Abstract
Background
Obesity and hypertension are reported among survivors of pediatric acute lymphoblastic leukemia (ALL). However, little is known about the trajectory of body mass index (BMI) and blood pressure over the course of ALL therapy.
Procedure
In a retrospective cohort of 183 pediatric ALL patients diagnosed from 2000-2008, prevalence, severity, and risk factors for obesity and hypertension were assessed during treatment.
Results
At diagnosis, 36% of patients were overweight and 19% were obese. Median BMI increased during induction therapy with a return to baseline soon after, but increased again over the first 22 months of maintenance therapy. At the end of therapy, 49% were overweight and 21% were obese. Increased BMI z-score at diagnosis was associated with increased z-score during maintenance (p<0.001). Elevated parental BMI was associated with elevated BMI at diagnosis. Median BMI z-score increased over the first 22 months of maintenance (p<0.001). Patients with high risk disease had lower BMI z-scores regardless of cranial radiotherapy exposure (p<0.001). Pre-hypertension was prevalent over the course of therapy (31.1% with systolic pre-hypertension and 18.6% with diastolic pre-hypertension). Hypertension was also highly prevalent with 41.5% meeting systolic criteria and 24.0% meeting diastolic criteria.
Conclusions
During ALL therapy, patients are at risk for early development of elevated BMI and blood pressure, which places them at potentially increased risk for future adverse health conditions. Future studies are needed to develop strategies to mitigate these risks, such as potential reduction of corticosteroid pulses or a family-based diet and exercise intervention during maintenance therapy.
Keywords: Obesity, body mass index, acute lymphoblastic leukemia, metabolic syndrome, pediatrics, hypertension
INTRODUCTION
Cure rates for acute lymphoblastic leukemia (ALL) now exceed 85% (1). Coincident with this improved survival are adverse long-term sequelae of treatment. These sequelae include components of the metabolic syndrome, specifically obesity and hypertension, which are particularly well-documented (2-8). Furthermore, it is well established that components of the metabolic syndrome (obesity, hypertension, hyperlipidemia, insulin resistance) increase risk for heart disease and diabetes (9).
Although obesity and hypertension are well-documented in long-term survivors of ALL (6, 10-12), their time of onset and the trajectory through therapy to long-term survivorship remain unclear. We hypothesize that the period of maintenance chemotherapy is when elevated body mass index (BMI) and blood pressure will manifest or become exacerbated due to the monthly pulses of high-dose corticosteroids. Therefore, in a cohort of patients with ALL we retrospectively collected data on BMI and blood pressure over the course of treatment and assessed the prevalence, severity, and risk factors for increased BMI and blood pressure. Identifying the period of risk during which metabolic abnormalities appear or worsen will inform the subsequent development of behavioral or therapeutic preventive interventions to mitigate these abnormalities
METHODS
Construction of the Cohort
Following Human Subjects Committee approval, we assembled a retrospective cohort from all pediatric patients ages 1 – 21 years at diagnosis treated for ALL in the Division of Hematology/Oncology at Vanderbilt University Medical Center between 2000-2008. Eligible patients were identified using a search of the electronic medical record. Inclusion criteria included a diagnosis of standard or high-risk precursor B-cell or T-cell ALL, with treatment on or according to Children’s Oncology Group (COG) ALL protocols. Figure 1 shows the basic treatment schema for these protocols. This cohort encompassed all ALL patients treated at Vanderbilt during that time period with the exception of a small subset of patients with very high risk ALL, infant ALL or patients with Down Syndrome. We excluded patients treated for very high risk ALL or infant ALL due to the very different treatment exposures and excluded children with Down syndrome due to differences in growth parameters making z-score comparisons impossible. Initially 215 patients were identified from the medical record. Of these, 15 were excluded due to transfer of their care during therapy, 12 were excluded due to a diagnosis of very high risk or infant ALL and 5 patients with Down Syndrome. This resulted in our study cohort of 183 patients.
Figure 1.
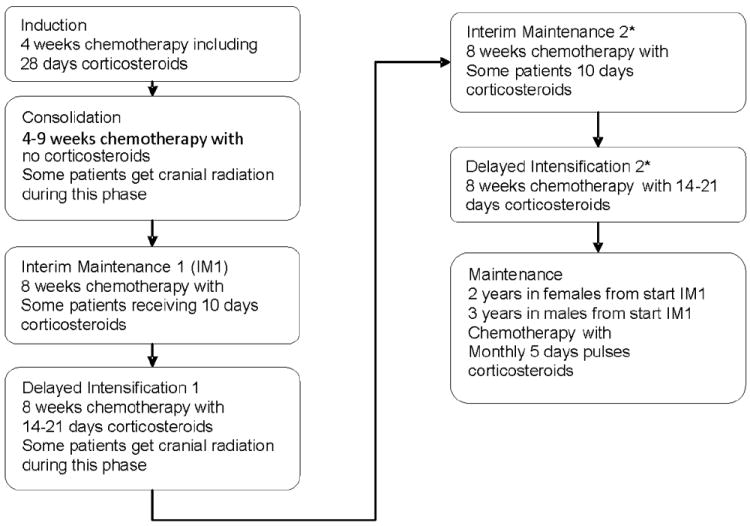
Schema for acute lymphoblastic leukemia therapy as per the Children’s Oncology Group protocols used by all patients in this study showing when in therapy patients receive cranial radiation and steroids. * Two IM and DI are given for limited patients.
Data collection
A medical record abstraction was conducted for each participant and included collection of demographic data and, for each course of chemotherapy through the end of therapy, height, weight, and blood pressure measurements. As males received an additional year of maintenance therapy, data was also collected for female patients every 3 months until 1 year off therapy. Family history of myocardial infarction, coronary artery bypass surgery, hypertension, hypercholesterolemia, cerebrovascular accident, and diabetes in first and second degree family members as well as parental height and weight were obtained for 140 of the 183 patients by means of a self-report questionnaire completed by a parent at the time of study entry.
Standardization of BMI and blood pressure data
Raw BMI values were converted to age and gender adjusted z-scores using the Centers for Disease Control and Prevention’s Year 2000 growth charts for patients 2 – 20 years (Epi Info 3.5) (13). Length for weight z-scores were substituted for patients who had measured values prior to turning 24 months of age. In patients over 20 years of age, z-score data for age 20 years were used.
Systolic and diastolic blood pressures were converted to height, age and gender- adjusted z-scores using the National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents guidelines for children 1 – 17 years old. (14). For patients who were over 17 years old, z-score data for 17 year olds were used. Parental BMI was calculated from height and weight reported on their survey at time of study entry.
Definitions
Patients were classified as overweight if their BMI z-scores exceeded the 85th percentile and obese if their BMI z-score exceeded the 95th percentile (13). Pre-hypertension was defined as an average blood pressure that exceeds the 90th percentile and hypertension as a repeated measurement exceeding the 95th percentile on three or more separate occasions (14).
Cumulative corticosteroid dose was computed at the time of the first maintenance course and again at the end of therapy and is expressed as prednisone-equivalent. For patients treated with dexamethasone and prednisone, the dexamethasone doses were multiplied by 6.67 and added to the prednisone dose.
Outcome variables
The primary outcome variables included BMI, systolic and diastolic blood pressure during maintenance therapy.
Statistical Analysis
For the analysis of BMI and blood pressure during maintenance therapy, mixed-effects models were used and are described below. In the BMI model, BMI z-score at diagnosis, time, gender, cumulative corticosteroid dose at the beginning of maintenance chemotherapy, cranial radiation-risk category (standard-risk, high risk - no radiation, high risk -radiation), and supplemental feeds during therapy were included. Because individual profiles of BMI z-scores often showed a convex shape, we included a time-squared term to account for this trend.
In the blood pressure model, BMI z-score at diagnosis, time, gender, age, anti-hypertensive medication use, and presence of steroid-induced hypertension (elevated blood pressures during continuous steroid therapy that required pharmacologic intervention) were included. Children less than 36 months of age at diagnosis were excluded from the blood pressure analyses due to concerns of falsely elevated blood pressure due to distress with measurement.
Family history data were available for 140 of 183 patients. To quantify the impact of the individual components, and due to the lack of an available validated instrument, we created a family history risk score to include in study analyses. The total number of first-degree and second-degree relatives were added who reported myocardial infarction, coronary artery bypass surgery, hypertension, hypercholesterolemia, cerebrovascular accident, or diabetes. Each of these conditions was given a score of 1, except myocardial infarction and coronary artery bypass surgery where a score of 0.5 was assigned, as the conditions often appear together. Scores from first-degree relatives were weighted twice as heavily as second-degree relatives in producing the composite risk score. An ordinary regression model was used to assess the contribution of family history risk score, with BMI z-score at diagnosis, family history risk score, age, gender, race, and average parental BMI included in the model.
For blood pressure analysis, ordinary regression models were developed with blood pressure z-score at diagnosis. BMI z-score at diagnosis, age, sex, race, average parental BMI, family history score, and hypertension (HTN) risk group included in the model. HTN risk groups included: 1) patients who never received antihypertensive medications; 2) those treated with such medications only for steroid-induced hypertension; and 3) those treated for hypertension non-steroid-induced. Blood pressure measurements at induction and consolidation were excluded from all analyses and other measurements included those taken at the beginning of a new course of chemotherapy, during which time the patients were not receiving corticosteroids.
The proportion of patients meeting criteria for pre-hypertension and for hypertension were computed. All data analyses were performed using R 2.10 (R Development Core Team, Vienna, Austria).
RESULTS
Characteristics of the Cohort
The median age at diagnosis of the cohort was 5.7 years, with more males (62%) than females and the majority (84%) were Caucasian. The median BMI z-score at diagnosis was 0.64, (74th percentile). Fifty-four (30%) patients received at least one type of supplemental feeding during their therapy with 37 (20%) requiring total parental nutrition (TPN), 39 (21%) receiving nasogastric (NG) tube feeds, and 1 patient requiring a gastrostomy tube. Twenty-three (13%) patients were treated with cranial radiotherapy. The median dose of corticosteroids received during maintenance was 5194mg/m2, which represents an average of 63% of the total amount of steroids patients received during therapy. Thirty patients received antihypertensive medications during therapy, of whom 21 (70%) received these medications only during periods of continuous corticosteroid use. Only 2 patients received antihypertensive medications during the maintenance phase of therapy. These data are shown in Table I.
Table I.
Characteristics of the Cohort
| Characteristic* | N (%)** |
|---|---|
| Age at diagnosis, years, median (range) | 5.7 (1.2, 20.9) |
| Male Gender | 113 (61.7) |
| Race | |
| Caucasian | 154 (84.2) |
| African American | 14 (7.6) |
| Hispanic | 11 (6.0) |
| Other | 4 (2.2) |
| Risk treatment category | |
| High risk | 108 (59.0) |
| Standard risk | 75 (41.0) |
| Diagnosis | |
| Pre B ALL | 167 (91.3) |
| T cell ALL | 16 (8.7) |
| CNS Status | |
| CNS 1 | 149 (81.4) |
| CNS 2 | 31 (16.9) |
| CNS 3 | 3 (1.6) |
| Supplemental feeds*** | 54 (29.5) |
| Total parental nutrition (TPN) | 37 (20.2) |
| Nasogastric (NG) tube feeds | 39 (21.3) |
| Gastrostomy tube | 1 (0.5) |
| Cranial radiotherapy | 23 (12.6) |
| Radiation dose (N=23) | |
| <1800 GY | 7 (30.4) |
| 1800 GY | 14 (60.9) |
| >1800 GY | 2 (8.7) |
| Anti-hypertensive medication during therapy | 30 (16.4) |
| Antihypertensive medication only while on continuous corticosteroids (N=30) | 21 (70.0) |
| Antihypertensive medication during maintenance (N=30) | 2 (6.7) |
| Completed ALL therapy at data censoring | 106 (57.9) |
| Completed ALL therapy through MC5 at censoring | 148 (80.9) |
CNS, central nervous system;
N=183 unless noted otherwise;
Except where noted otherwise;
Needing one or more of total parental nutrition, nasogastric or gastrostomy tube feeding during therapy.
BMI Analyses
BMI z-score increased (p<0.001) from induction (median = 0.64) to consolidation (median = 1.45), then decreased (p<0.001) by the start of delayed intensification (Median = 0.54) (Table II). This was followed by a steady increase in BMI over the early part of maintenance therapy (Tables II and III). At the end of therapy, the median z-score was stable at 1.06 in males and 0.96 in females. In addition, the percentage of overweight or obese and obese individuals increased from induction to consolidation, decreased to close to baseline by start of delayed intensification and then increased over the course of maintenance therapy (Table II). At the end of therapy, 50.8% of males and 46.8% of females were overweight/obese and 22% of males and 19.1% of females were obese.
Table II.
Changes in BMI, Systolic and Diastolic Blood Pressure over Therapy
| BMI z-scores over therapy and number of patients who were overweight or obese at each stage** | BMI z-score | % * Overweight or obese | % * Obese |
|---|---|---|---|
| Median (range) | N (%) | N (%) | |
| Induction | 0.64 (-3.40, 3.27) | 66 (36.1) | 35 (19.1) |
| Consolidation | 1.45 (-2.52, 4.01) | 112 (61.2) | 76 (41.5) |
| Delayed intensification (N=181) | 0.54 (-2.72, 4.36) | 63 (34.8) | 37 (20.4) |
| Start of maintenance therapy (N=175) | 0.67 (-2.01, 4.54) | 70 (40.0) | 39 (22.2) |
| Maintenance course 5 (N=148) | 0.98 (-2.81, 2.73) | 71 (47.9) | 35 (23.6) |
| Maintenance course 8 (N=81) | 1.11 (-2.49, 2.85) | 44 (54.3) | 24 (29.6) |
| End of therapy: male (N=59) | 1.06 (-2.80, 4.20) | 30 (50.8) | 13 (22.0) |
| End of therapy: female (N=47) | 0.96 (-2.34, 2.67) | 22 (46.8) | 9 (19.1) |
| Systolic and diastolic blood pressure z-scores at each stage of therapy | Systolic z-score | Diastolic z-score | |
| Median (range) | Median (range) | ||
| Induction (N=183/N=181) | 1.55 (-8.50, 5.61) | 0.98 (-1.61, 4.51) | |
| Consolidation (N=178) | 1.71 (-1.45, 5.85) | 1.42 (-0.83, 4.11) | |
| Delayed intensification (N=178) | 1.14 (-1.36, 4.65) | 0.78 (-1.32, 4.54) | |
| Start of maintenance therapy (N=170) | 0.74 (-2.31, 5.50) | 0.57 (-2.28, 5.04) | |
| Maintenance course 5 (N=145/ N=146) | 0.85 (-2.30, 5.25) | 0.48 (-1.38, 4.38) | |
| Maintenance course 8 (N=84) | 0.78 (-2.46, 3.20) | 0.53 (-1.54, 2.55) | |
| End of therapy: male (N=62) | 0.78 (-1.26, 3.97) | 0.47 (-1.68, 3.86) | |
| End of therapy: female (N=45) | 0.63 (-1.10, 5.09) | 0.48 (-0.83, 3.17) |
Overweight, ≥85th percentile; Obese, ≥95th percentile;
N=183 unless otherwise noted.
Table III.
Mixed-effects Model of Associations with BMI from the Start of Maintenance Therapy Until the End of Therapy
| Variable | Coefficient | 95% Confidence Interval | p-value |
|---|---|---|---|
| Intercept | 0.05 | (-0.53, 0.64) | 0.85 |
| BMI Z-score at Diagnosis | 0.61 | (0.52, 0.70) | <0.001 |
| Days Since Start of Maintenance (100 days) | 0.08 | (0.05, 0.11) | <0.001 |
| Days2* | -0.01 | (-0.01, -0.003) | <0.001 |
| Male Gender | 0.13 | (-0.09, 0.35) | 0.26 |
| High-Risk + No Radiation | -0.52 | (-0.76, -0.27) | < 0.001 |
| High-Risk + Radiation | -0.90 | (-1.29, -0.52) | < 0.001 |
| Received Supplemental Feeds** | -0.18 | (-0.42, 0.07) | 0.15 |
| Cumulative Composite | 0.02 | (-0.002, 0.03) | 0.08 |
| Corticosteroid Dose*** (per 1000mg/m2) |
Because individual profiles of BMI z-scores often showed a convex shape, we included a time-squared term Days2 to account for this trend;
Supplemental feeds, needing total parental nutrition, nasogastric, or gastrostomy tube feedings during therapy; Cumulative corticosteroid dose, cumulative steroid dosing prior to maintenance therapy; N=173.
Elevated BMI z-score throughout maintenance was associated with elevated BMI z-score at diagnosis (Coefficient = 0.61; p<0.001) (Table III). In addition, BMI z-score increased over the first 22 months of maintenance and then slightly decreased (p<0.001). Patients treated for high risk ALL had significantly lower BMI z-scores compared to those treated for standard risk ALL, regardless of radiation therapy. Gender, supplemental feeds, and corticosteroid dose were not associated with BMI z-score. Average trends of BMI z-score during maintenance therapy are shown in Figure 2 for risk-radiation groups.
Figure 2.

Estimated trend of BMI z-score in the 3 risk/radiation groups (treated on standard risk protocol / treated on high risk protocol, no radiation / treated on high risk protocol and cranial radiation) based on the mixed-effects model. Other covariates are fixed at male gender, no supplemental feeds, BMI z-score at start of induction of 0.67, and cumulative pre-maintenance steroid dose of 2921 mg/m2.
Average parental BMI at study entry was significantly associated with the patients’ BMI z-score at start of induction (P=0.02). Patient age, gender and race and family history risk score were not significantly associated with BMI z-score at diagnosis.
Blood Pressure Analysis
Systolic blood pressure was elevated at the start of induction and consolidation, with median z-scores of 1.6, and 1.7, respectively then decreased over therapy, but remained elevated above the 50th percentile with the median z-score between 0.6-0.9. The median systolic blood pressure remained stable though the end of therapy in all patients and through one year off therapy in females (Table II).
Diastolic pressures were elevated to a lesser degree, with median z-scores at induction and consolidation of 1.0 and 1.4, decreased during therapy, but remained elevated above the 50th percentile and ranged from 0.4-0.8. The median diastolic z-scores were also stable though the end of therapy in males and females and decreased in females during their first year off therapy (0.1-0.4) (Table II).
With a median of 13 measurements (25%,75% quartiles, 7-15) spaced at least one month apart, 57 patients (31.1%) met systolic criteria, and 34 (18.6%) met diastolic criteria for pre-hypertension; 27 (14.8%) met both systolic and diastolic criteria. These data did not change substantially when patients under 36 months were excluded from the analysis: 52 (28.9%) with systolic pre-hypertension, 26 (14.4%) with diastolic pre-hypertension and 22 (12.2%) with both systolic and diastolic pre-hypertension.
Seventy-six (41.5%) patients met criteria for systolic hypertension and 44 (24.0%) for diastolic hypertension; 36 (19.7%) met criteria for both. Again this did not change substantially when patients under 36 months of age were excluded: 66 (36.7%) still met criteria for systolic hypertension and 36 (20.0%) met criteria for diastolic hypertension; 27 (15%) for met criteria for both.
Age was inversely associated with systolic and diastolic blood pressure z-scores throughout maintenance therapy (p < 0.001). BMI z-score at diagnosis, gender, and hypertension risk group were not significantly associated with either systolic or diastolic z-score. We observed a downward trend in diastolic blood pressure throughout maintenance therapy (p = 0.03), but not in systolic blood pressure (p= 0.77); however, nonlinear trends were not significant in either model. Excluding patients who were less than 36 months at start of induction did not change the results.
DISCUSSION
Obesity, hypertension and other features of the metabolic syndrome have been reported in excess among survivors of pediatric ALL and risk factors include exposure to cranial radiation and corticosteroids (2-8, 10). This study was designed to evaluate the trajectory of BMI and blood pressure and to identify potential host and treatment-related risk factors during ALL therapy.
We demonstrated that BMI increases during the first 22 months of maintenance therapy, which is consistent with findings recently published from the Children’s Oncology Group (8). Furthermore, elevated parental BMI at study entry was associated with elevated participant BMI at induction, and elevated BMI z-score at induction was significantly associated with increased BMI z-score during maintenance. These data are consistent with other studies that have shown an elevated BMI at ALL diagnosis to be associated with long-term obesity (2, 7). Of note, the baseline BMI z-score was higher in our cohort than in other studies (3, 7), consistent with regional secular trends; 43% Tennessee school age children are overweight or obese vs. 36.1% in our cohort(15). Given the data regarding increased obesity in childhood ALL survivors (3), longitudinal follow-up is required to determine whether BMI plateaus or further continues to increase in the longer-term survivorship period.
Cumulative pre-maintenance corticosteroid dose was not associated with increases in BMI during maintenance therapy. However, there was a high degree of correlation between ALL risk group, age and corticosteroid dose. Monthly 5-day pulses of corticosteroids during maintenance therapy may well contribute to the increase in BMI over the course of maintenance; however, as all patients received equivalent doses of steroids during maintenance; this effect could not be evaluated in our model. Chow et al. have demonstrated an association between cumulative steroid dose and elevated BMI in long-term survivors (3).
In our cohort, treatment on high risk protocols, was associated with lower BMI z-score during maintenance therapy, which may reflect the more intensive treatment prior to maintenance. This may also reflect an age effect as most of the patients treated on high risk protocols were ≥ 10 years of age and previous studies have shown that younger age at diagnosis is associated with increased BMI during therapy (7, 10, 16).
Contrary to what has been reported in other studies (6, 10, 17, 18), we found that cranial radiotherapy exposure was independently associated with a decreased BMI z-score even when adjusting for treatment on a high risk protocol. This paradox may be explained by the fact that only 23 patients received cranial radiotherapy in the current cohort, with a mean dose of 17.4 Gy. Only two patients received doses in excess of 18 Gy. The largest analyses of BMI in ALL survivors are those of the Childhood Cancer Survivor Study (CCSS), (6, 10). In the initial analysis from the CCSS survivors treated with >20 Gy cranial radiotherapy were at significantly increased risk of elevated BMI as compared to matched siblings, but this relationship was not noted in survivors who received radiotherapy doses of 10-18 Gy (6). In a subsequent analysis with an additional ten years of follow-up, the increased risk for elevated BMI in survivors compared with siblings was noted to extend to those survivors also treated with cranial radiotherapy doses than 20 Gy (6, 10). Further follow-up of our cohort will be required in order to assess whether elevated BMI will be subsequently associated with the cranial radiotherapy received. In addition to the cranial radiotherapy dose, patients receiving radiation therapy in our cohort was highly correlated with treatment on a high risk protocol associated with increased mucositis. In fact, 56.5% of the patients who received cranial radiotherapy also received supplemental feeds, reflecting difficulty with weight gain in part due to mucositis. Female survivors of ALL have been shown to be at increased risk for obesity, compared with males (3, 6-8, 10), although this is not consistent across studies (2, 16). We did not identify an association between gender and BMI z-score during maintenance therapy, which may suggest that these gender-related differences become evident in the long-term survivorship period related to non-therapeutic risk factors.
Few studies have evaluated the association of parental BMI or family history of cardiovascular abnormalities with elevated BMI in ALL patients. In a recent study by Gofman and Ducore, there was no association between the patient’s BMI and family history of diabetes, thyroid disease, or hypertension (19). We found an association between parental BMI and participant BMI at baseline, but not with BMI over the course of maintenance Furthermore, family history of cardiovascular disease was not associated with BMI in our cohort. Two previous studies reported maternal BMI, but not paternal BMI, to be associated with obesity in ALL survivors (2, 20). Biologic correlates of BMI will be helpful in better defining the association of parental and patient BMI. This is supported by work from the CCSS, which reported inherited polymorphisms of the leptin receptor gene to be associated with increased risk of obesity in long-term survivors (21).
Obesity is an increasing problem in otherwise healthy children and adolescents in the US. We therefore examined the trajectory of BMI change in our cohort in context of that expected in the general population. Ogden et al. have reported that the prevalence of overweight and obese children has not significantly changed since 2000 (with the exception of increased obesity in boys ages 6-19 years) (22). Furthermore, this study reported that 32.1% of boys and 31.3% of girls were overweight or obese (22), which is much lower than the 50.8% of males and 46.8% of females in our cohort being overweight or obese at the end of therapy. Furthermore, the 2006 Tennessee Coordinated School Health Annual Report reported that between 2004-2006, 35% of Kindergarteners, 47% of 6th graders, 43% of high school students, and 43% of students overall were overweight or obese, again percentages lower than that seen in our cohort at the end of therapy (15). These data suggest ALL or its treatment does indeed contribute to the prevalence in excess of that expected from secular trends in the population. Regardless of the etiology, being overweight or obese increases risk for long-term health outcomes and thus needs to be addressed (23).
Systolic, and to a lesser extent, diastolic hypertension and pre-hypertension were noted in our cohort, with younger age at diagnosis associated with elevation in systolic and diastolic blood pressures. A higher prevalence of hypertension as opposed to pre-hypertension was noted in our cohort. This may reflect the large number of measurements in the data set (median = 13) and thus, a higher likelihood to identify three measurements above the 95th percentile rather than an average BP of greater than the 90th percentile. Our findings are consistent with the limited studies examining blood pressure changes in ALL patients or long-term survivors. (3, 5, 11, 12, 18).
This study had a few limitations. ALL treatment risk classification, age at diagnosis, steroid dose and cranial radiotherapy treatment were highly correlated with one another, making it difficult to separate the relative contributions of each on BMI. In addition, since all patients in the cohort had not completed therapy at the time of data censoring, it will be important to continue to follow this cohort to see if obesity and hypertension persist into the off-therapy period. As this was a retrospective study, the measurements of BMI and blood pressure were based on what our clinic nurses/care partners measured at the time of a clinic and were not able to be standardized. Prospectively designed studies with standardized measurements, including blood pressures taken outside of the clinic setting may be informative.
The current study has demonstrated that elevated BMI and blood pressure are highly prevalent during ALL therapy and furthermore, BMI increases the first 22 months of the two to three year period of maintenance therapy, suggesting that preventive interventions should not wait until the long-term survivorship period to consider therapeutic or behavioral preventive interventions. One potential consideration is the reduction of steroid pulses during maintenance. However, such a strategy could be associated with inferior disease-free survival and would require testing in large populations of children, which could be accomplished in the well-established clinical trials cooperative groups and consortiums.
Another approach, which is unlikely to impact disease-free survival, is the development of a behavioral intervention during maintenance, such as a combined diet and exercise program targeted towards the entire family. The period of maintenance therapy is ideal for such an intervention as it does not include intense myelosuppressive treatment and patients commonly return to normal daily routines. Such interventions may have benefits in established during therapy may promote healthy patterns in the long-term survivorship period for the child and their family and decrease the risk of long-term cardiovascular disease associated with obesity and hypertension. Diet and exercise interventions often do not produce lasting results, in part due to the difficulty getting patients to return to the clinic for either the program or for long-term reassessment (24). Patients with ALL provide an important group to study because patients reliably come to clinic regularly during therapy and are followed long-term. Furthermore, incorporating family members, which increases effectiveness of interventions (24) is also feasible in ALL population where parents and siblings are so intimately involved in the child’s care.
In conclusion, the medical advances that have resulted in high survival rates for ALL should not be overshadowed by risk for obesity and hypertension. Rather, behavioral and therapeutic interventions should be considered to prevent or mitigate risk.
Table IV.
Mixed-effects Model of Associations with Blood Pressure Over the Course of Therapy
| Variable | Systolic blood pressure | Diastolic blood pressure | ||
|---|---|---|---|---|
| Coefficient (95% CI)* | p-value | Coefficient (95% CI)* | p-value | |
| Intercept | 1.30 (1.02, 1.58) | < 0.001 | 1.18 (0.97, 1.39) | < 0.001 |
| BMI Z-score at | 0.05 (-0.05, 0.15) | 0.32 | -0.05 (-0.13, 0.02) | 0.17 |
| Diagnosis | ||||
| Days since start of maintenance** | -0.01 (-0.08, 0.06) | 0.77 | -0.05 (-0.10, -0.01) | 0.03 |
| Days2*** | -0.00048 (-0.0085, 0.0076) | 0.91 | 0.0015 (-0.0037, 0.0066) | 0.58 |
| Age | -0.07 (-0.10, -0.05) | < 0.001 | -0.07 (-0.09, -0.05) | < 0.001 |
| Male gender | 0.11 (-0.13, 0.34) | 0.39 | 0.10 (-0.08, 0.28) | 0.29 |
| Hypertension group 2**** | 0.02 (-0.34, 0.38) | 0.92 | 0.20 (-0.06, 0.47) | 0.14 |
| Hypertension group 3**** | 0.40 (-0.26, 1.05) | 0.24 | 0.33 (-0.17, 0.82) | 0.20 |
N=173; Measures taken during induction and consolidation are excluded from model as patients were on continuous steroids when measurements were taken;
CI, 95% confidence interval.
Coefficient for days corresponds to increment of 100 days;
Because individual profiles of BMI z-scores often showed a convex shape, we included a time-squared term Days2 to account for this trend;
Hypertension (HTN) risk group 1(reference value), never received blood pressure medications; HTN risk group 2, received blood pressure medicines only for steroids induced hypertension; HTN risk group 3, received blood pressure medicines for more than just steroid induced hypertension.
Acknowledgments
Supported in part by Vanderbilt CTSA grant 1 UL1RR024975 from NCRR/NIH
References
- 1.Horner MJ, R L, Krapcho M, et al. Seer Cancer Statistics Review, 1975-2006. Bethesda, MD: National Cancer Institute; 2009. http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Asner S, Ammann RA, Ozsahin H, et al. Obesity in long-term survivors of childhood acute lymphoblastic leukemia. Pediatric blood & cancer. 2008;51:118–122. doi: 10.1002/pbc.21496. [DOI] [PubMed] [Google Scholar]
- 3.Chow EJ, Pihoker C, Hunt K, et al. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 2007;110:2313–2320. doi: 10.1002/cncr.23050. [DOI] [PubMed] [Google Scholar]
- 4.Didi M, Didcock E, Davies HA, et al. High incidence of obesity in young adults after treatment of acute lymphoblastic leukemia in childhood. The Journal of pediatrics. 1995;127:63–67. doi: 10.1016/s0022-3476(95)70258-x. [DOI] [PubMed] [Google Scholar]
- 5.Kourti M, Tragiannidis A, Makedou A, et al. Metabolic syndrome in children and adolescents with acute lymphoblastic leukemia after the completion of chemotherapy. J Pediatr Hematol Oncol. 2005;27:499–501. doi: 10.1097/01.mph.0000181428.63552.e9. [DOI] [PubMed] [Google Scholar]
- 6.Oeffinger KC, Mertens AC, Sklar CA, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: A report from the childhood cancer survivor study. J Clin Oncol. 2003;21:1359–1365. doi: 10.1200/JCO.2003.06.131. [DOI] [PubMed] [Google Scholar]
- 7.Razzouk BI, Rose SR, Hongeng S, et al. Obesity in survivors of childhood acute lymphoblastic leukemia and lymphoma. J Clin Oncol. 2007;25:1183–1189. doi: 10.1200/JCO.2006.07.8709. [DOI] [PubMed] [Google Scholar]
- 8.Withycombe JS, Post-White JE, Meza JL, et al. Weight patterns in children with higher risk all: A report from the children’s oncology group (cog) for ccg 1961. Pediatric blood & cancer. 2009;53:1249–1254. doi: 10.1002/pbc.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 10.Garmey EG, Liu Q, Sklar CA, et al. Longitudinal changes in obesity and body mass index among adult survivors of childhood acute lymphoblastic leukemia: A report from the childhood cancer survivor study. J Clin Oncol. 2008;26:4639–4645. doi: 10.1200/JCO.2008.16.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talvensaari KK, Lanning M, Tapanainen P, et al. Long-term survivors of childhood cancer have an increased risk of manifesting the metabolic syndrome. The Journal of clinical endocrinology and metabolism. 1996;81:3051–3055. doi: 10.1210/jcem.81.8.8768873. [DOI] [PubMed] [Google Scholar]
- 12.van Waas M, Neggers SJ, Pieters R, et al. Components of the metabolic syndrome in 500 adult long-term survivors of childhood cancer. Ann Oncol. 2009 doi: 10.1093/annonc/mdp414. [DOI] [PubMed] [Google Scholar]
- 13.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for disease control and prevention 2000 growth charts for the united states: Improvements to the 1977 national center for health statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 14.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 15.Dunn M. Tennessee coordinated school health report. 2006:54. http://Tennessee.Gov/education/schoolhealth/doc/2006finalreport.pdf.
- 16.Armstrong GT, Sklar CA, Hudson MM, et al. Long-term health status among survivors of childhood cancer: Does sex matter? J Clin Oncol. 2007;25:4477–4489. doi: 10.1200/JCO.2007.11.2003. [DOI] [PubMed] [Google Scholar]
- 17.Sklar CA, Mertens AC, Walter A, et al. Changes in body mass index and prevalence of overweight in survivors of childhood acute lymphoblastic leukemia: Role of cranial irradiation. Medical and pediatric oncology. 2000;35:91–95. doi: 10.1002/1096-911x(200008)35:2<91::aid-mpo1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 18.Trimis G, Moschovi M, Papassotiriou I, et al. Early indicators of dysmetabolic syndrome in young survivors of acute lymphoblastic leukemia in childhood as a target for preventing disease. J Pediatr Hematol Oncol. 2007;29:309–314. doi: 10.1097/MPH.0b013e318059c249. [DOI] [PubMed] [Google Scholar]
- 19.Gofman I, Ducore J. Risk factors for the development of obesity in children surviving all and nhl. J Pediatr Hematol Oncol. 2009;31:101–107. doi: 10.1097/MPH.0b013e31818c0120. [DOI] [PubMed] [Google Scholar]
- 20.Shaw MP, Bath LE, Duff J, et al. Obesity in leukemia survivors: The familial contribution. Pediatric hematology and oncology. 2000;17:231–237. doi: 10.1080/088800100276406. [DOI] [PubMed] [Google Scholar]
- 21.Ross JA, Oeffinger KC, Davies SM, et al. Genetic variation in the leptin receptor gene and obesity in survivors of childhood acute lymphoblastic leukemia: A report from the childhood cancer survivor study. J Clin Oncol. 2004;22:3558–3562. doi: 10.1200/JCO.2004.11.152. [DOI] [PubMed] [Google Scholar]
- 22.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of high body mass index in us children and adolescents, 2007-2008. Jama. 303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 23.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 24.August GP, Caprio S, Fennoy I, et al. Prevention and treatment of pediatric obesity: An endocrine society clinical practice guideline based on expert opinion. The Journal of clinical endocrinology and metabolism. 2008;93:4576–4599. doi: 10.1210/jc.2007-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]


