Abstract
Cytochrome P450s (P450s) are involved in the metabolism of arachidonic acid (ARA), and ARA metabolites are associated with various cellular signaling pathways, such as blood hemostasis and inflammation. The present study demonstrates the expression of ARA-metabolizing P450s in the human megakaryocytic Dami cells using reverse transcriptase-polymerase chain reaction (RT-PCR) and immunublotting analysis followed by activity assays using ARA as a substrate. In addition to the previously identified CYP5A1, both protein and mRNAs of CYP1A1, 2U1, and 2J2 bands were detected. Ethoxyresorufin-O-deethylase (EROD) activity was observed in Dami cells, and its activity was significantly decreased after treatment with the P450 inhibitor SKF-525A when compared to the control groups (60% reduction, P < 0.001). CYP1A1 protein expression in Dami cells was induced by 3-methylenecholantheren. This increase in CYP1A1 protein level was correlated with enhanced EROD activity (fourfold increase vs. the control), as well as with increased metabolites, such as 20-hydroxyeicosatrienoic acid (20-HETE), 14, 15-EET (14-,15-epoxyeicosatrienoic acid), and 14, 15-dihydroxyeicosatrienoic acid (14, 15-DHET). The expression of soluble epoxide hydrolase, an enzyme responsible for the synthesis of DHETs from EETs, was confirmed by RT-PCR. Furthermore, 15 ARA metabolites, including 8,9-EET, 14,15-EET, and 20-HETE, were detected by LC-MS/MS in ARA-treated Dami cells, and their levels were decreased with the treatment of the SKF-525A. The present data suggest the possibility that the P450s play a role in the metabolism of ARA and other CYP-related substrates in human megakaryocytes and that P450 expression in megakaryocytic cell lines may predict their existences in platelets with functional activities.
Keywords: Arachidonic acid, Dami cells, P450s, Megakaryocytes, Platelets
Introduction
The cytochromes P450 (CYPs) constitute a superfamily of hemoprotein enzymes (Zhu and Silverman 2008). They are well recognized for their major roles in the synthesis, activation, and metabolism of many endogenous and xenobiotic compounds, such as hormones, fatty acids, drugs, and carcinogens (Spriet et al. 2009). It has been repeatedly reported that multiple P450s metabolize arachidonic acid (ARA) (Devos et al. 2010; Mitra et al. 2011; Theken et al. 2011; Xu et al. 2011). ARA is a fatty acid found in the human cell membrane, which is the precursor of eicosanoids that play important biological roles in the regulation of blood homeostasis and inflammation process (Imig 2012). ARA metabolites have also been reported to be involved in bone marrow haematopoiesis, and its abnormal regulation has been associated with the case of cardiovascular diseases (Lutton et al. 1989; Abraham et al. 1991; Pfister et al. 2010). In humans, ARA is epoxidized to epoxyeicosatrienoic acids (EETs) by CYP2C and CYP2J subfamilies, while CYP4A, 4F, and 1A subfamilies and CYP2U1 enzyme hydroxylate ARA to 20-HETE (Lasker et al. 2000; Pearson et al. 2009; Devos et al. 2010). The expression of ARA-matabolizing P450s varies among different tissues. For example, CYP2J2 is expressed in cardiac tissues more predominantly than in other tissues (Wu et al. 1996). Furthermore, CYP2C subfamily is found in significantly higher quantities in the liver and gastrointestinal tract than in bone marrow (Bieche et al. 2007). These differences in ARA-metabolizing P450 expression levels may lead to different levels of ARA metabolites, thus affecting tissues’ biological regulation.
Megakaryocytes are the bone marrow cells responsible for the production of blood platelets. In humans, megakaryocytes normally account for approximately 0.05−0.1% of all nucleated bone marrow cells. Each megakaryocyte produces 1,000–3,000 enucleate platelets through a thrombopoietin-mediated process (Deutsch and Tomer 2006). Although thromboxane synthase (CYP5A1) has been found to be highly expressed in megakaryocytes and platelets (Nakahata 2008), other P450 expressions in megakaryocytes and their daughter platelets are, as yet, poorly understood. Interestingly, ARA and some of its metabolites have been identified in platelets (Sheppard et al. 1992), suggesting the possible presence of ARA-metabolizing P450s in megakaryocytes. Since platelets, being cell fragments derived from the precursor megakaryocytes, cannot proliferate in the cell culture system due to a lack of nucleus, there are no cell lines available for the molecular study of platelets. The megakaryocytic cell line (Dami cells) has frequently been used for the study of platelets (Khetawat et al. 2000; Shi et al. 2010; Lev et al. 2011).The goal of the present study was to determine which P450s are expressed in Dami cells with the functional activity for the metabolism of ARA.
Materials and Methods
Chemicals and reagents.
Iscove's modified Dulbecco's medium, Moloney Murine Leukemia Virus Reverse Transcriptase, oligo(dT)18 primer, dithiothreitol (DTT), and first strand buffer were obtained from Invitrogen (Carlsbad, CA). D-Taq polymerase, 10× D-Taq buffer, and 2.5 mM dNTP were purchased from Sun Gen (Daejeon, Korea). Primary antibodies for CYP1A1, 2U1, and 2J2 and the chemiluminescence kit were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The primary antibody for actin was obtained from Cell Signaling Technology (Danvers, MA). Skimmed milk powder was obtained from BD Biosciences (San Jose, CA). Resorufin, 7-ethoxyresorufin (7-ER), SKF-525, protease inhibitor cocktail, and 3-methylenecholantheren (3-MC) were obtained from Sigma Aldrich (St. Louis, MO). Both 20-HETE and 20-HETE-d6 were obtained from Cayman (Ann Arbor, MI). All other chemicals and organic solvents for activity assays were of the highest grade available from commercial sources.
Cell culture.
The human megakaryocytic Dami cell line was obtained from the American Type Culture Collections (Rockville, MD). Dami cells were cultured in Iscove's modified Dulbecco's medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Cultures were maintained in a humidified atmosphere of 5% CO2 at 37°C as previously described (Khetawat et al. 2000). Dami cells were treated with 10 μM of 3-MC for 72 h in order to test the induction of CYP1A1 and determine the effect of CYP1A1 induction on ARA metabolism. For these experiments, 3-MC was dissolved in dimethyl sulfoxide (DMSO), and the final DMSO concentration in the culture medium was 0.1% (v/v). The control culture received the same volume of DMSO without 3-MC. After 3-MC treatment, the cells were incubated with 100 μM ARA for 12 h (Rehfeldt et al. 1993).
Reverse transcriptase-polymerase chain reaction.
RNAs from Dami cells, and human liver were extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Liver tissue samples were obtained from the Inje Pharmacogenomics Research Center Biobank (Inje University College of Medicine, Busan, Korea). The research protocol for the usage of human liver tissues was approved by the Institutional Review Board of Busan Paik Hospital (Inje University, Busan, Korea). Three micrograms of total isolated RNA was added to a reaction mixture containing 100 pmol oligo(dT), 2.5 mM dNTP, 0.1M DTT, 5× first strand buffer, 200 U of M-MLV reverse transcriptase and RNAase-free water for synthesis of cDNA. The reaction mixtures were incubated at 42°C for 50 min. Next, conventional PCR was performed by adding 330 ng of cDNA to a mixture containing 10 mM dNTPs, 25 mM MgCl2, 2× D-Taq polymerase buffer, 10 pmoles from each of the forward and reverse primers (Table 1), and 1.5 U of D-Taq DNA polymerase. The PCR products were separated on a 2% agarose gel and visualized using ethidium bromide staining.
Table 1.
PCR primer sequences and amplified product sizes for RT-PCR analysis
| Gene | Foreword primer (5′ to 3′) | Reverse primer (5′ to 3′) | Band size (bp) | Reference |
|---|---|---|---|---|
| CYP1A1 | GTAATCAGGGCCTCAAGAC | GACATTGGTCACTGATACC | 518 | Finnstrom et al. 2001 |
| CYP1A2 | CAGAATGCCCTCAACACCTTCTCCATCG | GTGATGTCCCGGACACTGTTCTTG | 430 | Finnstrom et al. 2001 |
| CYP1B1 | GTATATTGTTGAAGAGACAG | AAAGAGGTACAACATCACCT | 420 | Finnstrom et al. 2001 |
| CYP2C9 | AGGAAAAGCACAACCAACCA | TCTCAGGGTTGTGCTTGTC | 104 | Finnstrom et al. 2001 |
| CYP2C8 | AGATCAGAATTTTCTCACCC | AACTTCGTGTAAGAGCAACA | 158 | Finnstrom et al. 2001 |
| CYP2C19a | CCACATGCCCTACACAGATG | TGTAGCACAGAAGTGAGGGAAG | 159 | |
| CYP2D6 | AGGTGTGTCTCGAGGAGCCCATTTGGTA | GCAGAAAGCCCGACTCCTCCTTCA | 700 | Finnstrom et al. 2001 |
| CYP2J2 | AGAAGCCCTTATCCACAT | CTGAATGCGTTCCTCTAA | 186 | Finnstrom et al. 2001 |
| CYP2U1 | GCTCATCTCCATCGTGAC | TTTCTAGGCCTCGTGACATA | 320 | Finnstrom et al. 2001 |
| CYP4A11 | TCCTGTCTGCCCATATCCTG | CGGGCTTAGATTATGGTGCG | 332 | Finnstrom et al. 2001 |
| CYP4F2 | CGGAACCCATCACAACCCAGC | CTGGGCCCTGCCGAGAAGGGAA | 122 | Finnstrom et al. 2001 |
| CYP4F3 | TGCTGCACCCAAGACATTGTG | GCTCAGGGGCTCCACCCG | 200 | Finnstrom et al. 2001 |
| CYP3A4 | GTGTGGGGCTTTTATGATG | GGCGACTTTCTTTCATCCT | 558 | Finnstrom et al. 2001 |
| CYP3A5 | CCACCTACCTATGATGCC | TTGAAGAAGTCCTTGCGT | 510 | Finnstrom et al. 2001 |
| CYP5A1a | CAGATTCACACGGGAGGCA | GGACCTAGGGCAGATTTGGA | 342 | |
| AhRa | GGACATGGGTCCACTCTAAT | AGCCAGGAGGCAACTAGGAT | 322 | |
| EPHX2a | GAGTTGGTATTCTTGGAGG | TGTTTGGATTTGCTGG | 330 | |
| GAPDH | GCTCACTGGCATGGCCTTCCG | GTGGGCCATGAGGTCCACCAC | 310 | Finnstrom et al. 2001 |
aGene-specific primers were designed in the present study
Immunoblot analysis.
Dami cell cultures were centrifuged at 1,000 rpm for 3 min. Then, lysis buffer [50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1% NP40, 0.25% Na-deoxycholate, and protease inhibitor cocktail] was added to the Dami cell pellet. The mixture was vortexed vigorously, sonicated twice for 10 s, and the resulting lysate was incubated on ice for 50 min. The lysates were stored at −80°C until use. Then, 35 μg of protein was boiled at 95°C for 5 min with 4× loading buffer containing 0.1 M Tris–HCl (pH 6.8), 4% sodium dodecyl sulfate (SDS), 1.5% bromophenol blue, 20% glycerol, and 5% β-mercaptoethanol. The denatured proteins were separated with 12% SDS–polyacrylamide gel and then transferred to a nitrocellulose membrane in a buffer containing 25 mM Tris–HCl, 192 mM glycine, and 20% (v/v) methanol. The membrane was blocked by 5% skimmed milk in Tris-Buffered Saline supplemented with 0.1% Tween 20 solution. The membrane was probed with polyclonal goat anti-CYP1A1 IgG and anti-CYP2U1 IgG, and monoclonal mouse anti-CYP2J2 IgG, separately, and reproved with monoclonal rabbit anti-beta-actin IgG at 4°C overnight. Immunoreactive proteins were detected using the enhanced chemiluminescence method according to the manufacturer's instruction (GE Healthcare Bio-Sciences, Buckinghamshire, UK).
Determination of ethoxyresorufin O-deethylase activity.
Ethoxyresorufin O-deethylase (EROD) assays were performed on Dami cells. Intact cells were incubated with 2 μg of 7-ER in a TN assay buffer [0.1 M NaCl, 50 mM Tris (pH 7.8)] for 20 min. The amount of resorufin formed was measured at excitation/emission wavelengths of 545/575 nm (Perkin Elmer Victor 3V, MTX Lab Systems, Vienna, VA). The standard curve of known concentrations of resorufin was between 50 and 500 pmoles (Kennedy et al. 1993). After measurement, the cells were lysed, and the protein concentration was determined using the Bradford dye method (Martin et al. 1995). The resurfin formation was expressed as nanomoles per minute per milligram of protein.
ARA metabolism assay.
ARA metabolism in Dami cells was investigated by incubation of 1 × 106 Dami cells with 100 μM ARA for 12 h (Rehfeldt et al. 1993). Cells were then separated by centrifugation at 1,000 rpm for 3 min. ARA metabolites were extracted from Dami cells and cell media by ethyl acetate as previously reported (Zordoky et al. 2011). ARA and its metabolites were identified and quantified using a liquid chromatography–mass spectrometry (LC-MS/MS) system, with some modifications on the previous method (Shinde et al. 2012). Briefly, metabolites were separated on a reverse-phase column Atlantis dC18 (2.1 mm i.d. × 150 mm, 3 μm particle size; Waters, Ireland) with a solvent consisting of water (A) and acetonitrile containing 0.1% formic acid (B). The flow rate of 0.25 ml/min with gradient system was as follow: 0–5 min, 5% B; 5 min, 35% B; 15 min, 65% B; 20 min, 75% B; 24–28 min, 100% B; and 28.01 min, 5% B. ARA metabolites were identified using an API 5500 mass spectrophotometer (Applied Biosystems, Foster City, CA). Peak areas for all compounds were integrated using Analyst software (version 1.2; Applied Biosystems), and 20-HETE-6 (100 μg/ml) was used as internal standard. The detection limits of 14,15-EET, 14,15-dihydroxyeicosatrienoic acid (14,15-DHET) and 20-HETE were 20, 10, and 20 pg/ml, respectively.
Statistical analysis.
All values represent the mean ± standard deviation of triplicate reactions. Statistical significance was analyzed using a two-tailed Student’s t test. All statistical analyses were performed using the SAS program (version 9.1.3; SAS Institute, Cary, NC). Statistically significant differences as compared with the control groups are represented as *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
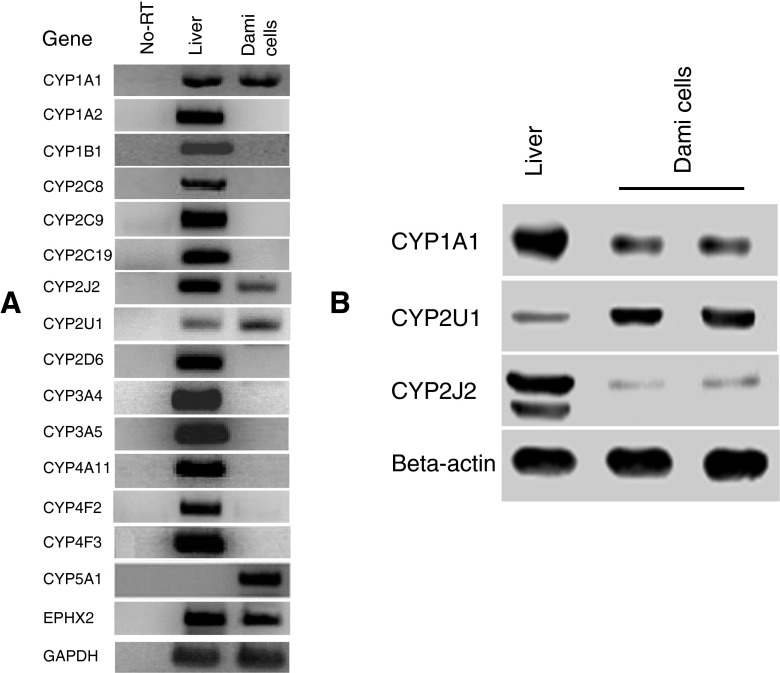
Using reverse transcriptase-polymerase chain reaction (RT-PCR) analysis, we screened the mRNA expression of 15 major P450s which are reported to metabolize ARA (Devos et al. 2010; Mitra et al. 2011; Theken et al. 2011; Xu et al. 2011). The screened ARA-P450s were as follows: CYP1A1, 1A2, 1B1, 2C8, 2C9, 2C19, 2D6, 2J2, 2U1, 4A11, 4F2, 4F3, 3A4, and 3A5 in addition to CYP5A1 which is known to be expressed in platelets. We detected mRNAs of CYP1A1, 2U1, and 2J2 in Dami cells, as well as the mRNA of CYP5A1 (Fig. 1). Specific amplification was confirmed as their predicted sizes as shown in Table 1. There were no detectable bands in the PCR reactions performed with RNA samples that were not treated with reverse transcriptase; these reactions served as a control to monitor genomic DNA contamination. Immunobloting analysis showed the detection of CYP1A1, 2U1, and 2J2 proteins, with bands size of 50–60 kDa, in Dami cells (Fig. 1B). The detected bands were in agreement with the theoretical size of 50–60 kDa for P450s and with the liver microsome sample used as a reference standard for the detection of P450s.
Figure 1.
Expression profiles of P450s in Dami cells. (A) RT-PCR was used to amplify each P450 with the specific primers listed in Table 1. The targeted genes were amplified as a single product of the expected size; no bands were detected in control samples in which the PCR was performed in RNA samples without reverse transcriptase treatment to confirm that there was no genomic DNA contamination. Liver tissue cDNA was used as a reference control for P450 detection. EPHX2, epoxide hydrolase 2. (B) Detection of CYP1A1, 2U1, and 2J2 proteins in Dami cells by immunublot analysis. Total protein lysates (35 μg) from Dami cells were loaded in each lane. Lysates prepared from liver microsomes were used as a reference standard. The immunoreactivity of actin was used as a loading control. Proteins were separated on a 13% SDS-polyacrylamide gel, transferred to nitrocellulose membrane, and detected by primary antibodies against CYP1A1, 2U1, and 2J2. Protein bands were visualized by the chemiluminescence method.
EROD assays of CYP1A1 were performed with Dami cells. The activity of resorufin formation was 112 ± 10 nmol/min/mg protein. To confirm the activity of P450, 40 μM of the P450 inhibitor SKF-525A was added to the reactions and compared with the non-SKF-525A-treated samples. Figure 2 shows that EROD activity decreased significantly with the addition of SKF-525A to Dami cells (60%, P < 0.001) when compared to the control groups. Because CYP1A1 (this study) and the nuclear Aryl hydrocarbon receptor (AhR) (Lindsey and Papoutsakis 2011) are both expressed in megakaryocytes, we investigated the inducibility of CYP1A1 in Dami cells using 3-MC which is an AhR agonist, after confirming the expression of AhR in Dami cells (Fig. 3A). Immunoblot analysis showed that the CYP1A1 protein level increased in Dami cells after 3-MC treatment (Fig. 3B), and that this increased protein level correlated with the increase in EROD activity (fourfold, P < 0.001) as compared with the DMSO-treated groups (Fig. 3C).
Figure 2.
EROD assays in Dami cells. The intact cells were incubated with 2 μg of 7-ER in a TN assay buffer for 20 min. The amount of resorufin formed was measured at excitation/emission wavelengths of 545/575 nm by comparison with a standard curve of known concentrations of resorufin. Further details are in the “Materials and Methods” section. EROD activity was expressed as nanomoles per minute per milligram of protein. Statistically significant differences as compared with the control group are indicated as ***P < 0.001, based on a two-tailed Student’s t test. Data are presented as the mean ± S.D. of reactions performed in triplicate.
Figure 3.
The effect of 3-MC on CYP1A1 expression and EROD activity. (A) Detection of Ah receptor by RT-PCR analysis in Dami cells. RT-PCR contains a set of AhR-specific primers as shown in Table 1. A specific band of AhR is marked by the arrow (322 bp). Liver tissue cDNA was used as a reference control. (B) Western blot analysis of CYP1A1. Dami cells were cultured with 10 μM of 3-MC (+3MC) for 72 h. The control group (-3MC) received the same volume of the solvent (DMSO) without 3-MC. After 3-MC treatment, 35 μg of total protein lysates from each group was loaded in a SDS-polyacrylamide gel for Western blot analysis of CYP1A1. Immunoblotting for actin (45 kDa) was performed as an internal control. Detailed procedures for the Western blot analysis are explained in the “Materials and Methods” section. (C) Effect of 3-MC on the EROD activity. EROD assay was performed in the 3-MC-treated and control Dami cells. The intact Dami cells were incubated with 2 μg of 7-ER in a TN assay buffer for 20 min. Details regarding the EROD assay are explained in the “Materials and Methods” section. EROD activity was expressed as nanomoles per minute per milligram of protein. Statistically significant changes as compared with the DMSO treatment group are indicated as ***P < 0.001. Pairwise comparisons were performed using a Student’s paired t test.
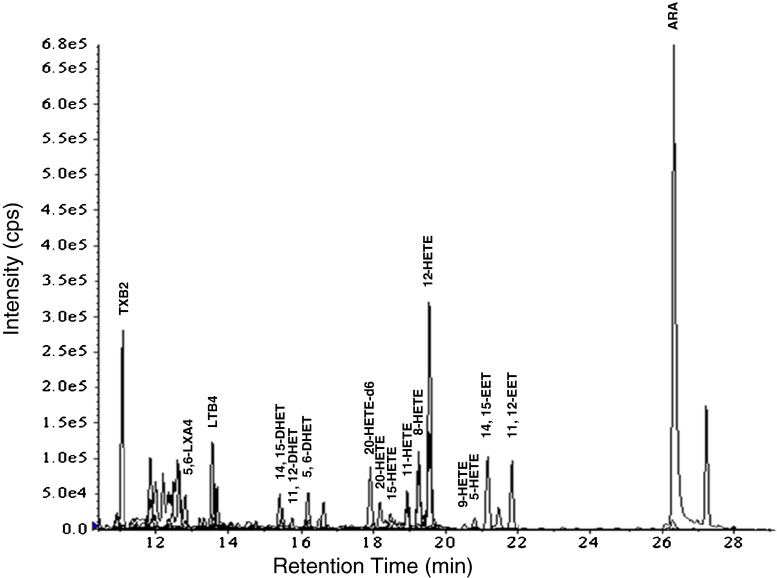
We found that 15 ARA metabolites were detected by LC-MS/MS in Dami cells (Fig. 4), including 5-HETE, 8-HETE, 9-HETE, 11-HETE, 12-HETE, 15-HETE, 20-HETE, 11,12-EET, 14,15-EET, 5,6-DHET, 11,12-DHET, 14,15-DHET, leukotriene B4 (LTB4), 5,6-lipoxin A4 (LXA4), and TXB2. Among the detected ARA metabolites in Dami cells, 20-HETE, 11,12-EET, and 14,15-EET have been reported to be mediated through the ARA-P450-metabolizing pathway in the kidney, liver, and vascular tissues (Lasker et al. 2000; Pearson et al. 2009). The expression of soluble epoxide hydrolase was confirmed by a specific RT-PCR (Fig 1A), indicating that the generation of DHETs from EETs would be from soluble epoxide hydrolase in Dami cells. Induction of CYP1A1 protein by 3-MC treatment significantly increased the levels of 20-HETE, 14,15-EET, and 14,15-DHET than in the control group (Fig. 5). These ARA metabolites were significantly reduced with the treatment of the SKF-525A (P < 0.05–0.001).
Figure 4.
Identification of ARA metabolites in Dami cells. The HPLC chromatogram shows the 15 identified ARA metabolites in Dami cells. The cells were incubated with 100 μM ARA for 12 h. ARA metabolites were then extracted by ethyl acetate (pH 3−4). Ethyl acetate was evaporated, and the residue was dissolved in 100 μl ethanol, followed by LC-MS/MS screening for ARA metabolites. Further details are included in the “Materials and Methods” section. 20-HETE-6 (100 μg/ml) was used as internal standard.
Figure 5.
The effect of 3-MC on ARA metabolism in Dami cells. Dami cells were treated with 10 μM 3-MC for 72 h. The control group of Dami cells received the same final volume of the solvent (DMSO) without 3-MC. After 3-MC treatment, 3-MC-treated Dami cells were divided into two groups. One group of Dami cells (1 × 106 cells) was incubated with 100 μM ARA for 12 h. The other group received 40 μM of SKF-525A in addition to ARA. ARA metabolites, 14,15-EET (A), 14,15-DHET (B), and 20-HETE (C) were identified by LC-MS/MS after liquid/liquid extraction by ethyl acetate. Further details are described in the “Materials and Methods” section. The internal standard utilized was 20-HETE-6 (100 μg/ml). Statistically significant differences as compared with the DMSO treatment group are indicated as *P < 0.05, **P < 0.01, and ***P < 0.001, based on two-tailed Student’s t tests. Data are presented as the mean ± S.D. of reactions performed in triplicate.
Discussion
Although ARA and its metabolites are important signal molecules in blood hemostasis, ARA metabolism by P450s in megakaryocytes and megakaryocytic Dami cells remains unclear. Megakaryocytic Dami cells have been used to study the biological function of megakaryocytes and platelets, because circulating platelets have no nucleus (Khetawat et al. 2000; Lev et al. 2011; Lee et al. 2012). In the current study, we investigated ARA-metabolizing P450s in Dami cells. In addition to CYP5A1, we found that CYP1A1, 2U1, and 2J2 were also expressed in Dami cells. CYP1A1, 2U1, and 2J2 have been reported to metabolize ARA and its derivatives (Devos et al. 2010; Gaedigk et al. 2006). Therefore, it can be suggested that these P450s may play a role in the metabolism of ARA and its related eicosanoid compounds in the megakaryocytes and the platelets.
The literature reports several instances of similarities in the P450 expression profiles of Dami cells and bone marrow. For example, in human bone marrows, CYP2U1 and 1A1 are expressed at high levels, but CYP3A and 2C are not present (Bieche et al. 2007). Similarly, in this study CYP1A1 and 2U1 were strongly expressed in Dami cells, while CYP3A4, 3A5, 2C8, 2C9, and 2C19 were not detectable. The similar expression profiles of P450s in bone marrow tissues and Dami cells may indicate that CYP2U1 and 1A1 in bone marrow could be derived, at least in part, from megakaryocytes; however, it cannot rule out the possibility that other bone marrow cell types can also express CYP1A1 and 2U1.
CYP1A1 expression was detected, and its expression was induced by 3-MC in Dami cells. This increase in protein expression correlated with the increase in EROD activity. These results suggest that there could be variations in CYP1A1 expression levels in megakaryocytes induced by environmental stimuli, such as cigarette smoking and other aromatic hydrocarbons with the potential to alter the metabolism of ARA as well as other CYP1A1 substrates. CYP1A1 metabolizes some drugs, such as theophylline and caffeine (Yang and Lee 2008; Amin et al. 2011). It is expressed more in cancer tissues and activates pro-carcinogenic compounds, such as polycyclic aromatic hydrocarbons (Levova et al. 2011). We found that EROD activity was inhibited by 40 μM SKF-525A, confirming that the reaction was mediated by P450s in megakaryocytic Dami cells. Because EROD activity is mediated by the CYP1A subfamily and CYP1B1 (Smith et al. 2011), EROD activity in Dami cells appears to be a result of CYP1A1. Our results showed that 14,15-EET and 14,15-DHET were increased in Dami cells after treatment with 3-MC. It is known that 14,15-EET is metabolized to 14, 15-DHET by epoxide hydrolases (Seidegard et al. 1984). This finding is consistent with a previous report that 14,15-EET synthesis increases after induction of the CYP1A subfamily by different AhR nuclear receptor agonists in human HepG2 cells (Diani-Moore et al. 2006). In the present study, the expression of soluble epoxide hydrolase was evidenced in Dami cells, and the experiment using the inhibitor of epoxide hydrolase enzyme would add additional information for further translation of the metabolic fate for EETs and DHETs. CYP4A and 4F subfamilies have been identified as the main P450s for 20-HETE production (Lasker et al. 2000; Pearson et al. 2009), although multiple studies showed that CYP1A1 hydroxylases ARA to 20-HETE (Aboutabl et al. 2009; Arnold et al. 2010). Furthermore, the formation of 20-HETE is inhibited by 7-ER in bone marrow (Abraham et al. 1991). These results potentially indicate an essential role for CYP1A1 in the formation of 20-HETE in bone marrow. Our results showed that induction of CYP1A1 by 3-MC in Dami cells increased the metabolism of ARA to 20-HETE. These results indicate that altered levels of the metabolite may cause variation in megakaryocyte and platelet functions. The relatively high expression of CYP1A1 in megakaryocytes could increase our understanding of hematologic diseases and hematotoxicity. Benzenes cause bone marrow toxicity and hematotoxicity (Hirabayashi and Inoue 2010), and the aryl hydrocarbon receptor (AhR) was found to mediate benzene-induced toxicity (Hayashibara et al. 2003). The potent carcinogen 3-MC is an alkylated derivative of benzo(a)anthracene. In this study, we confirmed the expression of AhR in Dami cells by RT-PCR and observed that 3-MC induced CYP1A1 expression, which is predicted due to the activation of AhR by 3-MC. Polyaromatic hydrocarbons identified in cigarette smoke revealed to activate AhR and induce CYP1A1 (Roth et al. 2001). Moreover, smoking showed to increase platelet aggregation (Li et al. 2011). Therefore, it is suggested to investigate the effect of CYP1A1 induction on platelet aggregation variations. The results of the present study indicate that Dami cells may be a good cell line for studying CYP1A1 induction and metabolism for human megakaryocytes and human platelets, at least in part. Pregnane X receptor (PXR) and constitutive androstane receptor (CAR) have been reported to be involved in the regulation of CYP2J2 (Siest et al. 2008; Ellfolk et al. 2009). However, CAR and PXR were not detected by RT-PCR in our hand (data not shown), prompting us to study the induction of CYP1A1 in the present study.
CYP2U1 is an extrahepatic P450. It is expressed more in the thyroid, brain, heart, and bone marrow than in the liver (Karlgren et al. 2005; Dutheil et al. 2009). We found that CYP2U1 was also expressed in Dami cells. Information on the molecular regulation of CYP2U1 is very limited, and there is still no specific inhibitor and inducer of CYP2U1. However, CYP2U1 metabolized ARA to 20-HETE (Devos et al. 2010), suggesting that 20-HETE, which was detected in Dami cells, could be produced, at least in part by CYP2U1.
CYP2J2 is highly expressed in the cardiovascular system (Wu et al. 1996). It affects blood hemostasis via the metabolism of ARA to EETs, which are known to decrease blood pressure and to inhibit platelet aggregation (Spiecker and Liao 2006). Western blot analysis of CYP2J2 revealed one major band with a molecular weight of 50–60 kDa in Dami cells. However, we noticed that CYP2J2 in liver tissue was revealed by two distinct bands. Wu et al. (1996) also showed two bands in the liver and major one band of CYP2J2 in other tissues in immunoblot analysis. These results may indicate that CYP2J2 in human heart, kidney, and the platelet precursor cells megakaryocytes share similar immunochemical reactions. CYP2J2 is suggested to contribute to the formation of epoxidation of ARA (Wu et al. 1996). Expression of CYP2J2 in megakaryocytes might play a role in the formation of ARA metabolites, and they may be involved in the biological function of megakaryocytes and platelets.
In summary, we identified ARA-metabolizing P450s and confirmed their expressions with functional activities in Dami cells. These P450s may play important roles in the metabolism of ARA as well as other xenobiotic compounds in megakaryocytes. Particularly, because CYP1A1 expression levels are largely induced by environmental polycyclic aromatic hydrocarbons, altered levels of CYP1A1 expression may cause variations in CYP1A1-mediated metabolism in megakaryocytes. The present information on P450 expressions in megakaryocytic Dami cells would further extend our knowledge on the roles of P450s in megakaryocytes as well as platelets.
Acknowledgments
We thank Ms. Woo-Young Kim and Ms. Il-Sun Jung for their help in Western blot analysis and cell cultures. For EROD assays, the expert technical assistance of the Department of Parasitology, Inje University College of Medicine, Inje University is greatly appreciated. The authors are particularly thankful for the generous help provided by Kyung-Suk Oh for ARA metabolite analysis. This work was supported by the 2012 Inje University research grant.
References
- Aboutabl ME, Zordoky BN, El-Kadi AO. 3-methylcholanthrene and benzo(a)pyrene modulate cardiac cytochrome P450 gene expression and arachidonic acid metabolism in male Sprague Dawley rats. Br. J. Pharmacol. 2009;158:1808–1819. doi: 10.1111/j.1476-5381.2009.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham NG, Feldman E, Falck JR, Lutton JD, Schwartzman ML. Modulation of erythropoiesis by novel human bone marrow cytochrome P450-dependent metabolites of arachidonic acid. Blood. 1991;78:1461–1466. [PubMed] [Google Scholar]
- Amin N, Byrne E, Johnson J, Chenevix-Trench G, Walter S, Nolte IM, Vink JM, Rawal R, Mangino M, Teumer A, Keers JC, Verwoert G, Baumeister S, Biffar R, Petersmann A, Dahmen N, Doering A, Isaacs A, Broer L, Wray NR, Montgomery GW, Levy D, Psaty BM, Gudnason V, Chakravarti A, Sulem P, Gudbjartsson DF, Kiemeney LA, Thorsteinsdottir U, Stefansson K, van Rooij FJ, Aulchenko YS, Hottenga JJ, Rivadeneira FR, Hofman A, Uitterlinden AG, Hammond CJ, Shin SY, Ikram A, Witteman JC, Janssens AC, Snieder H, Tiemeier H, Wolfenbuttel BH, Oostra BA, Heath AC, Wichmann E, Spector TD, Grabe HJ, Boomsma DI, Martin NG, van Duijn CM. Genome-wide association analysis of coffee drinking suggests association with CYP1A1/CYP1A2 and NRCAM. Mol. Psychiatry. 2011;17:1–14. doi: 10.1038/mp.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold C, Konkel A, Fischer R, Schunck WH. Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. Pharmacol. Rep. 2010;62:536–547. doi: 10.1016/s1734-1140(10)70311-x. [DOI] [PubMed] [Google Scholar]
- Bieche I, Narjoz C, Asselah T, Vacher S, Marcellin P, Lidereau R, Beaune P, de Waziers I. Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenet. Genomics. 2007;17:731–742. doi: 10.1097/FPC.0b013e32810f2e58. [DOI] [PubMed] [Google Scholar]
- Deutsch VR, Tomer A. Megakaryocyte development and platelet production. Br. J. Haematol. 2006;134:453–466. doi: 10.1111/j.1365-2141.2006.06215.x. [DOI] [PubMed] [Google Scholar]
- Devos A, Lino Cardenas CL, Glowacki F, Engels A, Lo-Guidice JM, Chevalier D, Allorge D, Broly F, Cauffiez C. Genetic polymorphism of CYP2U1, a cytochrome P450 involved in fatty acids hydroxylation. Prostaglandins Leukot. Essent. Fat. Acids. 2010;83:105–110. doi: 10.1016/j.plefa.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Diani-Moore S, Papachristou F, Labitzke E, Rifkind AB. Induction of CYP1A and cyp2-mediated arachidonic acid epoxygenation and suppression of 20-hydroxyeicosatetraenoic acid by imidazole derivatives including the aromatase inhibitor vorozole. Drug Metab. Dispos. 2006;34:1376–1385. doi: 10.1124/dmd.106.009498. [DOI] [PubMed] [Google Scholar]
- Dutheil F, Dauchy S, Diry M, Sazdovitch V, Cloarec O, Mellottee L, Bieche I, Ingelman-Sundberg M, Flinois JP, de Waziers I, Beaune P, Decleves X, Duyckaerts C, Loriot MA. Xenobiotic-metabolizing enzymes and transporters in the normal human brain: regional and cellular mapping as a basis for putative roles in cerebral function. Drug Metab. Dispos. 2009;37:1528–1538. doi: 10.1124/dmd.109.027011. [DOI] [PubMed] [Google Scholar]
- Ellfolk M, Norlin M, Gyllensten K, Wikvall K. Regulation of human vitamin D(3) 25-hydroxylases in dermal fibroblasts and prostate cancer LNCaP cells. Mol. Pharmacol. 2009;75:1392–1399. doi: 10.1124/mol.108.053660. [DOI] [PubMed] [Google Scholar]
- Finnstrom N, Bjelfman C, Soderstrom TG, Smith G, Egevad L, Norlen BJ, Wolf CR, Rane A. Detection of cytochrome P450 mRNA transcripts in prostate samples by RT-PCR. Eur. J. Clin. Investig. 2001;31:880–886. doi: 10.1046/j.1365-2362.2001.00893.x. [DOI] [PubMed] [Google Scholar]
- Gaedigk A, Baker DW, Totah RA, Gaedigk R, Pearce RE, Vyhlidal CA, Zeldin DC, Leeder JS. Variability of CYP2J2 expression in human fetal tissues. J. Pharmacol. Exp. Ther. 2006;319:523–532. doi: 10.1124/jpet.106.109215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashibara T, Yamada Y, Mori N, Harasawa H, Sugahara K, Miyanishi T, Kamihira S, Tomonaga M. Possible involvement of aryl hydrocarbon receptor (AhR) in adult T-cell leukemia (ATL) leukemogenesis: constitutive activation of AhR in ATL. Biochem. Biophys. Res. Commun. 2003;300:128–134. doi: 10.1016/S0006-291X(02)02793-6. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y.; Inoue T. Benzene-induced bone-marrow toxicity: a hematopoietic stem-cell-specific, aryl hydrocarbon receptor-mediated adverse effect. Chem. Biol. Interact. 184: 252–258; 2010. [DOI] [PubMed]
- Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol. Rev. 2012;92:101–130. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlgren M, Miura S, Ingelman-Sundberg M. Novel extrahepatic cytochrome P450s. Toxicol. Appl. Pharmacol. 2005;207:57–61. doi: 10.1016/j.taap.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Kennedy SW, Lorenzen A, James CA, Collins BT. Ethoxyresorufin-O-deethylase and porphyrin analysis in chicken embryo hepatocyte cultures with a fluorescence multiwell plate reader. Anal. Biochem. 1993;211:102–112. doi: 10.1006/abio.1993.1239. [DOI] [PubMed] [Google Scholar]
- Khetawat G, Faraday N, Nealen ML, Vijayan KV, Bolton E, Noga SJ, Bray PF. Human megakaryocytes and platelets contain the estrogen receptor beta and androgen receptor (AR): testosterone regulates AR expression. Blood. 2000;95:2289–2296. [PubMed] [Google Scholar]
- Lasker JM, Chen WB, Wolf I, Bloswick BP, Wilson PD, Powell PK. Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of Cyp4F2 and Cyp4A11. J. Biol. Chem. 2000;275:4118–4126. doi: 10.1074/jbc.275.6.4118. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Kwon JA, Cho SA, Jarrar YB, Shin JG. Effects of testosterone and 17beta-oestradiol on expression of the G protein-coupled receptor P2Y12 in megakaryocytic DAMI cells. Platelets. 2012;23:579–585. doi: 10.3109/09537104.2012.670812. [DOI] [PubMed] [Google Scholar]
- Lev PR, Goette NP, Glembotsky AC, Laguens RP, Meckert PM, Salim JP, Heller PG, Pozner RG, Marta RF, Molinas FC. Production of functional platelet-like particles by the megakaryoblastic DAMI cell line provides a model for platelet biogenesis. Platelets. 2011;22:28–38. doi: 10.3109/09537104.2010.515271. [DOI] [PubMed] [Google Scholar]
- Levova K, Moserova M, Kotrbova V, Sulc M, Henderson CJ, Wolf CR, Phillips DH, Frei E, Schmeiser HH, Mares J, Arlt VM, Stiborova M. Role of cytochromes P450 1A1/2 in detoxication and activation of carcinogenic aristolochic acid I: studies with the hepatic NADPH:cytochrome P450 reductase null (HRN) mouse model. Toxicol. Sci. 2011;121:43–56. doi: 10.1093/toxsci/kfr050. [DOI] [PubMed] [Google Scholar]
- Li WJ, Zhang HY, Miao CL, Tang RB, Du X, Shi JH, Ma CS. Cigarette smoking inhibits the anti-platelet activity of aspirin in patients with coronary heart disease. Chin. Med. J. 2011;124:1569–1572. [PubMed] [Google Scholar]
- Lindsey S, Papoutsakis ET. The aryl hydrocarbon receptor (AHR) transcription factor regulates megakaryocytic polyploidization. Br. J. Haematol. 2011;152:469–484. doi: 10.1111/j.1365-2141.2010.08548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutton JD, Schwartzman ML, Abraham NG. Cytochrome P450 dependent arachidonic acid metabolism in hemopoietic cells. Adv. Exp. Med. Biol. 1989;271:115–121. doi: 10.1007/978-1-4613-0623-8_13. [DOI] [PubMed] [Google Scholar]
- Martin SW, Stevens AJ, Brennan BS, Reis ML, Gifford LA, Rowland M, Houston JB. Regional drug delivery I: permeability characteristics of the rat 6-day-old air pouch model of inflammation. Pharm. Res. 1995;12:1980–1986. doi: 10.1023/A:1016260426830. [DOI] [PubMed] [Google Scholar]
- Mitra R, Guo Z, Milani M, Mesaros C, Rodriguez M, Nguyen J, Luo X, Clarke D, Lamba J, Schuetz E, Donner DB, Puli N, Falck JR, Capdevila J, Gupta K, Blair IA, Potter DA. CYP3A4 mediates growth of estrogen receptor-positive breast cancer cells in part by inducing nuclear translocation of phospho-Stat3 through biosynthesis of (+/−)-14,15-epoxyeicosatrienoic acid (EET) J. Biol. Chem. 2011;286:17543–17559. doi: 10.1074/jbc.M110.198515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata N. Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacol. Ther. 2008;118:18–35. doi: 10.1016/j.pharmthera.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Pearson T, Warren AY, Barrett DA, Khan RN. Detection of EETs and HETE-generating cytochrome P-450 enzymes and the effects of their metabolites on myometrial and vascular function. Am. J. Physiol. Endocrinol. Metab. 2009;297:647–656. doi: 10.1152/ajpendo.00227.2009. [DOI] [PubMed] [Google Scholar]
- Pfister SL, Gauthier KM, Campbell WB. Vascular pharmacology of epoxyeicosatrienoic acids. Adv. Pharmacol. 2010;60:27–59. doi: 10.1016/B978-0-12-385061-4.00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeldt W, Resch K, Goppelt-Struebe M. Cytosolic phospholipase A2 from human monocytic cells: characterization of substrate specificity and Ca(2+)-dependent membrane association. Biochem. J. 1993;293:255–261. doi: 10.1042/bj2930255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MD, Marques-Magallanes JA, Yuan M, Sun W, Tashkin DP, Hankinson O. Induction and regulation of the carcinogen-metabolizing enzyme CYP1A1 by marijuana smoke and delta (9)-tetrahydrocannabinol. Am. J. Respir. Cell Mol. Biol. 2001;24:339–344. doi: 10.1165/ajrcmb.24.3.4252. [DOI] [PubMed] [Google Scholar]
- Seidegard J, DePierre JW, Pero RW. Measurement and characterization of membrane-bound and soluble epoxide hydrolase activities in resting mononuclear leukocytes from human blood. Cancer Res. 1984;44:3654–3660. [PubMed] [Google Scholar]
- Sheppard KA, Greenberg SM, Funk CD, Romano M, Serhan CN. Lipoxin generation by human megakaryocyte-induced 12-lipoxygenase. Biochim. Biophys. Acta. 1992;1133:223–234. doi: 10.1016/0167-4889(92)90073-K. [DOI] [PubMed] [Google Scholar]
- Shi X, Cai W, Zhou Y, Zhang X, Xiong L, Li R, Yu X, Li W. IL-13 upregulates GPIIb expression in megakaryocytic cell lines via STAT6. Anat. Rec. 2010;293:1470–1476. doi: 10.1002/ar.21144. [DOI] [PubMed] [Google Scholar]
- Shinde DD, Kim KB, Oh KS, Abdalla N, Liu KH, Bae SK, Shon JH, Kim HS, Kim DH, Shin JG. LC-MS/MS for the simultaneous analysis of arachidonic acid and 32 related metabolites in human plasma: basal plasma concentrations and aspirin-induced changes of eicosanoids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012;911:113–121. doi: 10.1016/j.jchromb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Siest G, Jeannesson E, Marteau JB, Samara A, Marie B, Pfister M, Visvikis-Siest S. Transcription factor and drug-metabolizing enzyme gene expression in lymphocytes from healthy human subjects. Drug Metab. Dispos. 2008;36:182–189. doi: 10.1124/dmd.107.017228. [DOI] [PubMed] [Google Scholar]
- Smith MT, Zhang L, McHale CM, Skibola CF, Rappaport SM. Benzene, the exposome and future investigations of leukemia etiology. Chem. Biol. Interact. 2011;192:155–159. doi: 10.1016/j.cbi.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiecker M, Liao J. Cytochrome P450 epoxygenase CYP2J2 and the risk of coronary artery disease. Trends Cardiovasc. Med. 2006;16:204–208. doi: 10.1016/j.tcm.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Spriet I, Meersseman W, de Hoon J, von Winckelmann S, Wilmer A, Willems L. Mini-series: II. Clinical aspects. Clinically relevant CYP450-mediated drug interactions in the ICU. Intensive Care Med. 2009;35:603–612. doi: 10.1007/s00134-008-1383-2. [DOI] [PubMed] [Google Scholar]
- Theken KN, Deng Y, Kannon MA, Miller TM, Poloyac SM, Lee CR. Activation of the acute inflammatory response alters cytochrome P450 expression and eicosanoid metabolism. Drug Metab. Dispos. 2011;39:22–29. doi: 10.1124/dmd.110.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Moomaw CR, Tomer KB, Falck JR, Zeldin DC. Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J. Biol. Chem. 1996;271:3460–3468. doi: 10.1074/jbc.271.7.3460. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhang XA, Wang DW. The roles of CYP450 epoxygenases and metabolites, epoxyeicosatrienoic acids, in cardiovascular and malignant diseases. Adv. Drug Deliv. Rev. 2011;63:597–609. doi: 10.1016/j.addr.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Yang SH, Lee MG. Effects of cytochrome P450 (CYP) inducers and inhibitors on ondansetron pharmacokinetics in rats: involvement of hepatic CYP2D subfamily and 3A1/2 in ondansetron metabolism. J. Pharm. Pharmacol. 2008;60:853–861. doi: 10.1211/jpp.60.7.0006. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Silverman RB. Revisiting heme mechanisms. A perspective on the mechanisms of nitric oxide synthase (NOS), heme oxygenase (HO), and cytochrome P450s (CYP450s) Biochemistry. 2008;47:2231–2243. doi: 10.1021/bi7023817. [DOI] [PubMed] [Google Scholar]
- Zordoky BN, Anwar-Mohamed A, Aboutabl ME, El-Kadi AO. Acute doxorubicintoxicity differentially alters cytochrome P450 expression and arachidonic acid metabolism in rat kidney and liver. Drug Metab. Dispos. 2011;39:1440–1450. doi: 10.1124/dmd.111.039123. [DOI] [PubMed] [Google Scholar]