Abstract
Pregnancy-associated plasma protein-A (PAPP-A) enhances local IGF signaling through its ability to proteolyze inhibitory IGF binding proteins. In vivo, PAPP-A (like IGF) appears to exhibit antagonistic pleiotropy; ie, it has beneficial effects early in life but detrimental effects later in life. Accordingly, PAPP-A knockout (KO) mice are born as proportional dwarfs and have diminished reproductive vigor and reduced peak bone mass acquisition at puberty. On the other hand, PAPP-A KO mice live approximately 30% longer than their wild-type littermates, with decreased incidence and severity of age-related diseases and resistance to adverse responses of vascular injury. To be able to distinguish the impact of PAPP-A deficiency in the adult from that in early life, we developed a mouse model suitable for inducible Cre recombinase-mediated excision of the PAPP-A gene. In this study, we characterize the conditional PAPP-A KO mouse model for efficacy of tamoxifen-induced floxed PAPP-A excision in various tissues of adult mice and demonstrate a significant (P = .0001) reduction of neointimal formation in these mice after unilateral carotid artery ligation.
Pregnancy-associated plasma protein-A (PAPP-A) has been shown to enhance IGF signaling via its ability to proteolyze inhibitory IGF binding proteins, which thereby increases local IGF available to activate receptors (reviewed in Ref. 1). In vivo, PAPP-A appears to exhibit antagonistic pleiotropy, ie, it has beneficial effects early in life but detrimental effects later in life (2). This is probably related to its regulation of IGF signaling, because IGFs are critically involved in fetal growth, reproduction, and pubertal bone mass acquisition (3–5). On the other hand, IGFs have been associated with aging and several age-related diseases (6, 7). We hypothesized that PAPP-A, being a modulator of IGF action, would be important in achieving optimal fetal growth, optimal reproductive capability, and peak bone mass during puberty, while promoting aging and age-related disease. Accordingly, PAPP-A knockout (KO) mice are born as proportional dwarfs and have diminished reproductive vigor and reduced bone mass (8–10). However, PAPP-A KO mice live approximately 30% longer than their wild-type (WT) littermates along with having suppressed thymic atrophy, delayed occurrence of spontaneous cancers, and decreased incidence and severity of age-related degenerative diseases (11, 12). PAPP-A KO mice are also resistant to the development of neointimal formation after acute vascular injury and to atherosclerotic plaque progression when challenged with a high-fat diet (13, 14). Thus, PAPP-A has been proposed as a possible therapeutic target for a variety of age-related diseases and adverse injury–related responses.
The aforementioned PAPP-A KO mouse was generated through homologous recombination in embryonic stem (ES) cells (8). Given the antagonistic pleiotropic effects of PAPP-A, it was important to distinguish the impact of PAPP-A deficiency in the adult from that in early life. One approach taken was to rescue the dwarf phenotype in the PAPP-A KO mouse by crossing it with an IGF-II–overexpressing mouse. These mice, which were normal-sized at birth, maintained the longevity phenotype but did not have moderation in the reproductive or skeletal deficiencies (15). A more powerful approach is to conditionally knock out the gene in adult animals. To this end, we developed a mouse model suitable for inducible Cre recombinase–mediated excision of the PAPP-A gene. In this study, we characterize the conditional PAPP-A KO mouse model and demonstrate effective reduction of neointimal formation in these mice after unilateral carotid ligation.
Materials and Methods
Generation of floxed PAPP-A mice
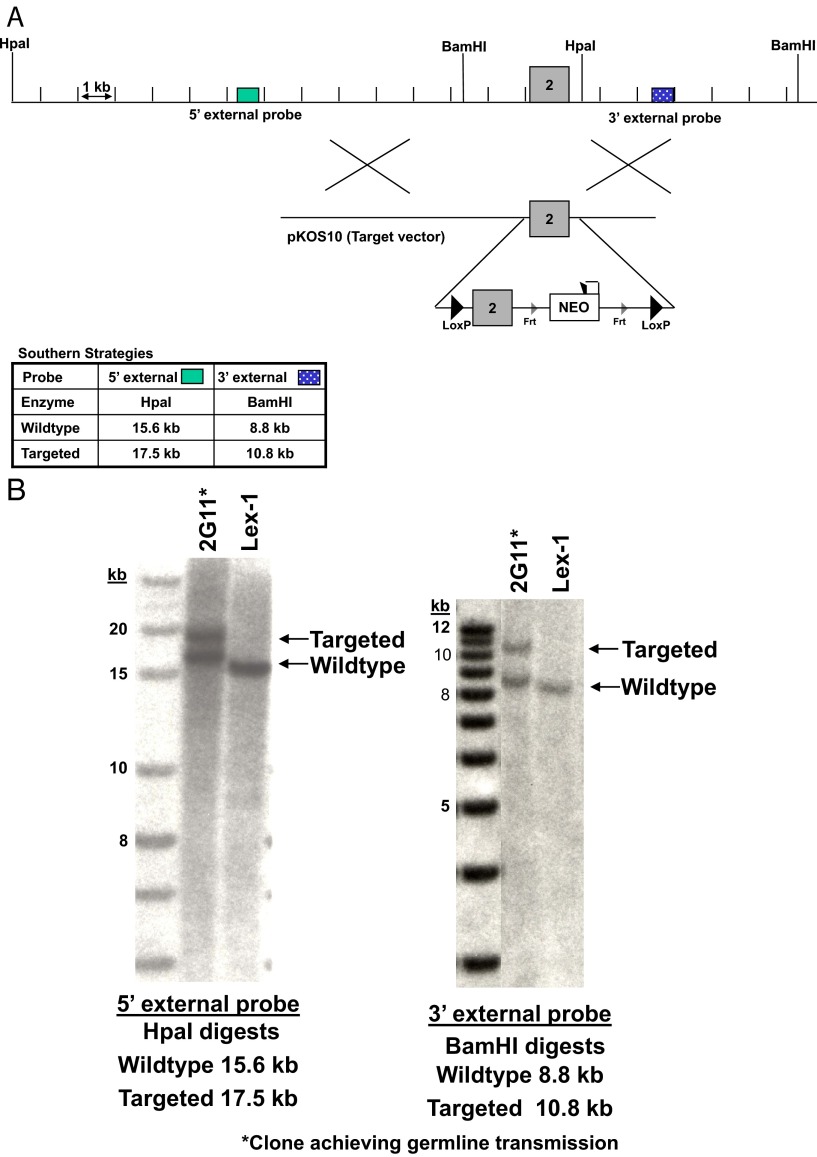
The floxed PAPP-A mice were generated at Lexicon Pharmaceuticals, Inc (The Woodlands, Texas). A schematic diagram for the engineered insertion of 2 LoxP sites flanking a genomic segment of PAPP-A, the “floxed” locus, is presented in Figure 1A. These LoxP sites, which are absent from the mammalian genome, act as recognition sites for Cre recombinase, a bacteriophage enzyme. They were placed in cis arrangement and oriented in the same direction for Cre-mediated deletion of the intervening DNA sequence and recombination. The conditional targeting vector was derived using the lambda KOS system (16). The lambda KOS phage library, arrayed into 96 superpools, was screened by PCR using exon 2–specific primers Pap-3 (5′-ACTTGCCTGCATCTACTTCC-3′) and Pap-4 (5′-GTTGTGATTCAGAGCACTGC-3′). The PCR-positive phage superpools were plated and screened by filter hybridization using the 389-bp amplicon derived from primers Pap-3 and Pap-4 as probes. One pKOS genomic clone, pKOS-10, was isolated from the library screen and confirmed by sequence and restriction analysis. Gene-specific arms (5′-ATTATCCAGATGTGACATTTATCAGAGAAGAAT-3′ and 5′-GATAGCTGTACAGACTGGAAAAATGCCATAAA-3′) were appended by PCR to a yeast selection cassette containing the URA3 marker. The yeast selection cassette and pKOS-10 were cotransformed into yeast, and clones that had undergone homologous recombination to replace a 1209-bp region containing exon 2 with the yeast selection cassette were isolated. This 1209-bp fragment was independently amplified by PCR and cloned into the intermediate vector pLFNeo, introducing flanking LoxP sites and a Neo selection cassette (Pap-pLFNeo). The yeast cassette was subsequently replaced with the Pap-pLFNeo selection cassette to complete the conditional PAPP-A targeting vector that has exon 2 flanked by LoxP sites. The NotI-linearized targeting vector was electroporated into 129/SvEvBrd (Lex-1) ES cells. G418/FIAU-resistant ES cell clones were isolated, and correctly targeted clones were identified and confirmed by Southern analysis using a 227-bp 5′ external probe (10/11), generated by PCR using primers Pap-10 (5′-GTGTAGGTATGTGCAATCATG-3′) and Pap-11 (5′-GCATGGAAAGTGTCCATTTGAA-3′) and a 241-bp 3′ external probe (12/13), amplified by PCR using primers Pap-12 (5′-CCAGATTCTGAAAGACGGTG-3′) and Pap-13 (5′-GCATAGGACTCTGAAAAGGC-3′). Southern analysis using probe 10/11 (Figure 1B) detected a 15.6-kb WT band and a 17.5-kb mutant band in HpaI-digested genomic DNA, whereas probe 12/13 detected a 8.8-kb WT band and a 10.8-kb mutant band in BamHI-digested gDNA. One targeted ES cell clone was microinjected into C57BL/6 (albino) blastocysts. The resulting chimeras were mated to C57BL/6 (albino) females to generate mice that were heterozygous for the PAPP-A conditional mutation.
Figure 1.
Targeted disruption of the PAPP-A gene locus. A, Targeting strategy used to disrupt the PAPP-A locus. Homologous recombination (represented by X) between the targeting vector and the PAPP-A gene results in the substitution of a LoxP-flanked exon 2 along with a Frt-flanked selection cassette. B, Southern hybridization indicating proper gene targeting in the ES cell clones. Clone 2G11 was used for blastocyst injections; Lex-1 represents untransfected ES cell DNA. These are spliced images.
Generation of conditional PAPP-A KO mice
Transgenic mice having a tamoxifen (Tam)-inducible Cre-mediated recombination system driven by the chicken β-actin promoter/enhancer coupled with the cytomegalovirus immediate-early enhancer (Tam-Cre mice) were purchased from The Jackson Laboratory (Bar Harbor, Maine). This mouse line, in which Cre is ubiquitously expressed, permits temporal regulation of Cre-mediated recombination in diverse tissues.
Mice homozygous for floxed PAPP-A (fPAPP-A) were bred with Tam-Cre transgenic mice. This mating produced mice heterozygous for fPAPP-A and positive (pos) or negative (neg) for Tam-Cre. These mice were then mated to produce offspring with 4 genotypes: homozygous fPAPP-A/pos, homozygous fPAPP-A/neg, WT/pos, and WT/neg. Mice were further checked for “leaky Cre,” ie, evidence of residual Cre recombinase activity. Only those mice without leaky Cre (65%) were used in experiments.
Cre-mediated excision and recombination were induced with ip injection of Tam (Sigma-Aldrich, St Louis, Missouri) in corn oil with 2% ethanol. The initial injection was with 6 mg of Tam/40 g body weight (bw) and then with 3 mg of Tam/40 g bw weekly for 3 weeks.
All procedures involving mice complied with the standards stated in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Mayo Clinic Institutional Animal Care and Use Committee.
Genotyping
Floxed PAPP-A primers were 5′-GTCCCAGAGGCTCCAATAGTAGC-3′ (forward) and 5′-GGAAGTTGTGATTCAGAGCACTGC-3′ (reverse), yielding a WT band at 488 bp and the flox mutant band at 572 bp. Primers for leaky Cre used the same forward as above with reverse 5′-GCTGGCCTGAGGATAACTTCG-3′, yielding a band at 166 bp. The PCR protocol was 95°C for 5 minutes, then 10 cycles of 94°C for 15 seconds, 65°C for 30 seconds, and 72°C for 40 seconds with a decrease of 1° per cycle in the annealing temperature, and then 30 cycles of 94°C for 15 seconds, 55°C for 30 seconds, and 72°C for 40 seconds. The final elongation was 72°C for 7 minutes with a hold at 4°C.
Tam-Cre primers were 5′-GCGGTCTGGCAGTAAAAACTATC-3′ (forward) and 5′-GTGAAACAGCATTGCTGTCACTT-3′ (reverse). The PCR protocol was 95°C for 5 minutes and then 94°C for 30 seconds, 51.7°C for 60 seconds, and 72°C for 60 seconds for 32 cycles, with a final elongation at 72°C for 7 minutes and a hold at 4°C. DNA from Tam-Cre–positive mice yielded a band at 100 bp.
Efficiency of inducible PAPP-A deletion
The efficiency of PAPP-A deletion in various tissues of fPAPP-A/pos mice treated with Tam was determined by a 3-primer end-point PCR. Primers were the following: common forward, 5′-TAGTTCCTCCAGCTTTTACCTTG-3′; intact reverse, 5′-ATTTGTCATACAGCCCTATGTG-3′; and excised reverse, 5′-AAAATGCCATAAACTATAGGG-3′. The PCR protocol was an initial denaturization step of 95°C for 3 minutes and then 32 cycles of 95°C for 10 seconds, 54°C for 30 seconds, a final elongation of 72°C for 7 minutes, and a hold at 4°C. End-products were visualized on a 2% agarose/ethidium bromide gel. A band at 288 bp denoted intact fPAPP-A and a band at 240 bp indicated the postexcision product.
Unilateral carotid ligation
The unilateral carotid ligation model induces arterial injury due to cessation of blood flow, resulting in rapid proliferation and migration of medial smooth muscle cells, which leads to neointimal formation (17). Mice (male and female) were anesthetized, a midline incision on the ventral surface of the neck was made, and the right and left common carotid arteries were isolated from the surrounding tissues. A suture was passed under the left vessel just proximal to the carotid bifurcation, and the artery was ligated; the right carotid artery was treated similarly (minus the ligation) and was considered the contralateral sham control.
Mice were killed 10 days after ligation, and the carotid arteries fixed in situ by perfusion with PBS-buffered formalin at physiological pressure. Individual arteries were removed, placed in PBS-buffered formalin, and fixed for 24 hours before paraffin embedding. Sections (5.0-μm-thick) were collected 0.5, 1.0, and 1.5 mm distal to a reference point (0.25 mm distal to the ligature). At each distance, Verhoff von Giessen (VVG)–stained (Accustain; Sigma-Aldrich) sections were evaluated using Adobe PhotoShop software (version 6.0.1; Adobe Systems, Inc, Mountain View, California). The neointimal area (area within the internal elastic lamina minus luminal area) for each animal was expressed as the mean of the neointimal area measured at 3 distinct distances. The medial area was quantified as the area defined by the external elastic lamina minus that defined by the internal elastic lamina.
Results
Conditional PAPP-A KO mouse model
fPAPP-A mice had body weights similar to those of WT mice, normal fertility, and no obvious phenotype alteration (data not shown). Thus, these mice exhibited none of the effects of deletion of the PAPP-A gene during fetal development and early postnatal growth.
Initial studies were done in fPAPP-A/pos mice to establish the dose of Tam and the dosing schedule needed to achieve efficient excision and recombination without toxicity. Toxicity was assessed by weight loss in older animals or failure to gain weight in younger, growing animals. We found that multiple rounds of ip Tam were needed for efficient induction of recombination in a broad spectrum of adult tissues and that 6 mg of Tam/40 g bw followed by weekly injections of 3 mg of Tam/40 g bw for 3 weeks was well tolerated.
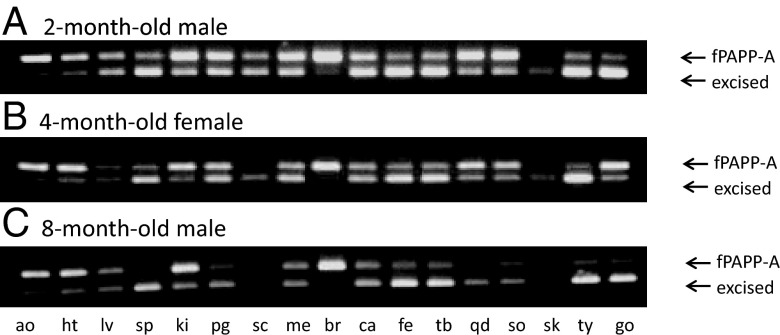
The relative effectiveness of Tam-induced fPAPP-A excision in various tissues was assessed by PCR. Representative results are presented in Figure 2. A band at 288 bp denoted intact fPAPP-A, and a band at 240 bp indicated the postexcision product. There appeared to be near-complete deletion of fPAPP-A in bone (calvaria, femur, and tibia), thymus, and testes. Apparent but more variable deletion was seen in aorta, heart, liver, spleen, kidney, fat (perigonadal and mesenteric), skeletal muscle (quadriceps and soleus), and ovaries. Brain fPAPP-A appeared to be resistant to Tam-induced deletion. Little fPAPP-A was detected in subcutaneous fat and skin. Importantly, there was a similar pattern of tissue-specific excision in mice that were 2, 4, and 8 months old. Treatment with vehicle alone (corn oil + 2% ethanol) had no effect on fPAPP-A PCR (data not shown). Thus, this model is suitable for inducible deletion of PAPP-A in adult mice.
Figure 2.
Inducible PAPP-A excision and recombination in various tissues. PCRs of Tam-induced fPAPP-A excision in a 2-month-old male mouse (A), a 4-month-old female mouse (B), and an 8-month-old male mouse, as described in Materials and Methods, are shown. The 288-bp band represents intact fPAPP-A, and the 240-bp band represents the postexcision product. ao, aorta; ht, heart; lv, liver; sp, spleen; ki, kidney; pg, perigonadal fat; sc, subcutaneous fat; me, mesenteric fat; br, brain; ca, calvaria; fe, femur; tb, tibia; qd, quadriceps; so, soleus; sk, skin; th, thymus; go, gonadal tissue.
Carotid ligation
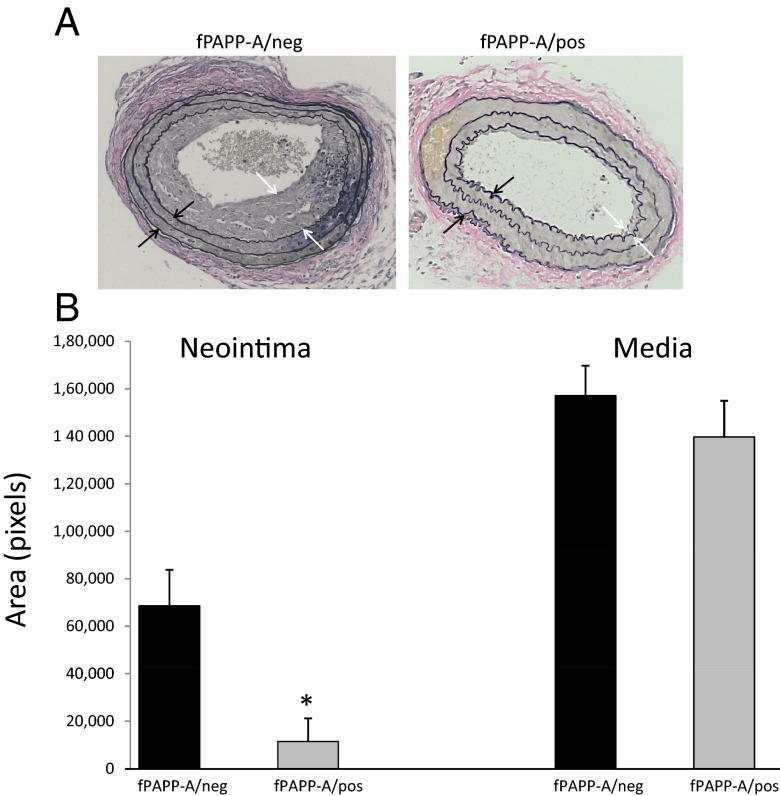
Five-week-old fPAPP-A/pos and fPAPP-A/neg mice were treated with Tam once a week for 4 weeks. Tam was given to both groups to control for any confounding effects of the Tam injections per se. After this treatment period, unilateral carotid ligation was performed. Ten days after the surgery, carotid arteries were harvested, fixed, sectioned, and stained with VVG. Images were analyzed for neointimal and medial areas. The results are presented in Figure 3. The formation of neointima in fPAPP-A/neg mice was significantly (P = .0001) reduced by 80% in fPAPP-A/pos mice compared with that in fPAPP-A/neg mice. There was no significant difference in medial area between the 2 groups. To control for a possible Cre effect independent of deletion of the target gene, PAPP-A, we performed carotid ligation in WT/pos and WT/neg mice. There was no significant difference in neointimal formation in these mice (P = .77)
Figure 3.
Neointimal and medial area 10 days after carotid ligation. A, VVG-stained cross sections of carotid. White arrows define the neointima. Black arrows define the media. B, fPAPP-A mice that are positive or negative for Tam-Cre. Results are means ± SEM of 10 to 12 mice. *, Significant effect of Tam-inducible Cre excision at P < .05.
Discussion
In this study, we generated and characterized a new model suitable for conditional KO (or knock-down) of PAPP-A in adult mice using a Tam-inducible system. We determined a dose of Tam (6 mg/40 g bw initially and then 3 mg/40 g bw) and a dosing schedule (once a week for 4 weeks) that balanced efficacy against possible toxicity. Higher doses of Tam produced evidence of morbidity. Increasing the number of weeks of treatment produced no discernible increase in effectiveness. It was also important to establish that Tam induction worked in adult mice to make a distinction between early life vs later life effects of PAPP-A deletion. We showed the same pattern of efficacy of Tam administration in 2-, 4-, and 8-month-old mice (Figure 2). This was not a trivial outcome because we and others found that in certain models tetracycline-controlled Cre recombination could not be induced in adult mice (Ref. 18 and our unpublished results).
The cytomegalovirus promoter driving Tam-Cre is ostensibly ubiquitous, but Cre-mediated PAPP-A excision and recombination efficiency in this mouse model appeared to differ among tissues, as assessed by PCR. Of the tissues tested, highly efficient PAPP-A gene deletion was seen in bone, thymus, and testes. Other tissues appeared to have more variable excision efficiency. Brain was the only tissue that appeared resistant to Tam-induced PAPP-A deletion. The reason for the variability is unclear. However, if the excision is cell type or region specific, it would probably confound interpretation of results from a whole-tissue preparation. Furthermore, this PCR was not designed to be quantitative and may underestimate effective deletion of PAPP-A in tissues, especially in situations where PAPP-A gene expression is up-regulated in response to inflammation or injury (13, 14, 19). In the example chosen for this study, vascular tissue appeared to have approximately 30% PAPP-A excision by PCR. The knockdown of the PAPP-A gene in vascular smooth muscle cells was associated with significantly inhibited injury-induced neointimal formation. These studies were controlled for possible side effects of Tam administration per se and any Cre effects independent of Cre-mediated PAPP-A deletion.
In summary, we now have a valuable model for age-related studies of PAPP-A that can be extricated from lifelong deletion of PAPP-A and any effects of this deficiency on fetal and early neonatal growth. In particular, this mouse model will be important for studies of aging and age-related disease that may involve multiple tissues. In addition, generation of mice with tissue-specific PAPP-A deletion by crossing fPAPP-A mice with transgenic mice using different promoters to drive Tam-inducible Cre recombinase would introduce other important and complementary models for addressing various issues of PAPP-A physiology and pathophysiology.
Acknowledgments
This work was supported by National Institutes of Health Grant HL074871 (to C.A.C.).
Disclosure Summary: D.R.P. is an employee of Lexicon Pharmaceuticals, Inc, and has received compensation in the form of salary and stock options. The other authors have nothing to disclose.
Footnotes
- bw
- body weight
- ES
- embryonic stem
- f
- floxed
- KO
- knockout
- neg
- negative
- PAPP-A
- pregnancy-associated plasma protein-A
- pos
- positive
- Tam
- tamoxifen
- VVG
- Verhoff von Giessen
- WT
- wild-type.
References
- 1. Conover CA. Key questions and answers about pregnancy-associated plasma protein-A. Trends Endocrinol Metab. 2012;23:242–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411 [Google Scholar]
- 3. Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82 [PubMed] [Google Scholar]
- 4. Baker J, Hardy MP, Zhou J, et al. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol. 1996;10:903–918 [DOI] [PubMed] [Google Scholar]
- 5. Govoni KE, Lee SK, Chung YS, et al. Disruption of insulin-like growth factor-I expression in type IIαI collagen-expressing cells reduces bone length and width in mice. Physiol Genomics. 2007;30:354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barbieri M, Bonafè M, Franceschi C, Paolisso G. Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab. 2003;285:E1064–E1071 [DOI] [PubMed] [Google Scholar]
- 7. Yang J, Anzo M, Cohen P. Control of aging and longevity by IGF-I signaling. Exp Gerontol. 2005;40:867–872 [DOI] [PubMed] [Google Scholar]
- 8. Conover CA, Bale LK, Overgaard MT, et al. Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development. 2004;131:1187–1194 [DOI] [PubMed] [Google Scholar]
- 9. Tanner SJ, Hefferan TE, Rosen CJ, Conover CA. Impact of pregnancy-associated plasma protein-A deletion on the adult murine skeleton. J Bone Miner Res. 2008;23:655–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mason EJ, Grell JA, Wan J, Cohen P, Conover CA. Insulin-like growth factor (IGF)-I and IGF-II contribute differentially to the phenotype of pregnancy associated plasma protein-A knock-out mice. Growth Horm IGF Res. 2011;21:243–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Conover CA, Bale LK, Mader JR, Mason MA, Keenan KP, Marler RL. Longevity and age-related pathology of mice deficient in pregnancy-associated plasma protein-A. J Gerontol A Biol Sci Med Sci. 2010;65:590–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vallejo AN, Michel JJ, Bale LK, Lemster BH, Borghesi L, Conover CA. Resistance to age-dependent thymic atrophy in long-lived mice that are deficient in pregnancy-associated plasma protein A. Proc Natl Acad Sci USA. 2009;106:11252–11257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Resch ZT, Simari RD, Conover CA. Targeted disruption of the pregnancy-associated plasma protein-A gene is associated with diminished smooth muscle cell response to insulin-like growth factor-I and resistance to neointimal hyperplasia after vascular injury. Endocrinology. 2006;147:5634–5640 [DOI] [PubMed] [Google Scholar]
- 14. Harrington SC, Simari RD, Conover CA. Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet. Circ Res. 2007;100:1696–1702 [DOI] [PubMed] [Google Scholar]
- 15. Bale LK, Conover CA. Disruption of insulin-like growth factor-II imprinting during embryonic development rescues the dwarf phenotype of mice null for pregnancy-associated plasma protein-A. J Endocrinol. 2005;186:325–331 [DOI] [PubMed] [Google Scholar]
- 16. Wattler S, Kelly M, Nehls M. Construction of gene targeting vectors from lambda KOS genomic libraries. Biotechniques. 1999;26:1150–1156, 1158,, 1160 [DOI] [PubMed] [Google Scholar]
- 17. Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–2244 [DOI] [PubMed] [Google Scholar]
- 18. Bäckman CM, Zhang Y, Malik N, et al. Generalized tetracycline induced Cre recombinase expression through the ROSA26 locus of recombinant mice. J Neurosci Methods. 2009;176:16–23 [DOI] [PubMed] [Google Scholar]
- 19. Conover CA, Bale LK, Harrington SC, Resch ZT, Overgaard MT, Oxvig C. Cytokine stimulation of pregnancy-associated plasma protein A expression in human coronary artery smooth muscle cells: inhibition by resveratrol. Am J Physiol Cell Physiol. 2006;290:C183–C188 [DOI] [PubMed] [Google Scholar]