Abstract
Kisspeptin signaling via its Gαq-coupled receptor GPR54 plays a crucial role in modulating GnRH neuronal excitability, which controls pituitary gonadotropins secretion and ultimately reproduction. Kisspeptin potently depolarizes GnRH neurons primarily through the activation of canonical transient receptor potential (TRPC) channels, but the intracellular signaling cascade has not been elucidated. Presently, we have established that kisspeptin activation of TRPC channels requires multiple membrane and intracellular signaling molecules. First, phosphatidylinositol-4,5-bisphosphate (PIP2) hydrolysis by phospholipase Cβ is required because whole-cell dialysis of Dioctanoylglycerol-PIP2 (DiC8-PIP2) inhibited the kisspeptin activation of TRPC channels, and the phosphatidylinositol 4-kinase inhibitor wortmannin, which attenuates PIP2 synthesis, prolonged TRPC channel activation. Using single cell RT-PCR, we identified that the mRNA for the PIP2-interacting TRPC channel subunit, TRPC4α, is expressed in GnRH neurons. Depletion of intracellular Ca2+ stores by thapsigargin and inositol 1,4,5-trisphosphate had no effect, indicating that the TRPC channels are not store-operated. Neither removing extracellular Ca2+ nor buffering intracellular Ca2+ with EGTA or BAPTA had any effect on the kisspeptin activation of the TRPC channels. However, the Ca2+ channel blocker Ni2+ inhibited the kisspeptin-induced inward current. Moreover, inhibition of protein kinase C by bisindolylmaleimide-I or calphostin C had no effect, but activation of protein kinase C by phorbol 12,13-dibutyrate occluded the kisspeptin-activated current. Finally, inhibition of the cytoplasmic tyrosine kinase cSrc by genistein or the pyrazolo-pyrimidine PP2 blocked the activation of TRPC channels by kisspeptin. Therefore, TRPC channels in GnRH neurons are receptor-operated, and kisspeptin activates TRPC channels through PIP2 depletion and cSrc tyrosine kinase activation, which is a novel signaling pathway for peptidergic excitation of GnRH neurons.
Mutations in G protein-coupled receptor 54 (GPR54) cause autosomal recessive idiopathic hypogonadism in humans, and deletion of GPR54 in mice results in defective sexual development and reproductive failure (1, 2). Kisspeptin-54 is the endogenous ligand of GPR54 (also known as Kiss1R), which is highly expressed in GnRH neurons (3–5). The Kiss-1 gene encodes a 145–amino-acid protein, which is proteolytically processed to kisspeptin-54 and several other smaller peptide fragments (3), and centrally administered kisspeptins robustly stimulate GnRH and gonadotropin secretion in both prepubertal and adult animals (6–10). Kisspeptin-54 and the smaller peptide fragments (eg, kisspeptin-14, -13, and -10) bind with low nanomolar affinities to rat and human GPR54 expressed in Chinese hamster ovary cells and stimulate phosphatidylinositol-4,5-bisphosphate (PIP2) hydrolysis, Ca2+ mobilization, arachidonic acid (AA) release, and ERK1, ERK2, and p38 MAPK phosphorylation (3, 11). Kisspeptin is the most potent and efficacious neurotransmitter to excite native GnRH neurons (12–17). Kisspeptin excites GnRH neurons via GPR54 coupling to the activation of canonical transient receptor potential (TRPC) channels and inhibition of inwardly rectifying K+ (Kir) channels (18–22).
GnRH neurons express the full complement of brain TRPC channels, but based on the biophysical properties, pharmacological profiling, and mRNA analysis, TRPC1, 4, and 5 appear to be the key players in mediating the excitatory effects of kisspeptin in GnRH neurons (18). Our recent quantitative PCR study showed that TRPC4 is the main transcript, which is 4-fold higher than TRPC1 and TRPC5 (23). TRPC channels can be activated by G protein-coupled receptors and receptor tyrosine kinases (24, 25). All mammalian TRPC channels require phospholipase C (PLC) for activation (26), and the PLC inhibitor U73122 inhibits the effects of kisspeptin in native GnRH neurons (18, 20). Therefore, it appears that Gq-coupled GPR54 activates PLCβ to signal downstream to facilitate the opening of TRPC channels in GnRH neurons. Although PLCβ metabolizes PIP2 to diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) and the TRPC3, 6, and 7 subfamily is DAG-sensitive (24, 25), the surrogate DAG signaling molecule 1-oleoyl-2-acetyl sn-glycerol (OAG) has only a minimal effect to activate an inward current in GnRH neurons (18). Moreover, the role of PIP2 hydrolysis by PLCβ and Ca2+ in facilitating TRPC channel opening has not been elucidated in GnRH neurons. Importantly, intracellular dialysis with aminoethoxydiphenylborane (2-APB), an IP3 receptor inhibitor, does not abrogate the effects of kisspeptin, suggesting that IP3 receptors are not involved. However, extracellular application of 2-APB and flufenamic acid, both potent blockers of TRPC channels, inhibits the effects of kisspeptin in GnRH neurons (18). Collectively, the findings indicate that TRPC channels are critical for mediating kisspeptin's actions. Therefore, it was of interest to investigate the signaling pathway underlying the kisspeptin-mediated activation of TRPC channels in GnRH neurons.
Materials and Methods
Animals and treatments
All animal treatments described in this study are in accordance with institutional guidelines based on National Institutes of Health standards and were performed with Institutional Animal Care and Use Committee approval at the Oregon Health and Science University. Transgenic female mice expressing enhanced green fluorescent protein (EGFP) under the control of the GnRH promoter (EGFP-GnRH) were used in these studies (27). Animals were group-housed until surgery at which time they were housed individually. All animals were maintained under controlled temperature and photoperiod (lights on at 6:00 am and off at 6:00 pm) and given free access to food and water. Adult (2–5 months old) females were ovariectomized under isoflurane inhalation anesthesia as described previously (18). Animals were used 8 to 11 days after ovariectomy.
Preparation of preoptic area GnRH slices
Mice were killed quickly by decapitation. The brain was rapidly removed from the skull, and a block containing the diagonal band-preoptic area (DB-POA) was immediately dissected. The DB-POA block was submerged in cold (4°C) oxygenated (95% O2, 5% CO2) high-sucrose cerebrospinal fluid (CSF) containing (in mM) 208 sucrose, 2 KCl, 26 NaHCO3, 10 glucose, 1.25 NaH2PO4, 2 MgSO4, 1 MgCl2, and 10 HEPES (pH7.4; 290 mOsm). Coronal slices (200 μm) from the DB-POA were cut on a vibratome during which time (10 minutes) the slices were bathed in high-sucrose CSF at 4°C. The slices were then transferred to an auxiliary chamber where they were kept at room temperature (25°C) in artificial CSF (aCSF) consisting of (in mM) 124 NaCl, 5 KCl, 2.6 NaH2PO4, 2 MgCl2, 2 CaCl2, 26 NaHCO3, 10 HEPES, and 10 glucose (pH 7.4; 310 mOsm) until recording (recovery for 2 hours). A single slice was transferred to the recording chamber at a time and was kept viable by continually perfusing with warm (35°C), oxygenated aCSF at 2 mL/min.
Visualized whole-cell patch recording
Whole-cell patch recordings were made under a Zeiss Axioskop FS upright microscope equipped with fluorescein isothiocyanate (FITC) filter set and infrared differential interference contrast imaging devices. GnRH neurons were identified by the method described in our previous papers (18, 28). Patch pipettes (A-M Systems, Seattle, Washington; 1.5 mm outer diameter borosilicate glass) were pulled on a Brown/Flaming puller (Sutter Instrument Co, Novato, California; model P-97). Recording pipettes were normally filled with the following internal solution (in mM): 128 potassium gluconate, 10 NaCl, 1 MgCl2, 11 EGTA, 10 HEPES, 2.5 ATP, 0.25 GTP adjusted to pH 7.3 with KOH (290 mOsm). To isolate the TRPC channel, 300μM Ba2+ was added to the aCSF to block Kir channels. In Figure 4, pipettes were filled with cesium-based internal solution to block potassium channels. To study the Ca2+ sensitivity of the kisspeptin-activated TRPC channel, the following BAPTA or EGTA/Ca2+ buffers were also used: 10mM BAPTA/0Ca2+, 0.05mM EGTA/0Ca2+, 0.5mM EGTA/0Ca2+ and 11mM EGTA/6.6mM Ca2+ (170nM free Ca2+). For normal internal Ca2+, no EGTA or BAPTA was added to the internal solution. The osmolarity of the above buffers was adjusted by potassium gluconate or gluconic acid, and the pH was adjusted to 7.2 to 7.3 with KOH or CsOH. Pipette resistances were 2.5 to 4 MΩ when filled with above internal solutions. In whole-cell configuration, access resistance was 10 to 25 MΩ. The access resistance was 80% compensated. Nominally Ca2+-free extracellular aCSF was prepared by replacing Ca2+ with Mg2+. Voltage-clamp experiments were performed with an Axopatch 1D amplifier (2-kHz low-pass filter; Axon Instruments, Foster City, California). The kisspeptin-activated inward TRPC current was recorded at a holding potential of −65 mV. Electrophysiological signals were digitized with Digidata 1322A (Axon Instruments) and the data recorded using Clampex version 9.2 software (Axon Instruments). The calculated liquid junction potential for potassium gluconate was about 15 mV. The reported membrane potentials were all corrected for the liquid junction potential.
Figure 4.
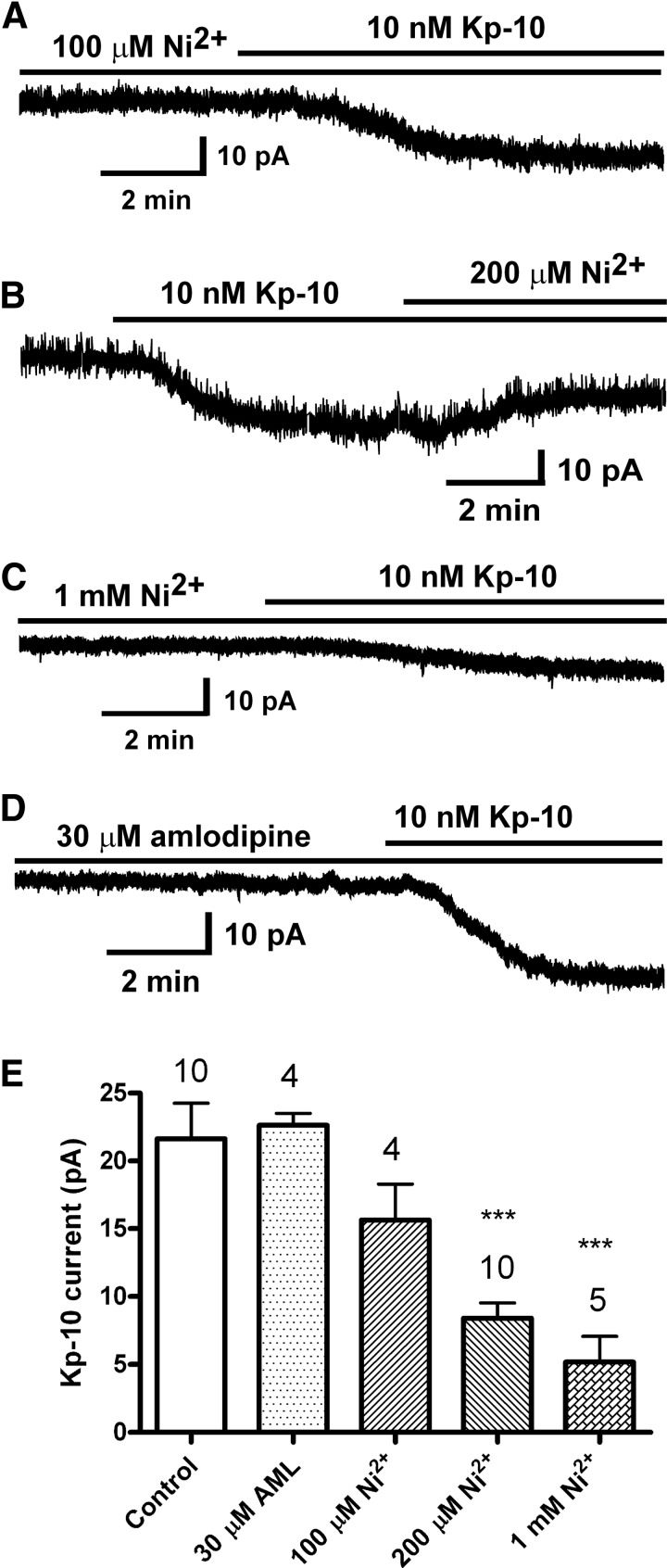
Effect of Ca2+ channel blockers on the kisspeptin activation of TRPC channels. A–D, Representative recordings showing that the kisspeptin (Kp-10)-activated inward current was inhibited by LVA Ca2+ channel blocker Ni2+ but not by HVA Ca2+ channel (L, N, P/Q) blocker amlodipine (AML). Vhold = −65 mV. E, Summary of the effects of Ca2+ channel blockers on the kisspeptin-activated inward current. Cells were recorded using a Cs+-based internal solution. ***, P < .001 versus vehicle control group (1-way ANOVA)
Electrophysiological solutions/drugs
aCSF was used in all cases for electrophysiological recording. In whole-cell voltage-clamp recordings, tetrodotoxin (TTX) was used to block sodium channels. For TRPC current recordings, 300μM Ba2+ was added to the bath to block the Kir channels. Different drug stock solutions were diluted at least 1000-fold in aCSF to their final concentrations in 20-mL syringes and were delivered by a Gilson Mini-Plus pump with a perfusion rate of 2 mL/min. The following chemicals or drugs (see Table 1) were used: kisspeptin-10 (mouse Kiss-1 [110-119]-NH2; Phoenix Pharmaceuticals, Belmont, California); TTX and thapsigargin (Alomone Laboratories, Jerusalem, Israel); phorbol 12,13-dibutyrate (PDBu), wortmannin, genistein, PP2 (3,4-chlorophenyl) 1-(1,1-dimethylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine) (Sigma-Aldrich, St Louis, Missouri); calphostin C, bisindolylmaleimide-I (BIS-I), PP3 (1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine), amlodipine, AA, and U0126 (Tocris, Ellisville, Missouri); myo-IP3 and OAG (Avanti Polar Lipids, Alabaster, Alabama); and Dioctanoylglycerol-PIP2 (DiC8-PIP2) (Echelon Biosciences, Salt Lake City, Utah). Thapsigargin, amlodipine, PDBu, wortmannin, genistein, PP2, PP3, calphostin C, BIS-I, and U0126 were dissolved in dimethylsulfoxide (DMSO). Kisspeptin-10, myo-IP3 trisodium salt, and TTX were dissolved in H2O. DiC8-PIP2 was dissolved in the pipette solution. AA was dissolved in 100% ethanol.
Table 1.
Targets and Selectivity of Drugs Used to Identify the Kisspeptin Signaling Pathway in GnRH Neurons
| Drugs/Chemicals | Targets and Selectivity |
|---|---|
| 2-APB | TRPC channel blocker; IP3R antagonist |
| Amlodipine | Broad-spectrum blocker of HVA calcium channels (L, N, P/Q) |
| AA | Metabolite of DAG by DAG lipase |
| BAPTA | Ca2+ chelator with fast association/on rate |
| BIS-I | Broad-spectrum PKC inhibitor of conventional and novel PKCs |
| Calphostin C | Broad-spectrum PKC inhibitor of conventional and novel PKCs |
| Cd2+ | Broad-spectrum calcium channel blocker |
| DiC8-PIP2 | Synthetic short-chain PIP2, an inhibitor of TRPC4α |
| EGTA | Ca2+ chelator with much slower on rate than BAPTA |
| Genistein | General inhibitor of tyrosine kinases including cSrc kinase |
| Myo-IP3 | IP3R agonist; causes calcium store depletion |
| Ni2+ | Selective calcium channel blocker of T-type |
| OAG | DAG surrogate; TRPC3/6/7 activator |
| PDBu | Potent PKC activator; less hydrophobic than PMA |
| PMA | Potent PKC activator |
| PP2 | Selective inhibitor of cSrc kinase |
| PP3 | Negative control for PP2 |
| Thapsigargin | Sarcoplasmic reticulum/ER Ca2+ ATPase inhibitor; causes calcium store depletion |
| U0126 | Selective inhibitor of the MAPKs MEK-1 and MEK-2 |
| Wortmannin | PI4K inhibitor at μM concentrations |
Electrophysiology data analysis
The steady-state responses at −65 mV of the kisspeptin-activated current were measured for comparison. Data were analyzed using Clampfit version 9.2 and GraphPad Prism version 4 software. Graphs were made with Macromedia FreeHand MX and SigmaPlot version 8 software. Comparisons between different treatments were performed using a 1-way ANOVA followed by Tukey's multiple-comparison test (see Figures 3–7) or an unpaired Student's t test (see Figure 2). Differences were considered significant if the probability of error was < 5%. All data are presented as mean ± SE.
Figure 3.
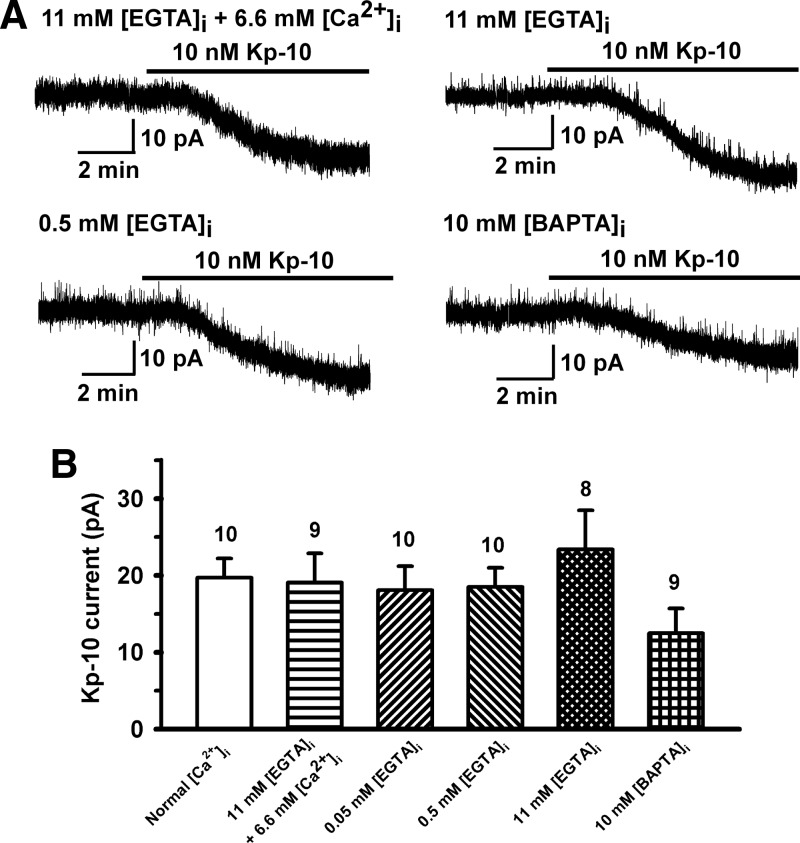
Role of intracellular Ca2+ on the kisspeptin activation of TRPC channels. A, Kisspeptin-10 (Kp-10)-induced currents were measured at a holding potential of −65 mV after whole-cell dialysis for about 15 minutes (12-18 minutes) with different Ca2+ buffers as indicated on the traces. B, Summary of the effects of different intracellular calcium buffers on the inward current. The calculated value was 170nM free [Ca2+]i for the internal solution containing 11mM EGTA and 6.6mM Ca2+ (pH 7.23). There was no Ca2+ added to the other 4 internal solutions shown in B. For normal [Ca2+]i, neither Ca2+ nor EGTA/BAPTA was added to the internal solution. P > .05 (1-way ANOVA).
Figure 5.
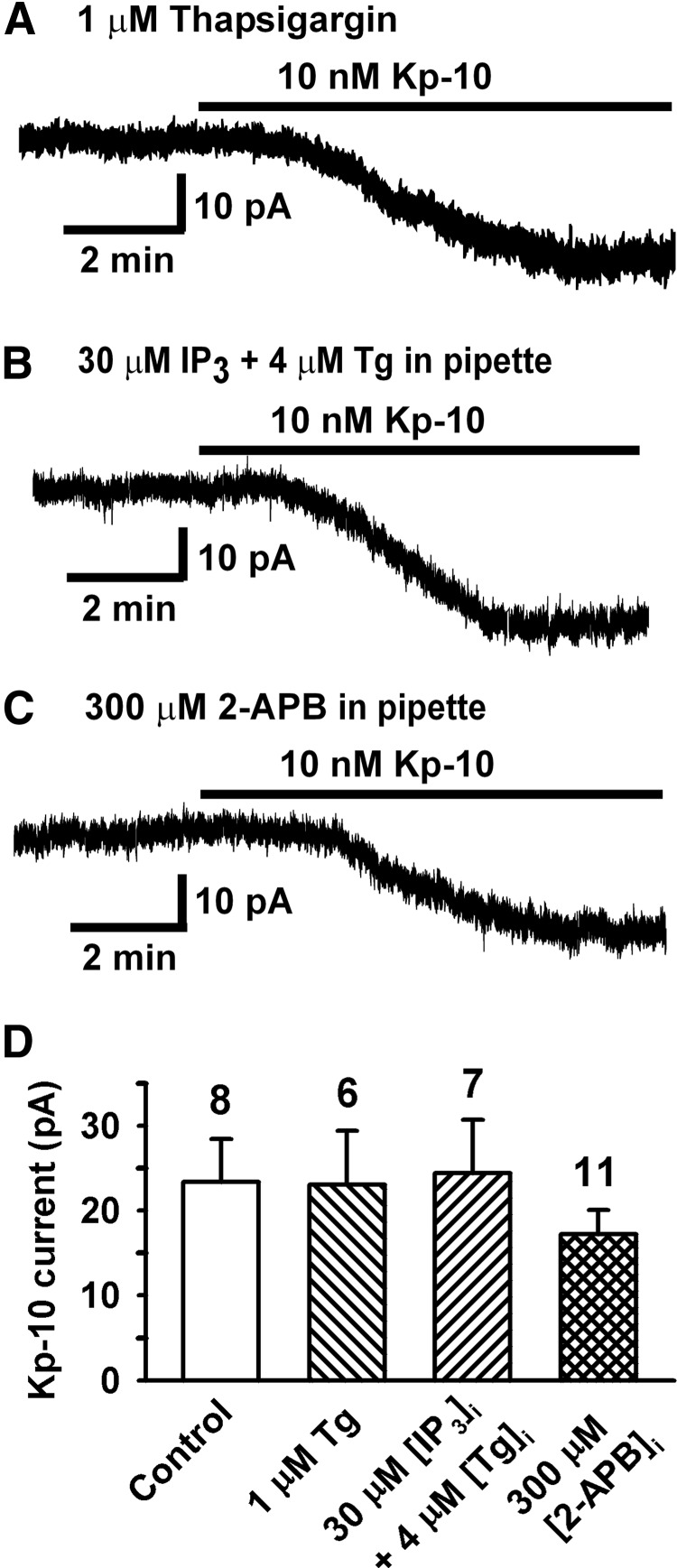
Ca2+ store depletion does not affect TRPC channel activation by kisspeptin. A–C, Representative recordings showing the kisspeptin (Kp-10)-induced inward current after a GnRH neuron had been exposed to thapsigargin (Tg, 1μM) for 10 minutes (A), internally dialyzed with 30μM myo-IP3 (IP3) and 4μM Tg for 13 minutes (B), or internally dialyzed with 2-APB (300μM) for 15 minutes (C). Vhold = −65 mV. D, Summary of the effects of Tg, IP3, and 2-APB on the kisspeptin-induced inward currents. The control represents kisspeptin response in the presence of vehicle. P > .05 (1-way ANOVA).
Figure 6.
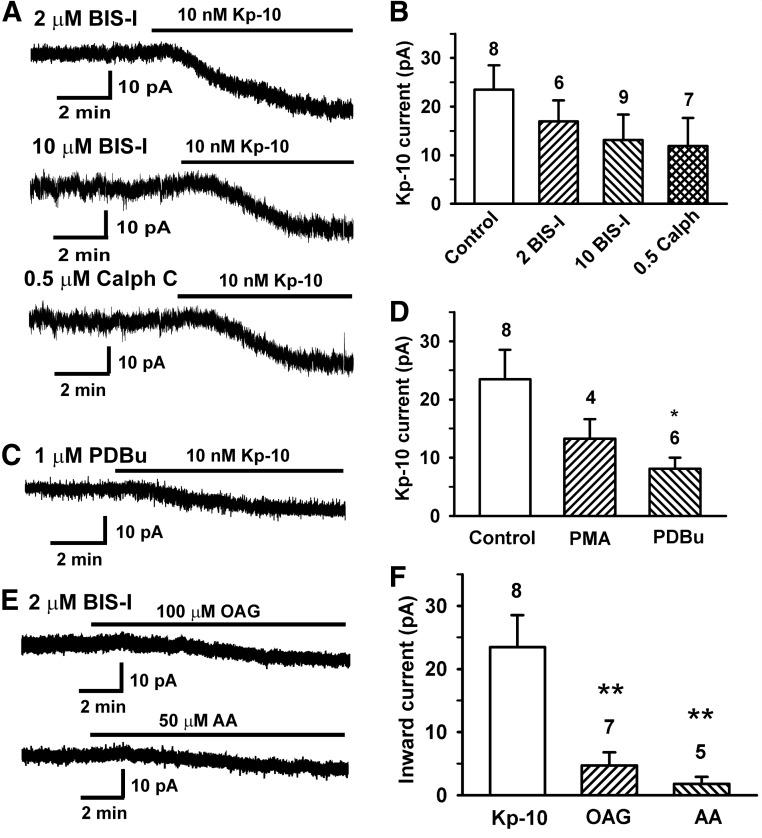
A–D, The PKC activator PDBu attenuates the kisspeptin-activation of the TRPC channels. A, Representative recordings showing the effects of PKC inhibitors BIS-I (2μM and 10μM) and calphostin C (0.5μM) on the kisspeptin (Kp-10)-activated current. B, Summary of the effects of PKC inhibitors on the kisspeptin-activated currents. P > .05 (1-way ANOVA). C, Representative recording showing the effect of PKC activator PDBu on the kisspeptin-activated current. Vhold = −65 mV. D, Summary of the effects of PKC activators PMA and PDBu on the kisspeptin-activated currents. In B and D, the control represents kisspeptin in the presence of vehicle. *, P < .05, PDBu in comparison with vehicle control (1-way ANOVA). E and F, DAG analog OAG and AA do not mimic the effects of kisspeptin. E, Representative recordings showing that 100μM OAG or 50μM AA induced a small inward current in GnRH neurons in the presence of 2μM BIS-I. Vhold = −65 mV. F, Summary of the inward currents induced by kisspeptin, OAG, and AA. **, P < .01 in comparison with kisspeptin-10 (1-way ANOVA).
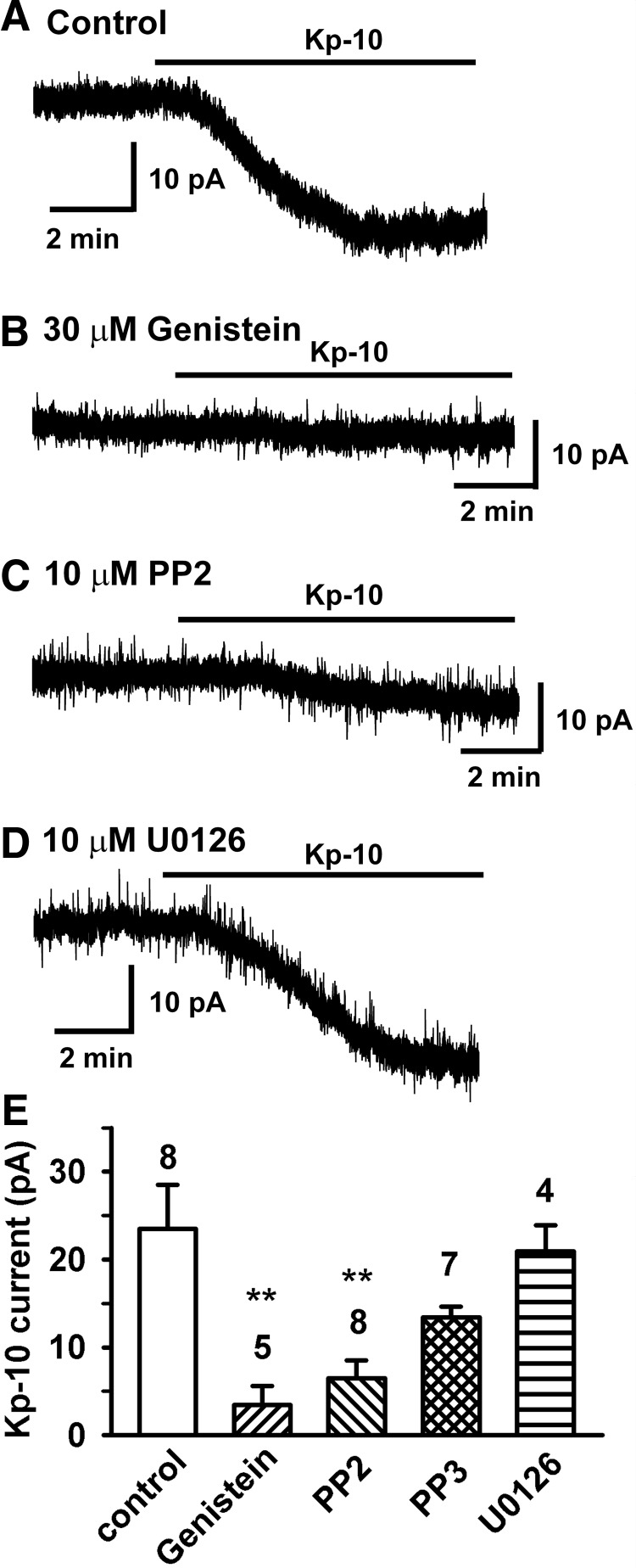
Figure 7.
Src kinase inhibitors abrogate the kisspeptin activation of TRPC channels in GnRH neurons. A–D, Representative recordings showing that the kisspeptin (Kp-10)-activated inward currents were inhibited by tyrosine kinase inhibitor genistein (30μM) and the cSrc kinase inhibitor PP2 (10μM) but not by MAPK inhibitor U0126. Vhold = −65 mV. E, Summary of the effects of genistein, PP2, PP3, and the MAPK inhibitor U0126 on the kisspeptin-induced currents. The control represents kisspeptin in the presence of vehicle. **, P < .01, genistein versus control and PP2 versus control (1-way ANOVA).
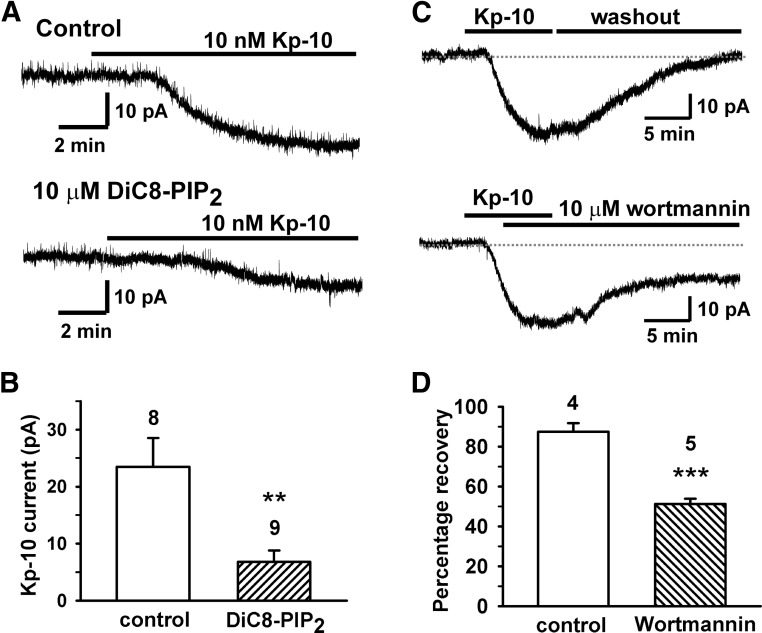
Figure 2.
PIP2 depletion is required for the kisspeptin activation of TRPC channels. A, Representative recordings showing that the DiC8-PIP2 (10μM) dialysis for 15 minutes inhibited the kisspeptin (Kp-10)-activated inward currents. Vhold = −65 mV. B, Mean kisspeptin-activated current without (control) or with DiC8-PIP2 dialysis. **, P < .01, DiC8-PIP2 versus control (Student's t test). Cell numbers are indicated. C, Representative recordings showing that the 10nM kisspeptin (Kp-10)-activated inward current fully recovered after 15 minutes of washing out of kisspeptin. However, in the presence of wortmannin (10μM), it only partially recovered after 15 minutes of washout of kisspeptin. D, Mean recovery of the TRPC current from kisspeptin activation and the effects of wortmannin. Recoveries were 87.5% ± 4.3% (n = 4) in control versus 51.1% ± 2.8% in the wortmannin groups (n = 5). The control group represents washout of kisspeptin in the absence of wortmannin. ***, P < .001, wortmannin versus control (Student's t test).
GnRH neuronal harvesting, single-cell reverse transcription, and PCR
Individual GnRH neurons were dispersed, patched and harvested, and subjected to single-cell reverse transcription (RT) and PCR as previously described in detail (18, 23). Briefly, single-cell PCR was performed using 2 to 3 μL cDNA template from each RT reaction in a 30-μL PCR mix. Fifty cycles of amplification were performed using a Bio-Rad C1000 Thermal Cycler (Bio-Rad, Hercules, California), and the PCR product was visualized with ethidium bromide on a 2% agarose gel. The mouse GnRH (accession number NM_008145) forward primer corresponded to nucleotides (nt) 21 to 40, and the reverse primer was complementary to nt 259 to 278. The mouse GPR54 (accession number NM_053244) forward primer corresponded to nt 1900 to 1917, and the reverse primer was complementary to nt 2125 to 2144. The full-length TRPC4 channel sequence, TRPC4α (NM_016984), is identical to TRPC4β (NM_001253682) except for an additional 251-bp fragment in exon 11. Two sets of primers were designed to detect TRPC4 in single neurons: primer set a (forward primer nt 1841-1860 and reverse primer nt 1956-1937) produced a 116-bp product and crosses the intron-exon boundary between exons 6 and 7; primer set b (forward primer nt 2426-2445 and reverse primer nt 2649-2666) was designed to produce a 241-bp product that crosses the intron-exon boundary between exons 10 and 11 and to incorporate part of the TRPC4α-specific sequence in exon 11 (Figure 1A). Several primer pairs were designed and tested for amplification efficiency, and the best pairs were used for further studies. The amplification efficiency of the TRPC4 and TRPC4α primers were determined as described previously (23).
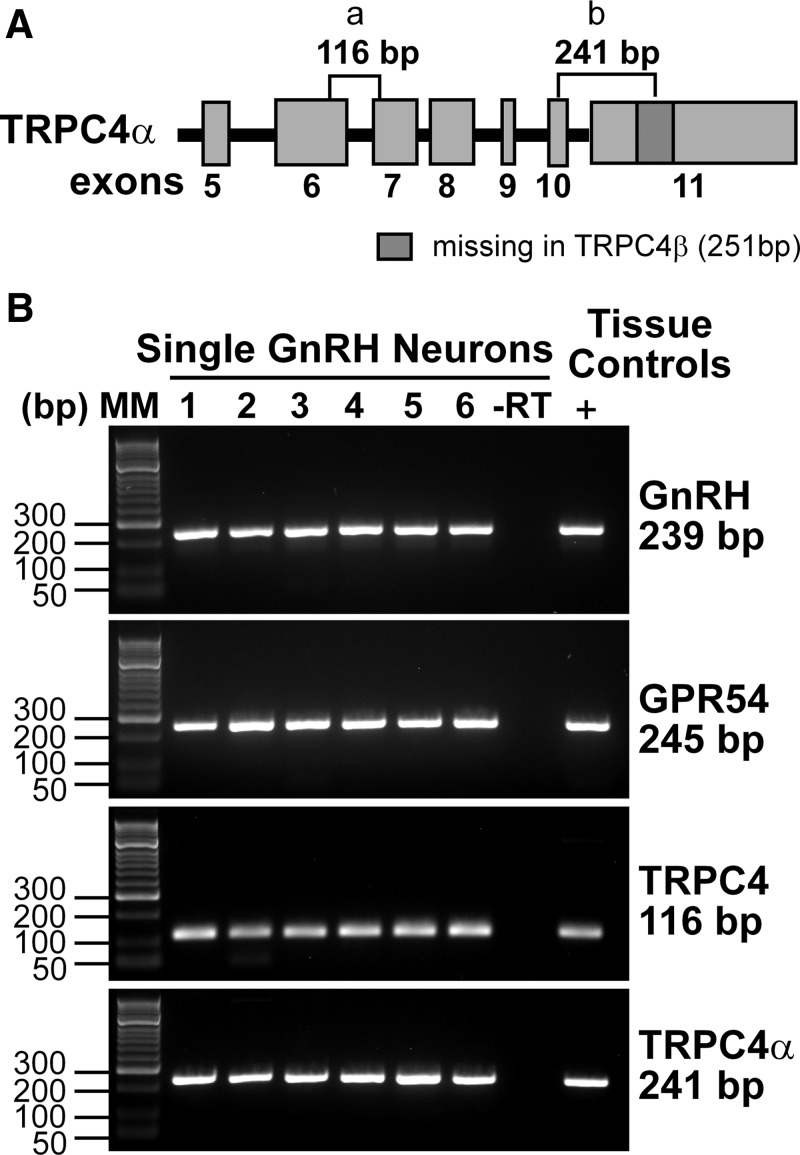
Figure 1.
GnRH neurons express TRPC4α, a PIP2-regulated isoform. A, Schematic diagram of TRPC4 gene structure and primer locations. The full-length TRPC4 channel, TRPC4α, is identical to TRPC4β except for the additional 251-bp fragment in exon 11. B, Representative gels illustrating the coexpression of GPR54, TRPC4, and TRPC4α mRNAs in selected GnRH neurons. The expected sizes for the PCR products are as follows: for GnRH, 239 bp; for GPR54, 245 bp; for TRPC4, 116 bp; and for TRPC4α, 241 bp. As a negative control, a cell subjected to RT-PCR but without reverse transcriptase (−RT) did not express any of the transcripts. POA tissue RNA was also included as positive control (+, with RT). Abbreviation: MM, molecular markers.
Results
PIP2 depletion is required for kisspeptin activation of the TRPC current
PIP2 is an important regulator of TRPC channels (29–32). TRPC1-containing heteromeric channels are activated by PIP2 (29, 30). In contrast, TRPC4 homomeric channels are inhibited by PIP2 in a splice isoform (TRPC4α)-dependent manner (33). Our recent studies found that TRPC4 is the dominant TRPC channel subtype expressed in GnRH neurons (23); therefore, we examined the expression of TRPC4 isoforms as well as the mRNA expression of the kisspeptin receptor GPR54. Our single-cell RT-PCR analysis of 74 cells from 5 animals revealed that the majority (72%) of GnRH neurons expressed TRPC4 mRNA and the full-length TRPC4α transcript was found in 40% of GnRH neurons (Figure 1B). In addition, the mRNA for the kisspeptin receptor GPR54 was expressed in 98% of GnRH neurons. To differentiate TRPC4α in individual GnRH neurons and meet the criterion for primers to cross introns, we had a very limited sequence range from which to design primer pairs (Figure 1A). For this reason, the transcription efficiency achieved with the TRPC4α primers (83%) was less than that obtained with the TRPC4 primers (100%). This technical difference may have influenced our ability to detect TRPC4α transcripts in GnRH neurons. We next examined the effect of PIP2 on the kisspeptin-activated inward current. Water-soluble DiC8-PIP2 was dissolved in the internal solution to a final concentration of 10μM, and after whole-cell dialysis for about 15 minutes, kisspeptin induced a much smaller inward current in 8 of 9 cells examined (5.6 ± 1.8 pA, n = 9, P < .01) (Figure 2). This indicates that the depletion of PIP2 is required for TRPC channel activation by kisspeptin. Then we asked whether PIP2 depletion is a mechanism by which kisspeptin activates the TRPC current. PIP2 depletion is the established mechanism underlying the Gq-coupled muscarinic M1 receptor-mediated inhibition of the M (KCNQ2/3) current (34). Micromolar concentrations of wortmannin potently inhibit phosphatidylinositol 4-kinase (PI4K), a critical enzyme responsible for the synthesis of PIP2 (35). It has been shown that wortmannin (10μM) strongly prevents the recovery of the M current from M1 receptor-mediated inhibition due to the inhibition of the resynthesis of PIP2 by PI4K (36). Therefore, we examined whether inhibition of PIP2 resynthesis by wortmannin treatment prolonged the kisspeptin-activated TRPC current. As shown in Figure 2, the kisspeptin-activated current under control conditions recovered by 87.5% ± 4.3% (n = 4) after 15 minutes of washing out kisspeptin. However, in the presence of wortmannin (10μM), the TRPC current was significantly prolonged such that it recovered by only 51.1% ± 2.8% over the same time period (n = 5, P < .001) (Figure 2, C and D). Therefore, PIP2 depletion is required but not sufficient for kisspeptin to activate the TRPC current in GnRH neurons.
Effect of Ca2+ on the kisspeptin activation of the TRPC current
The kisspeptin receptor GPR54 (or Kiss1R) is a Gq/11-coupled receptor. Its activation stimulates PLCβ and metabolizes PIP2 into 2 second messengers, DAG and IP3 (3, 37). The downstream messenger IP3 binds to the IP3 receptor (IP3R) on endoplasmic reticulum (ER) membranes to cause Ca2+ mobilization. To test whether an increase in intracellular Ca2+ concentration is required for kisspeptin to induce an inward current in GnRH neurons, cells were dialyzed with EGTA or BAPTA buffer with or without added Ca2+ for approximately 15 minutes. The estimated levels of intracellular Ca2+ for 11mM EGTA plus 6.6mM CaCl2 was 170nM (calculated by winmaxc version 2.0, http://www.stanford.edu/∼cpatton/maxc.html). The intracellular Ca2+ levels without or with low concentrations (0.5mM and 0.05mM) of EGTA in the pipette should be able to freely fluctuate in response to intracellular Ca2+ release from Ca2+ stores or Ca2+ influx through plasma membrane Ca2+ channels. However, with high concentrations of EGTA (11mM) or BAPTA (10mM) in the pipette, a rise in intracellular Ca2+ levels would be suppressed. None of these buffer conditions affected the kisspeptin-induced current (Figure 3). Removal of extracellular calcium by switching to nominally Ca2+-free aCSF had no effect on the kisspeptin-activated TRPC current (20.3 ± 4.2 pA, n = 6 vs control 23.4 ± 5.0 pA, n = 8, P > .05), which is consistent with our previous findings (18). However, our previous study showed that the calcium channel blocker Cd2+ (250μM) inhibited the kisspeptin current (18). Therefore, we examined the effects of low-voltage–activated (LVA) and high-voltage–activated (HVA) Ca2+ channel blockers on the kisspeptin-induced inward current. As shown in Figure 4, LVA Ca2+ channel blocker Ni2+ dose-dependently (100μM to 1mM) inhibited the kisspeptin-induced TPRC current, but HVA Ca2+ channel blocker amlodipine (30μM) had no effect. To examine the effects of depolarization-induced calcium entry from HVA Ca2+ channels, we applied a 600-millisecond pulse to +40 mV every minute to activate HVA Ca2+ channels and measured the kisspeptin current at −65 mV. However, these high-voltage pulses also had no effect on the kisspeptin-activated inward currents (23.0 ± 3.1 pA, n = 10 vs control 23.4 ± 5.0 pA, n = 8). Therefore, LVA (ie, T-type) Ca2+ and not HVA Ca2+ channels play a critical role in TRPC channel activation in GnRH neurons.
Calcium store depletion does not affect the kisspeptin activation of the TRPC current
TRPC channels can form either receptor-operated or store-operated calcium channels (SOCs) (38). SOCs are activated by the depletion of ER calcium stores via the activation of IP3Rs. Dialysis of high concentrations of EGTA alone or combined with blockade of the sarcoplasmic reticulum Ca2+ ATPase by thapsigargin can also deplete calcium stores and cause the activation of SOCs (39). Therefore, we examined the effects of thapsigargin or IP3 in combination with dialysis of a high concentration of EGTA on the kisspeptin-activated inward current. As shown in Figure 5, when GnRH neurons were pretreated with 1μM thapsigargin for 10 to 15 minutes, the kisspeptin-induced current was not affected. It has been shown that intracellular dialysis of IP3 and thapsigargin can accelerate the depletion of ER calcium stores and activate SOCs (39). To ensure the depletion of the IP3-sensitive calcium stores, 30μM myo-IP3 plus 4μM thapsigargin were included in the pipette and allowed to dialyze the cells for 10 to 15 minutes. However, the kisspeptin-activated current was not affected. In our previous studies, we showed that extracellularly applied 2-APB blocked the kisspeptin-activated TRPC current, whereas intracellular dialysis of 2-APB, an IP3R inhibitor, had no effect on the kisspeptin-activated TRPC current (18). To ensure that 2-APB reached an effective concentration, we increased the concentration from 0.1mM to 0.3mM and dialyzed the cells for 15 minutes before kisspeptin application. In agreement with our previous findings, blockade of IP3R did not affect the kisspeptin activation of TRPC current in GnRH neurons (18). Therefore, the kisspeptin activation of TRPC channels is not regulated by internal calcium stores, which argues that the TRPC channels in GnRH neurons are receptor-operated and not store-operated.
Effects of protein kinase C on the kisspeptin-activated TRPC current
Protein kinase C (PKC) is an important second messenger stimulated by Gq-coupled receptor signaling pathways. It has been shown that PKC activation can either inhibit or stimulate TRPC channels (26, 30, 40). Therefore, we examined the effects of BIS-I, a broad-spectrum PKC inhibitor, on the kisspeptin-activated TRPC current. To ensure a full inhibition of conventional PKC isoforms by BIS-I, GnRH neurons were pretreated with 2μM BIS-I for 45 to 90 minutes (mean time = 67 ± 12 minutes, n = 6) before kisspeptin application. However as shown in Figure 6, A and B, inhibition of conventional PKC isoforms by BIS-I had no effect on the kisspeptin-induced current (BIS 17.0 ± 4.3 pA, n = 6, vs control 23.4 ± 5.0 pA, n = 8; P > .05). Increasing BIS-I concentrations to 10μM to inhibit novel PKC isoforms also did not significantly affect the kisspeptin-induced TRPC current (68 ± 4 minutes, 13.4 ± 5.2 pA, n = 9, P > .05). Moreover, pretreatment with another broad-spectrum PKC inhibitor calphostin C (0.5μM) for 55 ± 10 minutes also had no significant effect on the kisspeptin-activated TRPC current (11.9 ± 5.8 pA, n = 7, P > .05). Next, we examined the effect of PKC activators PDBu and phorbol-12-myristate 13-acetate (PMA) on the kisseptin-activated current. Neurons were pretreated with PDBu (1μM) or PMA (1μM) for 10 minutes before kisspeptin application. As shown in Figure 6, C and D, PDBu pretreatment inhibited the kisspeptin-induced inward current (8.1 ± 1.9 pA, n = 6, P < .05) (Figure 6 D). However, PMA pretreatment had no significant effect (13.3 ± 3.4 pA, n = 4, P > .05). Therefore, PKC activation by PDBu inhibited the kisspeptin-activated TRPC currents in GnRH neurons.
OAG and AA do not mimic the effect of kisspeptin
We then asked whether kisspeptin activates TRPC channel through the direct action of the second messenger DAG or its metabolite AA. Previously, we found that the DAG analog OAG induced a small inward current in GnRH neurons (18). To prevent the activation of PKC by OAG, we examined the effect of OAG in the presence of PKC inhibitor BIS-I (2μM). As shown in Figure 6, E and F, OAG induced a small inward current (5.0 ± 0.7 pA, n = 7) even in the presence of PKC inhibitor. We also examined the effect of AA. Fifty micromolar AA induced a even smaller inward current in GnRH neurons (1.8 ± 1.1 pA, n = 5). Therefore, neither OAG nor AA mimic the effects of kisspeptin.
cSrc kinase inhibition prevents kispeptin activation of the TRPC current
cSrc tyrosine kinase has been reported to be activated by G protein-coupled receptors or epidermal growth factor receptor to regulate TRPC channel activity (41–45). Therefore, we first examined the effects of genistein, a general tyrosine kinase inhibitor (46), on the kisspeptin-activated TRPC channels. After pretreatment of GnRH neurons with genistein (30μM) for 30 to 60 minutes (53 ± 9 minutes), the kisspeptin-induced inward current was robustly attenuated (Figure 7; genistein 3.2 ± 2.0 pA, n = 5, vs vehicle control 23.0 ± 5.0 pA, n = 8; P < .01). Then we examined the effects of PP2 (10μM), a more selective cSrc tyrosine kinase inhibitor (47, 48). PP2 also inhibited the kisspeptin current (6.5 ± 2.0 pA, n = 8, P < .01). As a negative control, PP3 (10μM) did not significantly affect the kisspeptin current (13.5 ± 3.6 pA, n = 7, P > .05). To test whether cSrc kinase directly regulates TRPC channel activity or whether MAPK activation is also required, we measured the effects of kisspeptin in the presence of U0126 (10μM), which is a selective MEK1 and -2 inhibitor. As shown in Figure 7, MAPK inhibition had no effect on the kisspeptin response (20.9 ± 5.4 pA, n = 4, P < .05). Therefore, cSrc appears to be a key signaling molecule in the kisspeptin-mediated activation of TRPC channels.
Discussion
We have shown for the first time that kisspeptin activation of TRPC channels in GnRH neurons requires the depletion of PIP2 and activation of cSrc kinase. The release of calcium from intracellular stores and downstream activation of PKC are not required for kisspeptin's potent depolarization of GnRH neurons. Therefore, the kisspeptin activation of TRPC channels involves a novel signaling pathway for peptidergic excitation of GnRH neurons.
Kisspeptin activation of TRPC channels does not depend on calcium release from internal stores
Kisspeptin potently depolarizes GnRH neurons primarily through activation of a TRPC conductance, which ensures a sustained activation of GnRH neurons (18, 20). TRPC channels are differentially regulated by intracellular and extracellular calcium. TRPC5 and to a lesser extent TRPC4 channels expressed in HEK cells are potentiated by extracellular and intracellular calcium (49). In GnRH neurons, kisspeptin induces a transient elevation of intracellular calcium, which is thought to be due to intracellular calcium store release and has been hypothesized to play an important role in the kisspeptin-mediated depolarization (20). However, our previous and present results show that the activation of TRPC channels by kisspeptin are not affected by buffering intracellular calcium levels or by calcium store depletion. Buffering intracellular calcium levels to low nanomolar concentrations with high EGTA or BAPTA, which is known to suppress an elevation in free intracellular calcium from calcium store release or extracellular calcium influx, had no effect on the kisspeptin-mediated response. Therefore, a rise in intracellular calcium does not appear to play a critical role in the kisspeptin-mediated activation of TRPC channels but may be involved in Ca2+/calmodulin-dependent inhibition of high-voltage–gated Ca2+ channels (50). On the other hand, the kisspeptin-activated TRPC current was attenuated by calcium channel blockers Cd2+ and Ni2+ but not by HVA calcium channel blocker amlodipine (present results) (18). This would indicate that T-type calcium channels may be involved. However, reducing extracellular calcium to nominally calcium-free had no effect on the kisspeptin-activated TRPC current (present results) (18), an indication that very little calcium is needed to spark the opening of TRPC channels in GnRH neurons. This is consistent with the small, but persistent, T-type calcium channel window current around −65 mV in GnRH neurons (51). Therefore, with a sustained depolarization that exceeds that of classical neurotransmitters (eg, glutamate), kisspeptin excites GnRH neurons primarily through the opening of a cation-selective (TRPC) channel that is independent of intracellular calcium store release. However, TRPC channel activation does appear to depend on calcium influx through T-type calcium channels (Figure 8).
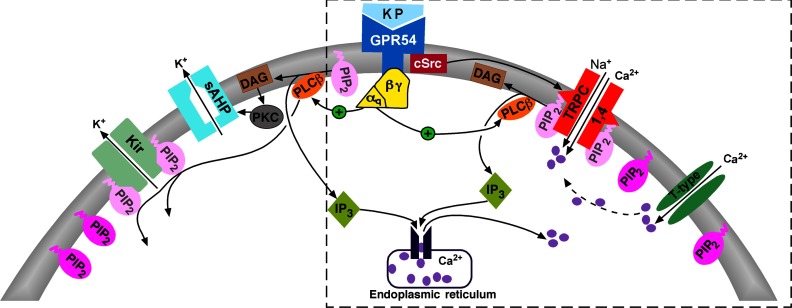
Figure 8.
Schematic diagram illustrating the main signaling pathways responsible for kisspeptin-induced depolarization and burst firing of GnRH neurons. Kisspeptin binds to the Gq-coupled GPR54 receptor to activate PLCβ, which catabolizes PIP2, potentiates TRPC channel activity, and inhibits the Kir channel activity. PKC, activated by the PIP2 hydrolysis product DAG, inhibits the activity of a calcium-activated slow afterhyperpolarization (sAHP) current (see Ref. 56). cSRC, which is activated by kisspeptin/GRP54 signaling, potentiates the activity of TRPC4 channels. Calcium entering the cell via T-type channels may facilitate the activation of TRPC channels. The present findings for the signaling pathway underlying TRPC4 channel activation are highlighted in the box.
TRPC channels in GnRH neurons are receptor-operated
TRPC channels can form either SOCs (ie, activated by depletion of calcium stores) or receptor-operated calcium channels (ie, activated by G protein-coupled receptors) (52). TRPC SOCs expressed in vascular smooth muscle cells are activated by PKC as well as intracellular calcium store depletion (53). Our present results show that depletion of intracellular calcium stores by intracellular dialysis of a combination of thapsigargin, IP3,and EGTA did not affect the kisspeptin-activated TRPC current. Similarly, Inhibition of IP3Rs by intracellular dialysis of 2-APB also had no effect (present results) (18). These cumulative results indicate that TRPC channels in GnRH neurons are not store-operated (38, 54). In addition, all TRPC receptor-operated calcium channels are inhibited by PKC activation in heterologous (HEK-293) cells (55). Indeed, the kisspeptin-activated TRPC current in GnRH neurons was significantly attenuated by PKC activation (ie, pretreatment with PDBu) but was not affected by PKC inhibition. In contrast, our recent study shows that kisspeptin inhibits a calcium-activated slow afterhyperpolarization current in GnRH neurons through a PKC pathway (56). Collectively, our findings would indicate that TRPC channels in GnRH neurons are receptor-operated channels and do not depend on intracellular calcium store release. This ensures fast and sustained depolarization of GnRH neurons.
PIP2 depletion is required for kisspeptin activation of the TRPC channels in GnRH neurons
The transcripts for all, with the exception of TRPC2, of the TRPC channels (the TRPC1, -4, and -5 family and the TRPC3, -6, and -7 family) are expressed in GnRH neurons (18). However, TRPC4 is the dominant TRPC channel expressed in GnRH neurons with a severalfold higher expression than TRPC1 and TRPC5 (18, 23). TRPC1 channels form heteromeric complexes with TRPC4 and/or TRPC5 channels. In addition TRPC4 and -5 can form homomultimer channels that are distinct from the heteromultimers (24). PIP2 is an important regulator of TRPC channels (29–32). Heteromeric channels expressing TRPC1 channels are activated by PIP2 (29, 30). However, homomeric TRPC4 channels, composed of the full-length TRPC4α, but not the truncated TRPC4β splice variant, are inhibited by PIP2 in HEK cells and vascular smooth muscle cells (33). Currently, TRPC4α was identified in a subpopulation of GnRH neurons, whereas intracellular dialysis with DiC8-PIP2 robustly inhibited the kisspeptin-activated TRPC current in essentially all of the neurons. Therefore, we deduced that the full-length isoform TRPC4α is responsible for kisspeptin activation of the TRPC current in most GnRH neurons. However, we cannot rule out that a subpopulation of GnRH neurons express TRPC4β. Whether or not PIP2 depletion would activate GnRH neurons expressing TRPC4β is currently not known and needs to be investigated in future experiments. Nevertheless, in the presence of micromolar concentrations of wortmannin, which inhibit the regeneration of PIP2 via antagonizing PI4K (35), the recovery of TRPC channels after kisspeptin activation was significantly prolonged. Therefore, the depletion of PIP2 is required for TRPC channel activation in GnRH neurons, which is similar to vascular myocytes and HEK cells expressing TRPC4α (33). In contrast to TRPC1, -4, and -5 channels, the TRPC3, -6, and -7 subfamily are activated by DAG (24). Presently, we confirmed our previous findings that the DAG analog OAG had only minor effects in GnRH neurons (18). Also, the DAG metabolite AA was ineffective. Collectively, these findings would indicate that PIP2 depletion is necessary for kisspeptin activation of TRPC current in GnRH neurons and maybe a critical point of physiological regulation as has been shown for other channels (34, 57).
cSrc tyrosine kinase activation is also required for kisspeptin activation of the TRPC channels in GnRH neurons
G protein-coupled receptors and epidermal growth factor receptors are coupled to activation of cSrc, which increases TRPC channel activity through phosphorylation of tyrosine residues (41–45). Epidermal growth factor has been found to cause rapid vesicular translocation and insertion of TRPC5 channels into the plasma membrane of hippocampal neurons (58). In the present experiments, the global tyrosine kinase inhibitor genistein (46) robustly inhibited the kisspeptin-induced inward current. Moreover, the selective cSrc kinase inhibitor PP2 (47, 48) also inhibited the kisspeptin currents. cSrc kinase can directly regulate TPRC4 channel activity through tyrosine phosphorylation, which causes rapid insertion of TRPC4 into the plasma membrane (43). Although we could not measure phosphorylation of TRPC4 channels by cSrc in GnRH neurons directly, downstream activation of another tyrosine kinase, MAPK, is not required because a selective MEK1/2 inhibitor, U0126, had no effect on the kisspeptin response, which is consistent with the findings of Liu et al (20). In contrast, kisspeptin signaling in Chinese hamster ovary (CHO) cells expressing rat or human GPR54 is coupled to ERK1/2 and p38 MAPK phosphorylation (3), suggesting different cellular signaling mechanism by kisspeptin in heterologous expression systems versus native GnRH neurons. Therefore, cSrc appears to be a key signaling molecule in the kisspeptin-mediated activation of TRPC channels in GnRH neurons (Figure 8).
In summary, previous studies have shown that kisspeptin robustly depolarizes and increases the firing of GnRH neurons through a combination of activating TRPC channels, inhibiting Kir channels, and inhibiting the slow afterhyperpolarization in GnRH neurons (18–20, 56, 59). Presently, we have elucidated the complex signaling pathway by which kisspeptin activates TRPC channels in native GnRH neurons through a combination of the depletion of PIP2 and activation of cSrc kinase (Figure 8). Kisspeptin activation of TRPC channels through PIP2 depletion and cSrc tyrosine kinase activation is a novel and potent signaling pathway for sustained excitation of GnRH neurons and as a consequence sustained GnRH neurosecretion characteristic of the preovulatory surge.
Acknowledgments
The technical assistance of Mr M. Richard Rollins is greatly appreciated. We also thank Drs Charles Roselli and Casey Nestor for their comments on the manuscript.
Research reported in this publication was supported by National Institutes Health R01 Grants NS 38809, NS 43330, and DK 68098. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AA
- arachidonic acid
- aCSF
- artificial CSF
- 2-APB
- aminoethoxydiphenylborane
- BIS-I
- bisindolylmaleimide-I
- CSF
- cerebrospinal fluid
- DAG
- diacylglycerol
- DB-POA
- diagonal band-preoptic area
- DiC8
- Dioctanoylglycerol
- ER
- endoplasmic reticulum
- GPR54
- G protein-coupled receptor 54
- HVA
- high-voltage–activated
- IP3
- inositol 1,4,5-trisphosphate
- IP3R
- IP3 receptor
- Kir
- inwardly rectifying K+
- LVA
- low-voltage–activated
- OAG
- 1-oleoyl-2-acetyl sn-glycerol
- PDBu
- phorbol 12,13-dibutyrate
- PIP2
- phosphatidylinositol-4,5-bisphosphate
- PI4K
- phosphatidylinositol 4-kinase
- PKC
- protein kinase C
- PLC
- phospholipase C
- PMA
- phorbol 12-myristate 13-acetate
- PP2
- 3,4-chlorophenyl 1-(1,1-dimethylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine
- PP3
- 1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine
- SOC
- store-operated calcium channel
- TRPC
- canonical transient receptor potential
- TTX
- tetrodotoxin.
References
- 1. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 3. Kotani M, Detheux M, Vandenbogaerde A, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636 [DOI] [PubMed] [Google Scholar]
- 4. Stafford LJ, Xia C, Ma W, Cai Y, Liu M. Identification and characterization of mouse metastasis-suppressor KiSS1 and its G-protein-coupled receptor. Cancer Res. 2002;62:5399–5404 [PubMed] [Google Scholar]
- 5. Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 7. Irwig MS, Fraley GS, Smith JT, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuronendocrinology. 2004;80:264–272 [DOI] [PubMed] [Google Scholar]
- 8. Thompson EL, Patterson M, Murphy KG, et al. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–858 [DOI] [PubMed] [Google Scholar]
- 9. Kinoshita M, Tsukamura H, Adachi S, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–4436 [DOI] [PubMed] [Google Scholar]
- 10. Messager S, Chatzidaki EE, Ma D, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ohtaki T, Shintani Y, Honda S, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617 [DOI] [PubMed] [Google Scholar]
- 12. Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH. GABA-and glutamate activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci. 1999;19:2037–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuehl-Kovarik MC, Pouliot WA, Halterman GL, Handa RJ, Dudek FE, Partin KM. Episodic bursting activity and response to excitatory amino acids in acutely dissociated gonadotropin-releasing hormone neurons genetically targeted with green fluorescent protein. J Neurosci. 2002;22:2313–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-type γ-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16:2872–2891 [DOI] [PubMed] [Google Scholar]
- 15. Han SK, Abraham IM, Herbison AE. Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology. 2002;143:1459–1466 [DOI] [PubMed] [Google Scholar]
- 16. Suter KJ. Control of firing by small (S)-α-amino-3-hydroxy-5methyl-isoxazolepropionic acid-like inputs in hypothalamic gonadotropin releasing-hormone (GnRH) neurons. Neuroscience. 2004;128:443–450 [DOI] [PubMed] [Google Scholar]
- 17. Iremonger KJ, Constantin S, Liu X, Herbison AE. Glutamate regulation of GnRH neuron excitability. Brain Res. 2010;1364:35–43 [DOI] [PubMed] [Google Scholar]
- 18. Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci. 2008;28:4423–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology. 2008;149:4605–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Constantin S, Caligioni CS, Stojilkovic S, Wray S. Kisspeptin-10 facilitates a plasma membrane-driven calcium oscillator in gonadotropin-releasing hormone-1 neurons. Endocrinology. 2009;150:1400–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kroll H, Bolsover S, Hsu J, Kim SH, Bouloux PM. Kisspeptin-evoked calcium signals in isolated primary rat gonadotropin-releasing hormone neurones. Neuroendocrinology. 2011;93:114–120 [DOI] [PubMed] [Google Scholar]
- 23. Bosch MA, Tonsfeldt KJ, Rønnekleiv OK. mRNA expression of ion channels in GnRH neurons: subtype-specific regulation by 17β-estradiol. Mol Cell Endocrinol. 2013;367:85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524 [DOI] [PubMed] [Google Scholar]
- 25. Ambudkar IS, Ong HL. Organization and function of TRPC channelosomes. Pflugers Arch. 2007;455:187–200 [DOI] [PubMed] [Google Scholar]
- 26. Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141:412–419 [DOI] [PubMed] [Google Scholar]
- 28. Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express KATP channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci. 2007;27:10153–10164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Albert AP. Gating mechanisms of canonical transient receptor potential channel proteins: role of phosphoinositols and diacylglycerol. Adv Exp Med Biol. 2011;704:391–411 [DOI] [PubMed] [Google Scholar]
- 30. Large WA, Saleh SN, Albert AP. Role of phosphoinositol 4,5-bisphosphate and diacylglycerol in regulating native TRPC channel proteins in vascular smooth muscle. Cell Calcium. 2009;45:574–582 [DOI] [PubMed] [Google Scholar]
- 31. Lemonnier L, Trebak M, Putney JW., Jr Complex regulation of the TRPC3, 6 and 7 channel subfamily by diacylglycerol and phosphatidylinositol-4,5-bisphosphate. Cell Calcium. 2008;43:506–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trebak M, Lemonnier L, DeHaven WI, Wedel BJ, Bird GS, Putney JW., Jr Complex functions of phosphatidylinositol 4,5-bisphosphate in regulation of TRPC5 cation channels. Eur J Physiol. 2008;457:757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Otsuguro K, Tang J, Tang Y, et al. Isoform-specific inhibition of TRPC4 channel by phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2008;283:10026–10036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suh BC, Hille B. Regulation of KCNQ channels by manipulation of phosphoinositides. J Physiol. 2007;582(Pt 3):911–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakanishi S, Catt KJ, Balla T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc Natl Acad Sci U S A. 1995;92:5317–5321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Suh BC, Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 2002;35:507–520 [DOI] [PubMed] [Google Scholar]
- 37. Castaño JP, Martínez-Fuentes AJ, Gutiérrez-Pascual E, Vaudry H, Tena-Sempere M, Malagón MM. Intracellular signaling pathways activated by kisspeptins through GPR54: do multiple signals underlie function diversity? Peptides. 2009;30:10–15 [DOI] [PubMed] [Google Scholar]
- 38. Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca2+ concentrations. Annu Rev Pharmacol Toxicol. 2009;49:395–426 [DOI] [PubMed] [Google Scholar]
- 39. Fierro L, Parekh AB. Substantial depletion of intracellular Ca2+ stores is required for macroscopic activation of the Ca2+ release-activated Ca2+ current in rat basophilic leukaemia cells. J Physiol. 2000;522:247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shi J, Ju M, Abramowitz J, Large WA, Birnbaumer L, Albert AP. TRPC1 proteins confer PKC and phosphoinositol activation on native heteromeric TRPC1/C5 channels in vascular smooth muscle: comparative study of wild-type and TRPC1−/− mice. FASEB J. 2012;26:409–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McGarrigle D, Huang X. GPCRs signaling directly through src-family kinases. Sci STKE. 2007;392:pe35. [DOI] [PubMed] [Google Scholar]
- 42. Tsai W, Morielli AD, Peralta EG. The m1 muscarinic acetylcholine receptor transactivates the EGF receptor to modulate ion channel activity. EMBO J. 1997;16:4597–4605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Odell AF, Scott JL, Van Helden DF. Epidermal growth factor induces tyrosine phosphorylation, membrane insertion, and activation of transient receptor potential channel 4. J Biol Chem. 2005;280:37974–37987 [DOI] [PubMed] [Google Scholar]
- 44. Kawasaki BT, Liao Y, Birnbaumer L. Role of Src in C3 transient receptor potential channel function and evidence for a heterogeneous makeup of receptor- and store-operated Ca2+ entry channels. Proc Natl Acad Sci U S A. 2006;103:335–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gamper N, Stockand JD, Shapiro MS. Subunit-specific modulation of KCNQ potassium channels by Src tyrosine kinase. J Neurosci. 2003;23:84–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Akiyama T, Ishida J, Nakagawa S, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595 [PubMed] [Google Scholar]
- 47. Hanke JH, Gardner JP, Dow RL, et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 2012;271:695–701 [DOI] [PubMed] [Google Scholar]
- 48. Lawrence DS, Niu J. Protein Kinase inhibitors: the Tyrosine-specific Protein Kinases. Pharmacol Ther. 1998;77:81–114 [DOI] [PubMed] [Google Scholar]
- 49. Blair NT, Kaczmarek JS, Clapham DE. Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. J Gen Physiol. 2009;133:525–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang XB, Spergel DJ. Kisspeptin inhibits high-voltage activated Ca2+ channels in GnRH neurons via multiple Ca2+ influx and release pathways. Neuroendocrinology. 2012;96:68–80 [DOI] [PubMed] [Google Scholar]
- 51. Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK. 17β-estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2009;29:10552–10562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Salido GM, Jardín I, Rosado JA. The TRPC ion channels: association with Orai1 and STIM1 proteins and participation in capacitative and non-capacitative calcium entry. Adv Exp Med Biol. 2011;704:413–433 [DOI] [PubMed] [Google Scholar]
- 53. Albert AP, Saleh SN, Peppiatt-Wildman CM, Large WA. Multiple activation mechanisms of store-operated TRPC channels in smooth muscle cells. J Physiol. 2007;583:25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058 [DOI] [PubMed] [Google Scholar]
- 55. Venkatachalam K, Zheng F, Gill DL. Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C. J Biol Chem. 2003;278:29031–29040 [DOI] [PubMed] [Google Scholar]
- 56. Zhang C, Ronnekleiv OK, Kelly MJ. Kisspeptin inhibits a slow afterhyperpolarization current via protein kinase C and reduces spike-frequency adaptation in GnRH neurons. Am J Physiol Endocrinol Metab. 2013;304:E1237–E1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shyng SL, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141 [DOI] [PubMed] [Google Scholar]
- 58. Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720 [DOI] [PubMed] [Google Scholar]
- 59. Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ. γ-Aminobutyric acid B receptor mediated inhibition of gonadotropin-releasing hormone neurons is suppressed by kisspeptin-G protein-coupled receptor 54 signaling. Endocrinology. 2009;150:2388–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]