Abstract
It is known that insulin resistance and type 2 diabetes mellitus are associated with increased fractures and that brown adipose tissue (BAT) counteracts many if not all of the symptoms associated with type 2 diabetes. By the use of FoxC2AD+/Tg mice, a well-established model for induction of BAT, or beige fat, we present data extending the beneficial action of beige fat to also include a positive effect on bone. FoxC2AD+/Tg mice are lean and insulin-sensitive and have high bone mass due to increased bone formation associated with high bone turnover. Inducible BAT is linked to activation of endosteal osteoblasts whereas osteocytes have decreased expression of the Sost transcript encoding sclerostin and elevated expression of Rankl. Conditioned media (CM) collected from forkhead box c2 (FOXC2)-induced beige adipocytes activated the osteoblast phenotype and increased levels of phospho-AKT and β-catenin in recipient cells. In osteocytes, the same media decreased Sost expression. Immunodepletion of CM with antibodies against wingless related MMTV integration site 10b (WNT10b) and insulin-like growth factor binding protein 2 (IGFBP2) resulted in the loss of pro-osteoblastic activity, and the loss of increase in the levels of phospho-AKT and β-catenin. Conversely, CM derived from cells overexpressing IGFBP2 or WNT10b restored osteoblastic activity in recipient cells. In conclusion, beige fat secretes endocrine/paracrine activity that is beneficial for the skeleton.
Recent advances in the characterization of brown adipose tissue (BAT) function in postnatal life suggest that, besides its role in nonshivering thermogenesis and energy dissipation, BAT is essential for the determination of insulin sensitivity and regulation of energy metabolism (1, 2). Genetic ablation of BAT in rodents results in diet-induced obesity, diabetes, and hyperlipidemia (3). Uncoupling protein 1 (UCP1) deficiency renders animals sensitive to cold and increases their susceptibility to diet-induced obesity with aging (4, 5). In humans, BAT activity correlates negatively with impairment in energy metabolism seen with aging, diabetes, and obesity (6). These conditions are associated with a decrease in bone mass, an increase of fat volume in bone marrow cavity, and an increase in fractures (reviewed in Ref. 7). In contrast, high BAT activity in healthy young women correlates positively with high bone mineral density (8), and there is a positive association between BAT volume, the amount of bone, and cross-sectional size of the femur in children and adolescents (9). In addition, the bone mass in women recovering from wasting diseases such as anorexia nervosa is higher in those who possess cold-induced BAT foci as compared with those who lost BAT function (10).
These observations suggest that energy metabolism regulates bone turnover. Indeed, bone homeostasis and remodeling are closely linked to the osteoblastic response to insulin (11, 12). Insulin induces osteoblastogenesis and receptor activator of nuclear factor kappa-B ligand (RANKL) production leading to high bone mass, which is associated with increased bone turnover (11, 12). In contrast, conditions of insufficient insulin signaling due to either deficiency in insulin production in type 1 diabetes or resistance to insulin in type 2 diabetes are associated with low bone turnover and correlate with high levels of circulating sclerostin, a negative regulator of bone remodeling, and low numbers of circulating osteoprogenitors (13–16).
It has been recognized that BAT may come from 2 different origins. The classical preformed BAT originates from Myf5-positive dermomyotomal progenitors, which also give rise to skin and muscle and function in nonshivering thermogenesis (17). In contrast, the Myf5-negative progenitors can differentiate to white adipocytes with function in energy storage or to beige adipocytes, which have characteristics of both brown and white fat cells (18). The BAT-like phenotype can be induced in white adipose tissue (WAT)-type adipocytes by several mechanisms comprising either endocrine action of fibroblast growth factor 21 (19) or irisin (20) or action of transcriptional regulators including forkhead box c2 (FOXC2) (21) and PR domain-containing protein 16 (22) and, as shown recently, SirT1-mediated deacetylation of peroxisome proliferator-activated receptor gamma (23).
Bone marrow adipose tissue (BMAT) represents another type of fat that plays an important role in modulation of marrow environment supporting bone remodeling. BMAT volume increases with aging, estrogen deficiency, diabetes, and anorexia nervosa, and this expansion correlates with a decrease in bone mass and increase in fractures (reviewed in Ref. 7). Interestingly, the metabolic profile of BMAT has both WAT and BAT characteristics in respect to the expression of gene markers and its function (24). BAT-like features of BMAT are compromised with aging and in diabetes, suggesting a positive correlation between the BAT-like metabolic profile of BMAT and bone mass (24).
The transcription factor FOXC2 promotes brown fat development by sensitizing cells to the β-adrenergic cAMP-protein kinase A pathway and by regulation of mitochondrial metabolism (21, 25). Targeted expression of FoxC2 in adipocytes under the control of fatty acid binding protein 4 (FABP4/aP2) promoter converts epididymal WAT into BAT-like or beige fat and results in mice that are lean, insulin-sensitive, and resistant to diet-induced obesity (21, 26). Consistent with the metabolic function, the levels of FOXC2, and mitochondria gene expression, which are decreased in adipocytes of type 2 diabetic patients, can be normalized in response to the antidiabetic therapy with the insulin sensitizer rosiglitazone (27).
In the present study, we have analyzed the endocrine/paracrine activity of beige fat toward regulation of bone mass using the FoxC2AD+/Tg murine model of induced BAT activity targeted by expression of FOXC2 in fat cells. We have demonstrated, for the first time, that beige fat produces factors that may be secreted to circulation or act directly in the bone marrow environment and induce osteoblast differentiation and osteocyte support for bone formation and bone turnover. Two of these factors, insulin-like growth factor binding protein 2 (IGFBP2) and wingless related MMTV integration site 10b (WNT10b), are of special interest because they function in the regulation of both bone remodeling and energy metabolism. Their potential contribution to the anabolic effect of beige fat on bone is presented in this manuscript.
Materials and Methods
Plasmids
The expression construct for human FOXC2 was prepared at the University of Gothenburg, and the Igfbp2 construct was purchased from OriGene Technologies, Inc (Rockville, Maryland), whereas the Wnt10b expression construct was based on cDNA obtained from Addgene (Cambridge, Massachusetts). All constructs were prepared in pcDNA3.1 vector (Invitrogen, Grand Island, New York).
Protein analysis
The following antibodies were used for immunochemistry and immunodepletion studies: anti-β-catenin (BD Biosciences, San Jose, California), anti-β-actin (Sigma-Aldrich, St. Louis, Missouri), anti-AKT and anti-phospho-AKT (Cell Signaling Technology, Beverly, Massachusetts), anti-WNT10b and IGFBP2 (Santa Cruz Biotechnology, Santa Cruz, California). Western blot relative band density was measured with ImageJ National Institutes of Health (NIH) software. UCP1 immunohistochemistry was performed using anti-UCP1 (Sigma) and the immunoperoxidase system provided by Vector Laboratories (Burlingame, California).
Gene expression analysis using quantitative real-time RT-PCR analysis
The reaction was prepared as described (24) using Power SYBR Green and processed with StepOne Plus System (Applied Biosystems, Carlsbad, California). Relative gene expression was determined by the ΔΔ−Ct method using 18S RNA levels for normalization. All primers used in this study are listed in Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Animals
FoxC2AD+/Tg mice were described previously (21). The colony of FoxC2AD+/Tg and wild-type (WT) (FoxC2AD+/+) mice were maintained at the University of Toledo Health Science Campus. The animal treatment and care protocols conformed to NIH Guidelines and were performed using a University of Toledo Health Science Campus Institutional Animal Care and Utilization Committee protocol. Fat and lean mass were evaluated using a minispec mq10 NMR analyzer (Bruker, Billerica, Massachusetts). Food consumption was determined by monitoring food intake of 4-month-old animals during a 1week period. For glucose (ip glucose tolerance test [IGTT]) and insulin (ip insulin tolerance test [IITT]) tolerance tests either 2 g/kg glucose or 0.75 U/kg insulin were injected ip after 4 hours fasting. Blood glucose was measured using the murine-specific AlphaTRAK system (Abbott Laboratories, North Chicago, Illinois). Random levels of serum insulin were measured at the University of Michigan Diabetes Research Center Core Facility (Ann Arbor, Michigan), whereas serum levels for IGF-1 and IGFBP2 were measured by both Arkansas Childrens' Hospital Research Institute (Little Rock, Arkansas) and by MECORE Laboratory (Bangor, Maine), and adiponectin by Maine Research Institute (Scarborough, Maine). Serum levels of bone-specific alkaline phosphatase (BALP) were measured using the alkaline phosphatase (ALP) diagnostic kit (Sigma-Aldrich) as described (28), and tartrate-resistant acid phosphatase form 5b (TRAP5b) using an ELISA provided by Immunodiagnostic Systems Inc (Scottsdale, Arizona).
Bone analysis
Microcomputed tomography (mCT) of the tibiae and L4 vertebrae was performed using the μCT-35 system (Scanco Medical AG, Bassersdorf, Switzerland) as previously described (29). The analysis of bone microstructure conformed to recommended guidelines (30).
To permit static and dynamic bone histomorphometry, 4-month-old animals were injected with 30 mg/kg tetracycline 7 and 2 days before they were killed, and undecalcified tibiae were embedded in methyl methacrylate, sectioned, and stained with either Goldner's trichrome or Von Kossa/McNeal by the Histology Core at the Department of Anatomy and Cell Biology, Indiana University (Indianapolis, Indiana). The histomorphometric examination was confined to the secondary spongiosa of proximal tibia and was performed using the Nikon NIS-Elements BR3.1 system. The measurements were collected under ×40 magnification from 6 representative fields per bone sample. The terminology and units used were those recommended by the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research (31).
Extraction of osteoblast- and osteocyte-enriched fractions
Cell fractions enriched in either osteoblasts or osteocytes were isolated by sequential collagenase digestion of femora bone, according to previously described protocol (32).
Cell culture experiments
Murine marrow-derived cell lines representing adipocytic (AD2 cells) and osteoblastic (U-33 cells) phenotypes have been previously described (33). The MLO-A5 cell line representing osteocytes was a kind gift of Dr. Bonewald (University of Missouri, Kansas City, Missouri). Primary bone marrow cultures were established from femur marrow aspirates and differentiated as described (34). The osteoclastogenesis assay was carried using primary bone marrow nonadherant cells as a source of osteoclast progenitors and either U-33/γ2 cells or adherent bone marrow cells as supporting osteoclast recruitment and differentiation (28). After 8 days of growth in the presence of 10−8M 1,25-hydroxyvitamin D3, osteoclasts were stained with the leukocyte acid phosphatase (TRAP+) kit (Sigma).
ALP activity in cell culture
ALP enzyme activity was measured as described (28) after normalization to cell number assessed by the Cell Titer 96 AQueous nonradioactive cell proliferation assay kit (Promega, Madison, Wisconsin).
Collection and testing activity of conditioned media
Conditioned media (CM) were collected either from AD2 cells transiently transfected with FoxC2, Igfbp2, or Wnt10b expression constructs or from the organ culture of epididymal fat after 3 days incubation with the media. For depletion studies, CM was incubated with 1 μg/ml anti-WNT10b or anti-IGFBP2 or nonspecific goat IgG for 2 hours at 4°C followed by 2 hours incubation with Protein A/G PLUS-Agarose beads (Santa Cruz Biotechnology).
Statistical analysis
Statistical analysis of cell culture experiments was conducted with 2-tailed Student's t test, whereas animal experiments were analyzed with SPSS Statistics version 17.0 software using 1-way ANOVA and post hoc Tukey's test. All data shown represent means and SD. A P value < .05 was considered significant.
Results
FoxC2AD+/Tg animals are lean and insulin-sensitive
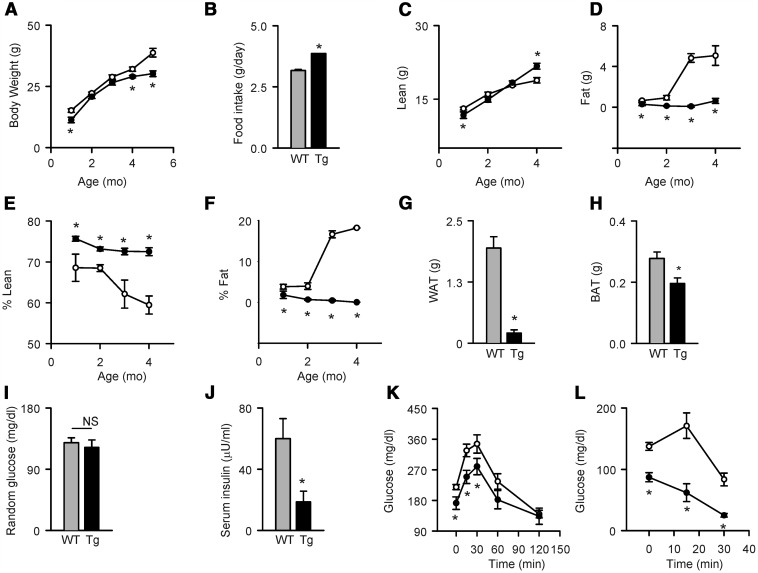
The murine model of FOXC2 transcriptional regulator ectopically expressed in adipocytes under the control of Fabp4/aP2 promoter/enhancer has been described previously (21). For the studies presented here, the phenotype of FoxC2AD+/Tg mice has been analyzed again with regard to the metabolic parameters, growth pattern, and gender differences (Figure 1). At 1 month of age, mice with FoxC2AD+/Tg genotype, both males (Figure 1) and females (not shown), have lower body weight than WT controls, and this difference increases with age progression (Figure 1A). Although smaller, the FoxC2AD+/Tg mice consume more food (Figure 1B). Along with higher caloric intake, these mice accrue up to 15% more lean mass than WT control over a 4-month growing period (Figure 1C). The mice are lean with nearly absent fat tissue as measured by nuclear magnetic resonance (Figure 1D). In WT controls, body composition changed with increasing age due to relative decrease in lean (Figure 1E) and relative increase in fat (Figure 1F) mass. In contrast, the balance between lean and fat mass remains similar over the 4-month growth period of FoxC2AD+/Tg mice (Figure 1, E and F). This suggests that FoxC2AD+/Tg mice are protected from age-related changes in body composition.
Figure 1.
Body composition and metabolic parameters of male FoxC2AD+/Tg (Tg) as compared with wild type (WT) control (n = 6 mice per group). A, Changes in body weight over a 5-month growth period. B, Daily food intake monitored over 1 week in 4-month-old mice. C–F, Changes in the lean (C) and fat mass (D) and percent contribution of lean (E) and fat (F) mass to body weight measured over a period of 4 months by nuclear magnetic resonance. G–H, Weights of epididymal WAT (G) and interscapular BAT (H) in 5-month-old mice. I–J, Random glucose (I) and insulin (J) levels in 4-month-old mice. K, Glucose disposal measured in IGTT in response to injection of 2 g/kg glucose. L, Glucose disposal measured in IITT in response to injection of 0.75 U/kg insulin. Open circles and gray bars represent WT, and black circles and black bars represent FoxC2AD+/Tg mice. *, P < .05 vs WT.
The reduced fat mass in 4-month-old FoxC2AD+/Tg is primarily due to significant reduction in the mass of epididymal WAT (Figure 1G); however, the mass of interscapular BAT is also reduced (Figure 1H), which differs from the original phenotype characterized by reduction in WAT but increase in BAT mass (21). This might be due to the fact that mice used in the present study were bred for more than 30 generations from original mice, which resulted in a decrease of initially high copy number of the FoxC2 transgene. This is frequently seen in mice with a high copy number of a transgenic construct that tends to be arranged as unstable multimers in a head to tail fashion and leads to the loss of transgene copies with increasing number of generations.
Despite increased food consumption (Figure 1B), FoxC2AD+/Tg mice maintained a level of serum glucose similar to WT mice (Figure 1I) paralleled with significantly reduced levels of insulin (Figure 1J); however, serum glucose levels after fasting were significantly reduced in FoxC2AD+/Tg mice as compared with WT (87.0 ± 13.1 mg/dl vs 137.3 ± 15.9 mg/dl) (Figure 1L). Glucose disposal, measured in either IGTT or IITT, is significantly higher in FoxC2AD+/Tg than in WT mice, indicating increased insulin sensitivity (Figure 1, K and L). On that note, the rapid glucose clearance in FoxC2AD+/Tg upon insulin challenge in IITT resulted in severe hypoglycemia within 30 minutes from insulin injection (Figure 1L). No gender differences were noted in the body composition and metabolic parameters of FoxC2AD+/Tg mice.
Ectopic expression of FoxC2 induces acquisition of beige phenotype in vivo in the epididymal WAT and in vitro in cells representing marrow adipocytes
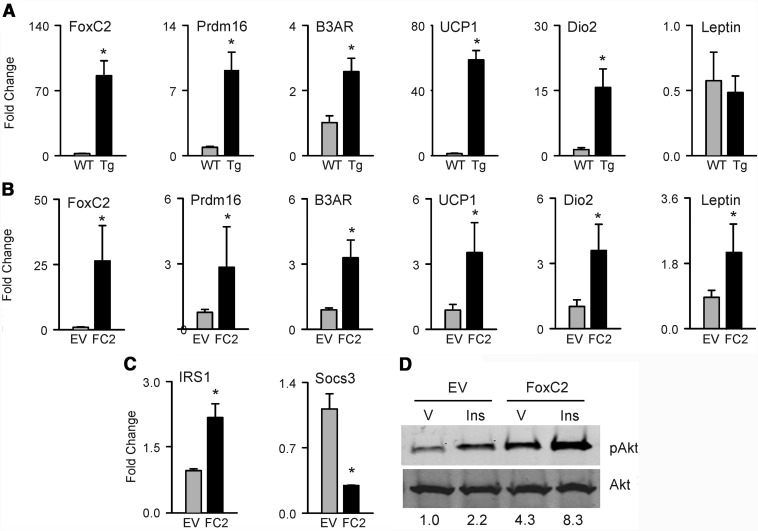
It was previously shown that ectopic expression of FoxC2 transcription factor in adipocytes induces BAT-like gene expression in WAT and enhances mitochondrial biogenesis (21, 25). Epididymal WAT of FoxC2AD+/Tg mice showed significantly increased expression of several markers of brown adipocytes (Figure 2A). Among tested markers, UCP1 and type II iodothyronine deiodinase (Dio2) showed the largest increase at 52- and 16-fold over age-matched WT controls, respectively, whereas the other markers of BAT including PR domain-containing protein 16 (Prdm16) and β3-adrenergic receptor (ADRB3) revealed modest, but consistent upregulation as compared with control mice. Interestingly, levels of leptin expression were similar in WAT of FoxC2AD+/Tg and WT mice.
Figure 2.
Metabolic profile of FOXC2-expressing adipocytes in vivo and in vitro. A, Quantitative real-time PCR analysis of BAT gene expression in epididymal WAT isolated from 6-month-old wild type (WT) and FoxC2AD+/Tg (Tg) mice (n = 4 mice per group). B, BAT gene expression in AD2 cells transfected with either empty vector (EV) or FoxC2 expression vector (FC2). C, Expression of insulin signaling genes in AD2 cells transfected with either empty vector or FoxC2 expression vector. D, Protein levels of pAKT in empty vector and FoxC2 expression vector transfected AD2 cells treated with either vehicle (V) or 100nM insulin (Ins) for 30 minutes after a 2-hour period of serum depletion. Values for relative band density measured with ImageJ NIH software are indicated below images. Gray bars represent WT mice or empty vector; black bars represent FoxC2AD+/Tg mice or FoxC2 expression vector. *, P < .05 vs WT mice or empty vector-transfected cells.
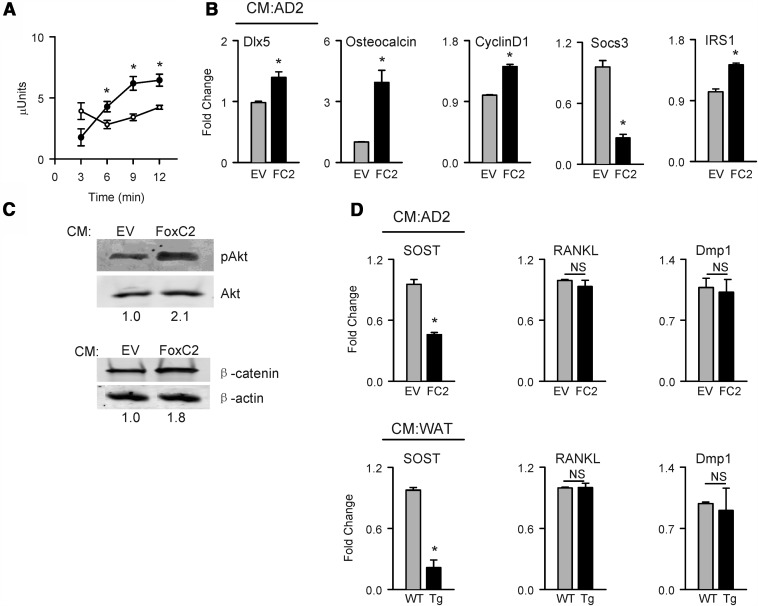
To assess whether BAT phenotype may be induced in marrow adipocytes, FoxC2 was ectopically expressed in AD2 cells representing immortalized marrow cells committed to the adipocyte lineage (33). Similar to its effect in WAT, FOXC2 induced the expression of BAT markers in marrow adipocytes. However, in contrast to WAT, FOXC2 increased the expression of leptin in AD2 cells (Figure 2B). Changes in BAT gene markers expression were proportional to the levels of FOXC2 expression in WAT and AD2 cells (Figure 2, A and B). Because FOXC2 increases insulin sensitivity in adipocytes as reported previously (21), we have tested whether it has the same effect on marrow adipocytes. As shown in Figure 2C, ectopic expression of FoxC2 in AD2 cells resulted in an increased expression of insulin receptor substrate 1 (IRS1), a positive regulator, and a decreased expression of suppressor of cytokine signaling 3 (Socs3), a negative regulator of insulin signaling. Insulin sensitivity was measured as protein levels of phosphorylated AKT (pAKT) in response to insulin challenge. In AD2 cells, FOXC2 increased basal levels of pAKT up to 4-fold and up to 8-fold upon stimulation with insulin, as compared with the empty vector control (Figure 2D). These data indicate that FOXC2 induces BAT phenotype and increases insulin sensitivity in both WAT and bone marrow adipocytes.
FoxC2AD+/Tg mice have high bone mass that is associated with increased bone formation rate and high bone turnover
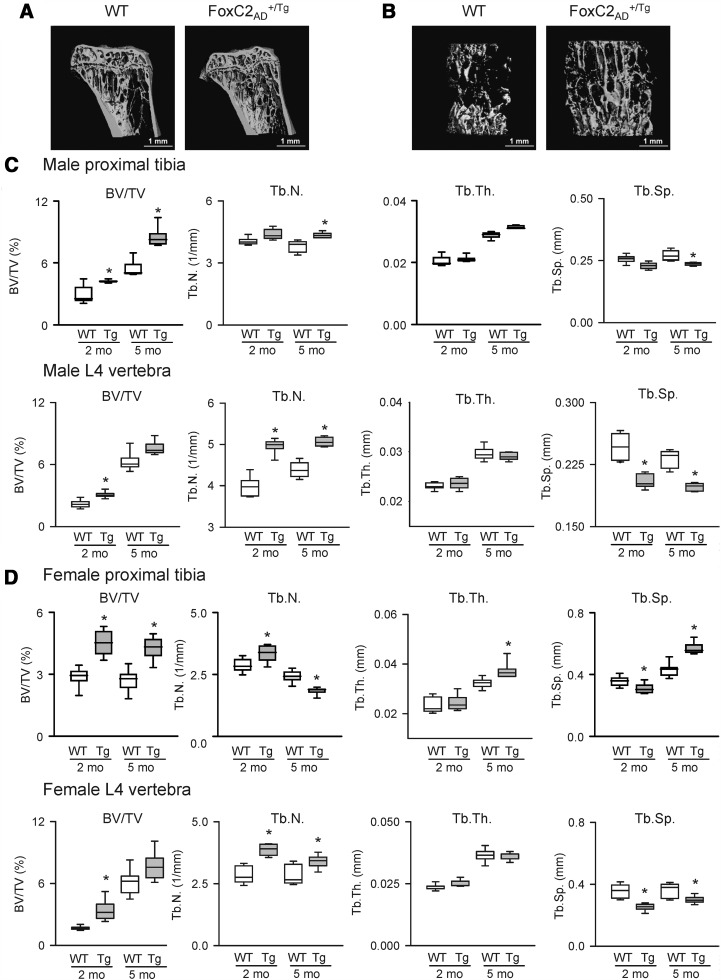
FoxC2AD+/Tg mice, males and females, have significantly higher trabecular bone mass as compared with age-matched WT animals (Figure 3 and Supplemental Table 2). The higher bone mass (bone volume/tissue volume) in proximal tibia was noticeable in 2-month-old animals and showed a sustained and progressive increase with animals' age. It was accompanied by an overall increase in the tissue volume of proximal tibia and increased trabecular bone volume (Supplemental Table 2). At 2 months of age, high bone mass in both males and females was associated with the high number of trabeculae, high connectivity, and decreased separation between trabeculae. The phenotype of high bone mass not only persisted but was even more pronounced with aging, although it was accompanied by different structural changes in males and females. Although high bone mass in 5-month-old males was still due to more numerous trabeculae, a high bone mass in 5-month-old females was due to much thicker trabeculae (Figure 3, C and D, and Supplemental Table 2). Female, but not male, bone had larger endosteal area, which correlated with increased strength to resist torsion and bending as measured by polar moment of inertia (Supplemental Table 3). High bone mass in long bones of FoxC2AD+/Tg males and females was restricted to the trabecular bone, because no differences were observed in either cortical thickness (Supplemental Table 2) or bone length (not shown) of tibia and femur between WT and FoxC2AD+/Tg mice.
Figure 3.
mCT analysis of trabecular bone in proximal tibia and L4 lumbar vertebra. A and B, mCT-generated coronal section images of trabecular bone in proximal tibia (A) and L4 vertebral body (B) of 5-month-old male wild type (WT) and FoxC2AD+/Tg (Tg) mice. C, Bone parameters of proximal tibia and L4 lumbar vertebra in 2- and 5-month-old males. D, Bone parameters of proximal tibia and L4 lumbar vertebra in 2- and 5-month-old females. Abbreviations: BV/TV, bone volume/tissue volume; Tb.N, trabecular number; Tb.Th, trabecular thickness; Tb.Sp, trabecular spacing. White boxes represent WT, and gray boxes represent FoxC2AD+/Tg mice; n = 4–10 animals per group. *, P < .05 vs age-matched WT control.
In the axial skeleton, the vertebral body was larger in size in FoxC2AD+/Tg mice than in WT animals and possessed increased trabecular bone mass due to a high number of trabeculae (Figure 3B and Supplemental Table 2). This feature was observed in both 2- and 5-month-old males and females. Interestingly, the increase in bone mass with age was rather due to an increase in the volumetric size of vertebra but not due to change in the density and thickness of trabeculae. No gender differences were observed with respect to the higher bone mass and structural differences in vertebra of FoxC2AD+/Tg mice. The difference between bone acquisition in vertebra and tibia can be attributed to both a difference in the sympathetic nervous system control of bones with different embryological origin (35) and the increased periosteal activity in vertebra of FoxC2AD+/Tg mice, which may result from the increased sensitivity to the sympathetic nervous system-controlled β-adrenergic/cAMP/protein kinase A signaling (21).
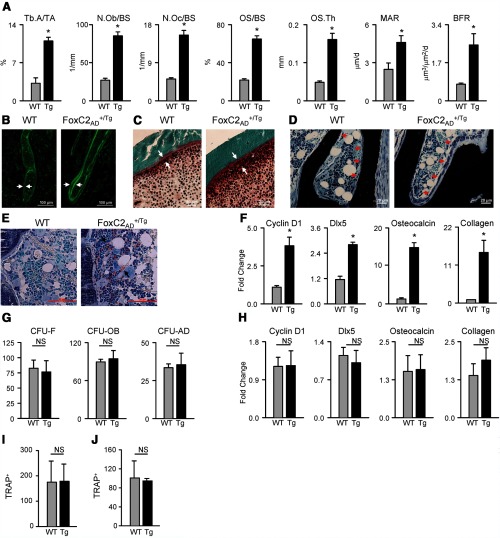
Histomorphometric analysis of trabecular bone in the proximal tibia confirmed mCT measurements of high bone mass (Figure 4A). The 3-fold increase in trabecular area (trabecular area/tissue area) in FoxC2AD+/Tg mice was associated with increased bone remodeling due to increased activity of its cellular components, osteoblasts and osteoclasts. The osteoblast number (per bone surface) in tibia of FoxC2AD+/Tg mice was 3.6-fold higher, and the trabecular surface occupied by active osteoblasts (osteoid surface/bone surface) was almost 3 times larger than in WT animals (22.6% vs 61.9%). At the same time, osteoclast number (per bone surface) was also elevated by 3-fold, indicating increased bone turnover in FoxC2AD+/Tg mice. Consistent with a high number of osteoblasts, the mineral apposition rate was increased by 2-fold and the bone formation rate (BFR) was increased by 3-fold in FoxC2AD+/Tg mice as assessed by dynamic histomorphometry of tetracycline-labeled bone (Figure 4, A and B). In addition, tetracycline labeling of newly deposited bone was relatively strong (Figure 4B) and extended to 64% of the trabecular surface in FoxC2AD+/Tg mice as compared with 30% in WT animals. Increased BFR corresponded to a 3-fold increase in the thickness of new osteoid (Figure 4C) and to the presence of activated cuboidal osteoblasts along the surface of trabeculae (Figure 4D). The number of adipocytes in proximal tibia of FoxC2AD+/Tg mice was elevated, whereas their average size was significantly smaller as compared with WT adipocytes (Supplemental Figure 1). Most importantly and in contrast to WT animals, UCP1 protein was present in the marrow of FoxC2AD+/Tg mice (Figure 4E). In conclusion, the bone phenotype of FoxC2AD+/Tg mice is associated with both high bone turnover, increased bone apposition, and expression of BAT marker UCP1 protein. In support of increased bone turnover, levels of both BALP and TRAP5b were significantly elevated in sera of FoxC2AD+/Tg mice (Table 1). No gender differences were noticed in measured parameters.
Figure 4.
Measurement of cellular bone compartments. A, Static and dynamic histomorphometry of trabecular bone in proximal tibia of 5-month-old male mice. Abbreviations: MAR, mineral apposition rate; N.Ob/BS, osteoblast number/bone surface; N.Oc/BS, osteoclast number/bone surface; OS/BS, osteoid surface/bone surface; OS.Th, osteoid thickness; Tb.A/TA, trabecular area/tissue area. B, Double tetracycline labeling of trabecular surface (magnification, ×10). C, Goldner's trichrome staining of osteoid (magnification, ×20). D, Microphotograph of activated osteoblasts on the surface of trabeculae. Staining with Von Kossa/McNeal to visualize mineralized bone (black) and osteoid and marrow cells (blue), respectively (magnification, ×40). E, UCP1 protein expression in proximal tibia, counterstained with methyl green (magnification, ×40). Black arrows indicate UCP1-positive staining. F, Osteoblast-specific gene expression in osteoblast-enriched fraction isolated from femora of 4-month-old males. G, Ex vivo analysis of the number of MSCs able to form fibroblast-like colonies (CFU-F), osteoblast colonies (CFU-OB), and adipocyte colonies (CFU-AD). H, Osteoblast-specific gene expression in ex vivo cultured primary bone marrow cells derived from WT and FoxC2AD+/Tg mice harvested from femora of 4-month-old males. I, Analysis of osteoclast progenitor number in the nonadherent fraction of bone marrow derived from WT and FoxC2AD+/Tg mice and cocultured with U-33/γ2 cells. J, Analysis of osteoclast progenitor differentiation potential in the presence of adherent primary bone marrow cells from WT and FoxC2AD+/Tg mice. Multinucleated cells (more than 2 nuclei) that stained positively for TRAP were considered as differentiated osteoclasts and were enumerated. Gray bars represent wild type (WT), and black bars represent FoxC2AD+/Tg (Tg) mice; n = 3 animals per group. *, P < .05 vs WT.
Table 1.
Bone Turnover Markers in Serum of WT and FoxC2AD+/TGa
Results are from n = 4 animals per group.
P > .01 vs WT.
P > .05 vs WT.
To confirm that osteoblasts in the bone of FoxC2AD+/Tg mice are highly activated, we analyzed their phenotype in vivo in the fraction of cells associated with endosteal bone surface. As shown in Figure 4F, the FoxC2AD+/Tg-derived osteoblasts had higher expression of cyclin D1, a marker of cell proliferation, and increased expression of distal-less homeobox 5, osteocalcin, and collagen, markers of osteoblast differentiation and maturation. This profile is consistent with the observed high number and activity of differentiated osteoblasts in FoxC2AD+/Tg mice (Figure 4A).
To determine whether the increase in the number of osteoblasts was due to intrinsic changes that would affect lineage commitment of the marrow mesenchymal stem cells (MSCs), the number of MSCs with a potential to form colonies and differentiate toward either osteoblast or adipocyte lineage was analyzed ex vivo using a colony-forming unit assay (33). As shown in Figure 4G, the number of MSCs with the ability to form fibroblast-like colonies and the number of colonies with potential to differentiate toward either osteoblasts or adipocytes were not different between FoxC2AD+/Tg and WT animals. Next, we analyzed the phenotype of FoxC2AD+/Tg osteoblast in ex vivo conditions. The mRNA expression levels of cyclin D1, distal-less homeobox 5 (Dlx5), osteocalcin, and collagen were not different in FoxC2AD+/Tg marrow MSCs as compared with WT cells (Figure 4H). These data excluded the possibility that the high number and increased activity of osteoblast in mice ectopically expressing FoxC2 in fat are due to intrinsic cellular changes at the level of MSC lineage commitment.
Similarly, the number of osteoclast progenitors and their differentiation did not differ between WT and FoxC2AD+/Tg animals as assessed in a coculture experiment of nonadherent bone marrow cells with either U-33/γ2 cells (Figure 4I) or adherent bone marrow cells derived from either WT and FoxC2AD+/Tg mice (Figure 4J).
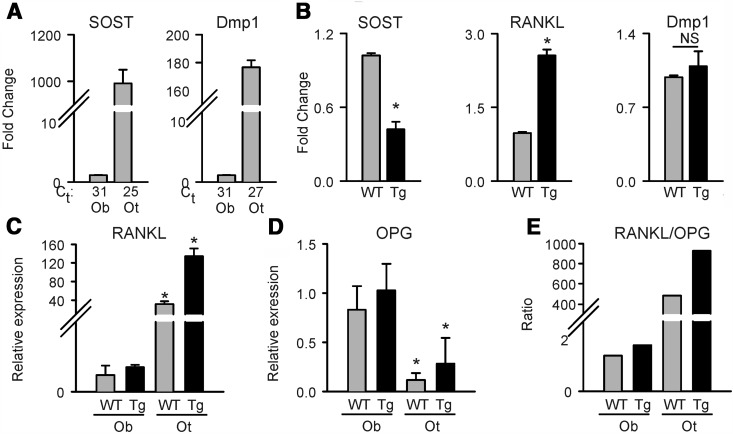
The rate of bone turnover is regulated by osteocytes, which produce 2 essential proteins, sclerostin and RANKL, for regulation of osteoblast and osteoclast functions, respectively. Because bone turnover is increased in FoxC2AD+/Tg mice, we compared the in vivo activity of FoxC2AD+/Tg with WT osteocytes. The enrichment of osteocytes isolates was assessed by the level of expression of Dmp1 and Sost gene markers (Figure 5A). As shown, the expression of Dmp1 and Sost in the osteocyte fraction was 2 to 3 orders of magnitude higher than the expression of these markers in osteoblast fraction, indicating relative homogeneity of analyzed cells (Figure 5A). When compared with osteocytes derived from WT animals, the isolates from bones of FoxC2AD+/Tg mice showed reduced expression of SOST by 2-fold and increased expression of RANKL by 3-fold (Figure 5B). Although SOST and RANKL expression were different, the expression of the Dmp1 marker of osteocyte maturation did not differ between FoxC2AD+/Tg and WT mice, indicating that osteocyte function, but not differentiation, is altered in FoxC2AD+/Tg mice (Figure 5B). Based on recent reports that osteoclast differentiation and function in bone remodeling is controlled by osteocytes rather than osteoblasts (36), we compared the levels of RANKL expression in the osteocyte and osteoblast fractions. As shown in Figure 5C, RANKL expression was almost 40-fold higher in the osteocyte fraction as compared with the osteoblast fraction in WT animals and over 120-fold higher in osteocytes of FoxC2AD+/Tg mice (Figure 5C). On that note, a similar difference in the RANKL expression as shown between WT osteocyte and osteoblast fraction has been recently reported (37). Next, we analyzed the levels of expression of osteoprotegerin (OPG), the RANKL decoy receptor and a negative regulator of osteoclastogenesis. Interestingly, OPG was expressed at similar levels as RANKL in osteoblasts derived from either WT or FoxC2AD+/Tg (Figure 5D), resulting in a RANKL to OPG ratio close to 1 (Figure 5E). In contrast, primary osteocytes expressed negligible levels of OPG, leading to a very high RANKL to OPG ratio (Figure 5, D and E). These results argue for a major role of osteocytes in regulation of bone remodeling and indicate that increased bone turnover in FoxC2AD+/Tg mice results from osteocyte support for osteoblast and osteoclast function.
Figure 5.
Analysis of primary osteoblasts and osteocytes isolated from femora of FoxC2AD+/Tg and WT mice. A, Determination of homogeneity of osteocyte (Ot)-enriched fraction as compared with osteoblast (Ob)-enriched fraction isolated from murine femora. Values below the graph represent the threshold cycle (Ct) at which the target transcript was detected in the real-time PCR. B, Sost, Rankl, and Dmp1 gene expression in osteocyte-enriched fraction. C, Relative expression of RANKL in osteoblast- and osteocyte-enriched fractions. D, Relative expression of OPG in osteoblast- and osteocyte-enriched fractions. E, RANKL to OPG ratio in primary osteoblasts and osteocytes. Gray bars represent wild type (WT), and black bars represent FoxC2AD+/Tg (Tg) mice; n = 3 animals per group. *, P < .05 vs WT mice.
The decreased expression of sclerostin, a negative regulator of osteoblast differentiation, and the increased expression of RANKL, a positive regulator of osteoclast differentiation, were consistent with increased osteoblast number and increased osteoclast number shown in Figure 4A and indicate that FoxC2AD+/Tg mice have increased bone remodeling due to altered activity of osteocytes. In conclusion, osteoblast and osteocyte activity is altered in FoxC2AD+/Tg mice, but this alteration results rather from systemic cues as opposed to intrinsic changes in the MSC or hematopoietic stem cells potential to differentiate toward either osteoblasts or osteoclasts, respectively.
Beige fat secretes factors that activate osteoblasts and modulate Sost expression in osteocytes
Because ectopic expression of FoxC2 in fat of the FoxC2AD+/Tg mice positively correlates with increased bone mass and increased in vivo bone-anabolic activity of osteoblasts and osteocytes, we tested the possibility that beige adipocytes release factors that regulate these activities. CM collected from AD2 adipocytes ectopically expressing FoxC2 (donor cells) and transferred to the cultures of the preosteoblastic U-33 cells (recipient cells) induced ALP activity (Figure 6A) and the expression of osteoblast-specific gene markers in the recipient cells (Figure 6B). This profile of expression was remarkably similar to the expression profile of osteoblasts isolated from the bone of FoxC2AD+/Tg mice (Figure 4F). Moreover, CM from FOXC2-expressing donor cells decreased the expression of suppressor of cytokine signaling 3 and increased the expression of insulin receptor substrate 1, indicating increased insulin sensitivity of recipient cells (Figure 6B). Consistently, the basal levels of both β-catenin and pAKT, two major cellular mediators of pro-osteoblastic and insulin signaling activity was increased in recipient osteoblastic cells (Figure 6C).
Figure 6.
The effect of CM harvested from either AD2 cells or WAT on osteoblastic U-33 and osteocytic MLO-A5 recipient cells after 3 days of incubation. A, The effect of AD2-derived CM on ALP activity in U-33 cells. B, The effect of AD2-derived CM on expression of osteoblast- and insulin signaling-specific gene markers in recipient U-33 cells. Open circles and gray bars represent CM derived from empty vector (EV)-transfected cells; black circles and black bars represent CM derived from FoxC2 (FC2)-transfected cells. C, Western blot analysis of CM derived from AD2 cells on pAKT and β-catenin levels in recipient U-33 cells. Values for relative band density were normalized to either AKT or β-actin and are provided below images. D, The effect of CM from AD2 cells (CM:AD2) and from cultured WAT (CM:WAT) on Sost, Rankl, and Dmp1 gene expression in recipient MLO-A5 osteocytes. WT, wild type mice; Tg-FoxC2AD+/Tg mice. *, P < .05 vs empty vector-transfected cells or WT mice.
The same CM from FOXC2-expressing adipocytes decreased expression of SOST in the osteocytic MLO-A5 cells; however, the expression of RANKL and DMP1 were not affected in the recipient cells (Figure 6D). A similar effect was observed when CM was collected from an organ culture of epididymal fat isolated from FoxC2AD+/Tg animals. Indeed, CM from beige fat decreased expression of SOST in the recipient osteocytic cells without effecting the expression of RANKL and DMP1 (Figure 6D). This suggests that, in contrast to sclerostin, elevated expression of RANKL in primary osteocytes of FoxC2AD+/Tg mice is not a result of a direct effect of factors secreted from beige fat but instead an indirect systemic effect. Another possibility is that the beige fat-induced mechanism leading to increased RANKL expression is not active in MLO-A5 cells.
Taken together, cells that acquire the beige fat phenotype due to ectopic expression of FoxC2 secrete factors that increase bone-forming activity either by directly activating osteoblast bone-forming capabilities or through increased support of osteocytes for osteoblasts as a result of decreased production of sclerostin.
Beige fat endocrine/paracrine activity comprises bone-anabolic factors including IGFBP2 and WNT10b
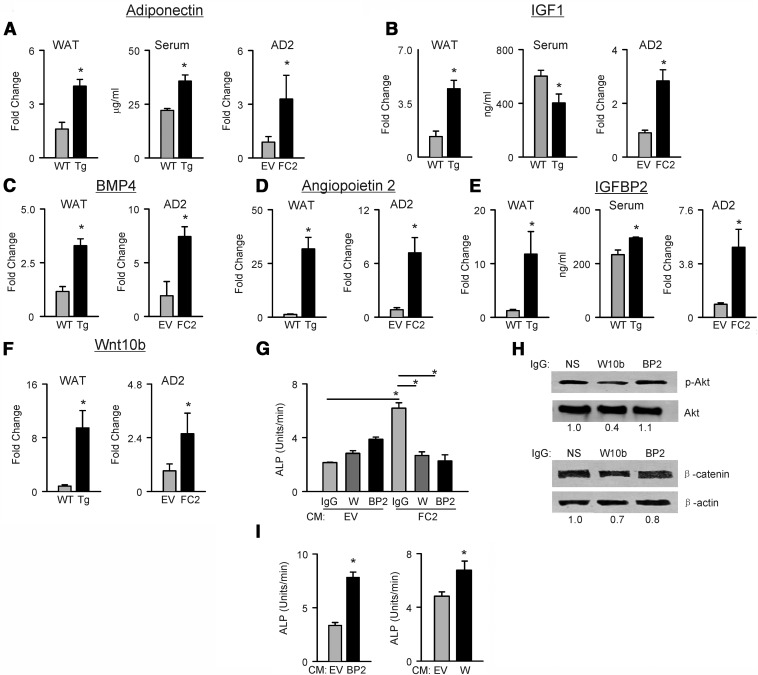
To characterize factors secreted by beige adipocytes, which may regulate osteoblast and osteocyte activity either in an endocrine or in a paracrine manner, we profiled fat tissue of FoxC2AD+/Tg mice and marrow adipocytes ectopically expressing FoxC2 for the expression of factors recognized for their bone-anabolic activity. If applicable, we determined the protein levels of these factors in the serum. Adiponectin, IGF-1, and IGFBP2 were selected as candidates for endocrine activity of fat to regulate bone mass, whereas activators of pro-osteoblastic WNT and bone morphogenic protein (BMP) signaling, and angiogenic factor angiopoietin 2, were selected as potential candidates for paracrine activity of bone marrow fat. As shown in Figure 7A, mice with inducible BAT activity have increased expression of adiponectin in fat and increased levels of adiponectin protein in circulation. Moreover, FOXC2 induced expression of adiponectin in AD2 cells, indicating that adiponectin levels can be also elevated in marrow of FoxC2AD+/Tg mice. Interestingly, the expression of IGF-1 in beige fat and AD2 cells ectopically expressing FoxC2 was significantly increased, although it was not reflected in the levels of circulating IGF-1, which were lower in FoxC2AD+/Tg as compared with WT animals (Figure 7B). The increased adiponectin and decreased IGF-1 levels in circulation may reflect an overall increase in insulin sensitivity of FoxC2AD+/Tg mice. Both inducible BAT and AD2 cells expressing FoxC2 showed large increases in the expression BMP4 (Figure 7C) and angiopoietin 2 (Figure 7D). These factors, if expressed locally in bone, may create an environment supporting osteoblast differentiation (BMP4) and nutrient availability (angiopoietin 2).
Figure 7.
The effect of FOXC2 on production of bone-anabolic factors. Gene expression was measured in WAT and AD2 cells, whereas protein levels were measured in sera, if applicable. A, Adiponectin. B, IGF-1. C, BMP4. D, Angiopoietin 2. E, IGFBP2. F, WNT10b. Gray bars reprsent wild type (WT), and black bars represent FOXC2-expressing AD2 cells or FoxC2AD+/Tg (Tg) mice. G, The effect of depletion of WNT10b or IGFBP2 from CM of AD2 cells on ALP activity in U-33 recipient cells. H, Effect of depleted CM on pAKT and β-catenin protein levels in U-33 recipient cells. Values for relative band density normalized to either AKT or β-actin are provided below images. I, Effect of CM derived from AD2 cells overexpressing either IGFBP2 or WNT10b on ALP activity in U-33 recipient cells. Abbreviations: EV, CM derived from cells transfected with empty vector; FC2, CM derived from cells transfected with FoxC2 expression vector; IgG, media depleted with nonspecific goat IgG; W, media depleted with WNT10b antibodies; BP2, media depleted with IGFBP2 antibodies. *, P < .05 vs empty vector-transfected cells or WT mice.
Because we have shown that activation of the osteoblastic phenotype by beige fat-derived CM increases pAKT levels in recipient cells (Figure 6C), we analyzed whether the transcript levels for IGFBP2 and WNT10b, 2 proteins whose activity is mediated by pAKT, are also elevated. As shown in Figure 7E, the expression of Igfbp2 transcript is 10-fold higher in epididymal fat of FoxC2AD+/Tg mice as compared with WT animals. This correlates with increased levels of circulating IGFBP2 protein. The expression of IGFBP2 is also increased in marrow adipocytes transfected with FoxC2, indicating that the marrow levels of IGFBP2 can be higher in FoxC2AD+/Tg mice. Similarly, the levels of Wnt10b transcripts are higher both in fat of FoxC2AD+/Tg mice and in transfected AD2 cells (Figure 7F). Immunodepletion of either IGFBP2 or WNT10b from CM derived from beige adipocytes resulted in the loss of pro-osteoblastic activity (Figure 6G) and failure to increase pAKT and β-catenin levels in recipient cells (Figure 7H). The pro-osteoblastic effect measured by ALP activity was restored upon exposing U-33 cells to CM collected from cells overexpressing either Igfbp2 or Wnt10b (Figure 7I).
Discussion
In the current study, we demonstrated that adipocytes with an induced BAT-like phenotype release factors that exert anabolic activity on the skeleton and increase bone remodeling. The anabolic activity of beige fat targets 2 types of bone cells, osteoblasts and osteocytes, although likely by different mechanisms. We identified several factors secreted in vivo by beige fat tissue and in vitro by FoxC2-overexpressing marrow adipocytes, which are recognized for their anabolic effect on bone either through a direct action on bone cells (WNT10b [38], IGFBP2 [39], IGF-1 [40], BMP4 [41], and adiponectin [42]) or through indirect action by regulating angiogenesis (angiopoietin 2 [43]).
We focused our analysis on 2 factors, IGFBP2 and WNT10b, as the best candidates for common regulators of energy metabolism and bone turnover. IGFBP2, which belongs to the family of IGF binding proteins, is synthesized and secreted by many tissues including several adipose depots. Its metabolic activity, independent of IGF-1, includes regulation of adipocyte differentiation and insulin sensitivity. Transgenic overexpression of IGFBP2 protects from diet-induced obesity and insulin resistance (44) and reverses diabetes in mice (45). In humans, serum levels of IGFBP2 are lower with aging, obesity, and type 2 diabetes (46, 47). IGFBP2 stimulates bone remodeling by enhancing osteoblast differentiation and osteoclastogenesis (39, 48). Similarly, WNT10b functions in both regulation of energy metabolism and regulation of bone homeostasis. Targeted expression of Wnt10b in adipose tissue renders diabetic animals lean and insulin-sensitive (49) and increases trabecular bone mass (50). In marrow MSCs, WNT10b supports osteoblast differentiation and inhibits adipogenesis (38).
A comparison of the FoxC2AD+/Tg mice phenotype with phenotypes of mice either ectopically expressing Wnt10b in fat (49, 50) or null in the expression of IGFBP2 (51) supports the major role of these two factors in conveying beige fat metabolic and bone-anabolic activity. Indeed, ectopic expression of WNT10b in fat cells under the Fabp4/aP2 promoter renders a phenocopy of FoxC2AD+/Tg animals including insulin sensitivity, protection from obesity, and high bone mass (49, 50), whereas a lack of IGFBP2 has an opposite effect and leads to obesity, insulin resistance, and low bone mass due to reduced skeletal remodeling (51).
Our results support the endocrine/paracrine activity of IGFBP2 and WNT10b on bone. We have demonstrated that both IGFBP2 and WNT10b, either secreted from beige fat or from FOXC2-expressing marrow adipocytes, have stimulatory effect on the osteoblastic phenotype. Because the glycoprotein WNT10b acts rather locally in a paracrine fashion, whereas IGFBP2 is released into the circulation from liver and adipose tissue, we suggest that peripheral inducible BAT exerts its endocrine anabolic activity on bone through IGFBP2, whereas the supportive role for osteoblastogenesis of beige marrow adipocytes includes local production of both IGFBP2 and WNT10b.
This study shows a direct link between energy metabolism and bone turnover and implicates that these two processes share similar regulatory mechanisms, especially insulin signaling. AKT kinase is activated by both insulin and IGFBP2 (39). Activation of AKT stabilizes β-catenin by inhibiting activity of glycogen synthase kinase 3 beta, which allows canonical WNT signaling to be activated (52). We have shown that factors secreted from beige adipocytes, including IGFBP2 and WNT10b, simultaneously activate AKT, increase levels of β-catenin, stimulate osteoblast differentiation, and increase insulin sensitivity in recipient osteoblasts. Because both WNT10b and IGFBP2 have the same effect on pAKT and β-catenin, and the absence of one protein cannot be substituted by the other in its pro-osteoblastic effect, it implies that both IGFBP2 and WNT10b signal through the same mechanism. It is also possible that besides their direct anabolic effect on bone cells, factors secreted from beige fat may sensitize osteoblasts and osteocytes to insulin and enhance their response to the anabolic effects of IGFBP2 and WNT10b.
These studies also indicate a new role of FOXC2 transcription factor in the regulation of IGFBP2 and WNT10b expression. Although we do not know at this point whether FoxC2 regulates the expression of these factors directly or indirectly, its role in the regulation of both metabolic activity of adipocytes and secreted bone-anabolic activity, suggests that FoxC2 plays a modulatory role in facilitating a dialog between fat and bone.
Another novel aspect of these studies is the demonstration that beige fat regulates osteocyte support for bone remodeling, which regulates skeletal homeostasis by removal of old or damaged bone and replacing it with new bone that is biomechanically strong. Osteocytes, which are residing in the mineralized compartment of bone tissue, orchestrate osteoblast bone-forming and osteoclast bone-resorbing activity by releasing factors including sclerostin and RANKL, respectively. Sclerostin, the product of the Sost gene, acts as a negative regulator of bone formation by inhibiting WNT signaling in osteoblasts (52). Osteocytes are also a major source in the bone of RANKL, the cytokine essential for their development (36). Our finding that endocrine activity of beige fat targets osteocytic expression of sclerostin and RANKL, the two most important proteins for regulation of bone remodeling, implicates that this type of fat is a powerful regulator of bone mass.
We have also addressed the question of whether BMAT can acquire beige fat activity. Isolation of intact marrow adipocytes from mice is extremely difficult due to the dispersion of these cells throughout the marrow and their fragility after extraction. Therefore, we used in our studies a substitute for marrow adipocytes, an immortalized AD2 cell line. We compared the activity of AD2 cells and WAT acquired upon FoxC2 ectopic expression and found that both types of adipocytes respond identically with respect to increased insulin sensitivity and production of bone-anabolic activity. This allows us to postulate that marrow fat plays a similar function locally in bone as peripheral beige fat by releasing paracrine factors that regulate osteoblast and osteocyte activity.
In conclusion, the finding of activity anabolic for bone of beige fat together with its beneficial role in regulation of energy balance suggest targeting this type of adipocyte to simultaneously treat metabolic and bone diseases.
Supplementary Material
Acknowledgments
We thank Larisa Fedorova for her help with UCP1 immunohistochemistry.
This work was supported by the National Institutes of Health (NIH) (AG028935 to B.L.-C., and AR45433 and DK092759 to C.J.R.) and the American Diabetes Association (ADA Amaranth Diabetes Fund 1-09 RA-95 to B.L.-C.). S.E. was supported by grants from the Swedish Research Council (Grants 2009-2590 and 2010-3281), The Knut and Alice Wallenberg Foundation, Sahlgrenska's University Hospital (LUA-ALF), EU grants (HEALTH-F2-2011-278373; DIABAT), The IngaBritt and Arne Lundgren Foundation, The Söderberg Foundation, The King Gustaf V, and Queen Victoria Freemason Foundation. This study used the Chemistry Laboratory of the Michigan Diabetes Research Center funded by NIH5P60 DK20572 from the National Institute of Diabetes and Digestive and Kidney Diseases.
B.L.-C. conceived and designed experiments, S.R. and Y.L. performed experiments, P.C. performed mCT analysis, and S.E. provided animals and reagents. All authors analyzed and discussed data. S.R., P.C., and B.L.-C. wrote the manuscript. S.E. and C.J.R. contributed to the writing.
Disclosure Summary: S.R., Y.L., P.J.C., and C.J.R. have nothing to declare, B.L.-C. is a consultant to Bristol-Myers Squibb, and S.E. is shareholder and consultant to Ember Therapeutics.
For editorial see page 2579
- ALK
- alkaline phosphatase
- BALP
- bone-specific alkaline phosphatase
- BAT
- brown adipose tissue
- BFR
- bone formation rate
- BMAT
- bone marrow adipose tissue
- BMP
- bone morphogenic protein
- CM
- conditioned media
- FABP4/aP2
- fatty acid binding protein 4
- FoxC2
- forkhead box c2
- IGFBP2
- insulin-like growth factor binding protein 2
- IGTT
- ip glucose tolerance test
- IITT
- ip insulin tolerance test
- mCT
- microcomputed tomography
- MSC
- mesenchymal stem cell
- Opg
- osteoprotegerin
- pAKT
- phosphorylated AKT
- RANKL
- receptor activator of nuclear factor kappa-B ligand
- TRAP5b
- tartrate-resistant acid phosphatase form 5b
- UCP1
- uncoupling protein 1
- WAT
- white adipose tissue
- Wnt10b
- wingless related MMTV integration site 10b.
References
- 1. Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab. 2010;11:268–272 [DOI] [PubMed] [Google Scholar]
- 2. Enerbäck S. Human brown adipose tissue. Cell Metab. 2010;11:248–252 [DOI] [PubMed] [Google Scholar]
- 3. Lowell BB, S-Susulic V, Hamann A, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742 [DOI] [PubMed] [Google Scholar]
- 4. Enerbäck S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94 [DOI] [PubMed] [Google Scholar]
- 5. Kontani Y, Wang Y, Kimura K, Inokuma KI, Saito M, Suzuki-Miura T, Wang Z, Sato Y, Mori N, Yamashita H. UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging Cell. 2005;4:147–155 [DOI] [PubMed] [Google Scholar]
- 6. Ouellet V, Routhier-Labadie A, Bellemare W, et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab. 2011;96:192–199 [DOI] [PubMed] [Google Scholar]
- 7. Lecka-Czernik B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone. 2012;50:534–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee P, Brychta RJ, Collins MT, et al. Cold-activated brown adipose tissue is an independent predictor of higher bone mineral density in women. Osteoporos Int. 2013;24:1513–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ponrartana S, Aggabao PC, Hu HH, Aldrovandi GM, Wren TA, Gilsanz V. Brown adipose tissue and its relationship to bone structure in pediatric patients. J Clin Endocrinol Metab. 2012;97:2693–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bredella MA, Fazeli PK, Freedman LM, et al. Young women with cold-activated brown adipose tissue have higher bone mineral density and lower Pref-1 than women without brown adipose tissue: a study in women with anorexia nervosa, women recovered from anorexia nervosa, and normal-weight women. J Clin Endocrinol Metab. 2012;97:E584–E590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fulzele K, Riddle RC, DiGirolamo DJ, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferron M, Wei J, Yoshizawa T, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM. Bone loss and bone turnover in diabetes. Diabetes. 1995;44:775–782 [DOI] [PubMed] [Google Scholar]
- 14. Reyes-García R, Rozas-Moreno P, López-Gallardo G, et al. Serum levels of bone resorption markers are decreased in patients with type 2 diabetes. Acta Diabetol. 2013;50:47–52 [DOI] [PubMed] [Google Scholar]
- 15. Gennari L, Merlotti D, Valenti R, et al. Circulating sclerostin levels and bone turnover in type 1 and type 2 diabetes. J Clin Endocrinol Metab. 2012;97:1737–1744 [DOI] [PubMed] [Google Scholar]
- 16. Manavalan JS, Cremers S, Dempster DW, et al. Circulating osteogenic precursor cells in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97:3240–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu J, Boström P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fisher FM, Kleiner S, Douris N, et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cederberg A, Grønning LM, Ahrén B, Taskén K, Carlsson P, Enerbäck S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–573 [DOI] [PubMed] [Google Scholar]
- 22. Seale P, Conroe HM, Estall J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qiang L, Wang L, Kon N, et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell. 2012;150:620–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50:546–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lidell ME, Seifert EL, Westergren R, et al. The adipocyte-expressed forkhead transcription factor Foxc2 regulates metabolism through altered mitochondrial function. Diabetes. 2011;60:427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim JK, Kim HJ, Park SY, et al. Adipocyte-specific overexpression of FOXC2 prevents diet-induced increases in intramuscular fatty acyl CoA and insulin resistance. Diabetes. 2005;54:1657–1663 [DOI] [PubMed] [Google Scholar]
- 27. Hakansson J, Eliasson B, Smith U, Enerbäck S. Adipocyte mitochondrial genes and the forkhead factor FOXC2 are decreased in type 2 diabetes patients and normalized in response to rosiglitazone. Diabetol Metab Syndr. 2011;3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang S, Kaw M, Harris MT, et al. Decreased osteoclastogenesis and high bone mass in mice with impaired insulin clearance due to liver-specific inactivation to CEACAM1. Bone. 2010;46:1138–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu L, Aronson J, Huang S, et al. Rosiglitazone inhibits bone regeneration and causes significant accumulation of fat at sites of new bone formation. Calcif Tissue Int. 2012;91:139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486 [DOI] [PubMed] [Google Scholar]
- 31. Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610 [DOI] [PubMed] [Google Scholar]
- 32. Kramer I, Halleux C, Keller H, et al. Osteocyte Wnt/β-catenin signaling is required for normal bone homeostasis. Mol Cell Biol. 2010;30:3071–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lecka-Czernik B, Gubrij I, Moerman EA, et al. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARγ2. J Cell Biochem. 1999;74:357–371 [PubMed] [Google Scholar]
- 34. Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology. 2007;148:2669–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bataille C, Mauprivez C, Haÿ E, et al. Different sympathetic pathways control the metabolism of distinct bone envelopes. Bone. 2012;50:1162–1172 [DOI] [PubMed] [Google Scholar]
- 36. Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nakashima T, Hayashi M, Fukunaga T, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234 [DOI] [PubMed] [Google Scholar]
- 38. Cawthorn WP, Bree AJ, Yao Y, et al. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone. 2012;50:477–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kawai M, Breggia AC, DeMambro VE, et al. The heparin-binding domain of IGFBP-2 has insulin-like growth factor binding-independent biologic activity in the growing skeleton. J Biol Chem. 2011;286:14670–14680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosen CJ. Insulin-like growth factor I and bone mineral density: experience from animal models and human observational studies. Best Pract Res Clin Endocrinol Metab. 2004;18:423–435 [DOI] [PubMed] [Google Scholar]
- 41. Abe E, Yamamoto M, Taguchi Y, et al. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J Bone Miner Res. 2000;15:663–673 [DOI] [PubMed] [Google Scholar]
- 42. Jiang X, Song D, Ye B, et al. Effect of intermittent administration of adiponectin on bone regeneration following mandibular osteodistraction in rabbits. J Orthop Res. 2011;29:1081–1085 [DOI] [PubMed] [Google Scholar]
- 43. Xue Y, Cao R, Nilsson D, et al. FOXC2 controls Ang-2 expression and modulates angiogenesis, vascular patterning, remodeling, and functions in adipose tissue. Proc Natl Acad Sci U S A. 2008;105:10167–10172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wheatcroft SB, Kearney MT, Shah AM, et al. IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes. 2007;56:285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hedbacker K, Birsoy K, Wysocki RW, et al. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 2010;11:11–22 [DOI] [PubMed] [Google Scholar]
- 46. Li Z, Picard F. Modulation of IGFBP2 mRNA expression in white adipose tissue upon aging and obesity. Horm Metab Res. 2010;42:787–791 [DOI] [PubMed] [Google Scholar]
- 47. Sabin MA, Russo VC, Azar WJ, Yau SW, Kiess W, Werther GA. IGFBP-2 at the interface of growth and metabolism: implications for childhood obesity. Pediatr Endocrinol Rev. 2011;8:382–393 [PubMed] [Google Scholar]
- 48. DeMambro VE, Maile L, Wai C, et al. Insulin-like growth factor-binding protein-2 is required for osteoclast differentiation. J Bone Miner Res. 2012;27:390–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wright WS, Longo KA, Dolinsky VW, et al. Wnt10b inhibits obesity in ob/ob and agouti mice. Diabetes. 2007;56:295–303 [DOI] [PubMed] [Google Scholar]
- 50. Bennett CN, Longo KA, Wright WS, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102:3324–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. DeMambro VE, Clemmons DR, Horton LG, et al. Gender-specific changes in bone turnover and skeletal architecture in igfbp-2-null mice. Endocrinology. 2008;149:2051–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ. Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012;492:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.