Abstract
To examine mirror neuron abnormalities in autism, high-functioning children with autism and matched controls underwent fMRI while imitating and observing emotional expressions. Although both groups performed the tasks equally well, children with autism showed no mirror neuron activity in the inferior frontal gyrus (pars opercularis). Notably, activity in this area was inversely related to symptom severity in the social domain, suggesting that a dysfunctional ‘mirror neuron system’ may underlie the social deficits observed in autism.
It has recently been proposed that dysfunction of the mirror neuron system (MNS) early in development could give rise to the cascade of impairments that are characteristic of autism spectrum disorders (ASD)1, including deficits in imitation, theory of mind and social communication. First discovered in the ventral premotor cortex (area F5) of the macaque, mirror neurons fire both while a monkey performs goal-directed actions and while it observes the same actions performed by others. This observation-execution matching system is thought to provide a neural mechanism by which others’ actions and intentions can be automatically understood2. The existence of an analogous MNS in humans has been demonstrated by a number of independent investigations2: MNS activity in the human homolog of area F5—the pars opercularis in the inferior frontal gyrus—has been consistently reported during imitation3, action observation4 and intention understanding5. Relevant to an MNS theory of autism is further evidence suggesting that the MNS, in concert with activity in limbic centers, may mediate our understanding of the emotional states of others6,7. Using functional magnetic resonance imaging (fMRI), we have previously described a neural network in which the insula acts as an interface between the frontal component of the MNS and the limbic system, thus enabling the translation of an observed or imitated facial emotional expression into its internally felt emotional significance6. Three recent studies using different electrophysiological techniques have reported preliminary evidence for abnormal MNS functioning during action imitation8 and observation9,10 in adults with ASD. However, a more definitive test of an MNS theory of autism would involve examining MNS activity in the context of a socio-emotional task and in a sample of children.
Here we used an event-related fMRI design to investigate neural activity during the imitation and observation of facial emotional expressions, in ten high-functioning children with ASD (9 males; 12.05 ± 2.50 years of age) and ten typically developing children (9 males; 12.38 ± 2.22 years of age) matched by age and IQ (Supplementary Table 1 online). Subjects and their parents provided written consent according to guidelines specified by the Institutional Review Board at the University of California, Los Angeles. Stimuli consisted of 80 faces expressing five different emotions: anger, fear, happiness, neutrality or sadness. Each face was presented for 2 s according to an optimized random sequence which included null events (that is, blank screens with fixation crosses at eye level) and temporal jittering to increase statistical efficiency. In two separate scans (with the order counterbalanced within each group), subjects either imitated or simply observed the faces presented via high-resolution, magnet-compatible goggles. For each subject, we acquired two sets of 96 whole-volume images using a 3.0-Tesla head-only scanner (Siemens). The images were realigned, spatially normalized and smoothed using Automated Image Registration. Random-effects analyses were implemented in SPM99. All children practiced the tasks outside the scanner, thus demonstrating that they were willing and able to comply with the task requirements. Subsequently, half the children in each group also performed both tasks during a videotaped session with an eye tracker. Analyses of these behavioral data showed no group differences in the amount of time spent fixating on the face and eye region, nor in how well the children imitated facial expressions (Supplementary Table 2 and Supplementary Methods online).
During the imitation of emotional expressions (versus null events), the typically developing children activated a neural network very similar to that previously observed in adults6 (Fig. 1a and Supplementary Table 2): there was extensive bilateral activation of striate and extra striate cortices, primary motor and premotor regions, limbic structures (amygdala, insula and ventral striatum) and the cerebellum. Notably, this group also showed strong bilateral activity within the pars opercularis of the inferior frontal gyrus (Brodmann’s area 44)—the site with previously identified mirror properties—as well as in the neighboring pars triangularis (Brodmann’s area 45), with strongest peaks in the right hemisphere. In the ASD group, we also observed robust activation in visual cortices (including the fusiform gyrus), premotor and motor regions of the face and the amygdala (Fig. 1b and Supplementary Table 3 online). This indicated that these children indeed attended to the stimuli and imitated the facial expressions. Unlike the typically developing children, however, the ASD group showed no activity in the mirror area in the pars opercularis (even when results were examined at the most liberal thresholds). Direct comparisons between the typically developing children and those with ASD confirmed that activity in the anterior component of the MNS was reliably greater in typically developing children (Fig. 1c). Consistent with the neural model previously proposed6, whereby the frontal component of the MNS modulates limbic system activity via the insula, typically developing children also showed reliably greater activity in insular and periamygdaloid regions as well as in the ventral striatum and thalamus (Supplementary Table 3). In contrast, children with ASD showed greater activity in left anterior parietal and right visual association areas (Supplementary Fig. 1 online).
Figure 1. Reliable activity during imitation of emotional expressions.
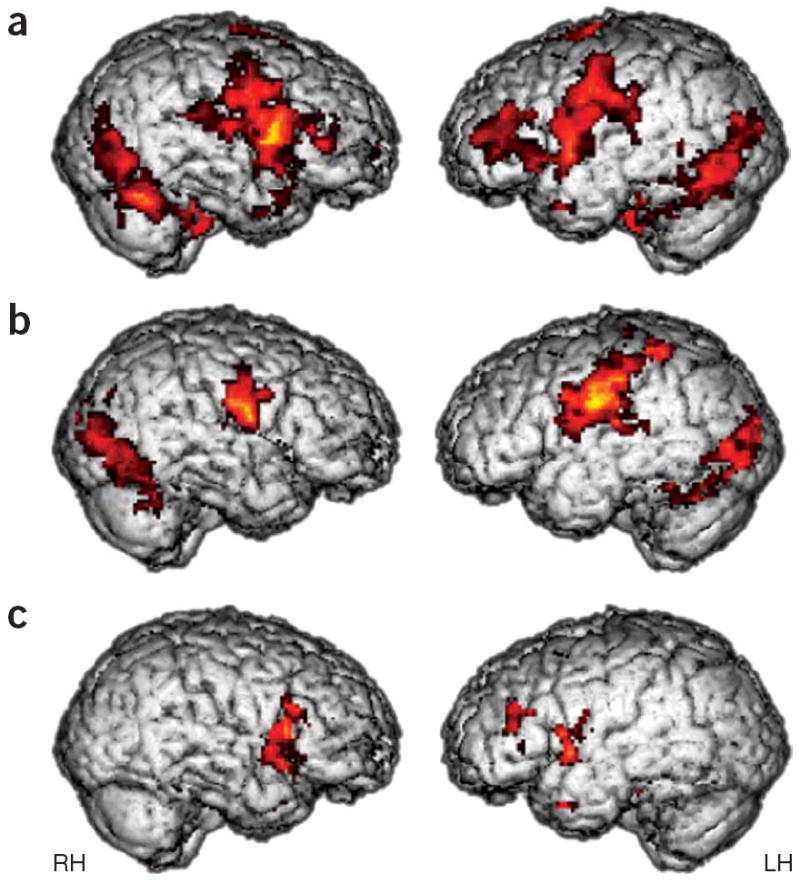
(a,b) Activity in bilateral pars opercularis (stronger in the right) of the inferior frontal gyrus is seen in the typically developing group (a) but not in the ASD group (b). A between-group comparison (c) revealed that this difference was significant (t > 1.83, P < 0.05, corrected for multiple comparisons at the cluster level). RH, right hemisphere; LH, left hemisphere.
Individuals with autism typically show deficits in understanding the emotional states of others; thus dysfunction in the MNS should be manifest not only when these individuals explicitly imitate emotional expressions but also when they merely observe emotions displayed by others. As predicted, activity in the right pars opercularis during the observation of facial expressions was reliably stronger in the typically developing group than in the ASD group (Fig. 2). Notably, this difference could not be attributed to a failure of the children with ASD to attend to the face stimuli, as both groups showed reliable activation in regions implicated in face processing, including the fusiform gyrus and the amygdala (Supplementary Fig. 2 and Supplementary Table 4 online). Moreover, we observed no group differences in these areas even when the data were explored at the most liberal thresholds.
Figure 2.
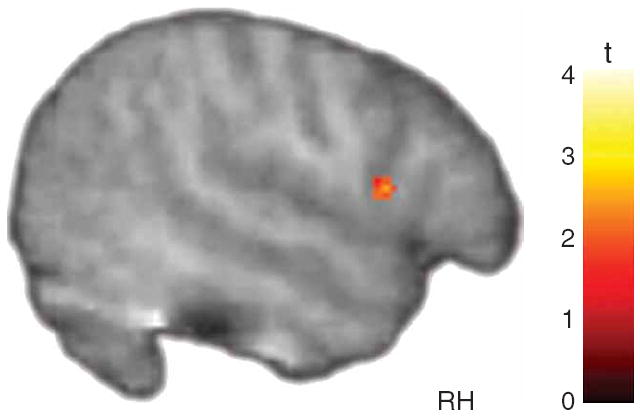
Mirror neuron system activity during observation of emotional expressions. The right pars opercularis showed significantly greater activity in typically developing children than in children with ASD (t > 1.83, P < 0.05, small volume corrected).
To further test the hypothesis that a dysfunctional MNS may underlie the social deficits characteristic of ASD, we examined the relationship between activity in regions with mirror neuron properties and symptom severity, as indexed by children’s scores on the Autism Diagnostic Observation Schedule–Generic (ADOS-G)11 and the Autism Diagnostic Observation Interview–Revised (ADI-R)12. Controlling for IQ, we found reliable negative correlations between activity in the pars opercularis and the children’s scores on the social subscales of the ADOS-G and ADI-R (Fig. 3): the greater the activity in this critical component of the MNS during imitation, the higher a child’s level of functioning in the social domain. Activity in other components of the normative network underlying emotion understanding via action representation (that is, the insula and limbic structures) was also negatively correlated with symptom severity (Supplementary Table 5 online).
Figure 3. Mirror neuron system activity and symptom severity.
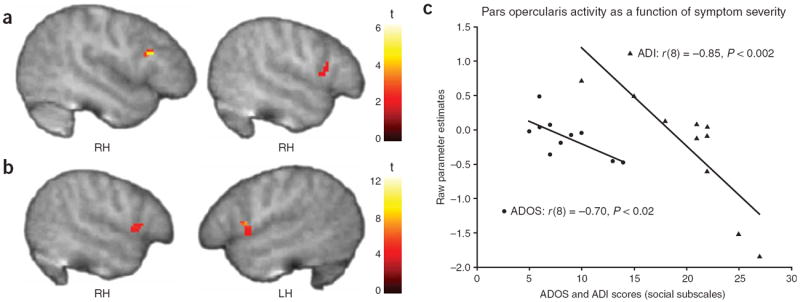
(a–c) Negative correlations were found in the ASD group between activity in the pars opercularis of the inferior frontal gyrus and scores on the social subscale of both ADOS-G (a,c) and ADI-R (b,c). t > 1.83, P < 0.05, corrected for multiple comparisons at the cluster level.
To our knowledge, this is the first developmental fMRI study to show reliable differences between typically developing children and children with ASD in the network comprising the frontal component of the MNS (namely, pars opercularis), the insula and the amygdala—that is, in the system thought to enable emotion understanding via action representation6. Although we were unable to monitor gaze fixation during scanning, a variable shown to affect brain activity in ASD13, it is extremely unlikely that our findings reflect between-group differences in the amount of time spent looking at the eye region. First, during neither the imitation or observation of facial expressions did we find group differences in the fusiform gyrus, a region that is differentially activated in individuals with ASD as a function of gaze fixation13. This suggests that—in keeping with other reports14,15—the inclusion of fixation crosses in the null events was successful at directing the subjects’ attention to this key region of the face, a conclusion supported by the fact that no group differences were observed in eye fixation time between the ASD and typically developing children who participated in the eye-tracking session outside the scanner. Second, activity in the fusiform gyrus did not correlate with activity in MNS areas in either condition. Third, in the children with ASD for whom eye-tracking data were available, there was no indication of a positive relationship between MNS activity in the pars opercularis and time spent fixating the eyes (see Supplementary Methods).
Similarly, despite the fact that we were unable to monitor task performance during scanning, and although imitation deficits in autism are well documented1, we believe it implausible that our findings merely reflect the children with ASD not performing the imitation task, or not performing it well. First, all the children with ASD were willing and able to perform the task just before scanning. Second, children with ASD who performed the task outside the scanner did so just as well as the typically developing children did, as rated by independent observers blind to diagnosis but familiar with autism symptomatology (see Supplementary Methods). Third, in the ASD group, we observed robust activity in primary motor and premotor areas of the face during the imitation task, with no evidence of between-group differences in these regions even at the most liberal statistical thresholds. Lastly, the children with ASD actually showed greater activity than did the typically developing children in right visual and left anterior parietal areas, regions shown to be modulated by visual and motor attention, respectively.
The evidence thus suggests that although both groups performed the imitation task as requested, the neural strategies adopted by typically developing children and those with ASD are quite different. Typically developing children can rely upon a right hemisphere–mirroring neural mechanism—interfacing with the limbic system via the insula—whereby the meaning of the imitated (or observed) emotion is directly felt and hence understood. In contrast, this mirroring mechanism is seemingly not engaged in children with ASD, who must then adopt an alternative strategy of increased visual and motor attention whereby the internally felt emotional significance of the imitated facial expression is probably not experienced. In line with previous findings in normal adults6,7, the fact that typically developing children showed increased MNS activity even when simply observing an emotional expression further indicates that this mirroring mechanism may underlie the remarkable ability to read others’ emotional states from a mere glance at their faces. The lack of MNS activity during both the imitation and the observation of emotional expressions in our sample of children with ASD provides strong support for the hypothesis that early dysfunction in the mirror neuron system may be at the core of the social deficits observed in autism.
Supplementary Material
Acknowledgments
Support for this work was provided by a grant from the National Institute of Child Health and Human Development (P01 HD035470). The authors thank the Brain Mapping Medical Research Organization, the Brain Mapping Support Foundation, the Pierson-Lovelace Foundation, the Ahmanson Foundation, the Tamkin Foundation, the Jennifer Jones-Simon Foundation, the Capital Group Companies Charitable Foundation, the Robson Family, the William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, the Northstar Fund and the National Center for Research Resources (grants RR12169, RR13642 and RR08655).
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
References
- 1.Williams JH, Whiten A, Suddendorf T, Perrett DI. Neurosci Biobehav Rev. 2001;25:287–295. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]
- 2.Rizzolatti G, Craighero L. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 3.Iacoboni M, et al. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- 4.Johnson-Frey SH, et al. Neuron. 2003;39:1053–1058. doi: 10.1016/s0896-6273(03)00524-5. [DOI] [PubMed] [Google Scholar]
- 5.Iacoboni M. In: Perspectives on Imitation: From Neuroscience to Social Science. Hurley S, Chater N, editors. MIT Press; Cambridge, Massachusetts: 2005. pp. 77–99. [Google Scholar]
- 6.Carr L, et al. Proc Natl Acad Sci USA. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leslie KR, Johnson-Frey SH, Grafton ST. Neuroimage. 2004;21:601–607. doi: 10.1016/j.neuroimage.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 8.Nishitani N, Avikainen S, Hari R. Ann Neurol. 2004;55:558–562. doi: 10.1002/ana.20031. [DOI] [PubMed] [Google Scholar]
- 9.Oberman LM, et al. Brain Res Cogn Brain Res. 2005;24:190–198. doi: 10.1016/j.cogbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Theoret H, et al. Curr Biol. 2005;15:R84–R85. doi: 10.1016/j.cub.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 11.Lord C, et al. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 12.Lord C, Rutter M, Le Couteur A. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 13.Dalton KM, et al. Nat Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadjikhani N, et al. Neuroimage. 2004;22:1141–1150. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Pierce K, Haist F, Sedaghat F, Courchesne E. Brain. 2004;127:2703–2716. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


