Abstract
Menopause is characterized by the rapid age-related decline of circulating 17β-estradiol (E2) levels in women, which can sometimes result in cognitive disorders such as impaired memory and increased anxiety. Hormone therapy (HT) is a widely used treatment for the adverse effects associated with menopause; however, evidence suggests that HT administered to postmenopausal women age 65 years and over can lead to increased risks for cognitive disorders. We hypothesized that these age-related changes in E2 action are due to posttranscriptional gene regulation by microRNAs (miRNAs). miRNAs are a class of small noncoding RNAs that regulate gene expression by binding to the 3′-untranslated region of target mRNAs and subsequently target these transcripts for degradation. In the present study, 3- and 18-month-old female rats were oophorectomized (OVX) and treated 1 week after surgery with 2.5 μg E2 once per day for 3 days. Total RNA was isolated from the ventral and dorsal hippocampus, central amygdala, and paraventricular nucleus. Our results showed that E2 differentially altered miRNA levels in an age- and brain region-dependent manner. Multiple miRNA target prediction algorithms revealed putative target genes that are important for memory and stress regulation, such as BDNF, glucocorticoid receptor, and SIRT-1. Indeed, quantitative RT-PCR analyses of some of the predicted targets, such as SIRT1, showed that the mRNA expression levels were the inverse of the targeting miRNA, thereby confirming the prediction algorithms. Taken together, these data show that E2 regulates miRNA expression in an age- and E2-dependent manner, which we hypothesize results in differential gene expression and consequently altered neuronal function.
Advanced age is accompanied by global changes in neuronal gene expression. Recent studies have demonstrated that microRNA (miRNA) expression also changes with age, an intriguing correlation suggesting that age-related miRNA fluctuations could be responsible for modulating the overall change in global gene expression (1–6). miRNAs are noncoding regulatory RNAs transcribed from intergenic or intragenic regions of the genome in an RNA polymerase II-dependent manner (7, 8). Primary miRNA (pri-miRNA) transcripts are sequentially cleaved by the nuclear enzyme drosha, and the cytoplasmic enzyme dicer, to form the functionally mature single-stranded form of the miRNA (9–11). Complementary binding of the mature miRNA to the 3′-untranslated region of a target mRNA, and its subsequent association with the RNA-induced silencing complex (RISC), leads to mRNA translational repression and/or degradation (12–14).
Current efforts are focused on elucidating potential target genes that are regulated by miRNAs; however, less is known about the upstream pathways that regulate miRNA expression, processing, and temporal activity in the brain. The gonadal steroid hormone 17β-estradiol (E2) is one factor that has been shown to regulate miRNA expression and biochemical processing in some systems, such as cancer cell models (15–20). Furthermore, E2 has been shown to be neuroprotective by decreasing the recovery time after stroke and improving learning and memory in aged rodents, primates, and humans, (21, 22); however, the beneficial effects observed in older women are largely dependent on the length of time between the onset of menopause and the administration of hormone therapy (23, 24). Aging adds an additional layer of complexity that alters these postmenopausal mechanisms (25–27).
Numerous studies clearly show that aging has detrimental consequences for the hippocampus, a brain region that is required for learning and memory as well as for mediating the physiological responses to perceived stressors. Multiple excitatory signaling pathways, growth factors, and steroid hormones govern hippocampal neuronal functions (28, 29). This brain structure has been extensively studied for its importance in memory formation; however, hippocampal neurons also interact broadly with other brain regions to mediate a variety of more complex behaviors (30). The hippocampus can be functionally divided into ventral and dorsal subdivisions, which primarily mediate emotion/stress and memory/cognition, respectively (31). In addition, these 2 divisions have distinct gene expression profiles, which are thought to delineate their specific functions (32, 33). Similarly, region-specific expression of miRNAs has been observed in the brain, suggesting that they have unique and specific neurological functions (34–40). For instance, miRNAs have been shown to play a critical role in neuronal development, survival, and synaptic plasticity (41–49). Consequently, disruption of miRNA activity has been associated with several neuropathological conditions such as Alzheimer's disease, schizophrenia, and general mood disorders (50–55).
In our studies, we hypothesized that E2 differentially regulates miRNA expression in the aged female hippocampus, leading to altered gene expression important for neuronal function. To test our hypothesis, we compared the hippocampal miRNA expression profile of 3-month-old (young adult) and 18-month-old (late middle age) female rats that underwent surgically induced menopause (ie, ovariectomy). After a 7-day period of E2 withdrawal, animals were then administered E2 or vehicle (control) for 3 days. Global changes in miRNA expression in the ventral hippocampus (vHIPP) were evaluated using a rat miRNA microarray. The analysis of global miRNAs confirmed the presence of brain region-specific miRNA composition. More importantly, our results also showed miRNA changes to be E2- and/or aging-dependent. Furthermore, we identified potential miRNA target genes that are important for cognition/memory (ie, sirtuin1 [SIRT1]and brain-derived neurotrophic factor [BDNF]), stress (ie, glucocorticoid receptor [GR]), and synaptic function (ie, γ-aminobutyric acid [GABA] A receptor 1 [GABRA1]). Collectively, our results showed that E2 treatment results in a fundamental shift in the composition of miRNA species in the aged brain.
Materials and Methods
Ethics statement
All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Loyola University Chicago, permit number 2009018. Surgeries were preformed under vaporized isoflurane anesthesia. After operation, animals were singly housed and provided with acetaminophen analgesic in their water for 3 days. All measures were taken to minimize suffering.
Animals
Female Fischer 344 rats (n = 20) were obtained from the National Institute of Aging colony (Taconic, Hudson, New York) at 3 and 18 months of age. The animals were allowed to acclimate to the housing facility for 7 days after arrival. Animals were housed 2 per cage and were allowed free access to food and water.
Surgery
One week after arrival, animals were deeply anesthetized with vaporized isoflurane and bilaterally ovariectomized. Briefly, the ovary and distal end of the uterine horn were pulled from the body cavity through a 1 cm incision made through the skin and body wall. The uterine horn was clamped with a hemostat and ligated proximal to the clamp. The entire ovary and distal uterine horn were then removed. Animals were singly housed and provided with acetaminophen analgesic in their water for 3 d postoperative. During this time, animals were weighed once/day and their water intake measured.
Treatment
Seven days after ovariectomy, animals in each age group were given an sc injection of either safflower oil (vehicle) (n = 10 per age group) or 2.5 μg E2 (n = 10 per age group) dissolved in safflower oil once per day for 3 days. This dose of E2 resulted in circulating plasma E2 concentrations of 72.27 ± 13.0 pg/ml in 3-month-old animals and 82.65 ± 32.14 pg/ml in the 18-month-old animals at the time of killing. There was no statistical difference between the treated groups (ie, young vs old), and these E2 levels are consistent with physiological levels observed during late diestrus/early proestrus (56). The animals were anesthetized and killed by rapid decapitation 24 hours after the last E2 injection. Brains were quickly removed and sagittally sectioned into left and right hemispheres, and the hippocampus was microdissected from the left side of the brain and then further separated into the ventral and dorsal subdivisions. The ventral and dorsal hippocampi were placed in separate microcentrifuge tubes containing QIAzol lysis reagent (QIAGEN, Venlo, the Netherlands) reagent for subsequent homogenization and RNA extraction. The right side of the brain was rapidly frozen in 2-methylbutane and sectioned at 200 μm on a freezing microtome, and the paraventricular nucleus (PVN) (−1.40 to −2.12 mm relative to bregma), central amygdala (CeA) (−2.12 to −2.80 mm relative to bregma), vHIPP (4.30 to 6.04 mm relative to bregma), and dorsal hippocampus (dHIPP) (−2.30 to −4.16 mm relative to bregma) were microdissected using a Palkovit's brain punch tool (0.75 mm; Stoelting, Inc, Wood Dale, Illinois).
RNA isolation
Total RNA was isolated from the vHIPP and dHIPP by the miRNeasy Mini Kit (QIAGEN). The tissue samples from all brain regions were placed in TRIzol reagent (Invitrogen Life Sciences, Carlsbad, California) for homogenization and RNA extraction. RNA isolation was performed according to the manufacturer's instructions and modified by the addition of GlycoBlue (Ambion, Austin, Texas) to the aqueous phase as a coprecipitant with isopropanol overnight at −20°C. All RNA samples were analyzed for quality by Nanodrop spectrophotometry and visualization of the RNA on 1.5% agarose gel.
miRNA microarray processing and analysis
Total RNA (5.0 μg) from the left side vHIPP in each treatment (n = 10) and age group (n = 10) was sent to LC Sciences (Huston, Texas) for rat miRNA microarray processing (miRBase 18 probe content). Multiple redundant regions were included in each chip, and each region comprised an miRNA probe region listed from Sanger miRBase release 18.0. Multiple controls were also included in each chip to control for quality of chip production, sample labeling, and assay condition. Analysis of the microarray data was initially performed by LC Sciences and further validated in our hands. The raw data from the microarray was normalized using quantile normalization, and the false discovery rate was set at 5%, which is considered highly stringent. miRNAs that were statistically significant between age and treatment group were validated in the ipsilateral and contralateral sides of the brain using quantitative RT-PCR (qRT-PCR). We also analyzed each of the normalization genes for evidence of E2 regulation, and samples were normalized to housekeeping genes that were not significantly altered by E2 or age (ie, RNU5G).
miRNA and mRNA cDNA synthesis
Total RNA (1.0 μg) was used to reverse transcribe both miRNA and mRNA cDNA synthesis. NCode miRNA First-Strand cDNA synthesis (Invitrogen) and SuperScript VILO cDNA synthesis kits were used for miRNA and mRNA, respectively, according to the manufacturer's instructions.
Quantitative real-time PCR
miRNA and mRNA qRT-PCR was performed with Fast Start Universal SYBR Green Master Mix (Roche, Penzberg, Germany) on an Eppendorf Realplex4. Forward primers for specific miRNAs were designed as described in the Ncode miRNA First-Strand cDNA synthesis kit handbook (Invitrogen) and using miRBase release 18 as a sequence reference. The small RNA, RNU5G, was used as a loading control and to normalize the data for analysis. The following program was used: 1) 95°C for 10 minutes, 2) 95°C for 20 seconds, 3) 59°C for 20 seconds, 4) 72°C for 10 seconds, and melting curve analysis. miRNA expression was analyzed using the delta delta Ct method as described previously (57). The following primers were used for analysis of selected miRNA target genes: SIRT1, 5′-GCGGCCGCGGATAGGTCCATA and 3′-TCCCACAGGAGACAGAAACCCCA; GR, 5′-CACCCATGATCCTGTCAGTG and 3′-AAAGCCTCCCTCTGCTAACC; GABRA1, 5′- AGACCAGGCTTGGGAGAGCGT and 3′- ACACGGGTCCGAAACTGGTGAC; BDNF, 5′-AGCCTCCTCTGCTCTTTCTGCTGGA and 3′-CTTTTGTCTATGCCCCTGCAGCCTT; DROSHA, 5′-GAAGTCACCGTGGAGCTGAGTA and 3′-ATCATTGCATGCTGACAGACATC; DICER1, 5′- GGGAAAGTCTGCAGAACAAAC and 3′-GGCTGTCTGAGCTCTTAGTTC; and AGO2, 5′-CCTGAGAAATGCCCTCGGAGAGTGA and 3′-GACCTCCAGCTCCACCTTGTCCCTG.
Statistics
All the miRNAs were analyzed by 2-way ANOVA within each brain region, with age and treatment as factors. A significant interaction between age and treatment was followed by a Tukey's post hoc test to determine statistically significant differences (P < .05) between groups. A separate Tukey's post hoc test was performed within groups that showed a statistically significant main effect of age and/or treatment. miRNA processing enzymes and target genes were analyzed by 1-way ANOVA within age groups, with treatment as the main factor followed by Tukey's post hoc test. All data are presented as mean ± SEM. Statistical significance was noted when P < .05. All F values and P values are detailed in Supplemental Tables 1–4, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Results
E2 differentially regulated miRNA expression in the vHIPP of aged female rats
Our first objective was to determine whether steady-state miRNA expression in the vHIPP was altered by age (3 vs 18 months) and/or E2 bioavailability. For this initial scan, we used a microarray platform that analyzed a total of 723 previously identified rat miRNAs of which 421 (58.2%) reached detectable levels. Figure 1 shows all of the miRNAs that reached a high threshold of detection and were significantly changed across all groups. Examination of the relative expression levels of the miRNAs expressed in the vHIPP revealed that the majority (288 miRNAs) are expressed at low (<500 signal intensity [SI]) levels (Figure 2A and Supplemental Table 1). We refined our analysis to include only those miRNAs that were expressed at moderate to very high expression levels (133 miRNAs >500 SI). Many of the miRNAs that had high or very high signal intensity in our array, such as miR-9, have previously been shown to be highly expressed in the brain and regulate important neuronal functions (39). Interestingly, some passenger strand miRNAs (formerly denoted as miR*) were among the highest expressed miRNAs, supporting previous studies indicating a functional role for passenger strand miRNAs (58). Notably, 58 additional miRNAs reached statistical significance; however, the signal intensity was low (<500 SI), and these miRNAs were excluded from further analysis. Of the subset that reached statistical significance and had a high signal intensity, 34 were significantly altered by E2 treatment regardless of age, 21 were significantly altered by age alone, and 9 were altered by E2 dependent upon age (Figure 2B). These 9 miRNAs were chosen for further validation using qRT-PCR.
Figure 1.
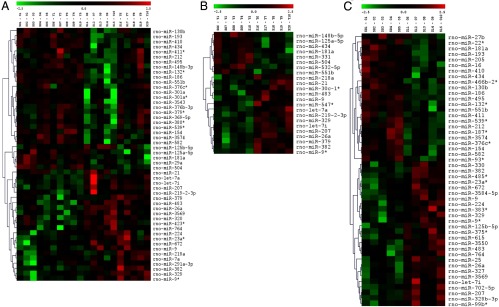
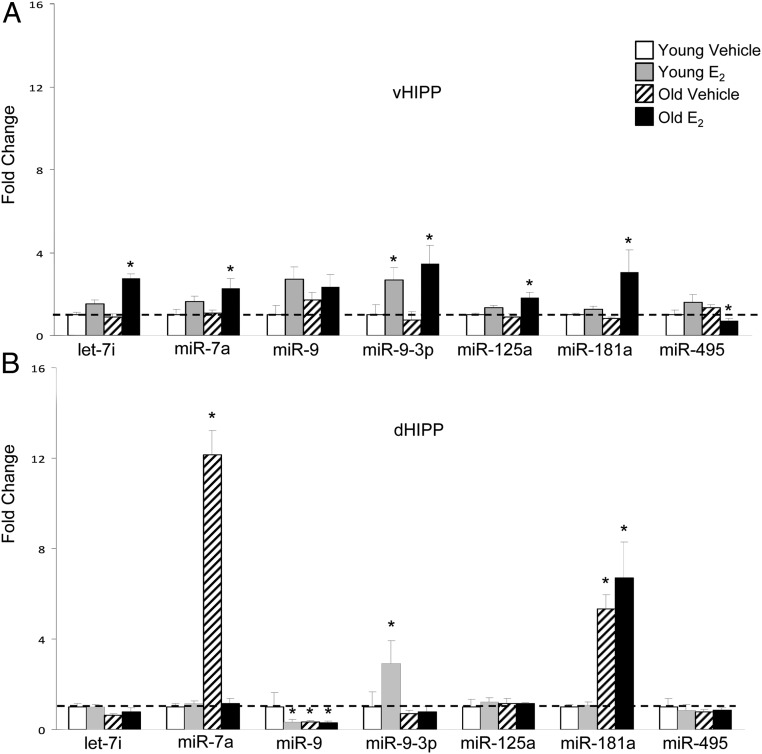
E2 differentially regulates miRNA expression in the vHIPP of young and old female rats. A–C, Heat maps depicting significantly regulated (P < .05) miRNAs in the vHIPP of 3 and 18-month-old (A), 3-month-old (B), and 18-month-old (C) female rats. The expression of an individual miRNA in each sample is shown using a color code where red and green represent high and low fold change, respectively. Samples O1 to O5 and Y1 to Y5 represent 18- and 3-month-old vehicle-treated animals, respectively. Samples O6 to O10 and Y6 to Y10 represent 18- and 3-month-old E2-treated animals, respectively.
Figure 2.
Summary of expression levels and E2/age-regulated miRNAs in the vHIPP of female rats. A, Pie chart depicting the number of miRNAs with very high (>20 000), high (10 000-20 000), moderate (500-10 000), and low (<500) signal in the vHIPP of 3- and 18-month-old ovariectomized female rats. B, A Venn diagram depicting the number of miRNAs regulated by E2, age, and E2/age combined by miRNA microarray analysis.
The vHIPP samples taken from the right hemisphere (contralateral the side used for the microarray) were used to validate the 9 miRNAs that were differentially regulated by E2 dependent upon age, as identified from the microarray. Of the miRNAs chosen for validation, miR-154 and miR-218 failed to reach statistical significance using qRT-PCR and were not pursued further. A significant interaction between treatment and age was observed for let-7i and miR-495 (Table 1) but not the other 5 miRNAs tested. However, a significant main effect of treatment was seen in 6 of the 7 miRNAs tested, and a main effect of age was observed in 2 of 7 miRNAs (see Table 1). E2 significantly increased (P < .05, let-7i, miR-7a, miR-9-3p, miR-125a, and miR-181a) or decreased (miR-495) steady-state expression levels of all of the 7 tested miRNAs in the old animals, whereas only miR-9-3p was increased in the young (Figure 3A). Notably, the only miRNA that was decreased due to E2 treatment in the vHIPP of either age group was miR-495 (Figure 3A). Moreover, unique to the vHIPP, there were no significant changes in any of the 7 E2-regulated miRNAs tested due to age alone (ie, vehicle-treated).
Table 1.
Statistical Analysis of miRNA Expression Levels in the vHIPP
| miRNA | Main Effect |
Interaction: Treatment × Age | |
|---|---|---|---|
| Treatment | Age | ||
| let-7i | Yes (F1,15 = 38.563, P < .001) |
Yes (F1,15 = 7.950, P = .013) |
Yes (F1,15 = 12.258, P = .003) |
| miR-7a | Yes (F1,16 = 7.528, P = .014) |
No | No |
| miR-9 | No | No | No |
| miR-9–3p | Yes (F1,14 = 11.249, P = .005) |
No | No |
| miR-125a | Yes (F1,16 = 14.574, P = .002) |
No | No |
| miR-181a | Yes (F1,16 = 5.109, P = .038) |
No | No |
| miR-495 | No | Yes (F1,16 = 6.677, P = .020) |
Yes (F1,16 = 11.472, P = .004) |
Figure 3.
E2 regulation of miRNA expression in the vHIPP and dHIPP. A and B, qRT-PCR validation of miRNAs in the vHIPP (A) and dHIPP (B) of 3- and 18-month-old ovariectomized rats treated with E2 or vehicle control. Results shown are mean fold change ± SEM as compared with young vehicle-treated animals. Data were analyzed by 2-way ANOVA with age and treatment as factors. Dissimilar symbols indicate a statistically significant difference between groups (P < .05).
E2 regulation of miRNA expression is brain region-specific
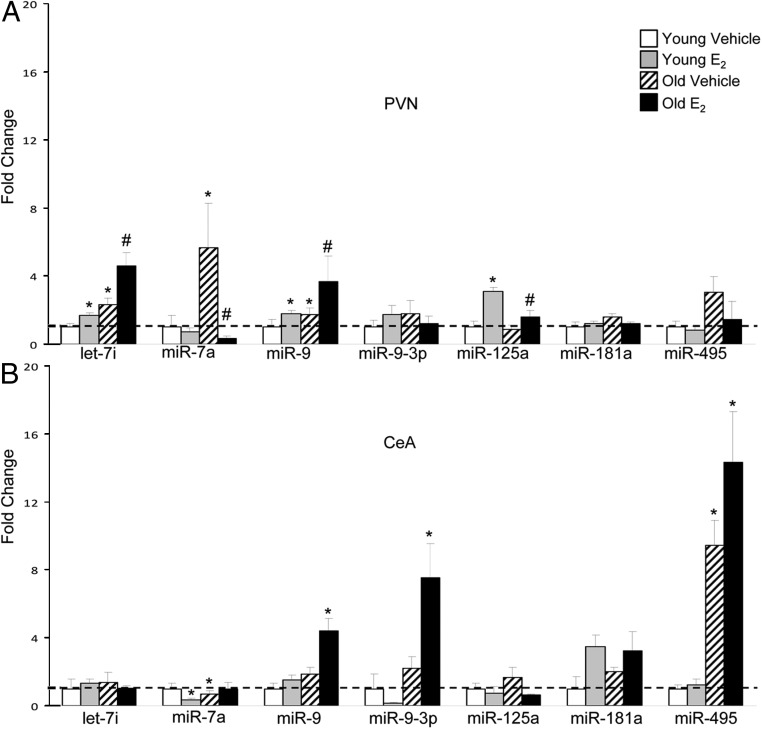
Similar to protein-coding RNA species (ie, mRNA), some studies have shown that miRNAs are expressed in a brain region-specific manner, suggesting they might play an important role in the function of that particular brain region (34, 35, 39). To determine whether E2 regulates the same miRNAs from the vHIPP in other brain regions, we examined 3 additional brain regions that are anatomically and functionally connected to the vHIPP: the dHIPP, the PVN, and the CeA. Across all brain regions, E2 significantly altered the expression levels of the greatest number of miRNAs tested in the vHIPP (2 in young animals and 7 in old animals, Figure 3A), whereas only 3 to 4 miRNAs were significantly changed after E2 treatment in each of the other 3 brain regions (Figures 3B and 4, A and B). Although we observed the greatest number of miRNA changes in the vHIPP, the largest magnitude (>12-fold increase) of E2-induced changes were in the dHIPP and CeA (Figures 3B and 4B). Taken together, miR-7a was the only miRNA that E2 consistently regulated in all 4 brain regions.
Figure 4.
E2 regulation of miRNA expression in the PVN and CeA. A and B, qRT-PCR validation of miRNAs in the PVN (A) and CeA (B) of 3- and 18-month-old ovariectomized rats treated with E2 or vehicle control. Results shown are mean fold change ± SEM as compared young vehicle-treated animals. Data were analyzed by 2-way ANOVA with age and treatment as factors. Dissimilar symbols indicate a statistically significant difference between groups (P < .05).
Dorsal hippocampus
Despite the anatomical proximity of the vHIPP and dHIPP, E2 effects in the dHIPP were substantively different from the vHIPP (Figure 3B). A significant interaction between age and treatment was observed for miR-7a (Supplemental Table 2) but not the other 7 miRNAs tested. A significant main effect of treatment was observed for 2 of 7 and a main effect of age for 5 of 7 miRNAs tested (Supplemental Table 2). Interestingly, 3 of the tested miRNAs showed a significant age-dependent increase in the absence of E2 (miR-7a, miR-9, and miR-181a, P < .05, Figure 3B), with miR-7a being increased by more than 12-fold by age alone. Notably, this observed age-related increase was completely abolished by E2 treatment in the old animals (Figure 3B). Unlike in the vHIPP, miR-9 was significantly decreased by age alone (P < .05), and E2 treatment did not further potentiate this effect (Figure 3B). Similarly, miR-181a was significantly increased with age alone (P < .01) and E2 had no additional effects (Figure 3B).
Paraventricular nucleus
In the PVN, there was a significant interaction between treatment and age for miR-7a only, similar to what we observed in the dHIPP (Supplemental Table 3). A significant main effect of treatment was observed for 4 of 7 and a main effect of age for 4 of 7 miRNAs tested (Supplemental Table 3). Age alone induced significant increases in let-7i and miR-7a, both of which were either potentiated by E2 treatment (ie, let-7i) or reversed to levels significantly below those observed in the young animals (ie, miR-7a) (Figure 4A). Similarly, E2 significantly increased miR-9 in the old animals as compared with the young (Figure 4A). miR-125a was not altered due to age alone; however, treatment with E2 significantly increased miR-125a in the young but did not increase it by the same magnitude in the old PVN (Figure 4A).
Central amygdala
In the CeA, there was a significant interaction between treatment and age in 3 of the 7 miRNAs (Supplemental Table 4). Main effects of treatment and age were seen in 1 of 7, and 3 of 7, miRNAs, respectively (Supplemental Table 4). Post hoc analysis showed that E2 significantly altered 1 miRNA in the young animals and 3 miRNAs in the old animals (Figure 4B). The analysis revealed a significant decrease in miR-7a due to age alone, and this old phenotype was mirrored in the young animals after E2 treatment (P < .01, Figure 4B.) Notably, the CeA is the only brain region where we observed a significant E2-mediated effect (decrease) on miR-7a in the young animals (Figure 4B). Both miR-9 and miR-9-3p were significantly increased by E2 in the old animals but not in the young (Figure 4B). Finally, there was a significant age-related increase in miR-495 (P < .001), which was significantly potentiated after E2 treatment (Figure 4B).
E2 had no effect on miRNA processing in the vHIPP
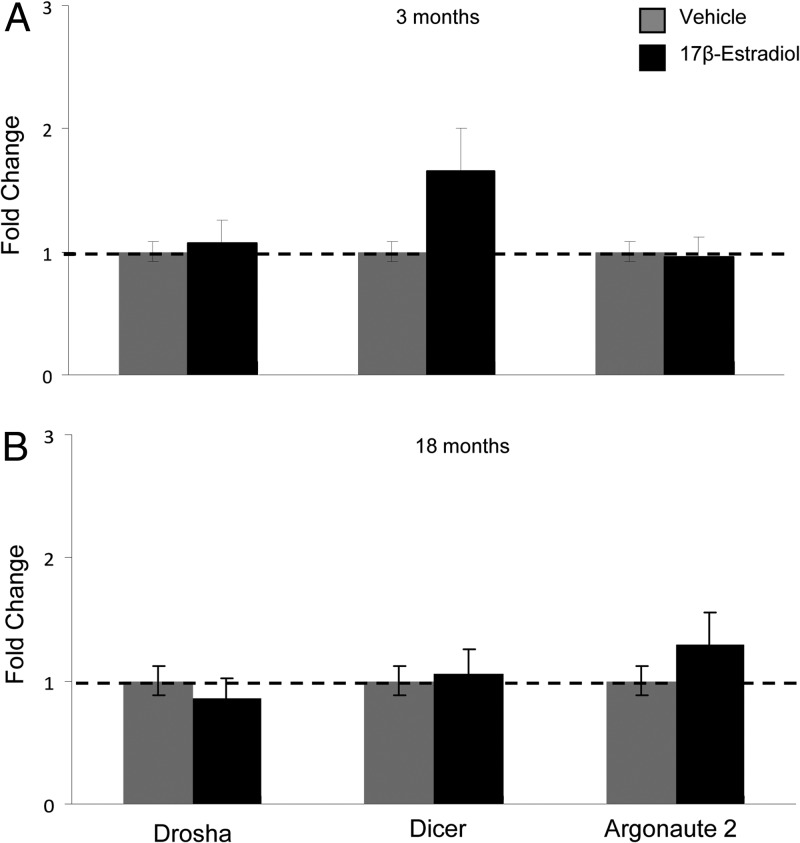
To begin to define the molecular mechanisms for E2 regulation of miRNA in the vHIPP, we measured 3 components of miRNA processing and regulation pathways: drosha, dicer, and argonaute 2 (AGO2) in our young and old animals. Our results showed that E2 treatment had no statistically significant effect on the steady-state expression levels of any of these genes in the vHIPP in either age group (Figure 5), although there was a strong trend for an E2-mediated increased dicer expression in the young animals.
Figure 5.
miRNA processing enzymes are not affected by E2 treatment. qRT-PCR analysis of drosha, dicer, and argonaute 2 in the 3-month-old (A) and 18-month-old (B) vHIPP. Results shown are mean fold change ± SEM as compared young vehicle-treated animals. Data were analyzed by 1-way ANOVA with treatment as a factor. No significant differences were noted (P > .05).
E2-regulated miRNA predicted target genes important for neuronal function.
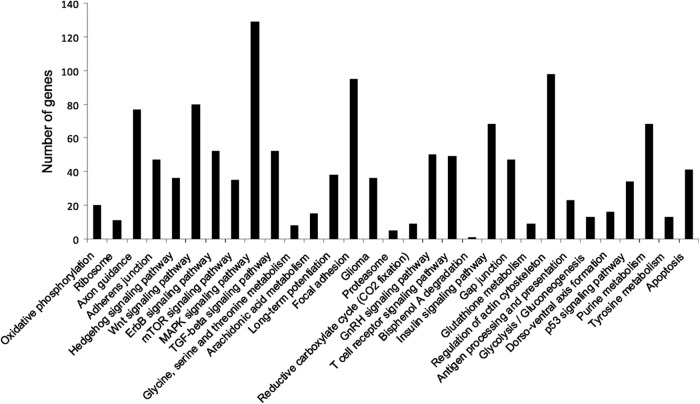
To understand the physiological impact of these E2-regulated miRNAs, we analyzed potential mRNA targets for each miRNA using 4 different target prediction programs (DIANA-mirpath, Targetscan, microRNA.org, and MicroCosm) (59–63). The predicted gene targets were represented in several cellular pathways including neuronal specific pathways, such as long-term potentiation and axon guidance (Figure 6). Putative targets were chosen for testing in our vHIPP tissue samples based on their known role in neuronal function and their algorithm score, as determined by the computer modeling programs (Figure 7A). Degradation of mRNA transcripts is one mechanism whereby miRNAs decrease the protein expression of their target genes (12, 13), and previous studies have demonstrated an inverse correlation of miRNA expression with their target mRNA expression (6). To determine whether there was an inverse correlation between the E2-regulated miRNAs in the vHIPP and their predicted targets, we measured SIRT1, GR, BDNF, and GABRA1 mRNA expression. Consistent with our expected outcomes, SIRT1 mRNA expression was significantly decreased in the vHIPP of young animals (Figure 7B), which inversely corresponded to SIRT1 predicted miRNAs, miR-9 (Figures 3A and 7B). Moreover, E2 significantly increased GR mRNA levels in the young animals, although miRNA-181a was unaffected by E2 in that same age group, suggesting that multiple miRNAs could target GR. E2 had no effect on GABRA1 or BDNF mRNA expression at either age (Figure 7B), although there was a strong trend for an E2-mediated increase in BDNF in the young animals.
Figure 6.
Predicted cellular pathways of potential target genes of identified miRNAs. DIANA-mirpath was used to predict KEGG cellular pathways impacted by potential target genes of significantly regulated miRNAs in the vHIPP of female rats. Results are depicted as the number of genes targeted by miRNAs (y-axis) in each cellular pathway (x-axis).
Figure 7.
Predicted gene targets of identified miRNAs are regulated by E2. A, Table of predicted miRNA gene targets determined based on algorithm scores of 3 computer prediction programs: Targetscan, microRNA.org, and MicroCosm. B and C, qRT-PCR for sirtuin 1 (SIRT1), glucocorticoid receptor (GR), GABA receptor A1 (GABRA1), and brain-derived neurotrophic factor (BDNF) in the 3-month-old (B), and 18-month-old (C) vHIPP. Results shown are mean fold change ± SEM as compared young vehicle-treated animals. Data were analyzed by one-way ANOVA with treatment as a factor. * indicates statistically significant difference (P < .05).
Discussion
Two novel findings emerged from these studies. First, we demonstrated that E2 is a critical regulator of mature miRNA expression levels in the brain and that the magnitude of E2 action differs according to specific brain regions. Second, we showed that even within a specific brain region, the effects of E2 on miRNA levels are temporally distinct. To our knowledge, this is the first evidence demonstrating E2 regulation of miRNA expression in the brain, although Morgan and Bale showed that blocking E2 synthesis altered miRNA expression in neonates (69). Therefore our data suggest that this may be an important mechanistic pathway for E2 modulation of neuronal target genes resulting in a fine tuning of gene expression. This concept of fine tuning has gained critical recognition as an important determinant of cellular function and is especially vital in the brain, because communication between neurons requires rapid modulation of gene expression at the synapses where local translation of proteins is crucial.
Our data underscore the concept that E2 can have completely different actions in an aged system compared with a young one, although more time points are needed to ascertain the linearity of these changes. Overall, we observed significant interactions between age and E2 treatment for 5 specific miRNAs: let-7i, miR-7a, miR-9, miR-9-3p, and miR-495. Interestingly, most of the brain regions had unique miRNAs that were significantly affected by both age and treatment with the notable exception of miR-7a, which was altered in 3 of the 4 brain regions analyzed. Putative gene targets for miR-7a include SIRT1, which is critically important for mediating anxiety and memory, among other things (43, 64). In this study, we measured a relatively short time frame between surgically induced menopause (ovariectomy) and hormone replacement (ie, 7 days), and the changes we observed might vary greatly under conditions with longer periods of E2 withdrawal. Longer periods of E2 withdrawal can alter multiple factors including estrogen receptor (ER) levels. ERα has been shown to physically associate with drosha and participate in the maturational processing of several miRNAs (65). Therefore, the length of E2 deprivation could have a significant impact on the expression of mature miRNAs leading to differential gene target expression profiles. Another important observation from these data was the revelation that large-scale global changes in miRNA expression levels were not observed, because only a small subset of the total 723 miRNAs were altered by age and hormone treatment. These results suggest that most miRNAs are likely key regulators of fundamental cellular processes that could be detrimental to have under the control of fluctuating steroid hormones.
The primary focus of our study was to determine the effects of E2 on miRNA expression between young and old animals, however the data revealed several miRNAs that were regulated by age alone. Importantly, our data show for the first time that age-dependent miRNA expression varied across brain regions, suggesting that the brain does not age uniformly. For instance, none of the miRNAs that we tested were significantly increased by age alone in the vHIPP. This observation was sharply contrasted by the results from the other 3 brain regions that each had 2 or 3 miRNAs that were regulated by age alone. Perhaps the most striking of these were the results of miR-7a, which had a 12-fold increase in the old dHIPP compared with that of young animals. Interestingly, this magnitude of effect was not observed for miR-7a in any of the other brain regions, suggesting that miR-7a could have significant biological consequences for dHIPP function in old animals. Most studies that have investigated age-dependent changes in mRNA and/or miRNA have been analyzed in only 1 brain region (prefrontal cortex) or in the entire brain as a whole (4, 6, 66–68, 70). Although these studies have provided important observations of age-dependent gene expression changes in the brain, the conclusions relating to brain region-specific functions and/or pathologies are limited by the brain samples used.
One of the more intriguing findings from our study was the observation that E2 differentially regulated miR-9 and miR-9-3p. These 2 functionally mature miRNAs are derived from the same primary miRNA transcript (pri-miRNA), yet typically 1 strand of the cleaved duplex is preferentially recruited to the RISC and becomes the biological effector, whereas the other strand undergoes degradation. Because the 2 strands are inverse complements, it is likely that they have vastly discrepant target genes and several studies have demonstrated biologically functional roles for selected miRNA passenger strands (72, 73). In our studies, E2 decreased miR-9 expression in the dHIPP of 3-month-old animals while simultaneously increasing the expression of miR-9-3p. Conversely, by 18 months of age, miR-9-3p was no longer significantly regulated by E2, yet the E2-induced reduction of miR-9 remained the same. The differential regulation of miR-9 and miR-9-3p suggest that a separate cohort of target genes may be affected in the aged vHIPP, which could have a significant impact on the hippocampal-mediated control of emotion/stress. Because miR-9/9-3p sequences are on the same primary miRNA transcript, it stands to reason that E2 regulates the processing of the pri-miRNA to favor the retention of miR-9-3p sequence over the miR-9 in the young brain. The miR-9-3p strand selection likely occurs after the enzyme dicer has cleaved the precursor miRNA (pre-miRNA) to form the mature miRNA duplex because the mature miRNA duplex still contains both miR-9/9-3p sequences. Therefore, one possibility is that E2 facilitates the retention of miR-9-3p by the RISC longer than miR-9. Alternatively, E2 could mediate the active degradation of miR-9 but not miR-9-3p; however, the mechanisms by which miRNAs are targeted for degradation are still largely unresolved.
The pleiotropic nature of E2 action suggests that the mechanisms underlying those actions are equally diverse. Conventional views dictate that a majority of E2 effects are due to direct transcriptional regulation of target genes. Consequently, the precise molecular mechanisms that mediate E2 actions on miRNA expression levels remain unresolved, especially in the in vivo system. One possibility is that E2 regulates miRNA processing, through modulation of key enzymes such as drosha or dicer, or by altering components of the RISC, such as argonaute 2. In our studies, E2 did not cause significant changes in any of these parameters. These observations led us to hypothesize that that E2 might act directly at the promoter level to modulate pri-miRNA transcription. E2 actions are mediated primarily by two high-affinity nuclear receptors, ERα and ERβ, and both subtypes are expressed in the vHIPP, although ERβ is expressed at higher levels and in different hippocampal divisions compared with ERα (74–76). Moreover, the temporal expression of ER subtypes in the brain has been well documented (72, 77), and it is exciting to postulate that some of the differential E2-mediated effects on miRNA expression are due to age-related changes in ER subtype expression. Interestingly, previous studies have shown that E2 can act through both ER subtypes to regulate miRNA expression in cancer cell models (18, 20). In these cell models, E2 has been shown not only to regulate miRNA transcription but also to play a role in the processing of the miRNAs via direct ERα interaction with components of the drosha processing complex (20).
The functional relevance of miRNAs is the focus of intensive investigation, and as such, defining the specific targets for these miRNAs is of paramount importance. We identified miRNAs uniquely regulated by E2 and then used several different computer prediction algorithms to pinpoint putative target genes. Sirtuin 1 (SIRT1), which is a protein deacetylase that first gained recognition for its effects on longevity (78–80), was identified as such a target gene. Recent research also showed SIRT1 to be important for energy balance, memory, anxiety, and neuroprotection in the brain (43, 64). Our data demonstrated an inverse correlation between miR-9 and SIRT1 expression at 3 months, which is consistent with a recent report revealing a complementary region on the SIRT1 3′-untranslated region with the miR-9 seed sequence (81). Two other putative miRNAs that target SIRT1 is miR-7a and miR-495. Compared with any miRNA tested, our data showed that miR-7a and miR-495 had the largest magnitudes of age-dependent change (ie, greater than 12-fold increase). Strikingly, E2 treatment completely abolished the age-related effect on miR-7a. This observation is in line with recent evidence in steroid hormone-regulated cancer models suggesting that SIRT1 interacts with the ER to regulate transcriptional activity (73). Overall, our observations raise the possibility that E2-activated ERs may be another pathway whereby SIRT1 activity is regulated in the brain. Other predicted gene targets in this study, such as GR and GABRA1, did not show a direct correlation between their expression level and the miRNA expression level. This observation may indicate that the E2-regulated miRNAs induced translational repression, as opposed to degradation of their target mRNA transcripts, or that multiple miRNAs are required to act in concert to achieve functional repression of an mRNA target. The mechanism that leads to miRNA-mediated mRNA degradation or translational repression is not well understood, although it is clear that both processes are equally viable mechanisms of miRNA action on their target genes (65, 71, 82). Taken together, our data clearly indicate that E2-regulated miRNA expression in the brain is age-dependent and the predicted targets of these miRNAs have important implications for neuronal function. Further validation of these and other potential targets are necessary, because computer algorithms are not well developed enough to accurately predict true targets of miRNAs in a specific cell type. Moreover, it is difficult to determine from these algorithms whether one miRNA or several miRNAs acting in concert would be required and sufficient to regulate a single mRNA.
Although our data show that E2-regulated miRNA expression is altered in the aged brain as opposed to the young brain, it is not certain at this time whether this is dysregulation that would result in a net increase in adverse effects or protective mechanisms. The in vivo paradigm presented here offers a broad picture of the impact E2 has on miRNA expression in the brain and how those effects might be altered by aging. There are inherent limitations using an in vivo paradigm, many of which preclude elucidating the precise molecular pathways of E2 action. Overall, our data reveal a novel temporal- and neuroanatomical-dependent regulation of miRNAs by E2, thereby furthering our understanding of the complex mechanisms for E2 in regulating neuronal functions.
Supplementary Material
Acknowledgments
This work was funded by National Institutes of Health Grant AG033605 (to T.R.P.).
Disclosure Summary: Y.S.R., N.N.M., Y.W., W.C.J.C., and T.R.P. have nothing to disclose and attest that there are no conflicts of interest.
For editorial see page 2570
- CeA
- central amygdala
- dHIPP
- dorsal hippocampus
- E2
- 17β-estradiol
- ER
- estrogen receptor
- miRNA
- microRNA
- pri-miRNA
- primary miRNA
- PVN
- paraventricular nucleus
- qRT-PCR
- quantitative RT-PCR
- RISC
- RNA-induced silencing complex
- SI
- signal intensity
- vHIPP
- ventral hippocampus.
References
- 1. Ibáñez-Ventoso C, Yang M, Guo S, Robins H, Padgett RW, Driscoll M. Modulated microRNA expression during adult lifespan in Caenorhabditis elegans. Aging Cell. 2006;5:235–246 [DOI] [PubMed] [Google Scholar]
- 2. Inukai S, de Lencastre A, Turner M, Slack F. Novel microRNAs differentially expressed during aging in the mouse brain. PLoS One. 2012;7:e40028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kato M, Chen X, Inukai S, Zhao H, Slack FJ. Age-associated changes in expression of small, noncoding RNAs, including microRNAs, in C. elegans. RNA. 2011;17:1804–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li N, Bates DJ, An J, Terry DA, Wang E. Up-regulation of key microRNAs, and inverse down-regulation of their predicted oxidative phosphorylation target genes, during aging in mouse brain. Neurobiol Aging. 2011;32:944–955 [DOI] [PubMed] [Google Scholar]
- 5. Persengiev S, Kondova I, Otting N, Koeppen AH, Bontrop RE. Genome-wide analysis of miRNA expression reveals a potential role for miR-144 in brain aging and spinocerebellar ataxia pathogenesis. Neurobiol Aging 2011;32:2316 e2317–2327 [DOI] [PubMed] [Google Scholar]
- 6. Somel M, Guo S, Fu N, et al. MicroRNA, mRNA, and protein expression link development and aging in human and macaque brain. Genome Res. 2010;20:1207–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee Y, Kim M, Han J, et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hutvágner G, McLachlan J, Pasquinelli AE, Bálint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838 [DOI] [PubMed] [Google Scholar]
- 10. Ketting RF, Fischer SE, Bernstein E, Sijen T, Hannon GJ, Plasterk RH. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001;15:2654–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee Y, Ahn C, Han J, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419 [DOI] [PubMed] [Google Scholar]
- 12. Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63 [DOI] [PubMed] [Google Scholar]
- 15. Castellano L, Giamas G, Jacob J, et al. The estrogen receptor-α-induced microRNA signature regulates itself and its transcriptional response. Proc Natl Acad Sci U S A. 2009;106:15732–15737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuokkanen S, Chen B, Ojalvo L, Benard L, Santoro N, Pollard JW. Genomic profiling of microRNAs and messenger RNAs reveals hormonal regulation in microRNA expression in human endometrium. Biol Reprod. 2010;82:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nothnick WB, Healy C, Hong X. Steroidal regulation of uterine miRNAs is associated with modulation of the miRNA biogenesis components Exportin-5 and Dicer1. Endocrine. 2010;37:265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paris O, Ferraro L, Grober OM, et al. Direct regulation of microRNA biogenesis and expression by estrogen receptor β in hormone-responsive breast cancer. Oncogene. 2012;31:4196–4206 [DOI] [PubMed] [Google Scholar]
- 19. Wickramasinghe NS, Manavalan TT, Dougherty SM, Riggs KA, Li Y, Klinge CM. Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells. Nucleic Acids Res. 2009;37:2584–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamagata K, Fujiyama S, Ito S, et al. Maturation of microRNA is hormonally regulated by a nuclear receptor. Mol Cell. 2009;36:340–347 [DOI] [PubMed] [Google Scholar]
- 21. Fernandez SM, Lewis MC, Pechenino AS, et al. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu F, Day M, Muñiz LC, et al. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343 [DOI] [PubMed] [Google Scholar]
- 23. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958 [DOI] [PubMed] [Google Scholar]
- 24. Zandi PP, Carlson MC, Plassman BL, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 2002;288:2123–2129 [DOI] [PubMed] [Google Scholar]
- 25. Choi SD, Steinberg EM, Lee HH, Naftolin F. The Timing Hypothesis remains a valid explanation of differential cardioprotective effects of menopausal hormone treatment. Menopause. 2011;18:230–236 [PubMed] [Google Scholar]
- 26. Hamilton RT, Rettberg JR, Mao Z, et al. Hippocampal responsiveness to 17β-estradiol and equol after long-term ovariectomy: implication for a therapeutic window of opportunity. Brain Res. 2011;1379:11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci. 2006;26:10332–10348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hibberd C, Yau JL, Seckl JR. Glucocorticoids and the ageing hippocampus. J Anat. 2000;197(Pt 4):553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Belujon P, Grace AA. Hippocampus, amygdala, and stress: interacting systems that affect susceptibility to addiction. Ann N Y Acad Sci. 2011;1216:114–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dong HW, Swanson LW, Chen L, Fanselow MS, Toga AW. Genomic-anatomic evidence for distinct functional domains in hippocampal field CA1. Proc Natl Acad Sci U S A. 2009;106:11794–11799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thompson CL, Pathak SD, Jeromin A, et al. Genomic anatomy of the hippocampus. Neuron. 2008;60:1010–1021 [DOI] [PubMed] [Google Scholar]
- 34. Amar L, Benoit C, Beaumont G, et al. MicroRNA expression profiling of hypothalamic arcuate and paraventricular nuclei from single rats using Illumina sequencing technology. J Neurosci Methods. 2012;209:134–143 [DOI] [PubMed] [Google Scholar]
- 35. Bak M, Silahtaroglu A, Møller M, et al. MicroRNA expression in the adult mouse central nervous system. RNA. 2008;14:432–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. He M, Liu Y, Wang X, Zhang MQ, Hannon GJ, Huang ZJ. Cell-type-based analysis of microRNA profiles in the mouse brain. Neuron. 2012;73:35–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hua YJ, Tang ZY, Tu K, et al. Identification and target prediction of miRNAs specifically expressed in rat neural tissue. BMC Genomics. 2009;10:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Juhila J, Sipila T, Icay K, et al. MicroRNA expression profiling reveals miRNA families regulating specific biological pathways in mouse frontal cortex and hippocampus. PLoS One. 2011;6:e21495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Olsen L, Klausen M, Helboe L, Nielsen FC, Werge T. MicroRNAs show mutually exclusive expression patterns in the brain of adult male rats. PLoS One. 2009;4:e7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Parsons MJ, Grimm CH, Paya-Cano JL, et al. Using hippocampal microRNA expression differences between mouse inbred strains to characterise miRNA function. Mamm Genome. 2008;19:552–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cohen JE, Lee PR, Chen S, Li W, Fields RD. MicroRNA regulation of homeostatic synaptic plasticity. Proc Natl Acad Sci U S A. 2011;108:11650–11655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Conaco C, Otto S, Han JJ, Mandel G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc Natl Acad Sci U S A. 2006;103:2422–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gao J, Wang WY, Mao YW, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Konopka W, Kiryk A, Novak M, et al. MicroRNA loss enhances learning and memory in mice. J Neurosci. 2010;30:14835–14842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lambert TJ, Storm DR, Sullivan JM. MicroRNA132 modulates short-term synaptic plasticity but not basal release probability in hippocampal neurons. PLoS One. 2010;5:e15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Magill ST, Cambronne XA, Luikart BW, et al. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc Natl Acad Sci U S A. 2010;107:20382–20387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schaefer A, O'Carroll D, Tan CL, et al. Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med. 2007;204:1553–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schratt GM, Tuebing F, Nigh EA, et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289 [DOI] [PubMed] [Google Scholar]
- 49. Wu D, Raafat A, Pak E, Clemens S, Murashov AK. Dicer-microRNA pathway is critical for peripheral nerve regeneration and functional recovery in vivo and regenerative axonogenesis in vitro. Exp Neurol. 2012;233:555–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–1541 [DOI] [PubMed] [Google Scholar]
- 51. Haramati S, Navon I, Issler O, et al. MicroRNA as repressors of stress-induced anxiety: the case of amygdalar miR-34. J Neurosci. 2011;31:14191–14203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miller BH, Zeier Z, Xi L, et al. MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proc Natl Acad Sci U S A. 2012;109:3125–3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moreau MP, Bruse SE, David-Rus R, Buyske S, Brzustowicz LM. Altered microRNA expression profiles in postmortem brain samples from individuals with schizophrenia and bipolar disorder. Biol Psychiatry. 2011;69:188–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Packer AN, Xing Y, Harper SQ, Jones L, Davidson BL. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington's disease. J Neurosci. 2008;28:14341–14346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sethi P, Lukiw WJ. Micro-RNA abundance and stability in human brain: specific alterations in Alzheimer's disease temporal lobe neocortex. Neurosci Lett. 2009;459:100–104 [DOI] [PubMed] [Google Scholar]
- 56. Tébar M, Ruíz A, Bellido C, Sánchez-Criado JE. Ovary mediates the effects of RU486 given during proestrus on the diestrous secretion of luteinizing hormone in the rat. Biol Reprod. 1996;54:1266–1270 [DOI] [PubMed] [Google Scholar]
- 57. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 58. Yang JS, Phillips MD, Betel D, et al. Widespread regulatory activity of vertebrate microRNA* species. RNA. 2011;17:312–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20 [DOI] [PubMed] [Google Scholar]
- 62. Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: targets and expression. Nucleic Acids Res. 2008;36:D149–D153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vlachos IS, Kostoulas N, Vergoulis T, et al. DIANA miRPath v.2.0: investigating the combinatorial effect of microRNAs in pathways. Nucleic Acids Res. 2012;40:W498–W504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Libert S, Pointer K, Bell EL, et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell. 2011;147:1459–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang QG, Raz L, Wang R, et al. Estrogen attenuates ischemic oxidative damage via an estrogen receptor α-mediated inhibition of NADPH oxidase activation. J Neurosci. 2009;29:13823–13836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Inukai S, de Lencastre A, Turner M, Slack F. Novel microRNAs differentially expressed during aging in the mouse brain. PloS one 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kumar A, Gibbs J, Beilina A, et al. Age-associated changes in gene expression in human brain and isolated neurons. Neurobiol Aging. 2013;34:1199–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25:294–297 [DOI] [PubMed] [Google Scholar]
- 69. Morgan CP, Bale TL. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci. 2011;31(33):11748–11755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lu T, Pan Y, Kao SY, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891 [DOI] [PubMed] [Google Scholar]
- 71. Yang LC, Zhang QG, Zhou CF, et al. Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus. PLoS One 5:e9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wu D, Gore AC. Changes in androgen receptor, estrogen receptor α, and sexual behavior with aging and testosterone in male rats. Horm Behav. 2010;58:306–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Moore RL, Dai Y, Faller DV. Sirtuin 1 (SIRT1) and steroid hormone receptor activity in cancer. J Endocrinol. 2012;213:37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-α and -β mRNA within the female rat brain. Brain Res Mol Brain Res. 1998;54:175–180 [DOI] [PubMed] [Google Scholar]
- 75. Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor β immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436:64–81 [PubMed] [Google Scholar]
- 76. Shima N, Yamaguchi Y, Yuri K. Distribution of estrogen receptor β mRNA-containing cells in ovariectomized and estrogen-treated female rat brain. Anat Sci Int. 2003;78:85–97 [DOI] [PubMed] [Google Scholar]
- 77. Walker DM, Juenger TE, Gore AC. Developmental profiles of neuroendocrine gene expression in the preoptic area of male rats. Endocrinology. 2009;150:2308–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128 [DOI] [PubMed] [Google Scholar]
- 79. Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–268 [DOI] [PubMed] [Google Scholar]
- 81. Schonrock N, Humphreys DT, Preiss T, Götz J. Target gene repression mediated by miRNAs miR-181c and miR-9 both of which are down-regulated by amyloid-β. J Mol Neurosci. 2012;46:324–335 [DOI] [PubMed] [Google Scholar]
- 82. Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–1124 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.