).
Figure 5.
Attenuation of CCK-induced NTS and AP pSyn immunoreactivity after fourth ventricle injection of the NMDAR antagonist d-CPP-ene. Left panel shows representative fluorescence images of NTS from the rats that received ip CCK (2 μg/kg) or NaCl immediately after fourth ventricle injection of d-CPP-ene (40 ng) or vehicle. Right panel shows pSyn immunoreactivity in the AP and NTS expressed as the average fluorescence intensity. Data are means ± SEM. Means sharing a common letter do not differ significantly.
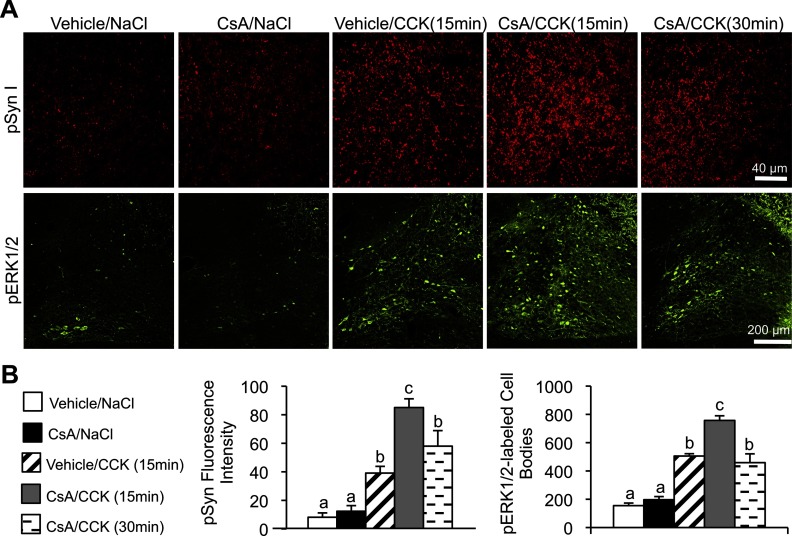
Hindbrain injection of calcineurin inhibitor enhances CCK-induced synapsin I phosphorylation and increases the potency of CCK to suppress food intake
Phosphorylated synapsin I at serines 62 and 67 is dephosphorylated by calcineurin (13, 31). To examine the effect of a calcineurin inhibitor on CCK-induced synapsin I phosphorylation, rats received a fourth ventricle injection of calcineurin inhibitor CsA prior to the ip CCK injection (Figure 6). We found that CsA treatment significantly increased (P < .01) synapsin I phosphorylation 15 minutes after the CCK injection compared with vehicle treatment. Whereas our previous time course revealed that CCK-induced synapsin I phosphorylation decreased to control levels by 30 minutes after the CCK injection, we found that synapsin I phosphorylation remained significantly (P < .01) elevated 30 minutes after CCK in CsA-treated rats. CsA treatment also significantly (P < .05) increased the number of pERK1/2-labeled cell bodies in the NTS 15 minutes after the CCK injection compared with vehicle treatment, and the CCK-induced increase in ERK1/2 phosphorylation remained elevated 30 minutes after CCK in CsA-treated rats (P < .05).
Figure 6.
Enhanced CCK-induced pSyn in the NTS after a fourth ventricle injection of CsA. A, Representatives images of hindbrain sections from rats injected ip with CCK (2 μg/kg) or saline depicting pSyn (red) and pERK1/2 (green) immunofluorescence 1 hour after fourth ventricle injection of CsA (12 μg) or vehicle. Rats were perfused either 15 or 30 minutes after the CCK injection. B, pSyn fluorescence intensity and number of pERK1/2-immunoreactive NTS neurons. Data are means ± SEM. Means sharing a common letter do not differ significantly.
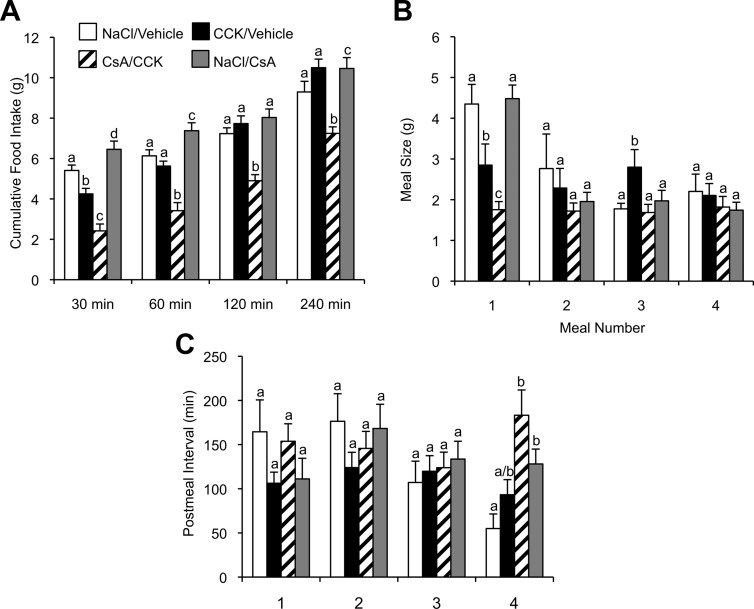
A separate group of animals were used to study the effect of CsA on CCK-induced suppression of food intake. In the absence of CsA, CCK significantly reduced cumulative food intake of overnight-fasted rats only for the first 30 minutes after the CCK injection. However, after the fourth ventricle injection of CsA, the reduction of cumulative food intake was sustained for 240 minutes after the CCK injection (Figure 7A). Meal pattern analysis revealed that CsA treatment significantly (P < .01) potentiated the ability of CCK to reduce the size of the first meal (Figure 7B). Notably, after CCK treatment in the absence of CsA, there was a rebound in the size of a later meal (meal 3). This rebound of meal size was absent in the presence of CsA. Coinjection of CsA and CCK did not significantly affect the postmeal interval of the first 4 meals compared with CCK injection alone (Figure 7C).
Figure 7.
Fourth ventricle injection of calcineurin inhibitor CsA potentiates CCK-induced reduction of food intake. A, Cumulative food intake in 16-hour food-deprived rats at 30, 60, 120, and 240 minutes after an ip of CCK (2 μg/kg) or saline administration preceded by a fourth ventricle injection of CsA (12 μg) or vehicle 1 hour prior to the ip injections. Meal size (B) and postmeal interval (C) for the first four meals after the return of food. Data are means ± SEM. Means sharing a common letter do not differ significantly.
Discussion
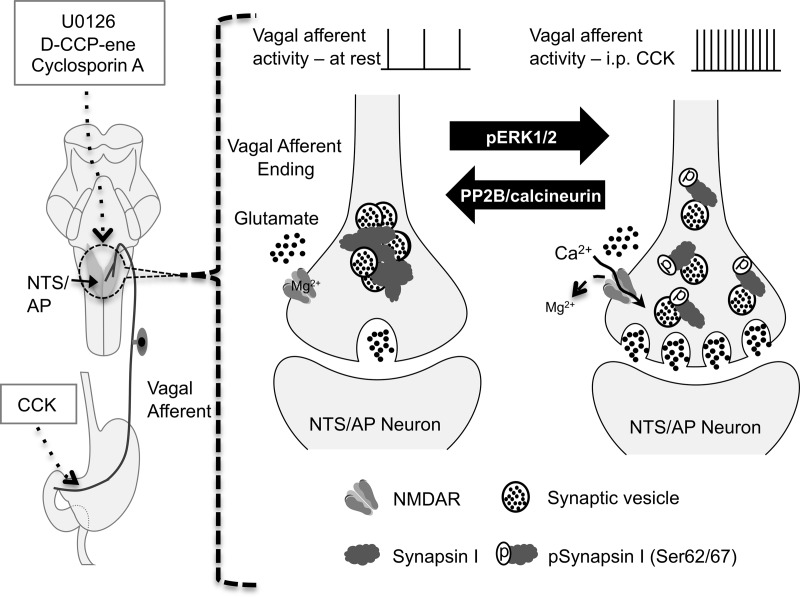
Previous reports indicated that CCK-induced reduction of food intake requires activation of NTS NMDARs (32), with consequent phosphorylation of ERK1/2 in vagal afferent endings and NTS neurons (12). The mechanisms by which NMDAR-mediated ERK1/2 phosphorylation enable reduction of food intake by CCK have not been determined. The synaptic protein, synapsin I, is a major substrate of pERK1/2, which selectively phosphorylates serines 62 and 67 (13). Our current results reveal a rapid and dramatic increase in synapsin I phosphorylation in the NTS after an ip CCK injection. We found that CCK-induced synapsin I phosphorylation is localized primarily, if not exclusively, to vagal afferent endings in the NTS. Destruction of C-type vagal afferents, which abolishes CCK-induced reduction of food intake, eliminates CCK-induced synapsin I phosphorylation in the NTS. In addition, CCK-induced synapsin I phosphorylation was attenuated by the fourth ventricle injection of an NMDAR antagonist or MEK inhibitor, indicating that CCK-induced synapsin I phosphorylation in vagal afferent terminals is downstream of NMDAR activation and ERK1/2 phosphorylation. Finally, inhibition of calcineurin, which dephosphorylates pSyn at pERK1/2-phosphorylated sites, enhances CCK-induced synapsin I phosphorylation and potentiates the reduction of food intake by CCK. Collectively our results support a model in which NMDAR activation leads to pERK1/2-catalyzed synapsin I phosphorylation in vagal afferent endings (Figure 8) and suggests synapsin I phosphorylation as a mechanism by which hindbrain NMDAR activation facilitates enhanced glutamate release from vagal afferent endings and thereby enhanced reduction of food intake by CCK.
Figure 8.
Schematic model of pERK1/2-mediated synapsin I phosphorylation triggered by the activation of NMDARs on the vagal afferent endings in the NTS/AP after vagal afferent stimulation by ip CCK. Left panel, Illustration of peripheral vagal afferent endings of the stomach and small intestine, the site of action of injected CCK. Also illustrated in the left side of the diagram is the hindbrain with NTS and AP shaded. Neurons in both the NTS and AP receive synaptic input from vagal afferent endings. Note that although CCK was injected ip, all other drugs (MEK inhibitor, U0126; NMDAR antagonist, d-CPP-ene; and calcineurin inhibitor, CsA) were injected into the fourth ventricle of the hindbrain. Right panel, Illustration of synaptic vesicles within the vagal afferent endings. At rest or at low levels of stimulation, vesicles are bound to each other and to actin by synapsin I in a reserve pool. At higher rates of discharge, as evoked by CCK, terminal membrane depolarization and the presence of glutamate result in calcium entry via the NMDAR channel, resulting in the phosphorylation of ERK1/2, which phosphorylates synapsin at serines 62 and 67 (pSynapsin I). Phosphorylation of synapsin I results in the unbinding of synaptic vesicles, allowing them to enter the readily releasable pool. Increasing the size of the this pool increases synaptic strength and prolongs the period over which the terminal can sustain transmitter release. The source(s) of glutamate necessary to activate NMDAR is not known but could come from vagal afferents themselves, axoaxonal synapses on vagal afferents, or glia.
Synapsins, which include mammalian synapsins I, II, and II, are highly conserved proteins comprising the most abundant constituents of synaptic vesicles in both vertebrates and invertebrates (33). Biochemical experiments revealed that synapsins tether synaptic vesicles to cytoskeletal actin, thereby preventing exocytosis and transmitter release (34–36). During neuronal activity, however, synapsins are phosphorylated in a site-selective manner by several activated kinases, including pERK1/2 (13). Phosphorylation of synapsin I at pERK1/2-specific phosphorylation sites has been shown to control the amount of neurotransmitter released by increasing the number of synaptic vesicles available for exocytosis (15–17). Experiments using a variety of in vitro and ex vivo preparations indicate that pERK1/2-catalyzed phosphorylation of synapsin I is necessary for the development of both short- and long-term forms of synaptic plasticity. For example, synapsin I phosphorylation by pERK1/2 contributes to the development of posttetanic potentiation (17) and is essential for development of long-term potentiation at some synapses (16). Hence, phosphorylation of synapsins, including synapsin I, is an important mechanism for modulating the functional influence of specific synapses.
Hay et al (37) reported increased synapsin I phosphorylation after potassium-induced depolarization of cultured vagal afferent neurons. However, they did not determine whether ERK1/2 phosphorylation contributed to the increase in synapsin I phosphorylation they observed. We previously reported that an ip injection of CCK results in the phosphorylation of ERK1/2 in vagal afferent endings in the NTS (12). In the current study, we found that CCK increased pSyn in C-type vagal afferent endings in the NTS. The antibody we used to detect phosphorylated synapsin I was selective for synapsin I phosphorylated at the pERK1/2 substrate sites, serines 62 and 67. After a CCK injection, pSyn was colocalized with pERK1/2 in vagal afferent endings. Moreover, the CCK-induced increase in vagal afferent synapsin I phosphorylation was prevented by the inhibition of ERK1/2 phosphorylation after a fourth ventricle injection of the MEK inhibitor, U0126. Thus, it is clear from our results that CCK-evoked activation of vagal afferents in vivo results in pERK1/2-catalyzed synapsin I phosphorylation in vagal afferent endings.
CCK reduces food intake by activating a subpopulation of vagal afferent neurons, which comprise 30%-38% of the total vagal afferent population (38, 39). Most, although not all, CCK-sensitive vagal afferents are unmyelinated C-type afferents (21), which are destroyed by capsaicin treatment (40). CCK-evoked activation of these vagal afferents is mediated by CCK1R activation (39, 41). In previously published experiments, in which we compared the prevalence of CCK-sensitive vagal afferents in the overall vagal afferent population with their prevalence in afferents that innervate the stomach and small intestine, we determined that most, perhaps all, CCK-sensitive afferents innervate the GI tract (21). We report here that CCK-induced increase in vagal afferent synapsin I phosphorylation was abolished by a CCK1R antagonist. Given that CCK1R expression appears to be specific for vagal afferents that innervate the GI tract, our results suggest that a CCK-induced increase in synapsin I phosphorylation is selective for GI vagal afferent endings.
It is well documented that the NTS and AP both are directly innervated by vagal afferent endings (42). Therefore, it is not surprising that CCK increased synapsin I phosphorylation in the AP as well as in the NTS. The AP, like the NTS, exhibits high concentrations of vanilloid receptors, as indicated by resniferotoxin binding (43). Moreover, silver staining after systemic capsaicin treatment reveals a marked degeneration of terminals in both the NTS and AP (40). We observed that increased synapsin phosphorylation in the NTS and AP after CCK was abolished in rats pretreated with capsaicin. Together with the above-cited observations, our findings are consistent with the interpretation that the CCK-induced increase in phosphorylated synapsin in the NTS and AP occurs in C-type vagal afferents endings.
Increased synapsin phosphorylation in the NTS and AP was attenuated when rats received a fourth ventricle NMDAR antagonist. Thus, phosphorylation of synapsin in the AP and NTS depends on NMDAR activation. Neurons in the AP project prominently to the medial and commissural NTS (42). Electrophysiological experiments indicate that these AP-to-NTS projections are predominantly excitatory (45) and that excitation of the NTS is mediated by both NMDA- and non-NMDA-type glutamate receptors (46). In addition, recordings in hindbrain slices suggest that AP stimulation can enhance NTS neuronal responses to solitary tract stimulation (47). Although it is clear that neurons in the AP are activated by direct vagal afferent input, activation of AP neurons also occurs in response to humoral stimuli arriving from the circulation. Hence, it conceivable that glutamate released by AP projections to the NTS could enhance synapsin phosphorylation in vagal afferent endings, thereby providing a presynaptic mechanism by which humoral stimuli acting on the AP might enhance vagal neurotransmission in the NTS.
Although NMDAR coupling to ERK1/2 phosphorylation is well established (48–50), a connection between presynaptic NMDAR activation and synapsin I phosphorylation has not previously been reported. However, activation of presynaptic NMDAR enhances neurotransmitter release in a variety of synaptic preparations (51), an effect that is consistent with pERK1/2-catalyzed synapsin I phosphorylation. Virtually all vagal afferent neurons express NMDARs (52–54), and the NMDAR NR1 subunit has been detected in vagal afferent terminals using electron microscopy (10). Although the functional importance of vagal afferent NMDARs has not been thoroughly investigated, Bach and Smith (55) recently reported that the activation of vagal afferent NMDARs enhances glutamate release from their terminals in the dorsal vagal motor nucleus. Bach and Smith did not determine whether NMDAR-mediated enhancement of glutamate release depended on ERK1/2 phosphorylation. However, our previously reported experiments demonstrated that fourth ventricle NMDAR antagonist administration prevents CCK-induced ERK1/2 phosphorylation in vagal afferent endings (12). Moreover, in the current study, we found that CCK-induced synapsin I phosphorylation in vagal afferent endings was also prevented by fourth ventricle NMDAR antagonism as well as by MEK inhibition. These results indicate that pERK1/2-catalyzed synapsin I phosphorylation in vagal afferent endings requires NMDAR activation. Because both presynaptic NMDAR and pERK1/2-catlyzed synapsin I phosphorylation have been shown independently to increase transmitter release, our current observations are consistent with the hypothesis that NMDAR activation triggers pERK1/2-catalyzed synapsin I phosphorylation, thereby increasing the release of sufficient neurotransmitter to enable the reduction of food intake.
Electrophysiological studies suggest that virtually all vagal afferents activated by electrical stimulation (56) or by CCK (57, 58) trigger glutamatergic excitatory postsynaptic currents in NTS neurons. Because synapsin phosphorylation enhances glutamate release from nerve terminals in areas like the hippocampus (16), it is reasonable to think that synapsin phosphorylation has similar effects on glutamate release from vagal afferent endings. It is not known, however, whether synapsin phosphorylation might facilitate the release of nonglutamate neuromodulators, for example neuropeptides, from vagal afferent terminals. A variety of neuropeptides are expressed in subpopulations of vagal afferents (59), and some of these peptides may participate in the control of food intake by CCK. For example, cocaine-amphetamine-regulated transcript (CART) mRNA is expressed by many vagal afferent neurons that express the CCK1 receptor transcript (60). CCK reportedly increases CART release from cultured vagal afferents and injection of CART into the fourth ventricle enhances the reduction of food intake by CCK (61). Therefore, it is conceivable that NMDAR-mediated synapsin phosphorylation might enhance the release of CART as well as of glutamate from vagal afferent endings. However, it is worth noting that spinal afferents that express neuropeptides reportedly do not express synapsins (62). Therefore, it appears that synapsins might not participate in neurotransmitter release from peptidergic spinal afferents. At this time there is virtually no information available concerning the role of synapsin phosphorylation in the release of peptide transmitters. Hence, any role of synapsins in the release of vagal neuropeptides and control of food intake awaits additional investigation.
Although the phosphorylation of serines 62 and 67 of synapsin I increases readily releasable neurotransmitter, dephosphorylation of these sites favors the binding of synaptic vesicles to actin and limits transmitter release (36). Dephosphorylation of serines 62 and 67 is mediated by protein phosphatase 2B, also known as calcineurin (13, 31). Consequently, reducing calcineurin activity would be expected to increase synapsin I phosphorylation and enhance transmitter release, with a consequent increased activation of postsynaptic neurons. Indeed, Ratnayaka et al (63), working with cultured hippocampal neurons, have demonstrated that calcineurin inhibition is accompanied by an increased readily releasable transmitter and increased synaptic strength. We found that fourth ventricle injection of the calcineurin inhibitor, cyclosporine A, increased and prolonged CCK-induced synapsin I phosphorylation and enhanced the reduction of food intake by CCK. Moreover, we observed that CsA increased the number of pERK1/2-immunoreactive NTS neurons after a CCK injection. Because calcineurin does not dephosphorylate pERK1/2, and its inhibition actually has been reported to attenuate ERK1/2 phosphorylation in some cells (64), it seems likely that increased pERK1/2 after CsA/CCK is related to stronger activation of NTS neurons after calcineurin inhibition. Together these effects are consistent with the participation of synapsin I phosphorylation in CCK-induced satiation via an enhanced activation of NTS neurons by vagal afferents. In addition, the fact that calcineurin treatment did not alter food intake in the absence of CCK would suggest that pERK1/2-catalyzed synapsin I phosphorylation may have the most impact on vagal afferent function during high levels of activation. Indeed, Chi et al (15), using cultured neurons, demonstrated that pERK1/2-catalyzed synapsin phosphorylation is critical for the synaptic function at high rates of neuronal discharge.
Activation of NMDARs and ERK1/2 phosphorylation are documented participants in neuroplastic mechanisms that adjust synaptic strength (65, 66). Likewise, increases in synapsin I phosphorylation contributes to plastic changes such as long-term potentiation (67, 68). Therefore, modulation of synapsin I phosphorylation via NMDAR activation is a potential mechanism for adjusting the strength of vagal afferent synapses involved in the control of food intake. In areas such as the hippocampus, neuroplastic changes that alter synaptic strength are modulated by endocrine signals, such as leptin (69, 70) and gonadal steroids (44, 71). Given that NMDARs appear to play a crucial role in these endocrine modulations of activity-dependent neural plasticity, it is tantalizing to speculate that NMDAR-dependent synapsin I phosphorylation in vagal afferent endings enables the modulation of vagal afferent satiation signals by nonvagal neuronal inputs and hormones.
In summary, our findings suggest that controlling synapsin I phosphorylation in vagal afferent endings may modulate the reduction of food intake by CCK. Variation of synapsin I phosphorylation, which is dependent on NMDAR-mediated phosphorylation of ERK1/2 and activation of calcineurin, could conceivably vary the strength of vagal afferent synapses activated by CCK and thereby vary the degree to which CCK reduces food intake. The nature of the inputs that might produce NMDAR-triggered changes in synapsin I phosphorylation remains to be identified.
Acknowledgments
The technical help of T. Duffy and N. Huston is gratefully acknowledged. We thank Drs Sue Ritter, Suzanne Appleyard, and James Peters for reading the manuscript and for their helpful suggestions.
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-52849 and the National Institute of Neurological Diseases and Stroke Grant NS-20561 (to R.C.R.).
Disclosure Summary: The authors declare no conflicts of interest, financial or otherwise.
Footnotes
- AP
- area postrema
- CART
- cocaine-amphetamine-regulated transcript
- CCK
- cholecystokinin
- CCK1R
- type 1 CCK receptor
- CsA
- cyclosporin A
- D-CPP-ene
- D-3-(2-carboxypiperazine-4-yl)-1-propenyl-1-phosphonic acid
- DMSO
- dimethylsulfoxide
- GI
- gastrointestinal
- IB4
- isolectin B4
- MEK
- mitogen-activated ERK kinase
- NMDAR
- N-methyl-D-aspartate-type glutamate receptor
- NTS
- nucleus of the solitary tract
- pERK1/2
- phosphorylation of ERK1/2
- pSyn
- pERK1/2-catalyzed phosphorylation of synapsin I.
References
- 1. Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol. 1985;249:R638–R641 [DOI] [PubMed] [Google Scholar]
- 2. Walls EK, Phillips RJ, Wang FB, Holst MC, Powley TL. Suppression of meal size by intestinal nutrients is eliminated by celiac vagal deafferentation. Am J Physiol. 1995;269:R1410–R1419 [DOI] [PubMed] [Google Scholar]
- 3. Schwartz GJ, Salorio CF, Skoglund C, Moran TH. Gut vagal afferent lesions increase meal size but do not block gastric preload-induced feeding suppression. Am J Physiol. 1999;276:R1623–R1629 [DOI] [PubMed] [Google Scholar]
- 4. Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Reg I. 2004;287:R87–RR96 [DOI] [PubMed] [Google Scholar]
- 5. Zheng H, Patterson LM, Rhodes CJ, et al. A potential role for hypothalamomedullary POMC projections in leptin-induced suppression of food intake. Am J Physiol Regul Integr Comp Physiol. 2010;298:R720–R728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allchin RE, Batten TF, McWilliam PN, Vaughan PF. Electrical stimulation of the vagus increases extracellular glutamate recovered from the nucleus tractus solitarii of the cat by in vivo microdialysis. Exp Physiol. 1994;79:265–268 [DOI] [PubMed] [Google Scholar]
- 7. Treece BR, Covasa M, Ritter RC, Burns GA. Delay in meal termination follows blockade of N-methyl-D-aspartate receptors in the dorsal hindbrain. Brain Res. 1998;810:34–40 [DOI] [PubMed] [Google Scholar]
- 8. Hung CY, Covasa M, Ritter RC, Burns GA. Hindbrain administration of NMDA receptor antagonist AP-5 increases food intake in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;290:R642–R651 [DOI] [PubMed] [Google Scholar]
- 9. Broussard DL, Wiedner EB, Li X, Altschuler SM. NMDAR1 mRNA expression in the brainstem circuit controlling esophageal peristalsis. Brain Res Mol Brain Res. 1994;27:329–332 [DOI] [PubMed] [Google Scholar]
- 10. Aicher SA, Sharma S, Pickel VM. N-methyl-D-aspartate receptors are present in vagal afferents and their dendritic targets in the nucleus tractus solitarius. Neuroscience. 1999;91:119–132 [DOI] [PubMed] [Google Scholar]
- 11. Gillespie BR, Burns GA, Ritter RC. NMDA channels control meal size via central vagal afferent terminals. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1504–R1511 [DOI] [PubMed] [Google Scholar]
- 12. Campos CA, Wright JS, Czaja K, Ritter RC. CCK-induced reduction of food intake and hindbrain MAPK signaling are mediated by NMDA receptor activation. Endocrinology. 2012;153(6):2633–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jovanovic JN, Benfenati F, Siow YL, et al. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc Natl Acad Sci USA. 1996;93:3679–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–329 [DOI] [PubMed] [Google Scholar]
- 15. Chi P, Greengard P, Ryan TA. Synaptic vesicle mobilization is regulated by distinct synapsin I phosphorylation pathways at different frequencies. Neuron. 2003;38:69–78 [DOI] [PubMed] [Google Scholar]
- 16. Kushner SA, Elgersma Y, Murphy GG, et al. Modulation of presynaptic plasticity and learning by the H-ras/extracellular signal-regulated kinase/synapsin I signaling pathway. J Neurosci. 2005;25:9721–9734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valente P, Casagrande S, Nieus T, et al. Site-specific synapsin I phosphorylation participates in the expression of post-tetanic potentiation and its enhancement by BDNF. J Neurosci. 2012;32:5868–5879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, hard cover edition Burlington, VT: Elsevier; 2007 [Google Scholar]
- 19. Ritter RC, Ladenheim EE. Capsaicin pretreatment attenuates suppression of food intake by cholecystokinin. Am J Physiol. 1985;248:R501–R504 [DOI] [PubMed] [Google Scholar]
- 20. Sutton GM, Patterson LM, Berthoud HR. Extracellular signal-regulated kinase 1/2 signaling pathway in solitary nucleus mediates cholecystokinin-induced suppression of food intake in rats. J Neurosci. 2004;24:10240–10247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peters JH, Ritter RC, Simasko SM. Leptin and CCK selectively activate vagal afferent neurons innervating the stomach and duodenum. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1544–R1549 [DOI] [PubMed] [Google Scholar]
- 22. Young RL, Cooper NJ, Blackshaw LA. Chemical coding and central projections of gastric vagal afferent neurons. Neurogastroenterol Motil. 2008;20:708–718 [DOI] [PubMed] [Google Scholar]
- 23. Li H, Nomura S, Mizuno N. Binding of the isolectin I-B4 from Griffonia simplicifolia to the general visceral afferents in the vagus nerve: a light- and electron-microscope study in the rat. Neurosci Lett. 1997;222:53–56 [DOI] [PubMed] [Google Scholar]
- 24. Kalia M, Sullivan JM. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol. 1982;211:248–265 [DOI] [PubMed] [Google Scholar]
- 25. Contreras RJ, Beckstead RM, Norgren R. The central projections of the trigeminal, facial, glossopharyngeal and vagus nerves: an autoradiographic study in the rat. J Auton Nerv Syst. 1982;6:303–322 [DOI] [PubMed] [Google Scholar]
- 26. Simasko SM, Ritter RC. Cholecystokinin activates both A- and C-type vagal afferent neurons. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1204–G1213 [DOI] [PubMed] [Google Scholar]
- 27. van de Wall EH, Duffy P, Ritter RC. CCK enhances response to gastric distension by acting on capsaicin-insensitive vagal afferents. Am J Physiol Regul Integr Comp Physiol. 2005;289:R695–R703 [DOI] [PubMed] [Google Scholar]
- 28. Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science. 1981;213:1036–1037 [DOI] [PubMed] [Google Scholar]
- 29. Yox DP, Ritter RC. Capsaicin attenuates suppression of sham feeding induced by intestinal nutrients. Am J Physiol. 1988;255:R569–R574 [DOI] [PubMed] [Google Scholar]
- 30. Yox DP, Stokesberry H, Ritter RC. Fourth ventricular capsaicin attenuates suppression of sham feeding induced by intestinal nutrients. Am J Physiol. 1991;260:R681–R687 [DOI] [PubMed] [Google Scholar]
- 31. Jovanovic JN, Sihra TS, Nairn AC, Hemmings HC, Jr, Greengard P, Czernik AJ. Opposing changes in phosphorylation of specific sites in synapsin I during Ca2+-dependent glutamate release in isolated nerve terminals. J Neurosci. 2001;21:7944–7953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wright J, Campos C, Herzog T, Covasa M, Czaja K, Ritter RC. Reduction of food intake by cholecystokinin requires activation of hindbrain NMDA-type glutamate receptors. Am J Physiol Regul Integr Comp Physiol. 2011;301:R8–R455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cesca F, Baldelli P, Valtorta F, Benfenati F. The synapsins: key actors of synapse function and plasticity. Prog Neurobiol. 2010;91:313–348 [DOI] [PubMed] [Google Scholar]
- 34. Bahler M, Greengard P. Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature. 1987;326:704–707 [DOI] [PubMed] [Google Scholar]
- 35. Benfenati F, Bahler M, Jahn R, Greengard P. Interactions of synapsin I with small synaptic vesicles: distinct sites in synapsin I bind to vesicle phospholipids and vesicle proteins. J Cell Biol. 1989;108:1863–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ceccaldi PE, Grohovaz F, Benfenati F, Chieregatti E, Greengard P, Valtorta F. Dephosphorylated synapsin-I anchors synaptic vesicles to actin cytoskeleton: an analysis by videomicroscopy. J Cell Biol. 1995;128:905–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hay M, Hoang CJ, Hasser EM, Price EM. Activation of metabotropic glutamate receptors inhibits synapsin I phosphorylation in visceral sensory neurons. J Membr Biol. 2000;178:195–204 [DOI] [PubMed] [Google Scholar]
- 38. Broberger C, Holmberg K, Shi TJ, Dockray G, Hokfelt T. Expression and regulation of cholecystokinin and cholecystokinin receptors in rat nodose and dorsal root ganglia. Brain Res. 2001;903:128–140 [DOI] [PubMed] [Google Scholar]
- 39. Simasko SM, Wiens J, Karpiel A, Covasa M, Ritter RC. Cholecystokinin increases cytosolic calcium in a subpopulation of cultured vagal afferent neurons. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1303–R1313 [DOI] [PubMed] [Google Scholar]
- 40. Ritter S, Dinh TT. Capsaicin-induced neuronal degeneration: silver impregnation of cell bodies, axons, and terminals in the central nervous system of the adult rat. J Comp Neurol. 1988;271:79–90 [DOI] [PubMed] [Google Scholar]
- 41. Lankisch TO, Tsunoda Y, Lu Y, Owyang C. Characterization of CCK(A) receptor affinity states and Ca(2+) signal transduction in vagal nodose ganglia. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1002–G1008 [DOI] [PubMed] [Google Scholar]
- 42. Shapiro RE, Miselis RR. The central neural connections of the area postrema of the rat. J Comp Neurol. 1985;234:344–364 [DOI] [PubMed] [Google Scholar]
- 43. Szallasi A, Nilsson S, Farkas-Szallasi T, Blumberg PM, Hokfelt T, Lundberg JM. Vanilloid (capsaicin) receptors in the rat: distribution in the brain, regional differences in the spinal cord, axonal transport to the periphery, and depletion by systemic vanilloid treatment. Brain Res. 1995;703:175–183 [DOI] [PubMed] [Google Scholar]
- 44. Vierk R, Glassmeier G, Zhou L, et al. Aromatase inhibition abolishes LTP generation in female but not in male mice. J Neurosci. 2012;32:8116–8126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cai Y, Hay M, Bishop VS. Synaptic connections and interactions between area postrema and nucleus tractus solitarius. Brain Res. 1996;724:121–124 [DOI] [PubMed] [Google Scholar]
- 46. Aylwin ML, Horowitz JM, Bonham AC. Non-NMDA and NMDA receptors in the synaptic pathway between area postrema and nucleus tractus solitarius. Am J Physiol. 1998;275:H1236–H1246 [DOI] [PubMed] [Google Scholar]
- 47. Bonham AC, Hasser EM. Area postrema and aortic or vagal afferents converge to excite cells in nucleus tractus solitarius. Am J Physiol. 1993;264:H1674–H1685 [DOI] [PubMed] [Google Scholar]
- 48. Chandler LJ, Sutton G, Dorairaj NR, Norwood D. N-methyl D-aspartate receptor-mediated bidirectional control of extracellular signal-regulated kinase activity in cortical neuronal cultures. J Biol Chem. 2001;276:2627–2636 [DOI] [PubMed] [Google Scholar]
- 49. Kanterewicz BI, Urban NN, McMahon DB, et al. The extracellular signal-regulated kinase cascade is required for NMDA receptor-independent LTP in area CA1 but not area CA3 of the hippocampus. J Neurosci. 2000;20:3057–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bertotto ME, Maldonado NM, Bignante EA, et al. ERK activation in the amygdala and hippocampus induced by fear conditioning in ethanol withdrawn rats: modulation by mk-801. Eur Neuropsychopharmacol. 2011;21(12):892–904 [DOI] [PubMed] [Google Scholar]
- 51. Duguid IC, Smart TG. Presynaptic NMDA Receptors. In: Van Dongen AM, ed. Biology of the NMDA Receptor. Boca Raton, FL: 2009, chapter 14 [PubMed] [Google Scholar]
- 52. Shigemoto R, Ohishi H, Nakanishi S, Mizuno N. Expression of the mRNA for the rat NMDA receptor (NMDAR1) in the sensory and autonomic ganglion neurons. Neurosci Lett. 1992;144:229–232 [DOI] [PubMed] [Google Scholar]
- 53. Czaja K, Ritter RC, Burns GA. N-methyl-D-aspartate receptor subunit phenotypes of vagal afferent neurons in nodose ganglia of the rat. J Comp Neurol. 2006;496:877–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Czaja K, Ritter RC, Burns GA. Vagal afferent neurons projecting to the stomach and small intestine exhibit multiple N-methyl-D-aspartate receptor subunit phenotypes. Brain Res. 2006;1119:86–93 [DOI] [PubMed] [Google Scholar]
- 55. Bach EC, Smith BN. Presynaptic NMDA receptor-mediated modulation of excitatory neurotransmission in the mouse dorsal motor nucleus of the vagus. J Neurophysiol. 2012;108:1484–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Andresen MC, Mendelowitz D. Sensory afferent neurotransmission in caudal nucleus tractus solitarius—common denominators. Chem Senses. 1996;21:387–395 [DOI] [PubMed] [Google Scholar]
- 57. Baptista V, Zheng ZL, Coleman FH, Rogers RC, Travagli RA. Cholecystokinin octapeptide increases spontaneous glutamatergic synaptic transmission to neurons of the nucleus tractus solitarius centralis. J Neurophysiol. 2005;94:2763–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Appleyard SM, Bailey TW, Doyle MW, et al. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci. 2005;25:3578–3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhuo H, Ichikawa H, Helke CJ. Neurochemistry of the nodose ganglion. Prog Neurobiol. 1997;52:79–107 [DOI] [PubMed] [Google Scholar]
- 60. Broberger C, Holmberg K, Kuhar MJ, Hokfelt T. Cocaine- and amphetamine-regulated transcript in the rat vagus nerve: a putative mediator of cholecystokinin-induced satiety. Proc Natl Acad Sci USA. 1999;96:13506–13511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. De Lartigue G, Dimaline R, Varro A, Raybould H, De la Serre CB, Dockray GJ. Cocaine- and amphetamine-regulated transcript mediates the actions of cholecystokinin on rat vagal afferent neurons. Gastroenterology. 2010;138:1479–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Morris JL, Konig P, Shimizu T, Jobling P, Gibbins IL. Most peptide-containing sensory neurons lack proteins for exocytotic release and vesicular transport of glutamate. J Comp Neurol. 2005;483:1–16 [DOI] [PubMed] [Google Scholar]
- 63. Ratnayaka A, Marra V, Bush D, Burden JJ, Branco T, Staras K. Recruitment of resting vesicles into recycling pools supports NMDA receptor-dependent synaptic potentiation in cultured hippocampal neurons. J Physiol. 2012;590:1585–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gary-Gouy H, Sainz-Perez A, Bismuth G, Ghadiri A, Perrino BA, Dalloul A. Cyclosporin-A inhibits ERK phosphorylation in B cells by modulating the binding of Raf protein to Bcl2. Biochem Biophys Res Commun. 2006;344:134–139 [DOI] [PubMed] [Google Scholar]
- 65. El Gaamouch F, Buisson A, Moustie O, et al. Interaction between αCaMKII and GluN2B controls ERK-dependent plasticity. J Neurosci. 2012;32:10767–10779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317 [DOI] [PubMed] [Google Scholar]
- 67. Giachello CN, Fiumara F, Giacomini C, et al. MAPK/ERK-dependent phosphorylation of synapsin mediates formation of functional synapses and short-term homosynaptic plasticity. J Cell Sci. 2010;123:881–893 [DOI] [PubMed] [Google Scholar]
- 68. Vara H, Onofri F, Benfenati F, Sassoe-Pognetto M, Giustetto M. ERK activation in axonal varicosities modulates presynaptic plasticity in the CA3 region of the hippocampus through synapsin I. Proc Natl Acad Sci USA. 2009;106:9872–9877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Moult PR, Harvey J. NMDA receptor subunit composition determines the polarity of leptin-induced synaptic plasticity. Neuropharmacology. 2011;61:924–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Grillo CA, Piroli GG, Junor L, et al. Obesity/hyperleptinemic phenotype impairs structural and functional plasticity in the rat hippocampus. Physiol Behav. 2011;105:138–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Smith CC, McMahon LL. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J Neurosci. 2006;26:8517–8522 [DOI] [PMC free article] [PubMed] [Google Scholar]