Abstract
The field of oxidative stress, and the study of oxidatively damaged DNA, in particular, is a subject of intense, and growing interest. This has, in part, benefited from the availability of kits from commercial suppliers which are advertised as reporting on markers of oxidative stress. Such widespread use has inevitably led to an increase in the number of concerns, amongst experts in the field, editors and referees, over appropriateness of terminology and methodology. Thus, the widely used term “oxidative DNA damage” is misleading as it implies that the damage, i.e. the lesion per se, is oxidative and thus capable of oxidising other substrates. We would encourage the use of such terms as ‘oxidatively damaged DNA’, ‘oxidatively generated DNA damage’, ‘oxidatively-derived damage to DNA’ or ‘oxidation-induced DNA damage’ to describe the consequence of the interaction of reactive oxygen species with DNA. One of the most studied nucleic acid-derived biomarkers of oxidative stress is 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG). Yet, in the literature, this compound has been referred to using a number of different terms, sometimes leading to confusion over the designation of the modified nucleobase or (2′-deoxy)ribonucleoside. Standardisation of nomenclature would not only simplify literature searches, but also clarify the lesion in question. Herein, we provide justification for our preferred nomenclature, and suggest a number of steps by which we may work towards standardisation of calibration, and with it improved inter-laboratory agreement, for assays of 8-oxodG, in order to achieve accurate measurements.
Introduction
Despite being a relatively young field, the study of oxidative stress has attracted huge interest. With the advent of simple, and relatively inexpensive assays (sometimes from commercial suppliers) a growing number of groups have been able to assess oxidatively-generated DNA damage in mammalian cells. Whilst this is good for raising the profile of the field of oxidative stress research, it has led to an increasing number of issues when the work is written up for publication and included in grant applications. In particular, it is evident to experts in the field, editors and referees alike that there is often uncertainty concerning what is appropriate and accurate terminology, when describing studies concerning the effects of oxidatively-generated DNA damage. For this reason, we wish to raise a number of points for discussion, incorporating our recommendations on this subject. The aim is to support those embarking on studies involving oxidatively-generated damage to DNA nucleobases, and to produce greater uniformity across the field. We do not wish to be dogmatic, but to present a well-argued rationale for our recommendations.
Terminology
The term “oxidative DNA damage” has been used extensively, and presently draws more than 2600 hits in PubMed. However, it can be misleading, as it implies that the damage i.e. the lesion per se, is oxidative and thus capable of oxidising other substrates. Most oxidised bases, with the exception of five hydroxyl radical-mediated thymine hydroperoxides including 5-(hydroperoxymethyl)uracil and cis and trans 5-(6)-hydroperoxy-6-(5)-hydroxy-5,6-dihydrothymine, do not exhibit oxidising properties. The same point may be made regarding the lesions themselves and also the corresponding repair enzymes, so the term oxidative lesion, and oxidative DNA glycosylase are similarly incorrect. In the interest of accuracy, we would encourage the use of such terms as ‘oxidatively damaged DNA’, or ‘oxidatively generated DNA damage’, to describe the consequence of the interaction of reactive oxygen species with DNA. At the same time, we accept that the term ‘oxidative DNA damage’ is simple, widely used, and understoood by the community.
Nomenclature
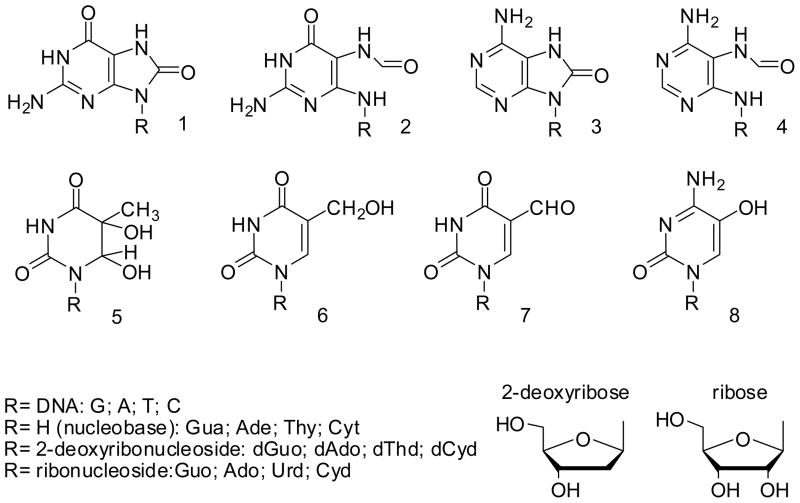
We would also like to draw attention to the nomenclature used to describe modified nucleobases, 2′-deoxyribonucleosides and ribonucleosides. Although a single letter should represent nucleobases in DNA (e.g. G, C, A, T), it is preferable to write the complete abbreviation when referring to monomers e.g. Gua for the nucleobase, dGuo for the 2′-deoxyribonucleoside, and Guo for the ribonucleoside (see Table 1 and Figure 1). The oxidatively-generated DNA product which has received, by far, the most attention is the modified guanine nucleobase, defined in full as, 8-oxo-7,8-dihydroguanine (which should be abbreviated as 8-oxoGua; Figure 1A). This is our recommended name, based upon advice from the International Union of Pure and Applied Chemistry (IUPAC) which states that, based upon current nomenclature, (di)hydro- prefixes are non-detachable, meaning that they are always immediately before the parent name (1). On this basis, 7,8-dihydro-8-oxoguanine and 7-hydro-8-oxoguanine would be incorrect. Furthermore, 8-oxo-2′-deoxyguanine is a confused name derived from a combination of both nucleobase and 2′-deoxyribonucleoside. For clarification, the 7,8-dihydro description is used to indicate the saturation of the double bond between N7 and C8 atoms of the parent unmodified guanine, from which the damaged nucleobase is derived (hence 8-oxo-7,8-dihydroguanine is the 8-oxo-substituted derivative of 7,8-dihydroguanine). 8-Hydroxyguanine is a frequently used term, and indeed one used by Chemical Abstracts. However this is, in fact, a rather minor tautomer at physiological pH (2) compared to the predominant 6,8-diketo form, as inferred from NMR studies of the lesion in duplex DNA (3, 4) and as the 2′-deoxyribonucleoside (5), which is further confirmed by theoretical calculations (6–8). By implication, 8-oxo, rather than 8-hydroxy, would be the form present in greatest amounts in biological systems, and hence the more accurate term to use when describing this lesion in vivo. Of course, this does not prevent the use of 8-hydroxy, if that is the specific tautomeric form in question (ref: Chatilialoglu et al. JACS Comparison of isoelectric 8-HO-G and 8-NH2-G derivatives in redox processes). There is precedent for this proposal: the malondialdehyde-, or base propenal-derived modification of guanine, known as ‘M1G’ [3-(2′-deoxy-β-D-erythro-pentofuranosyl)pyrimido[1,2-α]-purin-10(3H)-one], is a ring-closed species as a nucleobase at pH 7, but undergoes ring-opening at alkaline pH (9), and also when base-paired with dC in duplex DNA (10), when it becomes the N2-(3-oxo-1-propenyl)-dG (OPD) adduct. Nonetheless, the name remains M1G (or M1dG, for the 2′-deoxribonucleoside equivalent), since this is the form predominating at pH 7 (9, 10). Hence the proposal to define the names, based upon the major structure at pH 7, and biological context. The above advice also applies to 5,6-saturation products of thymine (Thy), cytosine (Cyt) and uracil (Ura). For example, 5,6-dihydroxy-5,6-dihydrothymine (thymine glycol, ThyGly or Thyg; see Table 1).
Table 1.
Name and abbreviation of common oxidatively damaged nucleobases of DNA.
| Name | Abbreviation | Structure (see Figure 1) |
|---|---|---|
| 8-Oxo-7,8-dihydroguanine | 8-oxoGua | 1 |
| 2,6-Diamino-4-hydroxy-5-formamidopyrimidine | FapyGua | 2 |
| 8-Oxo-7,8-dihydroadenine | 8-oxoAde | 3 |
| 4,6-Diamino-5-formamidopyrimidine | FapyAde | 4 |
| 5,6-Dihydroxy-5,6-dihydrothymine | ThyGly or Thyg | 5 |
| 5-(Hydroxymethyl)uracil | 5-HMU | 6 |
| 5-formyluracil | 5ForU | 7 |
| 5-hydroxycytosine | 5-OHCyt | 8 |
Figure 1.
Structure of oxidatively damaged nucleobases of DNA that have been detected so far in cells.
The corresponding 2′-deoxyribonucleoside of 8-oxoGua is 8-oxo-7,8-dihydro-2′-deoxyguanosine (Figure 1B), abbreviated as 8-oxodG or 8-oxodGuo. For completeness, it is worth noting that the ribonucleoside equivalent is 8-oxo-7,8-dihydroguanosine, abbreviated as 8-oxoGuo (Figure 1C).
Conclusions
An immediate benefit of achieving harmony in the terms used to describe oxidatively-modified DNA, and its constituents, would be the simplification of literature searches. One keyword could be used, instead of multiple variants thereof. A further benefit, evident to authors and readers alike, would be the removal of any doubt as to what lesion is being discussed. For example, confusion between modified free nucleobase, ribonucleoside and 2′-deoxyribonucleoside hampers meaningful interpretation, leading to suggestions that modified 2′-deoxyribonucleosides, in extracellular matrices, are products of base excision repair. By the same token, confusion over the choice of abbreviation, for example, 8-oxoGua, 8-oxoG and 8-oxoGuo, can make it unclear as to whether the modified nucleobase or ribonucleoside is being described. Standardisation of nomenclature would address this problem. Therefore, whilst trivial, or common, names may continue to be used, and feature prominently in existing literature, we strongly recommend a progressive move towards consensus on the use of 8-oxo-7,8-dihydroguanine, as indicated by IUPAC, and supported by others in the field (for example, Griffiths et al. (13)).
The year 2009 marked the 25th anniversary of Kasai and Nishimura’s publication concerning 8-oxoGua in Nucleic Acids Research (15), which was closely followed by another, describing the formation of 8-oxoGua in DNA (16). It is therefore perhaps pertinent that we are considering some of the issues surrounding the nomenclature and measurement of this and related lesions. The widespread, and increasing, interest in this particular marker of oxidative stress may be explained, in part, by its apparent omnipresence in cellular DNA in vivo, its biological significance, and its relatively straight forward quantification, using techniques such as HPLC-EC (11), and isotope-dilution LC-MS/MS (17). This has provided a great impetus to the study of oxidatively-damaged DNA, with emphasis moving from damage per se, to other downstream biological events, such as repair and mutagenesis. This also explains why major efforts have been made, for example via the European Standards Committee on Oxidatively Damaged DNA (ESCODD) and European Standards Committee on Urinary (DNA) Lesion Analysis (ESCULA; http://escula.org) networks, to resolve major discrepancies between the data provided by the available chemical and biochemical methods of measurement of 8-oxoGua and that can vary between reports by up to 103. Clearly, we have come a long way in twenty-five years, but there remains a great deal that we do not understand about this molecule, that is an ubiquitous marker of several oxidations reactions mediated by hydroxyl radical, one-electron oxidants and singlet oxygen (18).
Acknowledgments
The authors are very grateful to Dr. Gerry Moss, President, IUPAC Division VIII, for his useful comments in the drafting of this document.
MSC, SL, PM, RO, KB and MDE are partners of ECNIS (Environmental Cancer Risk, Nutrition and Individual Susceptibility), a network of excellence operating within the European Union 6th Framework Program, Priority 5:”Food Quality and Safety” (Contract No 513943).
JC is member of EU network COST Action CM0603 “Free Radical in Chemical Biology (CHEMBIO-RADICAL).
References
- 1.Panico R, Powell WH, Richer J-C, editors. A Guide to IUPAC Nomenclature of Organic Compounds, recommendations 1993. Blackwell Scientific Publications; Oxford: 1993. [Google Scholar]
- 2.Culp SJ, Cho BP, Kadlubar FF, Evans FE. Structural and conformational analyses of 8-hydroxy-2′-deoxyguanosine. Chem Res Toxicol. 1989;2:416–422. doi: 10.1021/tx00012a010. [DOI] [PubMed] [Google Scholar]
- 3.Oda Y, Uesugi S, Ikehara M, Nishimura S, Kawase Y, Ishikawa H, Inoue H, Ohtsuka E. NMR studies of a DNA containing 8-hydroxydeoxyguanosine. Nucleic Acids Res. 1991;19:1407–1412. doi: 10.1093/nar/19.7.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kouchakdjian M, Bodepudi V, Shibutani S, Eisenberg M, Johnson F, Grollman AP, Patel DJ. NMR structural studies of the ionizing radiation adduct 7-hydro-8-oxodeoxyguanosine (8-oxo-7H-dG) opposite deoxyadenosine in a DNA duplex. 8-Oxo-7H-dG(syn).dA(anti) alignment at lesion site. Biochemistry (Mosc) 1991;30:1403–1412. doi: 10.1021/bi00219a034. [DOI] [PubMed] [Google Scholar]
- 5.Cho BP, Kadlubar FF, Culp SJ, Evans FE. 15N nuclear magnetic resonance studies on the tautomerism of 8-hydroxy-2′-deoxyguanosine, 8-hydroxyguanosine, and other C8-substituted guanine nucleosides. Chem Res Toxicol. 1990;3:445–452. doi: 10.1021/tx00017a010. [DOI] [PubMed] [Google Scholar]
- 6.Aida M, Nishimura S. An ab initio molecular orbital study on the characteristics of 8-hydroxyguanine. Mutat Res. 1987;192:83–89. doi: 10.1016/0165-7992(87)90101-1. [DOI] [PubMed] [Google Scholar]
- 7.Venkatesmarlu D, Leszczynski J. Tautomerism equilibria in 8-oxopurines: implication for mutagenesis. J Comput Aided Mol Des. 1998;12:373–382. doi: 10.1023/a:1008067110965. [DOI] [PubMed] [Google Scholar]
- 8.Jang YH, Goddard WA, 3rd, Noyes KT, Sowers LC, Hwang S, Chung DS. First principles calculations of the tautomers and pK(a) values of 8-oxoguanine: implications for mutagenicity and repair. Chem Res Toxicol. 2002;15:1023–1035. doi: 10.1021/tx010146r. [DOI] [PubMed] [Google Scholar]
- 9.Niedernhofer LJ, Riley M, Schnetz-Boutaud N, Sanduwaran G, Chaudhary AK, Reddy GR, Marnett LJ. Temperature-dependent formation of a conjugate between tris(hydroxymethyl)aminomethane buffer and the malondialdehyde-DNA adduct pyrimidopurinone. Chem Res Toxicol. 1997;10:556–561. doi: 10.1021/tx960191c. [DOI] [PubMed] [Google Scholar]
- 10.Schnetz-Boutaud NC, Saleh S, Marnett LJ, Stone MP. The exocyclic 1,N2-deoxyguanosine pyrimidopurinone M1G is a chemically stable DNA adduct when placed opposite a two-base deletion in the (CpG)3 frameshift hotspot of the Salmonella typhimurium hisD3052 gene. Biochemistry (Mosc) 2001;40:15638–15649. doi: 10.1021/bi011242u. [DOI] [PubMed] [Google Scholar]
- 11.Floyd RA, Watson JJ, Wong PK, Altmiller DH, Rickard RC. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radic Res Commun. 1986;1:163–172. doi: 10.3109/10715768609083148. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths HR, Moller L, Bartosz G, Bast A, Bertoni-Freddari C, Collins A, Cooke M, Coolen S, Haenen G, Hoberg AM, Loft S, Lunec J, Olinski R, Parry J, Pompella A, Poulsen H, Verhagen H, Astley SB. Biomarkers. Mol Aspects Med. 2002;23:101–208. doi: 10.1016/s0098-2997(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 15.Kasai H, Nishimura S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984;12:2137–2145. doi: 10.1093/nar/12.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dizdaroglu M. Application of capillary gas chromatography-mass spectrometry to chemical characterization of radiation-induced base damage of DNA: implications for assessing DNA repair processes. Anal Biochem. 1985;144:593–603. doi: 10.1016/0003-2697(85)90158-7. [DOI] [PubMed] [Google Scholar]
- 17.Ravanat JL, Duretz B, Guiller A, Douki T, Cadet J. Isotope dilution high-performance liquid chromatography-electrospray tandem mass spectrometry assay for the measurement of 8-oxo-7,8-dihydro-2′-deoxyguanosine in biological samples. J Chromatogr B Biomed Sci Appl. 1998;715:349–356. doi: 10.1016/s0378-4347(98)00259-x. [DOI] [PubMed] [Google Scholar]
- 18.Cadet J, Douki T, Ravanat JL. Oxidatively generated damage to the guanine moiety of DNA: mechanistic aspects and formation in cells. Acc Chem Res. 2008;41:1075–1083. doi: 10.1021/ar700245e. [DOI] [PubMed] [Google Scholar]