Abstract
Kisspeptin (Kiss1) signaling to GnRH neurons is widely acknowledged to be a prerequisite for puberty and reproduction. Animals lacking functional genes for either kisspeptin or its receptor exhibit low gonadotropin secretion and infertility. Paradoxically, a recent study reported that genetic ablation of nearly all Kiss1-expressing neurons (Kiss1 neurons) does not impair reproduction, arguing that neither Kiss1 neurons nor their products are essential for sexual maturation. We posited that only minute quantities of kisspeptin are sufficient to support reproduction. If this were the case, animals having dramatically reduced Kiss1 expression might retain fertility, testifying to the redundancy of Kiss1 neurons and their products. To test this hypothesis and to determine whether males and females differ in the required amount of kisspeptin needed for reproduction, we used a mouse (Kiss1-CreGFP) that has a severe reduction in Kiss1 expression. Mice that are heterozygous and homozygous for this allele (Kiss1Cre/+ and Kiss1Cre/Cre) have ∼50% and 95% reductions in Kiss1 transcript, respectively. We found that although male Kiss1Cre/Cre mice sire normal-sized litters, female Kiss1Cre/Cre mice exhibit significantly impaired fertility and ovulation. These observations suggest that males require only 5% of normal Kiss1 expression to be reproductively competent, whereas females require higher levels for reproductive success.
Reproduction in mammals requires active GnRH neurons, which represent the final output of the brain to the pituitary. Over the past decade, evidence has accumulated to support the idea that GnRH neurons themselves require direct stimulation by kisspeptin to drive reproduction, both to initiate puberty and coordinate reproductive function in the adult. First, mutations in the genes that encode either kisspeptin (KISS1/Kiss1) or its receptor (KISS1R/Kiss1r) thwart sexual maturation in mice and humans (1–4), and individuals bearing such mutations exhibit hypogonadotropic hypogonadism and infertility. Second, kisspeptin acts directly on GnRH neurons to excite their activity (2, 5–7). Third, exogenous administration of GnRH rescues gonadotropin secretion in humans and mice with KISS1R/Kiss1r mutations (4). However, a recent study cast doubt on whether Kiss1 neurons are absolutely necessary for reproductive function (8). In that study, Kiss1-expressing cells in the brain (and elsewhere in the body) were congenitally ablated in Kiss1-Cre mice by a diphtheria toxin-mediated strategy, yet female mice that bore this lesion exhibited normal puberty onset and fertility. These results suggest either that Kiss1 neuron-dependent signaling in the brain is dispensable for reproduction or that some residual action of Kiss1-GnRH signaling persists in those animals. The authors of the ablation study suggest that compensatory mechanisms rescue reproduction when Kiss1 neurons are ablated during development. Indeed, studies of other pathways have shown that successful compensation occurs with neuronal ablation during neonatal life, but not in adulthood. For example, when agouti-related peptide (AgRP)/neuropeptide Y (NPY) neurons are destroyed in neonatal mice, there is little effect on feeding, whereas similar ablations in adults produce unremitting anorexia and death (9). However, unlike the mutations in Kiss1 and Kiss1r, mutations in Agrp and Npy produce only a mild phenotype, consistent with the idea that other neuromodulators compensate for the loss of AgRP and NPY function. We dispute the idea that compensation is sufficient to explain the phenotype of mice with putative ablations of their Kiss1 neurons and argue that expression of Kiss1 by a few remaining neurons explains their reproductive phenotype.
In light of the controversy about the requirement for kisspeptin in reproduction, we hypothesized that kisspeptin signaling is safeguarded by production of kisspeptin far in excess of what is required to support reproductive function. There is precedence for the notion of superfluity in other cellular and molecular features of the neuroendocrine reproductive axis. For example, ∼10% of the normal population of GnRH neurons is sufficient for reproduction (8, 10–12), so it seems plausible that an overproduction of kisspeptin represents yet another failsafe to guarantee success. To test this hypothesis, we examined the reproductive phenotype of mice with a 95% reduction in Kiss1 transcript levels and showed that males are virtually normal, whereas females are subfertile.
Materials and Methods
Animals
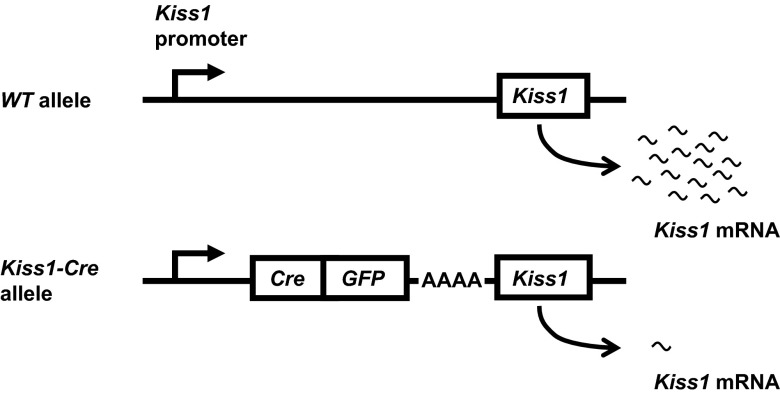
To execute these studies, we used wild-type (WT), Kiss1 knockout (KO) (3), and Kiss1-CreGFP knock-in mice described previously (13). To create the Kiss1-Cre knock-in mouse, a Cre-GFP cassette with a polyadenine (poly-A) tail was inserted upstream of the translation start site for Kiss1, which retained an in-frame coding sequence (Figure 1). The poly-A tail was engineered to derail RNA polymerase from the DNA template before reaching the Kiss1 gene, resulting in low transcript levels. Heterozygous Kiss1-Cre mice (Kiss1Cre/+) were backcrossed onto the C57BL/6J background 2 or 5 generations and bred to homozygosity to produce Kiss1Cre/Cre mice. WT littermates were always used as controls to reduce effects of genetic background. Animals were housed in a 14-hour light, 10-hour dark cycle. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Washington, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Kiss1 KO mice and their corresponding WT controls in experiment 3 were group housed at the Massachusetts General Hospital Center for Comparative Medicine in a temperature-controlled environment in a 12-hour light, 12-hour dark cycle, and food and water were provided ad libitum. All procedures were approved by the Subcommittee on Research Animal Care of Massachusetts General Hospital.
Figure 1.
Kiss1-CreGFP construct knocked into the Kiss1 locus to reduce Kiss1 transcription. Top, Schematic of WT Kiss1 allele. Bottom, Schematic of Kiss1-CreGFP allele described in detail in Ref. 13. The polyadenine tail dramatically reduces transcription of the Kiss1 gene.
Experimental design
Experiment 1: effects of Kiss1 transgene on Kiss1 transcript and hormones
The purpose of this experiment was to assess levels of Kiss1 mRNA and gonadotropins in Kiss1Cre/Cre and littermate control mice. To maximize Kiss1 expression in the mediobasal hypothalamus (MBH), mice of each genotype were gonadectomized (GDX) (n = 6–8 females and 4–5 males per group). To measure Kiss1 expression in the anteroventral periventricular nucleus (AVPV), a different subset of female mice (n = 6 per group) were GDX and received an implant containing 20 μL of 1 mg/mL 17β-estradiol (E2) dissolved in sesame oil. Implants were made from SILASTIC tubing having 1.47 mm inner diameter and 1.96 mm outer diameter, cut to 15 mm in length (Dow Corning, Elizabethtown, Kentucky) and sealed with silicone adhesive sealant 2.5 mm away from each end. One week after surgery, blood was collected from the orbital sinus for gonadotropin measurements. Brains were removed, after which the MBH or AVPV/preoptic area was cut in a mouse brain matrix (Zivic Labs, Pittsburgh, Pennsylvania), frozen on dry ice, and stored at −80°C until RNA was extracted with a QIAGEN (Valencia, California) RNeasy lipid tissue kit. Quantitative RT-PCR (qRT-PCR) was performed as described below in the Quantitative RT-PCR section.
Experiment 2: detection of kisspeptin by immunohistochemistry
To determine whether kisspeptin levels were reduced in Kiss1Cre/Cre animals, we stained sections of the arcuate nucleus (ARC) from WT, Kiss1Cre/Cre, and Kiss1 KO mice with a sheep kisspeptin antibody from Alain Caraty (AC053). This antibody was raised in sheep against a 14–amino-acid peptide fragment close to the N terminus of mouse Kp-52. Peptide PPVEGPAGRQRPLC (mouse Kp-52: 5–18) was synthesized and coupled to keyhole limpet hemocyanin at its C-terminal end (GenCust, Dudelange, Luxembourg) and used as the immunogen in the sheep as previously described (14). Specific anti-kisspeptin antibodies were further purified from the recovered serum on an affinity column with the corresponding peptide (Eurogentec, Seraing, Belgium). Control experiments to assess specificity of the AC053 antisera were performed either by preadsorption with murine Kp-52 peptide or by staining hypothalamic brain sections from adult Kiss1 KO male mice. In both conditions, AC053 staining was completely absent from the brain sections.
Mice (n = 4 per group) were castrated and perfused 1 week later with 4% paraformaldehyde (PFA). Brains were postfixed overnight in paraformaldehyde, cryopreserved in 30% sucrose in PBS, and sectioned at 35 μm. Free-floating sections were incubated at a 1:3000 concentration of AC053 antibody for 5 days at 4°C. The secondary antibody was an Alexa-555 donkey antisheep antibody (1:250) (Invitrogen, Carlsbad, California). Sections were imaged with a Zeiss LSM5 Pascal confocal microscope.
Experiment 3: bioassay for endogenous kisspeptin signaling in Kiss1Cre/Cre mice
The purpose of this experiment was to determine whether the low levels of kisspeptin activity in Kiss1Cre/Cre mice could stimulate GnRH/LH release. To assess this possibility, we challenged WT and Kiss1Cre/Cre mice with senktide, an agonist of the neurokinin-3 receptor (NK3R), which is expressed by Kiss1 neurons. Because senktide stimulates GnRH/LH release only if kisspeptin signaling is intact (15, 16), we could deduce that if we observed an increase in LH levels in response to senktide, there must be residual kisspeptin signaling capacity in the Kiss1Cre/Cre mice. To confirm that senktide is unable to stimulate LH release in animals that lack a functional Kiss1 gene, we injected WT or Kiss1 KO mice (n = 5–12 per group) sc with 30 nmol senktide (Tocris Bioscience, Ellisville, Missouri) in 0.1 mL PBS or PBS alone. All animals were confirmed to be in diestrus the morning of treatment by vaginal cytology. Blood was collected 30 minutes after injection by submandibular bleed or cardiac puncture and processed for LH RIAs by the University of Virginia Research in Reproduction Ligand Assay Core.
To test whether senktide stimulates LH in Kiss1Cre/Cre mice, we injected either senktide (600 pmol in 2 μL from Tocris Bioscience) or vehicle (sterile saline) into the lateral cerebral ventricle of WT and Kiss1Cre/Cre female mice (n = 7–10 per group) (15). Before the injection, female mice were cycled for at least 2 consecutive estrous cycles and injected on diestrus. Blood was collected from the orbital sinus 30 minutes after the injection between 11:30 am and 3:00 pm.
Experiment 4: development of secondary sexual characteristics in Kiss1Cre/Cre mice
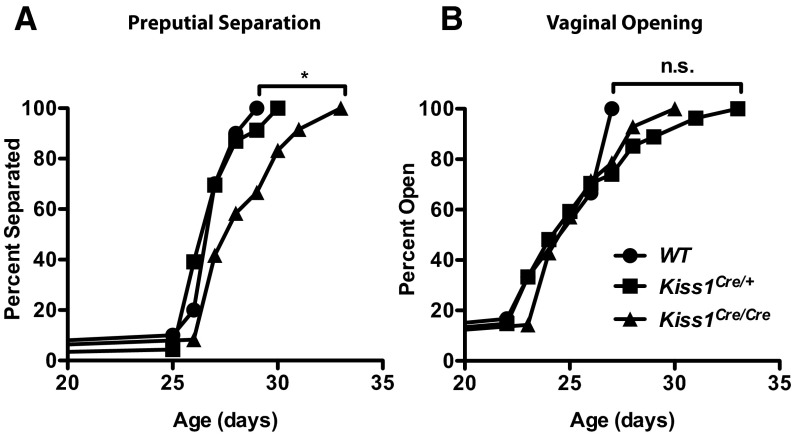
The purpose of this experiment was to determine whether Kiss1Cre/Cre mice had delayed development of secondary sexual characteristics, which depend on testosterone (T) or E2. Starting on postnatal day 21, Kiss1Cre/Cre, Kiss1Cre/+, and WT mice (n = 6–26) were checked daily for vaginal opening (VO) or preputial separation (PPS), while the experimenter was unaware of the animal's genotype. VO or PPS were considered to be the first of 3 consecutive days that the vagina was open or the foreskin separated from the penis. Body weight was also measured daily between days 21 and 35, then once weekly until the animals were 8 weeks old.
Experiment 5: fertility assessment
The purpose of this experiment was to determine whether Kiss1Cre/Cre mice were fertile (as determined by mating over the course of 1 typical estrous cycle). Kiss1Cre/Cre or WT littermates were paired for 5 consecutive days with a WT mate of proven fertility. Beginning 18 days later, the cages were checked daily for pups for the subsequent 10 days, and the number of pups was counted.
Experiment 6: search for compensation in Kiss1Cre/Cre mice
We reasoned that as a result of the profound reduction of kisspeptin expression in Kiss1Cre/Cre mice, Kiss1 neurons compensate by increasing auto-stimulation or reducing auto-inhibition by up-regulating levels of neurokinin B (NKB) and NK3R (the NKB receptor) or down-regulating levels of dynorphin, respectively (17). To this end, we measured the expression of the genes that encode NKB, NK3R, and dynorphin (Tac2, Tacr3, and Pdyn, respectively) by qRT-PCR in the MBH from males collected in experiment 1.
Quantitative RT-PCR
Concentrations of RNA were measured with a NanoDrop 1000 spectrophotometer (Thermo Scientific, Asheville, North Carolina), and samples were diluted to equal concentrations. Kiss1 reactions were amplified with the use of TaqMan Gene Expression Assays (Applied Biosystems/Life Technologies, Foster City, California), with a primer/probe set (Mm03058560_m1) designed to span the intron between the 2 coding exons of the Kiss1 gene and one-step Brilliant II qRT-PCR Master Mix (Agilent Technologies, Santa Clara, California). Tac2, Tacr3, Pdyn, and Actb were amplified by using one-step SYBR Brilliant II qRT-PCR Master Mix and the primer sets listed in Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). All qRT-PCR experiments were conducted with an MX3000P PCR machine (Stratagene, La Jolla, California). For PCR protocols, see Supplemental Table 2. Each control, standard, and unknown was amplified in duplicate. Negative controls included: 1) reactions without reverse transcriptase, 2) reactions without RNA, and 3) RNA from complete Kiss1-KO animals (3). The standard curve was made by serial dilution of RNA from WT GDX animals and assigning arbitrary values based on known concentrations of total RNA. The efficiency of all standard curves was between 85% and 115%. For each unknown, values were derived from the standard curve. Then, each unknown value was normalized to values from amplification of Actb to control for small variations in RNA concentration among samples. Finally, these normalized values were reported as a percentage of the mean WT value. Actb expression did not change in response to treatments or genotypes.
Intracerebroventricular injections
Intracerebroventricular injections were conducted as previously described (18, 19). Briefly, on the day before vaginal smears began (at least 8 days before the injection), a hole was made in the skull while mice were under isoflurane anesthesia. The hole was punched at a point that was 1.5 mm lateral and 0.5 mm posterior to bregma, with a 27-gauge needle covered with polyethylene tubing, leaving 3.5 mm of the needle's tip exposed. On the day of the injections, 2 μL of either senktide or saline was injected over a period of 30 seconds through the hole. After the injection, the needle was kept in place for 1 minute to limit backflow through the needle's track.
Hormone assays
Serum measurements for LH, FSH, and T were conducted in duplicate at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core. For specific assay information, reportable ranges and intra-assay coefficients of variation, see Supplemental Table 3.
Statistical analysis
GraphPad Prism software was used for all analyses, except for the Fisher's exact test, for which look-up tables were used (20). All data are represented as the mean ± SEM. For normally distributed data, an ANOVA was used for comparisons among more than 2 groups with Tukey's post hoc test. For hormone assays wherein some samples fell outside the reportable range, a Kruskal-Wallis nonparametric test was used. A 2-way ANOVA (group vs treatment) with Bonferroni correction was used to analyze LH levels in experiment 3. In experiment 4, differences between genotypes were assessed by survival analysis with the log-rank Mantel-Cox test for VO and PPS and 2-way repeated-measures ANOVA for body weight. In experiment 5, comparison of the fraction of mice within each group that had pups was made with the Fisher's exact test. For comparison of the number of pups per litter, data were included only if animals had at least 1 pup. In contrast, the average number of corpora lutea (CL) did include animals without CL. A comparison of litter size among genotypes was made with an unpaired t test.
Results
Quantification of Kiss1 transcript in Kiss1Cre/Cre animals
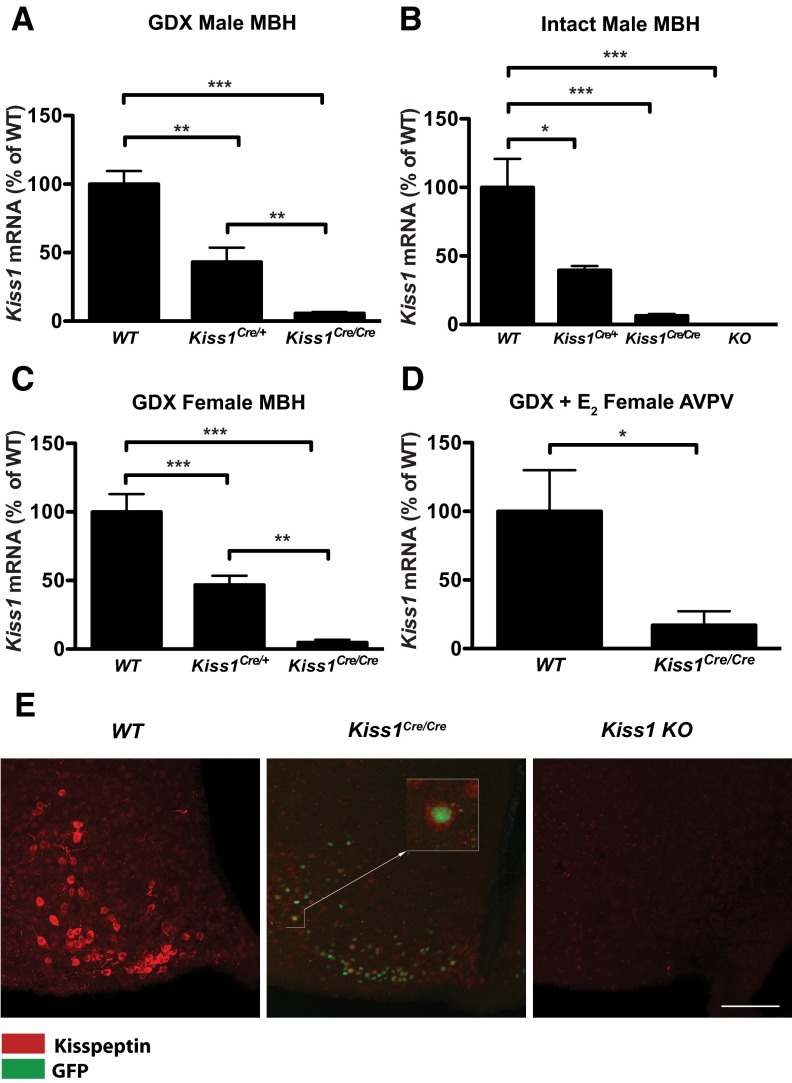
To quantify the extent to which Kiss1 transcript was suppressed in Kiss1Cre/+ and Kiss1Cre/Cre mice, we performed qRT-PCR on the MBH of intact males, GDX males and females, and on the AVPV/preoptic area of GDX E2-treated females. In GDX males, Kiss1Cre/+ mice had 43.1% ± 4.5% of the Kiss1 transcript found in WT mice, and Kiss1Cre/Cre males had only 5.7 ± 0.4% of the WT transcript in the MBH (P < .0001 by 1-way ANOVA; P < .05 for all comparisons by Tukey's post hoc test) (Figure 2A). The same pattern was observed in the MBH of intact males (Figure 2B) and GDX females (Figure 2C) (intact males P < .005; GDX females P < .0001 by 1-way ANOVA). The level of Kiss1 transcript in the MBH was approximately 10 times higher in GDX vs intact animals for each of the 3 genotypes (Supplemental Table 4), consistent with the known response of Kiss1 expression to gonadal steroids (21). In the preoptic area/AVPV region, GDX E2-treated Kiss1Cre/Cre females had 17.0% ± 10.2% of the Kiss1 transcript found in their WT littermates treated in the same manner (Figure 2D; P < .05 with 2-tailed t test). Neither the samples from Kiss1 KO animals (3) nor those lacking reverse transcriptase had any amplification after 45 cycles, confirming that the amplification product was derived from Kiss1 mRNA. LH levels confirmed the sex steroid treatment of the animals (eg, GDX males had elevated LH levels relative to intact males and GDX E2-treated females had LH levels below the limit of detection). For LH levels in intact and GDX males, see Adult phenotype of Kiss1Cre/Cre mice below (Figure 5B and Supplemental Table 4). These findings demonstrate that the Kiss1 transcript is dramatically reduced in Kiss1Cre/Cre animals, albeit present at very low levels.
Figure 2.
Kiss1 gene expression is dramatically reduced in Kiss1Cre/Cre mice. A–D, qRT-PCR of Kiss1 mRNA normalized first to Actb mRNA, then expressed as a percentage of WT levels. E, Immunohistochemistry for kisspeptin (red) in the ARC of the hypothalamus of castrated male WT, Kiss1Cre/Cre and complete Kiss1 KO animals (from left to right). In the center panel, nuclei express GFP driven by the Kiss1 promoter (see Figure 1). Scale bar, 100 μm. *, P < .05; **, P < .005; ***, P < .0005.
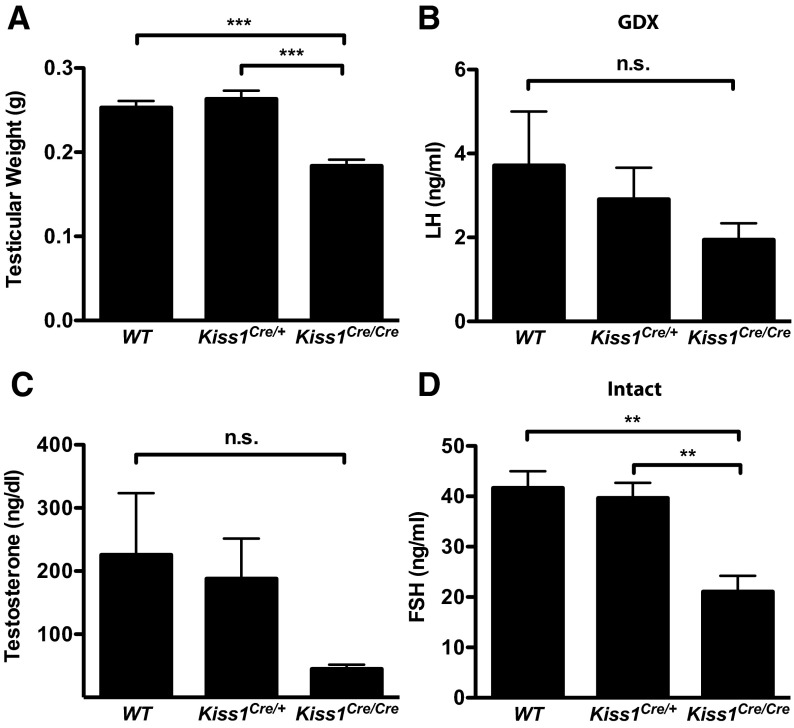
Figure 5.
Phenotype of adult Kiss1Cre/Cre males. A, Testicular weight (n = 10-12). B, LH in GDX mice (n = 4-5). C, T (n = 9-22). D, FSH in intact mice (n = 7-19). **, P < .005; ***P < .0001; A and D were analyzed with 1-way ANOVA with Tukey's multiple-comparison test. B and C were analyzed with Kruskal-Wallis nonparametric test because some samples were out of the range of the assay. Abbreviation: n.s., not statistically significant.
Detection of kisspeptin by immunohistochemistry in Kiss1Cre/Cre males
To determine whether kisspeptin peptide levels were reduced in parallel to the Kiss1 transcript in Kiss1Cre/Cre mice, we performed immunohistochemistry on tissue from the ARC from GDX WT, Kiss1Cre/Cre, and Kiss1 KO mice (n = 4 per group). Cell bodies and fibers were visible through the rostral to caudal extent of the ARC in WT mice (shown in red, Figure 2E). Sections of the ARC from Kiss1Cre/Cre mice had a few faint kisspeptin-labeled cells, indicating the presence of a diminished number of kisspeptin cells with reduced peptide levels. These kisspeptin-labeled cells (cytoplasmic staining) were positive for nuclear green fluorescent protein (GFP) (Figure 2E, center), the expression of which is driven by the Kiss1 promoter (Figure 1). No staining was visible in the ARC of Kiss1 KO animals (Figure 2E, right), indicating the specificity of the antibody. These results indicate that kisspeptin peptide expression was profoundly reduced (as was the number of identifiable cells) but not eliminated in the Kiss1Cre/Cre mice, because traces of kisspeptin remained in cells that would normally express kisspeptin.
Kiss1 neurons stimulate GnRH/LH release in Kiss1Cre/Cre mice
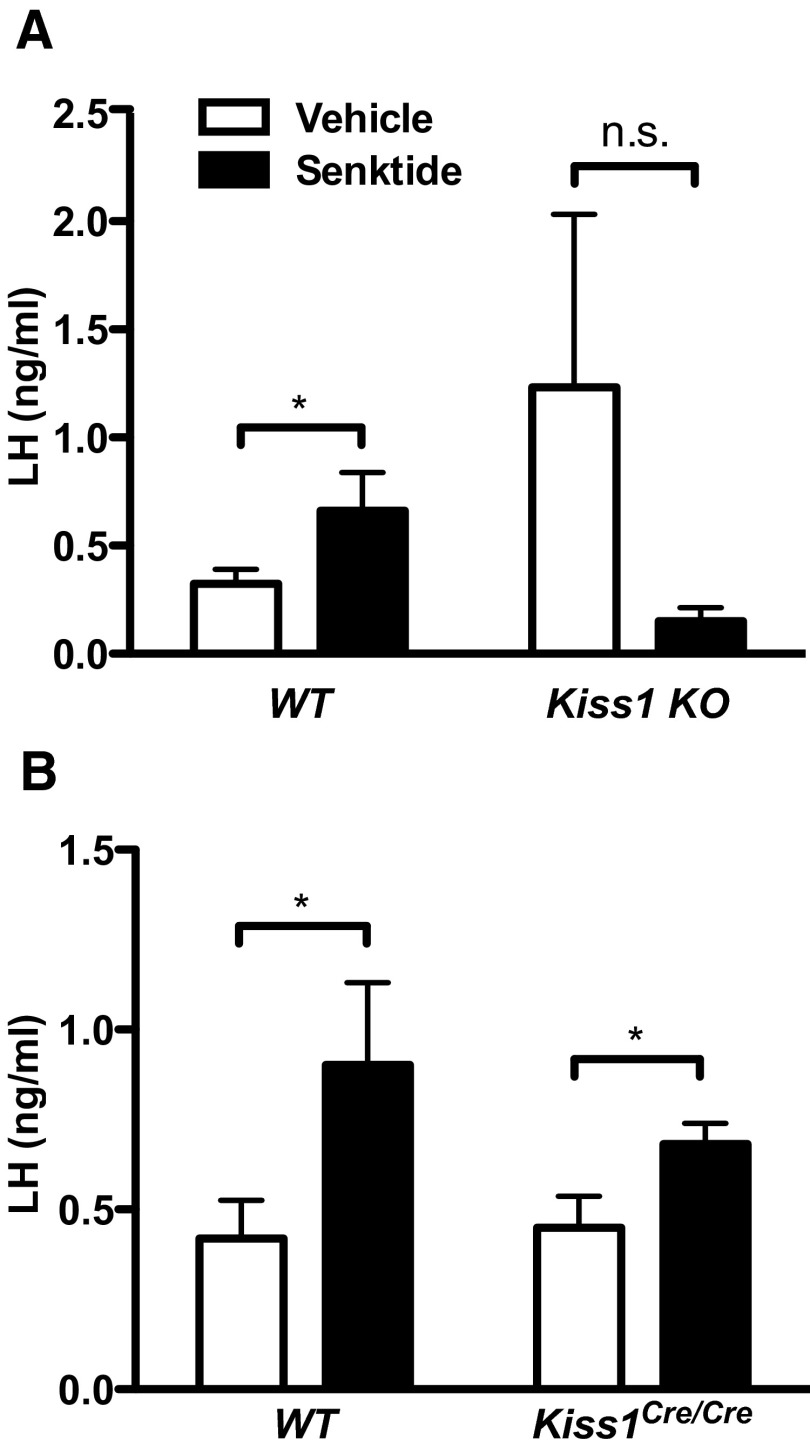
To determine whether the remaining kisspeptin produced in Kiss1Cre/Cre animals is functional and sufficient to drive GnRH (and LH) release, we challenged females with senktide, an NK3R agonist that triggers LH secretion only if kisspeptin signaling is intact (15, 16). Senktide stimulates kisspeptin neurons by activating NK3R receptors, which are expressed by these cells (22, 23). Although it has previously been shown that Kiss1 receptor is required for senktide stimulation of LH, whether kisspeptin itself is required has not been directly tested. Thus, we confirmed that senktide cannot stimulate LH in Kiss1 KO animals (Figure 3A, P < .05 by 2-way ANOVA for interaction between treatment and genotype). In contrast, senktide increased serum levels of LH in both WT and Kiss1Cre/Cre females at diestrus (Figure 3B). Two-way ANOVA revealed a significant effect of treatment, but not genotype or interaction (P < .01 by 2-way ANOVA). These results demonstrate that Kiss1Cre/Cre animals produce sufficient amounts of kisspeptin to induce GnRH/LH release.
Figure 3.
Kisspeptin is physiologically active in Kiss1Cre/Cre female mice. A, Senktide cannot stimulate LH in Kiss1 KO mice (n = 5-12). B, Senktide stimulates LH in Kiss1Cre/Cre mice, indicating that kisspeptin is present and functional (n = 7-10). *, P < .05 by 1-tailed t test within each genotype because we specifically sought to determine whether senktide would cause an increase in LH. Two-way ANOVA showed an interaction between genotype and treatment in A (P < .05) and an effect of treatment in B (P < .01). Abbreviation: n.s., not statistically significant.
Development of secondary sexual characteristics in Kiss1Cre/Cre mice
Kiss1 KO female mice have delayed VO, and most KO males lack PPS, indicating a defect in pubertal maturation, which reflects insufficient levels of sex steroids (3). In contrast, mice with ∼97% ablation of their Kiss1 neurons exhibited normal VO (8), suggesting that minute amounts of kisspeptin suffice for development of secondary sexual characteristics at puberty. Therefore, we sought to determine whether the residual kisspeptin remaining in Kiss1Cre/Cre mice would be sufficient to drive sexual maturation. Kiss1Cre/Cre males had a 1.5-day delay in the mean day of PPS in comparison with their WT and Kiss1Cre/+ littermates (Figure 4A; log-rank Mantel-Cox test, P < .05). Despite this delay, all males achieved PPS. These findings suggest that Kiss1Cre/Cre males have a slight deficit in T secretion but have sufficient redundancy or compensatory mechanisms to exhibit a relatively normal phenotype. All female Kiss1Cre/Cre mice attained VO with the same time course as their WT and Kiss1Cre/+ littermates (Figure 4B).
Figure 4.
Sexual maturation in Kiss1Cre/Cre mice. PPS (A) and VO (B) were checked daily in WT, Kiss1Cre/+, and Kiss1Cre/Cre males and females, respectively, as markers for circulating gonadal steroids (n = 6-26). Circles represent WT, squares represent Kiss1Cre/+, and triangles represent Kiss1Cre/Cre mice. *, P < .05 by log-rank Mantel-Cox test. Abbreviation: n.s., not statistically significant.
Circulating E2 can influence body weight through its effects on appetite (24, 25). Therefore, we weighed animals daily during pubertal maturation and found that Kiss1Cre/Cre female mice were slightly, but consistently, heavier than either their WT or Kiss1Cre/+ counterparts, from postnatal days 21 to 47 (∼0.8 g/d difference in mean weights; 2-way repeated-measures ANOVA revealed significant effects of genotype, P < .05; data not shown). No significant differences in body weight were found between genotypes in males or adult females weighed at a single time point. These results demonstrate that drastic reductions in Kiss1 expression cause only very subtle delays in the development of secondary sexual characteristics.
Adult phenotype of Kiss1Cre/Cre mice
The finding that Kiss1Cre/Cre mice exhibit secondary sexual characteristics suggests that they produce physiologically adequate levels of gonadotropins to sustain sex steroid synthesis, despite having greatly reduced Kiss1 expression. We sought to determine whether residual expression of kisspeptin is sufficient to sustain adult reproductive function.
Males
Underweight gonads are one hallmark of hypogonadotropic hypogonadism, including that of mice having mutations in the Kiss1/KISS1 gene (2–4). The testes of Kiss1Cre/Cre animals had modestly reduced weights (by ∼25%) relative to their WT and Kiss1Cre/+ littermates (P < .0001 by 1-way ANOVA) (Figure 5A), in marked contrast to the dramatic reduction of testicular weight in Kiss1 KO animals (3). Despite reduced testicular size, Kiss1Cre/Cre testicular morphology appeared normal when examined histologically and contained abundant spermatozoa, like those of WT animals (data not shown).
WT, Kiss1Cre/+, and Kiss1Cre/Cre males responded to GDX by increasing LH levels (P < .0005 for 2-way ANOVA effect of treatment and P < .05 Mann-Whitney U test comparing intact with GDX animals; Supplemental Table 4). Although plasma LH was slightly lower in GDX Kiss1Cre/Cre males relative to WT and Kiss1Cre/+ littermates, this reduction was not statistically significant (Figure 5B; n = 4–5 per group). A second experiment comparing LH levels between WT and Kiss1Cre/Cre GDX males also did not reveal a statistically significant difference (WT = 4.19 ± 0.82 ng/mL; Kiss1Cre/Cre = 2.54 ± 0.4 ng/mL; n = 8–9 per group). Levels of LH in intact animals were also normal in Kiss1Cre/Cre males compared with WT controls (data not shown). Although LH values for intact animals were above the limit of sensitivity for the assay, they were close to the limit of detection, which may not provide sufficient resolution to discriminate among genotypes. In contrast, intact Kiss1Cre/Cre males had significantly lower serum levels of FSH compared with their WT and Kiss1Cre/+ littermates (Figure 5D; P < .005 by 1-way ANOVA; n = 7–19 per group). Although T in Kiss1Cre/Cre males appeared to be reduced relative to WT and Kiss1Cre/+ littermates (Figure 5C), this difference was not statistically significant due to variability in WT and Kiss1Cre/+ animals (Kruskal-Wallis nonparametric test; n = 9–22 per group).
Given that Kiss1Cre/Cre males displayed only mild signs of hypogonadism, we suspected that they would be fertile. When paired with WT females, Kiss1Cre/Cre males succeeded in impregnating their mates, which delivered normal-sized litters (Table 1; n = 9–14 per group). There were no significant differences in either the rate of fertility or litter size between Kiss1Cre/Cre and WT males. These results indicate that adult Kiss1Cre/Cre males maintain virtually all of their reproductive capacity despite having only a small fraction of available kisspeptin.
Table 1.
Fertility Resultsa
| Percent Fertile | Mean Litter Size | |
|---|---|---|
| WT males | 79 (11/14) | 8.0 ± 0.6 |
| KissCre/Cre males | 56 (5/9) | 7.6 ± 0.9 |
| WT females | 78 (7/9) | 8.3 ± 0.6 |
| KissCre/Cre females | 27 (3/11)b | 4.7 ± 2.0b |
Kiss1Cre/Cre animals or their WT littermates were paired with fertile WT mates of the opposite sex for 5 consecutive days, after which the mates were removed. The number of fertile animals is expressed as a percentage of the total number of animals mated per group. Litter size does not include data from animals without offspring.
P < .05 by Fisher's exact test for percent fertile and 2-tailed t test for mean litter size.
Females
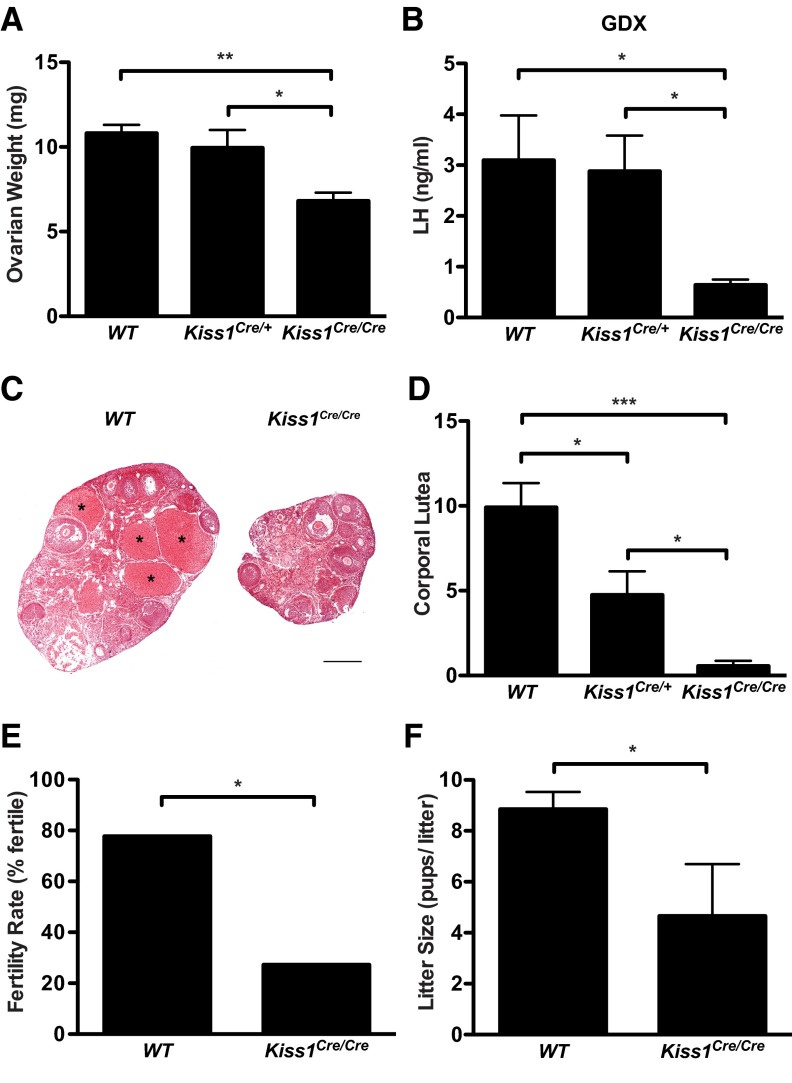
Similar to males, Kiss1Cre/Cre females had significantly reduced gonadal weight relative to WT and Kiss1Cre/+ littermates (Figure 6A; P < .005 by 1-way ANOVA; n = 6–8 per group; Kiss1Cre/Cre ovarian weight was 63% of WT). Kiss1Cre/Cre females had normal LH levels at diestrus (Figure 3), consistent with intact LH levels of Kiss1 KO mice (3) and progressed through the estrous cycle, although they occasionally had extended periods of estrus. GDX Kiss1Cre/Cre females had significantly lower levels of LH compared with their WT and Kiss1Cre/+ littermates (Figure 6B; P < .05 by 1-way ANOVA; n = 6–8 per group), which had normal LH levels.
Figure 6.
Phenotype of adult Kiss1Cre/Cre females. A, Combined ovarian weight (n = 6-8). B, LH in OVX mice (n = 6-8). C, Photomicrographs from WT (left) and Kiss1Cre/Cre (right) ovaries. Scale bar, 500 μm. *, CL. D, Mean number of CL in both ovaries obtained by averaging 2 sets of sections from each ovary analyzed by 2 experimenters blind to genotype (n = 6-7). E, Percentage of females that are fertile of all females paired with WT males (n = 9-11). (It is not possible to calculate error bars from a percentage). F, Mean number of pups per litter includes only data from females that had pups (n = 3-7). *, P < .05; **, P < .005; ***, P < .0001 by 1-way ANOVA with Tukey's multiple-comparison test (A, B, and D), Fisher's exact test (E), and 2-tailed t test (F).
In addition to being smaller, ovaries of Kiss1Cre/Cre mice showed markedly reduced numbers of CL relative to WT and Kiss1Cre/+ littermates, indicating significantly reduced ovulation (P < .0001 by 1-way ANOVA; n = 6–7 per group; Figure 6, C and D). Only 3 of 6 Kiss1Cre/Cre mice had any CL compared with the plethora of CL present in all WT animals (Figure 6D). Kiss1Cre/+ mice were also compromised in their ability to ovulate, showing an intermediate number of CL relative to WT and Kiss1Cre/Cre mice, suggesting that Kiss1 gene dose correlates with ovulation. However, Kiss1Cre/Cre ovaries contained antral and preovulatory follicles (Figure 6C), suggesting that they receive sufficient gonadotropin drive to support follicular maturation.
Predictably, compromised ovulation correlated with compromised fertility in Kiss1Cre/Cre females. When paired with a WT male, ∼80% of WT females became pregnant and delivered pups. In contrast, only ∼30% of Kiss1Cre/Cre mice sustained pregnancy (P < .05 by Fisher's exact test; n = 9–11 per group; Figure 6E). Furthermore, the Kiss1Cre/Cre mice that had pups had significantly reduced litter sizes compared with WT littermates (unpaired t test P < .05, Table 1 and Figure 6F). These results indicate that although Kiss1Cre/Cre females maintain some degree of fertility, a dramatic loss of kisspeptin compromises their reproductive success.
Expression of genes that regulate Kiss1 neuronal activity
We reasoned that as a result of the profound reduction of kisspeptin expression in Kiss1Cre/Cre mice, Kiss1 neurons might compensate by up-regulating the expression of the stimulatory co-neurotransmitter NKB and its receptor NK3R, which is also expressed by Kiss1 neurons, or down-regulating the expression of the autoinhibitory coneurotransmitter dynorphin (26). Thus, we measured and compared the expression of Tac2 (NKB), Tacr3 (NK3R), and Pdyn (dynorphin) mRNA in the MBH of WT and Kiss1Cre/Cre males. Because these genes are regulated by sex steroids (22, 23), we also compared their expression in the intact and GDX state as an internal control. As expected, we observed an increase in expression of all 3 genes with GDX (P < .01 by 2-way ANOVA for effect of treatment). There was no significant difference in the expression of Tac2, Tacr3, or Pdyn between WT and Kiss1Cre/Cre animals in the MBH, in either the intact or GDX states (Supplemental Figure 1).
Discussion
These results demonstrate that Kiss1Cre/Cre mice sustain some level of reproductive function despite having a 95% reduction of Kiss1 expression, corroborating the results, but not the conclusion, of the work by Mayer and Boehm (8). Male Kiss1Cre/Cre mice with 5% of the normal Kiss1 transcript and meager amounts of kisspeptin protein in their MBH can sire normal-sized litters. These results argue that WT male mice produce substantially more kisspeptin than is required for normal reproduction. Notwithstanding, experimental disruption of Kiss1 expression does appear to cause impairment of some aspects of reproduction, most notably in females. Although Kiss1Cre/Cre females can become pregnant and produce viable litters, they have fewer offspring. Thus, we conclude that WT females possess an abundance of Kiss1 expression, a failsafe to guard reproductive success, just like males. However, females are more sensitive to disruptions of Kiss1 signaling than males, perhaps reflecting the additional complexity required for ovulation.
How is fertility maintained despite a major impairment of Kiss1 signaling in the brain? First, kisspeptin stimulates sustained firing of GnRH neurons at vanishingly low concentrations (5, 6), testifying to the remarkable sensitivity of the Kiss1 receptor (Kiss1r) signaling cascade. Second, the GnRH system itself is highly redundant. Classic experiments by Krieger et al (12) demonstrated that transplants of only a few GnRH neurons into GnRH-deficient mice are sufficient to increase gonadotropin levels, gonadal development, and spermatogenesis in males. Similar results were obtained from studies in mice with compromised GnRH neuronal migration (11). Males with only 12% of the normal constituency of GnRH neurons are just as fertile as their WT counterparts. Although tiny amounts of GnRH can sustain normal levels of LH, males with compromised production of either GnRH or kisspeptin have impaired FSH secretion, which is consistent with the reduction in gonadal weight found in Kiss1Cre/Cre males (11, 27) (Figure 5, A and D). Although reduced circulating levels of FSH could decrease epididymal sperm count, a lack of FSH does not compromise male fertility (27), which is consistent with our results in Kiss1Cre/Cre males.
Similar to males, WT females appear to have great redundancy in their Kiss1 signaling. Kiss1Cre/Cre females bearing only 5% of the normal Kiss1 transcript in the ARC and 17% in the AVPV attain VO at the normal age (Figure 4B), indicating their ability to produce timely and normal amplification of estradiol secretion at puberty. Kiss1Cre/Cre females exhibit normal cyclicity in vaginal cytology, and at diestrus, Kiss1Cre/Cre mice display levels of LH that are indistinguishable from WT animals (Figure 3B). These results are consistent with those reported by Mayer and Boehm (8), who found that female mice with 95% to 98% reduction in Kiss1 mRNA had VO, estrous cycling, and normal levels of LH. The intact LH results are also consistent with those of Kiss1 KO animals, which have normal LH levels in the intact state, pointing to the presence of kisspeptin-independent GnRH/LH release (1). However, when Kiss1Cre/Cre females are challenged to increase LH secretion after ovariectomy, they have reduced LH levels relative to WT animals (Figure 6B), suggesting that kisspeptin plays an important role in driving maximal GnRH/LH output. These observations suggest that low levels of kisspeptin are sufficient to sustain basal pulsatile LH release and steroidogenesis in females, as is the case with GnRH.
Why are Kiss1Cre/Cre females subfertile? Several studies have shown that kisspeptin plays a crucial role in generating the preovulatory LH surge (28–30). Our results also suggest that the neural mechanisms that trigger the LH surge and ovulation require a considerable boost from kisspeptin. Although Kiss1Cre/Cre females exhibit follicular development, they produce few CL (Figure 6, C and D), indicating a disruption of ovulation. Furthermore, the Kiss1 mRNA content in hypothalami correlates with the number of CL in the ovaries (Figures 2C and 6D), suggesting a gene-dose effect of Kiss1 on ovulation. That is, the magnitude of the kisspeptin surge plausibly correlates with the magnitude of the GnRH/LH surge and thus the number of follicles that ovulate. Consistent with this idea, female mice with ∼12% of the normal allotment of GnRH neurons lack an estradiol/progesterone-induced LH surge, whereas those with ∼34% of GnRH neurons have an intermediate phenotype and a blunted LH surge (11). Taken together, these observations are consistent with the idea that a surge in kisspeptin drives the preovulatory surge in GnRH and that normal WT females require most of their available kisspeptin to generate a robust and successful GnRH/LH surge.
If it were true that females required >50% of the natural endowment of Kiss1 to drive normal ovulation and fertility, as our results suggest, why were the animals described by Mayer and Boehm (8), which lacked ∼97% of their Kiss1 neurons, fertile? We provide 3 possible explanations. First, it's possible that developmental compensation occurs (eg, through rewiring of inputs to GnRH neurons) when Kiss1 neurons are congenitally ablated (in the Mayer and Boehm study), but not when Kiss1 transcript is dramatically reduced (in our study). We find this explanation to be unlikely. Although compensation has been reported to occur when AgRP neurons are congenitally ablated (9), congenital disruptions of the NPY or AgRP genes cause only a mild phenotype, unlike the dramatic infertility reported to occur in Kiss1 KO animals (2, 3). Furthermore, it is difficult to imagine how the greater insult caused by cell death (including a reduction in NKB and dynorphin in the arcuate and dopamine in the AVPV) could have a more mild effect on reproduction than the partial insult caused by transcriptional suppression. Second, differences in the approach to assessing fertility might explain the discrepancies in results. We noticed that most Kiss1Cre/Cre females did not become pregnant when mated for only 5 days, but some did after 2 months (data not shown). Therefore, if the mice in the Mayer and Boehm (8) study had reduced frequency of ovulation, as suggested by their prolonged estrous cycles, this effect could be masked by a prolonged mating period. Although it was not revealed whether those mice had reduced ovulation efficiency, they were reported to have normal-sized litters, suggesting that at least those animals had relatively normal numbers of ovulations per cycle. Third, variation in the genetic background strains of the mice used in the different studies could account for differences in how much Kiss1/kisspeptin is required to generate the GnRH/LH surge. The Kiss1Cre/Cre mice were on a mixed genetic background, which was ∼75% C57BL/6J and ∼25% 129/Sv. The genetic background of the mice used in the Mayer and Boehm (8) study was not reported, so we cannot make a direct comparison. Nevertheless, reproductive capacity differs remarkably among strains of mice (31), with strains that are particularly sensitive to disruptions in fertility having 10 times less Kiss1 mRNA in the hypothalamus relative to C57BL6/J animals (32). Therefore, genetic background differences could well contribute to the discrepancies of our findings.
One limitation of our study is that we do not know exactly how much kisspeptin peptide is released in Kiss1Cre/Cre animals. However, we demonstrated that sufficient kisspeptin is released to trigger an LH response to the NK3R agonist senktide (Figure 3B), which requires Kiss1 signaling to stimulate LH (Figure 3A) (15, 16). We also showed that kisspeptin is reduced in Kiss1Cre/Cre mice with immunohistochemistry (Figure 2E) and that some kisspeptin is still present in neurons that normally express Kiss1, as demonstrated by faint kisspeptin immunolabeling surrounding GFP-expressing nuclei (Figure 2E). Taken together, our results are consistent with the idea that dramatically reduced levels of kisspeptin are sufficient to sustain endogenous gonadotropin release. However, having an oversupply of kisspeptin would not rule out other mechanisms of compensation for the reduction in kisspeptin production.
We reasoned that animals might compensate for reduced Kiss1 expression by increasing the activity of Kiss1 neurons, perhaps by allowing one or both of the Kiss1 cotransmitters to remediate the situation as part of a backup system. There are other examples of compensation in the hypothalamus after insults (33–36), so we sought specific evidence for adaptation. In our case, we envisioned that one possible remedy for the reduction of kisspeptin might be either to increase autostimulation by amplifying NKB/NK3R signaling or decrease autoinhibition by reducing dynorphin synthesis (17), either of which might maximize release of residual kisspeptin. We did not find evidence for changes in the expression of genes that encode NKB, NK3R, or dynorphin. Based on these data, we cannot rule out a role for NKB or dynorphin signaling in compensation because we did not limit gene expression analysis to Kiss1 neurons, and these genes are expressed in other neurons of the MBH (22, 37).
How else might Kiss1Cre/Cre animals compensate for diminished Kiss1 expression? First, GnRH neurons could become more sensitive to kisspeptin with its chronic loss. This seems plausible, because some (but not all) Kiss1 KO animals have an augmented LH response to kisspeptin administration (3, 38). Second, the pituitary could become sensitized to GnRH. However, this is not likely because the pituitary responds normally to GnRH when GnRH levels are present at low levels (11). Third, some kisspeptin-independent mechanisms may contribute to GnRH secretion. We know that the residual gonadotropin secretion that persists in both Kiss1 and Kiss1r KO mice is blocked by a GnRH antagonist (1); thus, we can deduce that some GnRH secretion remains even in the face of complete ablation of Kiss1 signaling, which could be driven by other stimulatory inputs to GnRH neurons, such as glutamate. Indeed, Kiss1 KO mice exhibit increased sensitivity to N-methyl-D-aspartate (38), suggesting that in the absence of kisspeptin input, GnRH neurons become more sensitive to some excitatory inputs. Fourth, it is conceivable that mating-induced ovulation occurs through the noradrenergic system, because this occurs in certain conditions in animals that are not normally reflex ovulators (39). Finally, Kiss1 signaling in peripheral tissues could play a role in fertility and compensation may occur outside the brain. Nevertheless, any such mechanism is insufficient to compensate fully for the absence of kisspeptin signaling, as demonstrated by infertility in animals lacking functional Kiss1 and Kiss1r genes (1–4). Collectively, these observations demonstrate that animals require kisspeptin for reproduction and synthesize this peptide in excess to ensure reproductive success.
Supplementary Material
Acknowledgments
We are grateful to John Incardona and Tanya Swarts at National Oceanic and Atmospheric Administration for sharing their confocal microscope, Yingtzang Tien for ovarian histology, Greg Martin at the Keck Microscopy Facility for assistance with imaging ovaries, and Dr Michael Griswold at Washington State University for examining testicular histology. We thank Dr Michelle Gottsch, Samuel Hobbs, and Katey Feng for technical assistance, Drs Albert Quintana and Sasha Kauffman for advice, and Dr. William Colledge for sending brain tissue. We are grateful to Aleisha Schoenfelder, Beth Marcinko, and Lisa Geddis for performing the RIAs and to Core Director Dr Dan Haisenleder.
This research was supported by National Institutes of Health (NIH) Grant 2 R01 HD049651 (to R.A.S.), U54 HD028138 (to S.B.S.), and JSPS KAKENHI 23780282 (to R.M.M.). We acknowledge support from the Developmental Biology Training Grant at the University of Washington (NIH T32HD007183). We are grateful to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH (Specialized Cooperative Centers Program in Reproduction and Infertility Research) Grant U54-HD28934)
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 2573
- AgRP
- agouti-related peptide
- ARC
- arcuate nucleus
- AVPV
- anteroventral periventricular nucleus
- CL
- corpora lutea
- E2
- 17β-estradiol
- GDX
- gonadectomized
- GFP
- green fluorescent protein
- KO
- knockout
- MBH
- mediobasal hypothalamus
- NKB
- neurokinin B
- NK3R
- neurokinin-3 receptor
- NPY
- neuropeptide Y
- PPS
- preputial separation
- qRT-PCR
- quantitative RT-PCR
- T
- testosterone
- VO
- vaginal opening
- WT
- wild-type.
References
- 1. Chan YM, Broder-Fingert S, Wong KM, Seminara SB. Kisspeptin/Gpr54-independent gonadotrophin-releasing hormone activity in Kiss1 and Gpr54 mutant mice. J Neuroendocrinol. 2009;21:1015–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. d'Anglemont de Tassigny X, Fagg LA, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A. 2007;104:10714–10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lapatto R, Pallais JC, Zhang D, et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–4936 [DOI] [PubMed] [Google Scholar]
- 4. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 5. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 6. Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Irwig MS, Fraley GS, Smith JT, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272 [DOI] [PubMed] [Google Scholar]
- 8. Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci. 2011;14:704–710 [DOI] [PubMed] [Google Scholar]
- 9. Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685 [DOI] [PubMed] [Google Scholar]
- 10. Gibson MJ, Charlton HM, Perlow MJ, Zimmerman EA, Davies TF, Krieger DT. Preoptic area brain grafts in hypogonadal (hpg) female mice abolish effects of congenital hypothalamic gonadotropin-releasing hormone (GnRH) deficiency. Endocrinology. 1984;114:1938–1940 [DOI] [PubMed] [Google Scholar]
- 11. Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology. 2008;149:597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krieger DT, Perlow MJ, Gibson MJ, et al. Brain grafts reverse hypogonadism of gonadotropin releasing hormone deficiency. Nature. 1982;298:468–471 [DOI] [PubMed] [Google Scholar]
- 13. Gottsch ML, Popa SM, Lawhorn JK, et al. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology. 2011;152:4298–4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caraty A, de Reviers MM, Pelletier J, Dubois MP. Reassessment of LRF radioimmunoassay in the plasma and hypothalamic extracts of rats and rams. Reprod Nutri Dev. 1980;20:1489–1501 [DOI] [PubMed] [Google Scholar]
- 15. García-Galiano D, van Ingen Schenau D, Leon S, et al. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology. 2012;153:316–328 [DOI] [PubMed] [Google Scholar]
- 16. Ramaswamy S, Seminara SB, Plant TM. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology. 2011;94:237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30:3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hohmann JG, Teal TH, Clifton DK, et al. Differential role of melanocortins in mediating leptin's central effects on feeding and reproduction. Am J Physiol Regul Integr Comp Physiol. 2000;278:R50–R59 [DOI] [PubMed] [Google Scholar]
- 19. Krasnow SM, Fraley GS, Schuh SM, Baumgartner JW, Clifton DK, Steiner RA. A role for galanin-like peptide in the integration of feeding, body weight regulation, and reproduction in the mouse. Endocrinology. 2003;144:813–822 [DOI] [PubMed] [Google Scholar]
- 20. Siegel S. Nonparametric Statistics for the Behavioral Sciences. New York, NY: McGraw-Hill; 1956 [Google Scholar]
- 21. Smith JT, Dungan HM, Stoll EA, et al. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984 [DOI] [PubMed] [Google Scholar]
- 22. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Navarro VM, Gottsch ML, Wu M, et al. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152:4265–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beatty WW, O'Briant DA, Vilberg TR. Effects of ovariectomy and estradiol injections on food intake and body weight in rats with ventromedial hypothalamic lesions. Pharmacol Biochem Behav. 1975;3:539–544 [DOI] [PubMed] [Google Scholar]
- 25. Fleming A. Effects of estrogen and prolactin on ovariectomy-induced hyperphagia and weight gain in female rats. Behav Biol. 1977;19:417–423 [DOI] [PubMed] [Google Scholar]
- 26. Navarro VM. New insights into the control of pulsatile GnRH release: the role of Kiss1/neurokinin B neurons. Front Endocrinol (Lausanne). 2012;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204 [DOI] [PubMed] [Google Scholar]
- 28. Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kinoshita M, Tsukamura H, Adachi S, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–4436 [DOI] [PubMed] [Google Scholar]
- 30. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vasudevan K, Raber J, Sztein J. Fertility comparison between wild type and transgenic mice by in vitro fertilization. Transgenic Res. 2010;19:587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Quennell JH, Howell CS, Roa J, Augustine RA, Grattan DR, Anderson GM. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology. 2011;152:1541–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clifton DK, Sawyer CH. LH release and ovulation in the rat following depletion of hypothalamic norepinephrine: chronic vs acute effects. Neuroendocrinology. 1979;28:442–449 [DOI] [PubMed] [Google Scholar]
- 34. Clifton DK, Sawyer CH. Positive and negative feedback effects of ovarian steroids on luteinizing hormone release in ovariectomized rats following chronic depletion of hypothalamic norepinephrine. Endocrinology. 1980;106:1099–1102 [DOI] [PubMed] [Google Scholar]
- 35. Pinto S, Roseberry AG, Liu H, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115 [DOI] [PubMed] [Google Scholar]
- 36. Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Navarro VM, Ruiz-Pino F, Sánchez-Garrido MA, et al. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci. 2012;32:2388–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. d'Anglemont de Tassigny X, Ackroyd KJ, Chatzidaki EE, Colledge WH. Kisspeptin signaling is required for peripheral but not central stimulation of gonadotropin-releasing hormone neurons by NMDA. J Neurosci. 2010;30:8581–8590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Everett JW. Provoked ovulation or long-delayed pseudopregnancy from coital stimuli in barbiturate-blocked rats. Endocrinology. 1967;80:145–154 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.