Abstract
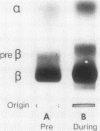
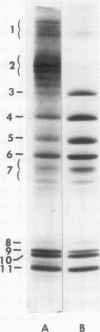
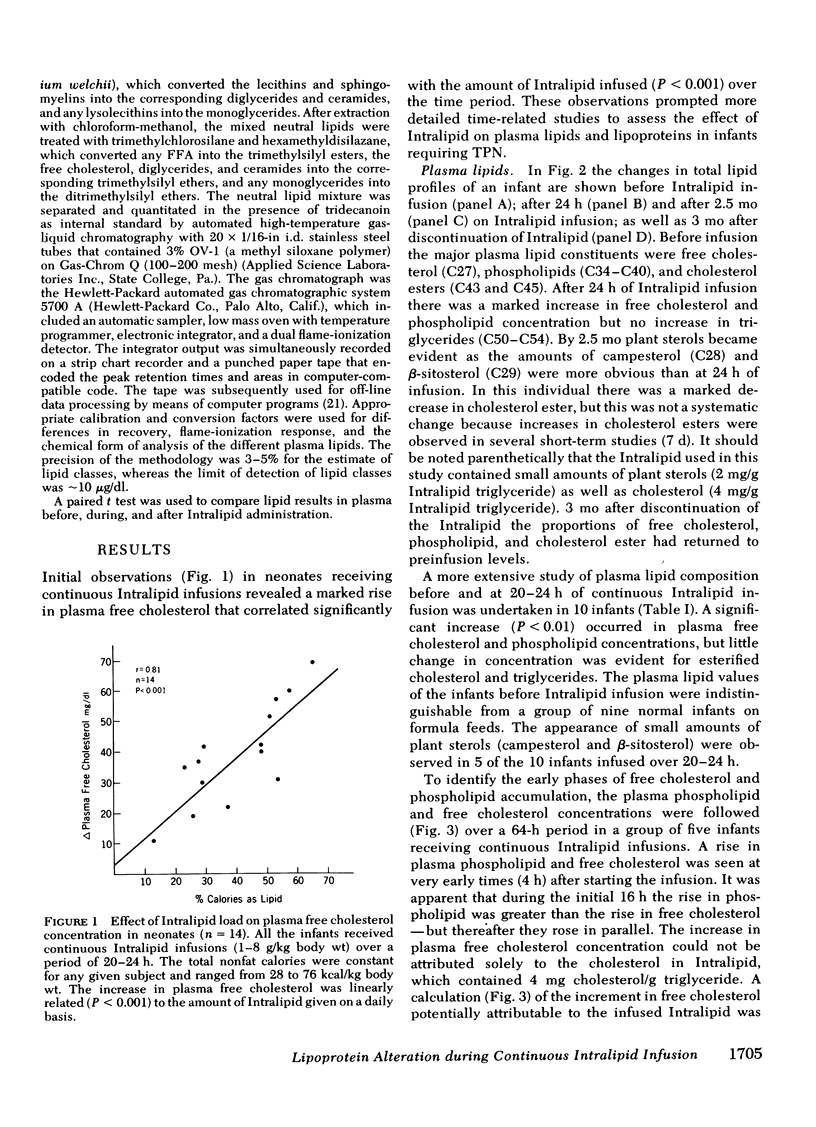
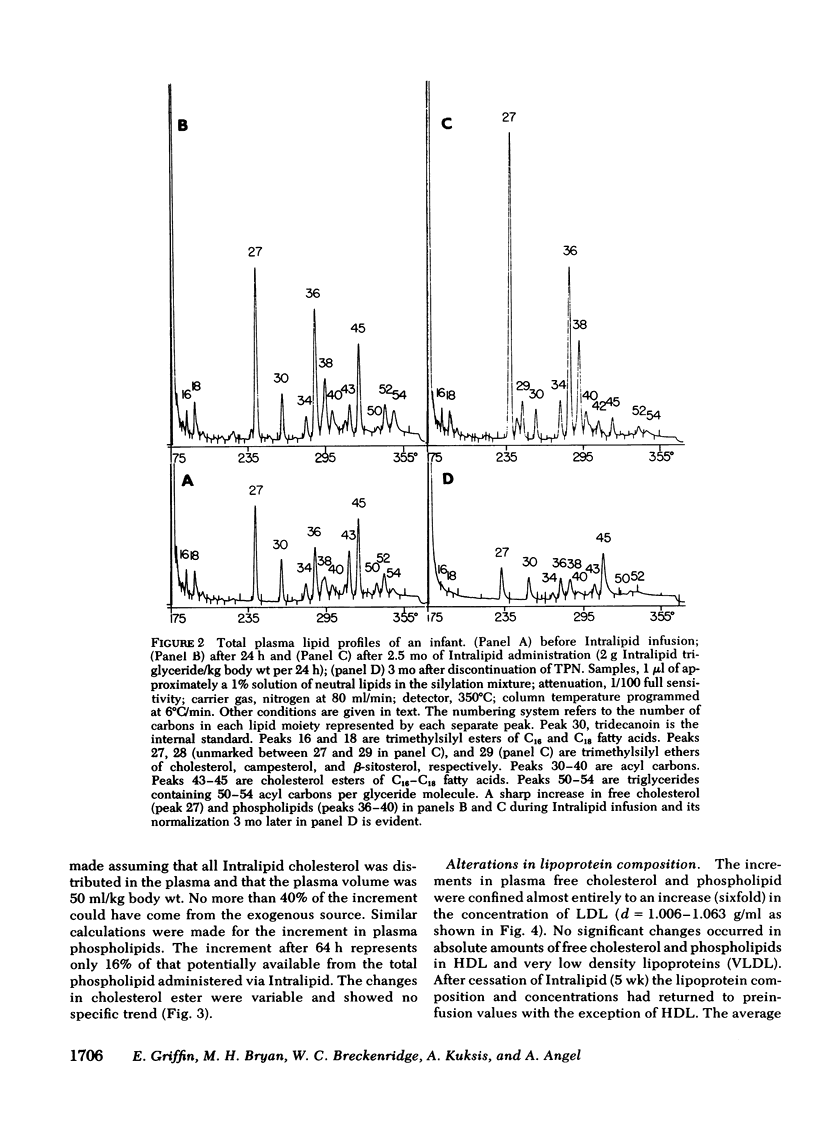
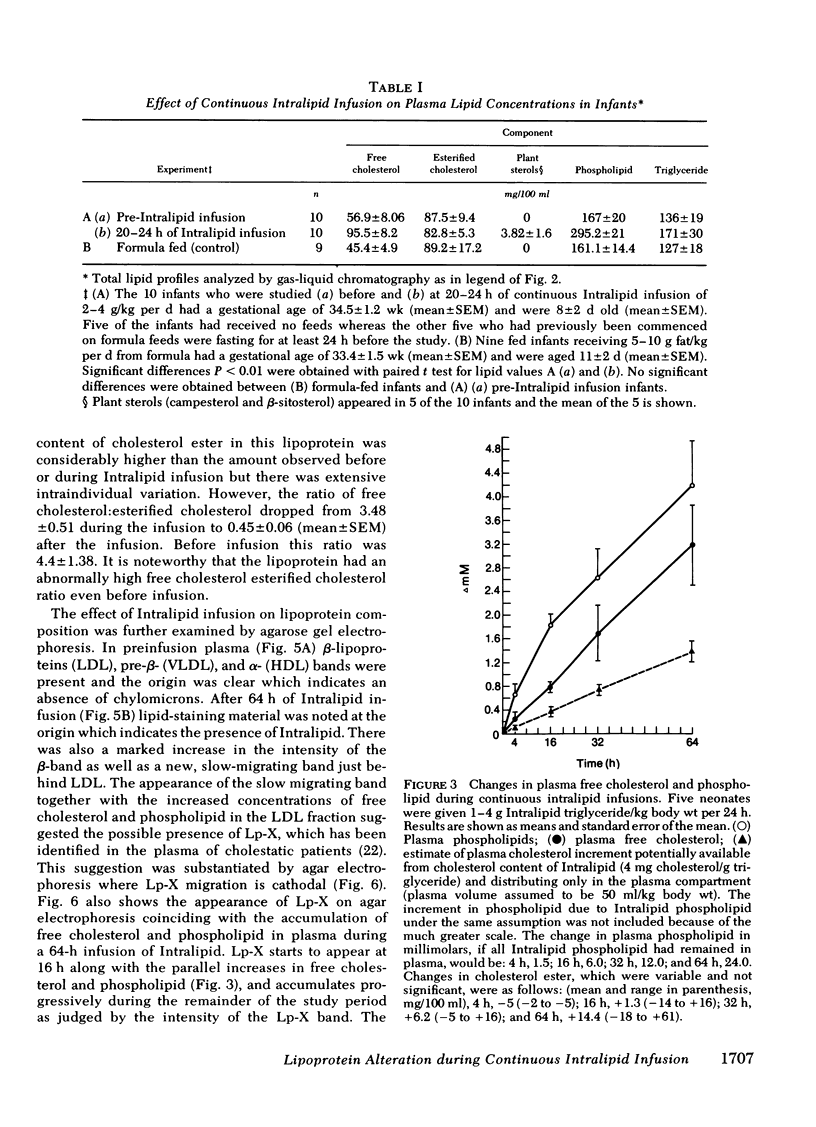
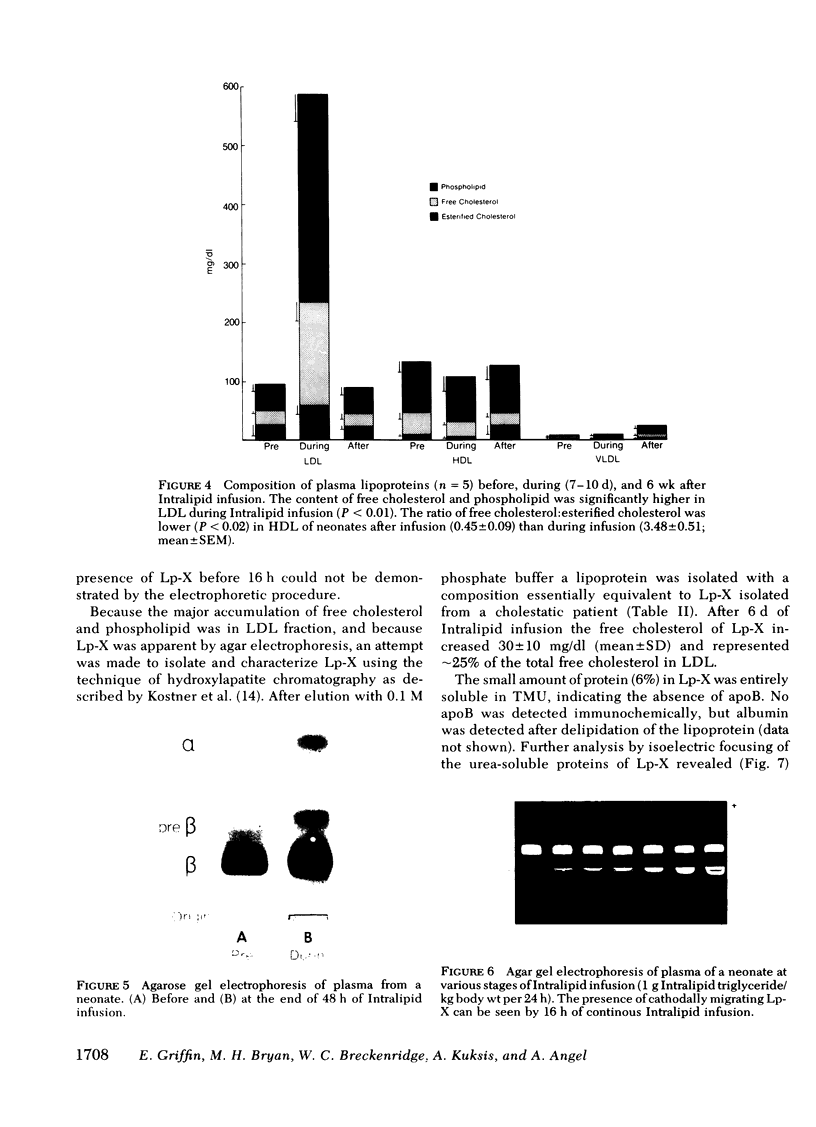
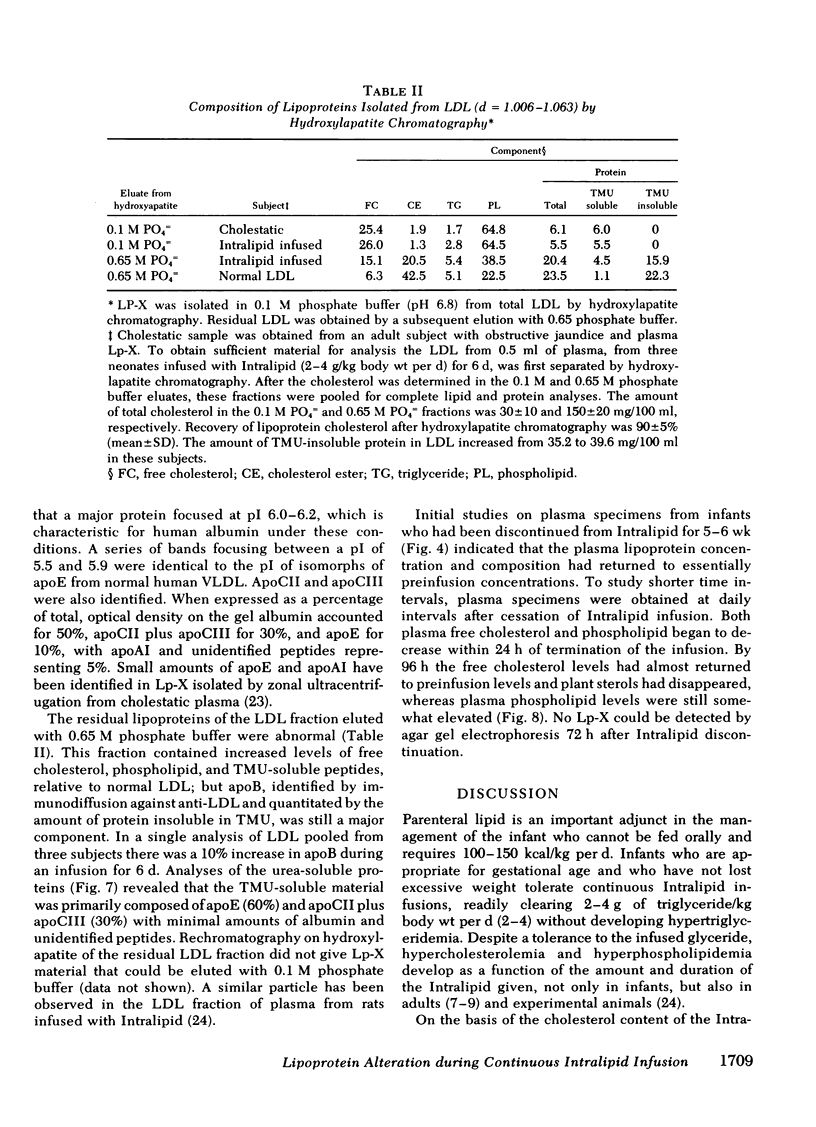
The development of hyperphospholipidemia and hypercholesterolemia was studied in infants that required total parenteral nutrition and given a continuous infusion of Intralipid, (1-4 g/kg body wt per 24 h. Detailed studies were carried out on infusion periods lasting 1-10 d. After 24 h there was a marked increase in plasma free cholesterol (68%) and phospholipid (77%) concentrations. Based on the amount of cholesterol in Intralipid, and the rate of infusion, it was estimated that at least 50% of the plasma cholesterol increment during 64-h infusions was derived from endogenous sources. By contrast, the hyperphospholipidemia could be attributed to the Intralipid as the rise in plasma was calculated to be equivalent to only 16% of the exogenous phospholipid infused. Approximately 10% of the phospholipid in Intralipid was in a triglyceride-free mesophase form with a free cholesterol:phospholipid molar ratio of 0.063. There were no systematic changes in plasma concentrations of cholesterol ester or triglyceride during Intralipid infusions. The increase in free cholesterol and phospholipid was localized in the low density lipoproteins (d = 1.006-1.063 g/ml). The presence of lipoprotein X (Lp-X) in the low density lipoprotein fraction was demonstrated by electrophoresis in agar and by isolation and chemical characterization with hydroxylapatite chromatography. Isoelectric focusing of urea-soluble protein of Lp-X revealed that albumin and apolipoproteins CII and CIII were major components, whereas apolipoprotein E and AI were minor constituents. The abnormal lipoprotein was apparent by 16 h during 64 h of infusion. After 6 d of continuous infusions the free cholesterol in Lp-X was 30±10 mg/dl (mean±SD), which represents a total Lp-X mass of 90 mg/dl. After cessation of the infusion, Lp-X, as monitored by electrophoresis in agar, disappeared within 72-96 h. Thus, during infusion of Intralipid in infants at rates commonly employed, the capacity of the clearance mechanisms for phospholipid are exceeded, which causes the accumulation of phospholipid and free cholesterol in the form of Lp-X particles. It is suggested that mesophase phospholipids in Intralipid may play a significant role in this process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew G., Chan G., Schiff D. Lipid metabolism in the neonate. I. The effects of Intralipid infusion on plasma triglyceride and free fatty acid concentrations in the neonate. J Pediatr. 1976 Feb;88(2):273–278. [PubMed] [Google Scholar]
- BYERS S. O., FRIEDMAN M. Independence of phosphatide induced hypercholesteremia and hepatic function. Proc Soc Exp Biol Med. 1956 Jul;92(3):459–462. doi: 10.3181/00379727-92-22510. [DOI] [PubMed] [Google Scholar]
- Breckenridge W. C., Kakis G., Kuksis A. Identification of lipoprotein X-like particles in rat plasma following Intralipid infusion. Can J Biochem. 1979 Jan;57(1):72–82. doi: 10.1139/o79-010. [DOI] [PubMed] [Google Scholar]
- Cooper R. A., Arner E. C., Wiley J. S., Shattil S. J. Modification of red cell membrane structure by cholesterol-rich lipid dispersions. A model for the primary spur cell defect. J Clin Invest. 1975 Jan;55(1):115–126. doi: 10.1172/JCI107901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards P. A. Effect of plasma lipoproteins and lecithin-cholesterol dispersions on the activity of 3-hydroxy-3-methylglutaryl-coenzyme A reductase of isolated rat hepatocytes. Biochim Biophys Acta. 1975 Oct 21;409(1):39–50. doi: 10.1016/0005-2760(75)90078-8. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN M., BYERS S. O. Production of hypercholesteremia in the rabbit by infusion of phosphatide or neutral fat. Proc Soc Exp Biol Med. 1957 Mar;94(3):452–455. doi: 10.3181/00379727-94-22975. [DOI] [PubMed] [Google Scholar]
- Felker T. E., Hamilton R. L., Havel R. J. Secretion of lipoprotein-X by perfused livers of rats with cholestasis. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3459–3463. doi: 10.1073/pnas.75.7.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget P. P., Fernandes J., Begemann P. H. Utilization of fat emulsion during total parenteral nutrition in children. Acta Paediatr Scand. 1975 May;64(3):377–384. doi: 10.1111/j.1651-2227.1975.tb03851.x. [DOI] [PubMed] [Google Scholar]
- Friedman Z., Danon A., Stahlman M. T., Oates J. A. Rapid onset of essential fatty acid deficiency in the newborn. Pediatrics. 1976 Nov;58(5):640–649. [PubMed] [Google Scholar]
- Greenblatt D. J., Kock-Weser J. Drug therapy. Clinical Pharmacokinetics (first of two parts). N Engl J Med. 1975 Oct 2;293(14):702–705. doi: 10.1056/NEJM197510022931406. [DOI] [PubMed] [Google Scholar]
- Gustafson A., Kjellmer I., Olegård R., Victorin L. Nutrition in low-birth-weight infants. I. Intravenous injection of fat emulsion. Acta Paediatr Scand. 1972 Mar;61(2):149–158. doi: 10.1111/j.1651-2227.1972.tb15919.x. [DOI] [PubMed] [Google Scholar]
- Hatch F. T. Practical methods for plasma lipoprotein analysis. Adv Lipid Res. 1968;6:1–68. [PubMed] [Google Scholar]
- Jakoi L., Quarfordt S. H. The induction of hepatic cholesterol synthesis in the rat by lecithin mesophase infusions. J Biol Chem. 1974 Sep 25;249(18):5840–5844. [PubMed] [Google Scholar]
- Kane J. P. A rapid electrophoretic technique for identification of subunit species of apoproteins in serum lipoproteins. Anal Biochem. 1973 Jun;53(2):350–364. doi: 10.1016/0003-2697(73)90081-x. [DOI] [PubMed] [Google Scholar]
- Kostner G. M., Petek W., Holasek A. Immunochemical measurement of lipoprotein-X. Clin Chem. 1974 Jun;20(6):676–681. [PubMed] [Google Scholar]
- Kuksis A., Breckenridge W. C., Myher J. J., Kakis G. Replacement of endogenous phospholipids in rat plasma lipoproteins during intravenous infusion of an artificial lipid emulsion. Can J Biochem. 1978 Jun;56(6):630–639. doi: 10.1139/o78-095. [DOI] [PubMed] [Google Scholar]
- Kuksis A., Myher J. J., Marai L., Geher K. Determination of plasma lipid profiles by automated gas chromatography and computerized data analysis. J Chromatogr Sci. 1975 Sep;13(9):423–430. doi: 10.1093/chromsci/13.9.423. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maguire G. F., Breckenridge W. C. Agarose gel electrophoresis of plasma lipoproteins using the Durrum cell. Clin Biochem. 1975 Jun;8(3):161–168. doi: 10.1016/s0009-9120(75)91675-6. [DOI] [PubMed] [Google Scholar]
- Manzato E., Fellin R., Baggio G., Walch S., Neubeck W., Seidel D. Formation of lipoprotein-X. Its relationship to bile compounds. J Clin Invest. 1976 May;57(5):1248–1260. doi: 10.1172/JCI108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcel Y. L., Vezina C. A method for the determination of the initial rate of reaction of lecithin: cholesterol acyltransferase in human plasma. Biochim Biophys Acta. 1973 Jun 21;306(3):497–504. doi: 10.1016/0005-2760(73)90188-4. [DOI] [PubMed] [Google Scholar]
- McConathy W. J., Alaupovic P., Curry M. D., Magnani H. N., Torsvik H., Berg K., Gjone E. Identification of lipoprotein families in familial lecithin: cholesterol acyltransferase deficiency. Biochim Biophys Acta. 1973 Dec 20;326(3):406–418. doi: 10.1016/0005-2760(73)90141-0. [DOI] [PubMed] [Google Scholar]
- Pagnan A., Havel R. J., Kane J. P., Kotite L. Characterization of human very low density lipoproteins containing two electrophoretic populations: double pre-beta lipoproteinemia and primary dysbetalipoproteinemia. J Lipid Res. 1977 Sep;18(5):613–622. [PubMed] [Google Scholar]
- Patsch J. R., Aune K. C., Gotto A. M., Jr, Morrisett J. D. Isolation, chemical characterization, and biophysical properties of three different abnormal lipoproteins: LP-X1, LP-X2, and LP-X3. J Biol Chem. 1977 Mar 25;252(6):2113–2120. [PubMed] [Google Scholar]
- Reisner A. H., Nemes P., Bucholtz C. The use of Coomassie Brilliant Blue G250 perchloric acid solution for staining in electrophoresis and isoelectric focusing on polyacrylamide gels. Anal Biochem. 1975 Apr;64(2):509–516. doi: 10.1016/0003-2697(75)90461-3. [DOI] [PubMed] [Google Scholar]
- Ritland S., Gjone E. Quantitative studies of lipoprotein-X in familial lecithin: cholesterol acyltransferase deficiency and during cholesterol esterification. Clin Chim Acta. 1975 Mar 10;59(2):109–119. doi: 10.1016/0009-8981(75)90017-0. [DOI] [PubMed] [Google Scholar]
- Ritland S. Quantitative determination of the abnormal lipoprotein of cholestasis, LP-X, in liver disease. Scand J Gastroenterol. 1975;10(1):5–15. [PubMed] [Google Scholar]
- Rose H. G., Juliano J. Regulation of plasma lecithin:cholesterol acyltransferase in man. I. Increased activity in primary hypertriglyceridemia. J Lab Clin Med. 1976 Jul;88(1):29–43. [PubMed] [Google Scholar]
- Seidel D., Alaupovic P., Furman R. H. A lipoprotein characterizing obstructive jaundice. I. Method for quantitative separation and identification of lipoproteins in jaundiced subjects. J Clin Invest. 1969 Jul;48(7):1211–1223. doi: 10.1172/JCI106085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel D., Wieland H., Ruppert C. Improved techniques for assessment of plasma lipoprotein patterns. I. Precipitation in gels after electrophoresis with polyanionic compounds. Clin Chem. 1973 Jul;19(7):737–739. [PubMed] [Google Scholar]
- Shennan A. T., Bryan M. H., Angel A. The effect of gestational age on intralipid tolerance in newborn infants. J Pediatr. 1977 Jul;91(1):134–137. doi: 10.1016/s0022-3476(77)80465-4. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y. The removal of cholesterol from Landschütz ascites cells by high-density apolipoprotein. Biochim Biophys Acta. 1973 Nov 29;326(2):232–244. doi: 10.1016/0005-2760(73)90249-x. [DOI] [PubMed] [Google Scholar]
- Thompson G. R., Jadhav A., Nava M., Gotto A. M., Jr Effects of intravenous phospholipid on low density lipoprotein turnover in man. Eur J Clin Invest. 1976 Jun 21;6(3):241–248. doi: 10.1111/j.1365-2362.1976.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Thompson G. R., Segura R., Hoff H., Gotto A. M., Jr Contrasting effects on plasma lipoproteins of intravenous versus oral administration of a triglyceride-phospholipid emulsion. Eur J Clin Invest. 1975 Sep 12;5(5):373–384. doi: 10.1111/j.1365-2362.1975.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Vogel W. C., Bierman E. L. Post-heparin serum lecithinase in man and its positional specificity. J Lipid Res. 1967 Jan;8(1):46–53. [PubMed] [Google Scholar]
- Wallentin L., Vikrot O. Lecithin:cholesterol acyl transfer in plasma of normal persons in relation to lipid and lipoprotein concentration. Scand J Clin Lab Invest. 1975 Nov;35(7):669–676. [PubMed] [Google Scholar]
- Zieve F. J., Zieve L. Post-heparin phospholipase and post-heparin lipase have different tissue origins. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1480–1485. doi: 10.1016/0006-291x(72)90239-2. [DOI] [PubMed] [Google Scholar]