Abstract
Uterine sarcomas and mixed epithelial–mesenchymal uterine tumors are a heterogeneous group of rare tumors for which there are very few diagnostic markers available. As aberrant microRNA (miRNA) expression patterns represent putative diagnostic cancer markers, we aimed to identify miRNA expression profiles of the major uterine sarcoma subtypes and mixed epithelial–mesenchymal tumors of the uterus. Eighty-eight miRNAs were assessed by quantitative RT-PCR in cancerous and non-cancerous tissue samples collected from 29 patients with endometrial sarcoma, leiomyosarcoma, and mixed epithelial–mesenchymal tumors. Tumor and control samples significantly (P < 0.05) differed in the expression of miR-23b, miR-1, let-7f, and let-7c in endometrial sarcomas, and miR-1, let-7c, miR-133b, let-7b, miR-143, let-7a, let-7d, let-7e, let-7g, miR-222, let-7i, and miR-214 in mixed epithelial–mesenchymal tumors. All the significantly changed miRNAs were down-regulated in the malignant tissues as compared to their normal counterparts. This may suggest their tumor suppressor role in these malignancies. No statistically significant changes in miRNA expression levels were found between leiomyosarcoma tumors and controls. The identified miRNAs warrant further studies as valuable candidate markers for the differential diagnosis of uterine sarcomas from benign uterine lesions and between uterine sarcoma subtypes.
Electronic supplementary material
The online version of this article (doi:10.1007/s13277-013-0748-5) contains supplementary material, which is available to authorized users.
Keywords: Uterine sarcoma, Carcinosarcoma, Adenosarcoma, Endometrial sarcoma, Leiomyosarcoma, microRNA, Biomarkers
Introduction
Uterine sarcomas are a heterogeneous group of rare tumors that constitute up to 8 % of cancers of the uterine corpus and 1 % of all tumors of the female genital tract [1, 2]. Uterine sarcomas are classified as mesenchymal tumors (endometrial sarcomas and leiomyosarcomas) and mixed epithelial–mesenchymal tumors (including adenosarcomas and carcinosarcomas) [3]. Carcinosarcomas were reclassified as a dedifferentiated or metaplastic form of endometrial carcinoma but are still included in the 2003 World Health Organization (WHO) classification [3, 4]. Uterine sarcomas are among the most lethal uterine malignancies with worse prognosis than other gynecologic malignancies; 5-year survival rate is below 50 % for stage I and less than 30 % for the remaining stages [2]. This group of tumors must be considered in a differential diagnosis of patients with abnormal uterine bleeding. Preoperative diagnosis of uterine sarcomas, including imaging-based diagnosis, is extremely difficult because there are no unambigous pathognomonic features of this group of tumors. Since uterine sarcomas are rarely diagnosed preoperatively, the initial treatment is often based on an inappropriate diagnosis.
microRNAs (miRNAs, miRs) are approximately 22-nucleotide long, non-coding RNAs that regulate the translation of the coding mRNAs. These molecules are generally considered to be negative regulators of gene expression [5], yet they may also function as inducers of translation [6]. miRNAs are involved in a variety of both normal and pathological biological processes. They are key players in the regulation of carcinogenesis where they may play a role of either tumor inducers (so-called oncomirs) or supressors [7]. Accumulating data on miRNAs’ expression in different tumor types points to miRNAs as putative diagnostic, prognostic, and predictive markers.
This study aimed at identification of miRNA expression patterns in uterine sarcomas and mixed epithelial–mesenchymal uterine tumors.
Materials and methods
Ten patients treated for endometrial sarcoma, 8 for leiomyosarcoma, and 11 treated for mixed epithelial–mesenchymal tumors at the Maria Sklodowska-Curie Memorial Cancer Centre and Institute of Oncology in Warsaw, between February 2009 and December 2010, were enrolled. The selected patients’ characteristics are presented in Table 1. The study was approved by the Independent Ethics Committee of the Maria Sklodowska-Curie Memorial Cancer Centre and Institute of Oncology in Warsaw and all patients gave their informed consent. Tissue specimens were divided into two parts: one part was examined histologically and the other was frozen in liquid nitrogen and stored at −70 °C until RNA isolation. In addition to 29 tumor tissue samples, 12 samples of normal uterine tissue were also collected from patients enrolled in the study.
Table 1.
Individual patient data
| Patient | Age (years) | Tumor type | Tumor histology/histological grade | Samples analyzed | ||
|---|---|---|---|---|---|---|
| Mesenchymal tumors | Endometrial sarcomas | 6 | 76.7 | Recurrent | Endometrial stromal sarcoma, low grade | T |
| 13 | 60.1 | Recurrent | Undifferentiated endometrial sarcoma | T | ||
| 16 | 43.3 | Primary | Undifferentiated endometrial sarcoma | T | ||
| 17 | 74.8 | Primary | Undifferentiated endometrial sarcoma | T, C | ||
| 31 | 51.0 | Primary | Endometrial stromal sarcoma, low grade | T, C | ||
| 36 | 64.6 | Primary | Undifferentiated endometrial sarcoma | T, C | ||
| 38 | 44.8 | Primary | Endometrial stromal sarcoma, low grade | T, C | ||
| 52 | 78.1 | Primary | Undifferentiated endometrial sarcoma | T | ||
| 62 | 53.4 | Primary | Undifferentiated endometrial sarcoma | T, C | ||
| 64 | 68.9 | Primary | Undifferentiated endometrial sarcoma | T | ||
| Smooth muscle tumors | 2 | 52.6 | Recurrent | Leiomyosarcoma/G3 | T, C | |
| 12 | 56.3 | Primary | Leiomyosarcoma/G3 | T, C | ||
| 23 | 63.2 | Recurrent | Leiomyosarcoma/G2 | T | ||
| 26 | 45.8 | Recurrent | Leiomyosarcoma/G3 | T | ||
| 28 | 51.5 | Recurrent | Leiomyosarcoma/G2 | T | ||
| 35 | 57.5 | Recurrent | Leiomyosarcoma/G2 | T | ||
| 63 | 40.5 | Recurrent | Leiomyosarcoma/G3 | T | ||
| 90 | 59.1 | Recurrent | Leiomyosarcoma/G2 | T | ||
| Mixed epithelial and mesenchymal tumors | 3 | 75.5 | Recurrent | Carcinosarcoma heterologousa | T | |
| 11 | 61.4 | Primary | Carcinosarcoma homologousb | T, C | ||
| 14 | 23.2 | Primary | Adenosarcoma homologous | T, C | ||
| 18 | 66.4 | Primary | Carcinosarcoma heterologous | T | ||
| 24 | 66.6 | Primary | Carcinosarcoma heterologous | T, C | ||
| 25 | 61.0 | Recurrent | Carcinosarcoma homologous | T | ||
| 30 | 64.3 | Primary | Carcinosarcoma heterologous | T, C | ||
| 66 | 55.1 | Primary | Carcinosarcoma homologous | T, C | ||
| 67 | 59.7 | Primary | Adenosarcoma homologous | T | ||
| 71 | 55.5 | Recurrent | Adenosarcoma homologous | T | ||
| 86 | 53.8 | Recurrent | Adenosarcoma homologous | T |
Abbreviations: T tumor sample, C control, i.e., a sample of normal uterine tissue obtained from the same patient
aHeterologous tumor—representing malignant counterparts that normally do not occur in the uterus
bHomologous tumor—representing malignant counterparts of tissues indigenous to the uterus
Human uterine sarcoma cell lines MES-SA (CRL-1976, derived from a poorly differentiated uterine sarcoma) and SK-UT-1 (derived from mixed mesodermal tumor) purchased from the American Type Tissue Collection, were cultured in the McCoy’s 5A and Dulbecco’s modified Eagle’s medium, respectively, supplemented with 10 % of FCS (Gibco) and gentamycin (Sigma). Cells were seeded at 50 × 103, in 75-cm2 bottles and passaged every 4–5 days by trypsinisation. 5 × 106 cells were collected, washed three times in PBS at 4 °C, snap-frozen and stored at –70 °C until RNA isolation.
Total RNA was isolated from approximately 50 mg of pulverized (with the Microdismembrator II, B Braun Biotech International) tissues as well as from harvested MES-SA and SK-UT-1 cell lines using miRNeasy Mini Kit with on-column DNase digestion (Qiagen), according to the manufacturer’s protocol. RNA quantity was assessed using the NanoDrop 2000 Spectrophotometer (ThermoScientific), while its quality was visually assessed following gel electrophoresis using FlashGel System (Lonza). RNA was extracted from 41 tissue samples (29 tumors and 12 control samples, i.e., fragments of normal uterine tissues). Reverse transcription was performed on 1 μg total RNA with the RT2 miRNA First Strand Kit (Qiagen) and used for Human Cancer microRNA PCR Arrays (MAH-102 from SABiosciences, miRNA list available at http://www.sabiosciences.com/mirna_pcr_product/HTML/MAH-102A.html).
Quantitative real-time PCR (qPCR) analysis was performed with the RT2 Real-Time PCR Master Mix (SABiosciences) in the 7500 Fast Real-Time PCR System (Applied Biosystems) following the manufacturer’s protocol (SABiosciences). The collected data were analyzed using threshold-cycle (Ct) values for the miRNAs with the SDS 2.1 software (Applied Biosystems). The normalized miR expression values in uterine sarcomas and mixed epithelial–mesenchymal uterine tumors as well in the cell lines were determined with DataAssist software (Applied Biosystems).
The Wilcoxon signed-rank test was used to test significance of difference in miRs’ expression between the tumor tissues and control samples. The statistical analysis was carried out using the R software (version 2.14.1, http://www.R-project.org). P values were corrected for multiple hypotheses using Benjamini–Hochberg algorithm. The differences were considered statistically significant at P < 0.05. Expression levels for the selected miRs were graphed using R software.
Results
Four potential reference genes for RT-qPCR application, namely SNORD44, SNORD47, SNORD48, and RNU6-2, were evaluated for normalization in 41 tissue samples (29 tumors and 12 fragments of normal uterine tissues) as well as in the two human sarcoma cell lines: MES-SA and SK-UT-1. Based on the expression stability values obtained in the data analysis using the geNorm™ algorithm (integrated into DataAssist software), SNORD47 and RNU6-2 were chosen as reference genes.
miRNAs isolated from 41 fresh-frozen specimens obtained from patients with uterine sarcomas and mixed epithelial–mesenchymal uterine tumors were tested for the expression of 88 types of miRNA known to be involved in carcinogenesis. Expression levels were analyzed using DataAssist software and values were normalized to SNORD47 and RNU6-2.
Fold changes in the median expression values of miRs that were significantly differentially expressed between tumor (n = 29) and control (n = 12) samples are presented in Table 2. The fold changes in the miRs’ expression values in the MES-SA and SKUT-1 cell lines related to the control uterine tissue samples, compared to the results obtained in clinical samples, are provided in Electronic supplementary material (ESM) Supplementary Fig. 1.
Table 2.
Fold changes (FC) in the median expression values of the significantly (P < 0.05) differentially expressed miRs in all the analyzed tumors (n = 29) compared to control (n = 12) samples, P value for differential expression between tumors and controls from Wilcoxon signed rank test adjusted for multiple hypotheses testing
| miR | FC | P value |
|---|---|---|
| miR-10b | 0.04 | 0.029 |
| miR-1 | 0.05 | 0.022 |
| miR-23b | 0.07 | 0.009 |
| miR-125b | 0.08 | 0.022 |
| miR-143 | 0.09 | 0.019 |
| let-7c | 0.12 | 0.000 |
| let-7b | 0.15 | 0.002 |
| miR-150 | 0.18 | 0.029 |
| miR-27b | 0.21 | 0.012 |
| let-7a | 0.21 | 0.004 |
| miR-193a-5p | 0.25 | 0.023 |
| let-7e | 0.25 | 0.006 |
| let-7f | 0.29 | 0.015 |
| let-7d | 0.35 | 0.011 |
| miR-126 | 0.37 | 0.029 |
| miR-214 | 0.50 | 0.006 |
Subsequently, the expression data were analyzed within the three groups of uterine tumors—endometrial sarcomas (n = 10), leiomyosarcomas (n = 8), and mixed epithelial–mesenchymal tumors (n = 11)—compared to all the control (n = 12) samples. Four miR genes, namely miR-23b, miR-1, let-7f, and let-7c, were found to be down-regulated in endometrial sarcomas when compared to the control samples (see Fig. 1). The expression levels of 12 miRs, miR-1, let-7c, miR-133b, let-7b, miR-143, let-7a, let-7d, let-7e, let-7g, miR-222, let-7i, and miR-214, which were down-regulated in mixed epithelial–mesenchymal tumors are presented in Fig. 2. The expression of the above-mentioned miRs’ presented in Figs. 1 and 2 significantly differed (P < 0.05) between the tumor and control samples. No statistically significant changes in miRNA expression levels were found between leiomyosarcoma tumors and controls.
Fig. 1.
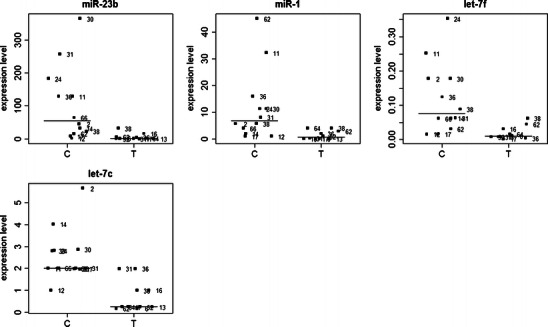
Differential expression of miR-23b, miR-1, let-7f, and let-7c in the normal uterine fragments (controls, C) and in endometrial sarcoma tumors (T). Lines indicate median values
Fig. 2.
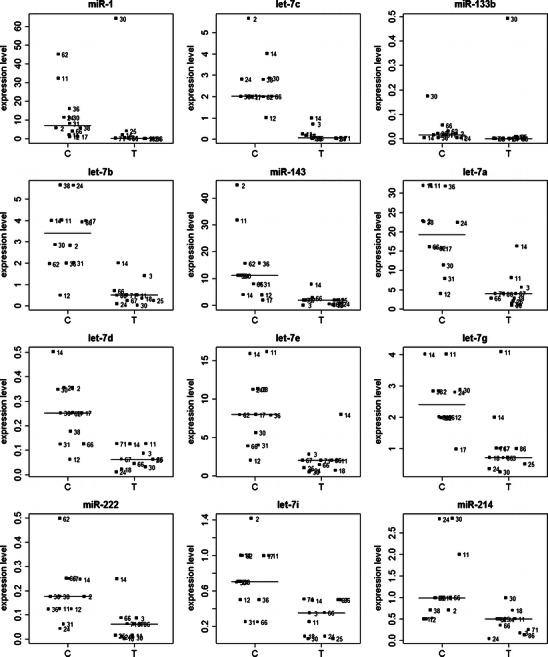
Differential expression of miR-1, let-7c, miR-133b, let-7b, miR-143, let-7a, let-7d, let-7e, let-7g, miR-222, let-7i, and miR-214 in the normal uterine fragments (controls, C) and in the mixed epithelial–mesenchymal uterine tumors (T). Lines indicate median values
The fold change values in the median expression of all the analyzed miRs between tumor and control samples, stratified by the disease type, are presented in ESM Supplementary Table 1.
Discussion
Uterine sarcomas present a significant therapeutic problem. Clinical data demonstrate the aggressive behavior of these tumors, with a high recurrence rate, and despite development of new adjuvant treatments patient outcomes have not improved. The benefits of chemotherapy are limited, and radiotherapy can result in a lower rate of local recurrence but still has no effect on patients’ overall survival [8]. Implementation of targeted therapy and individualized treatment of uterine sarcoma patients appears necessary [4]. Different course of this disease is associated not only with the histopathological variety of the tumors, as even patients with the same diagnosis may present different disease course. However, while individualized treatment should be based on preoperative diagnosis, the differential diagnosis of uterine tumors frequently causes problems [9].
Our goal was to identify alterations in miRNA expression in pure mesenchymal tumors and mixed epithelial–mesenchymal tumors of the uterus. The vast majority of 88 miR molecules analyzed here was down-regulated in the analyzed tumors when compared to normal uterine fragments. Pan et al. [10] observed a significantly lower number of miRNAs expressed in leiomyomas compared with normal myometrium, and a further reduction of miRNA expression in T-LSMC, a transformed culture of leiomyoma and SKLM-1, a leiomyosarcoma cell line, provided grounds to the authors’ hypothesis on the biological significance of these miRNAs and their target genes in the pathogenesis of leiomyosarcoma. Our observations were consistent with this hypothesis and extended it to uterine leiomyosarcomas as well as to endometrial sarcomas and mixed epithelial–mesenchymal tumors.
To our knowledge, miRNA signatures of uterine pure mesenchymal and mixed epithelial–mesenchymal tumors of different subtypes have not been previously studied. In our study, four miRs, i.e., miR-23b, miR-1, let-7f, and let-7c, were found to be down-regulated in endometrial sarcomas vs. control samples.
In the only other study to have examined miR expression in mixed uterine tumors, Ratner et al. [11] found miRNA signatures that discriminated carcinosarcomas from uterine carcinomas. In carcinosarcomas, they revealed up-regulated miR-19a and miR-19b—compared to endometrioid tumors, miR-182—compared to papillary serous carcinomas, miR-301, miR-20b, and miR-487b—jointly compared to endometrioid and papillary serous tumors. The down-regulated miRs in carcinosarcomas included miR-133a—as compared to endometrioid tumors, miR-22—as compared to papillary serous carcinomas, and miR-518b—as compared to endometrioid and papillary serous tumors, jointly. In accordance, we found mixed tumors to over-express miR-19a, miR-301a, and miR-20b; however, this has not reached statistical significance. The remaining miR molecules (miR-19b, miR-182, miR-487b, miR-133a, miR-22, and miR-518b) warrant further investigation. As we showed here, mixed epithelial–mesenchymal tumors compared to the normal uterine fragments, expressed decreased levels of 12 miRs (miR-1, let-7c, miR-133b, let-7b, miR-143, let-7a, let-7d, let-7e, let-7g, miR-222, let-7i, and miR-214).
Pan et al. [10] documented miR-20a, miR-21, and miR-206 to be up-regulated and miR-142-5p to be down-regulated in leiomyomas obtained from Caucasians as normalized to normal myometrium. The results obtained by Wang et al. [12] indicate that uterine leiomyomas, when compared to the matched myometrium, have a homogenous and specific miRNA signature, involving significantly over-expressed let-7 family, miR-21, miR-23b, and miR-27a as well as down-regulated miR-29b, miR-32, miR-144, and miR-212. Table 3 summarizes a comparison between our results and those of Pan et al. [10] and Wang et al. [12] on leiomyomas, and reveals that a decrease in miR-29b and miR-212 expression is commonly shared by leiomyomas, uterine sarcomas, and mixed epithelial–mesenchymal tumors. Studies in a mouse model of rhabdomyosarcoma have shown that reconstitution of miR-29b/c [13] and miR-206 [14] inhibits tumor growth and stimulates muscle differentiation, suggesting their role of a tumor suppressor. Correspondingly, miR-29b was down-regulated in all tumors examined in our study. However, an unexpected trend (though insignificant) towards miR-206 up-regulation was observed in endometrial sarcomas and mixed epithelial–mesenchymal tumors. Similarly Pan et al. [15] demonstrated miR-206 to be up-regulated in ectopic endometrium compared to the paired eutopic endometrium of women with endometriosis. In a recent study, Jin et al. [16] demonstrated that angiotensin II (Ang II) increased the expression of miR-132 and miR-212 cluster (miR-132/212) in rat vascular smooth muscle cells (RVSMC) in vitro, and in the aortas of Ang II-infused mice in vivo. These authors observed a positive feedback loop mechanism between cyclic AMP-response element binding protein activation and miR-132 expression and revealed that a subset of genes down-regulated by Ang II was a predicted target of miR-132. A key role of miR-132/212 in Ang II-induced monocyte chemoattractant protein-1 (MCP-1) gene expression in RVSMC was discovered. In RVSMC, Jin et al. [16] proved that phosphatase and tensin homolog (PTEN) is a target of miR-132, and that the induction of MCP-1 occurs via PTEN repression. Mutational inactivation of PTEN is not a common event in uterine sarcomas [17, 18], though in carcinosarcomas it may play a tumorogenic role [19]. Our data do not support the repression of PTEN in uterine sarcomas and mixed epithelial–mesenchymal tumors via miR-132/212, as both members of this cluster were down-regulated in all the tumors analyzed in our study.
Table 3.
Comparison of our results with those obtained by Pan et al. [10] and Wang et al. [12] for leiomyomas
| miR | Leiomyomas | Endometrial sarcomas | Leiomyosarcomas | Mixed epithelial–mesenchymal tumors |
|---|---|---|---|---|
| miR-20a | ↑ [10] | ↑ | ↓ | ↑ |
| miR-21 a | ↑ [10, 12] | ↓ | ↓ | ↓ |
| miR-206 | ↑ [10] | ↑ | ↓ | ↑ |
| miR-142-5p | ↓ [10] | ↑ | ↓ | ↑ |
| let-7 familya | ↑ [12] | ↓ | ↓ | ↓ |
| miR-23b a | ↑ [12] | ↓ | ↓ | ↓ |
| miR-27a a | ↑ [12] | ↓ | ↓ | ↓ |
| miR-29b | ↓ [12] | ↓ | ↓ | ↓ |
| miR-32 a | ↓ [12] | ↑ | ↑ | ↑ |
| miR-144 a | ↓ [12] | – | – | – |
| miR-212 | ↓ [12] | ↓ | ↓ | ↓ |
“↑” – increased, “↓” – decreased, and “–” unchanged expression levels in tumors vs control samples
amiRs that are differentially expressed between malignant and benign lesions
Importantly, the distinct patterns of expression of miR-21, let-7 family, miR-23b, and miR-27a between our samples and leiomyomas examined by Pan et al. [10] and Wang et al. [12] make these molecules putative markers distinguishing uterine sarcomas and mixed epithelial–mesenchymal tumors from leiomyomas. All these miRs were up-regulated in leiomyomas and commonly down-regulated in the tumors analyzed in our study. miR-21 is a proapoptotic oncomir [20], regulating multiple programs that enhance cell proliferation, apoptosis, and tumor invasiveness by targeting, e.g., PTEN, PDCD4, and RECK [21]. Its increased expression has been observed in many tumors, including endometrial cancer [22] and cervical cancer [23]. Our findings on miR-21 are similar to those in eutopic endometrium of women with endometriosis by Pan et al. [15] who demonstrated a slight down-regulation of miR-21 in these tissues compared to normal endometrium. miR-23b also functions as an oncomir, by targeting proline oxidase (POX), which suppresses proliferation and induces apoptosis through generation of reactive oxygen species [24] and by inhibiting invasion via suppressing several prometastatic genes [25]. An oncomir activity of miR-27a has also been observed. It down-regulates prohibitin (PHB, anti-proliferative protein), as documented in gastric adenocarcinoma cells [26], and targets Sprouty2 (Spry2, tumor supressor, an antagonist of the Ras/MAPK signaling pathway) in pancreatic adenocarcinoma cells [27]. In view of the above, our results on the three oncomirs, miR-21, miR-23b, and miR-27a, to be under-expressed in tumor samples may suggest that their oncogenic role is relative. Yet our findings on a common decrease in the expression of the let-7 family members (let-7a, let-7b, let-7c, let-7d, let-7e, let-7f, let-7g and let-7i) in tumor samples support the notion of let-7 family members as tumor suppressors influencing survival [28 and references therein]. Most or all let-7 family members are down-regulated in many cancer types, including sarcomas [29].
Additional putative markers distinguishing uterine sarcomas and mixed epithelial–mesenchymal tumors from leiomyomas are miR-32 and miR-144 molecules. According to Pan et al. [10] both miRs are down-regulated in leiomyomas. MiR-32 was commonly up-regulated in all the tumors analyzed in our study while the expression of miR-144 remained unchanged when compared to the control samples. miR-32 may have an anti-apoptotic activity, as in human leukemia 60 (HL60) cells it was shown to reduce the expression of BCL2L11 encoding the proapoptotic protein Bim [30]. miR-144 (functioning in a cluster with miR-451) promotes erythropoiesis [31], down-regulates insulin receptor substrate 1 (IRS1) [32] and represses NRF2 [33], a key regulator of oxidative stress response in erythrocytes. miR-144 confers protection against simulated ischemia/reperfusion-induced cardiomyocyte death via targeting CUG triplet repeat-binding protein 2 (CUGBP2)–COX-2 pathway [34].
This preliminary study also provides hints for the future investigations on the altered miRs that have not reached the statistical significance in our small series of samples. The fold change values of the average expression of all the analyzed miRs between tumor and control samples are provided in ESM Supplementary Table 1. In addition, our results on miR expression in the uterine sarcoma cell lines, SK-UT-1 and ME-SA, presented in ESM Supplementary Fig. 1, demonstrate a marked discrepancy between the profiles of cell lines and the matched clinical material and prove that cell lines are inadequate controls for miRNA studies in uterine sarcomas.
To conclude, we identified miRNAs, which are deregulated in uterine sarcomas and mixed epithelial–mesenchymal uterine tumors. While we recognize the low number of tissues profiled by a histopathological subtype as a limitation of our study, this is the first report on differential miRNA signatures across the subtypes of uterine sarcomas. By identifying significant differences in miRs’ expression levels in tumors compared to normal uterine tissues (Figs. 1 and 2)—though, perhaps due to the small series of samples, no specific miRNA subset classifying these subtypes was distinguished—our findings provide insights into the biology of these tumors. Some of these molecules, when further validated, may prove to have a value of biomarkers for uterine lesions. Profiling miR expression in uterine sarcomas and mixed uterine tumors and other uterine entities may provide a valuable tool for the differential diagnosis between uterine tumor subtypes and between malignant and benign uterine pathologies.
Electronic supplementary material
(DOC 199 kb)
The fold changes (RQ)—as generated with DataAssist software—in the miRs’ expression values in the SKUT-1 (a) and MES-SA (b) cell lines, compared to the leiomyosarcoma (n = 8) and mixed epithelial–mesenchymal uterine (n = 11) tumors, respectively. The control uterine tissue samples (n = 12) were used as a reference. (PNG 55 kb)
(PNG 66 kb)
Acknowledgments
We thank Anna Leonowicz for her excellent technical assistance. This work was supported by the grant of the Polish Ministry of Science and Higher Education no. NN407 134 039.
Conflicts of interests
None
References
- 1.Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989–1999. Gynecol Oncol. 2004;93:204–208. doi: 10.1016/j.ygyno.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 2.Major FJ, Blessing JA, Silverberg SG, Morrow CP, Creasman WT, Currie JL, et al. Prognostic factors in early-stage uterine sarcoma. A gynecologic oncology group study. Cancer. 1993;71:1702–1709. doi: 10.1002/cncr.2820710440. [DOI] [PubMed] [Google Scholar]
- 3.Tavassoeli FA, Deville P. Pathology and genetics of tumours of the breast and female genital organs. Lyon: IARC Press; 2003. [Google Scholar]
- 4.D’Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116:131–139. doi: 10.1016/j.ygyno.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 7.Drakaki A, Iliopoulos D. MicroRNA gene networks in oncogenesis. Curr Genomics. 2009;10:35–41. doi: 10.2174/138920209787581299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amant F, Coosemans A, Debiec-Rychter M, Timmerman D, Vergote I. Clinical management of uterine sarcomas. Lancet Oncol. 2009;10:1188–1198. doi: 10.1016/S1470-2045(09)70226-8. [DOI] [PubMed] [Google Scholar]
- 9.Nasierowska-Guttmejer A, Bakula-Zalewska E. Principles of histopathological diagnosis of uterine sarcomas. Gin Onkol. 2007;5:54–60. [Google Scholar]
- 10.Pan Q, Luo X, Chegini N. Differential expression of microRNAs in myometrium and leiomyomas and regulation by ovarian steroids. J Cell Mol Med. 2008;12:227–240. doi: 10.1111/j.1582-4934.2007.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Ratner ES, Tuck D, Richter C, Nallur S, Patel RM, Schultz V, et al. MicroRNA signatures differentiate uterine cancer tumor subtypes. Gynecol Oncol. 2010;118:251–257. doi: 10.1016/j.ygyno.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T, Zhang X, Obijuru L, Laser J, Aris V, Lee P, et al. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer. 2007;46:336–347. doi: 10.1002/gcc.20415. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Garzon R, Sun H, Ladner KJ, Singh R, Dahlman J, et al. NF-kappaB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell. 2008;14:369–381. doi: 10.1016/j.ccr.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taulli R, Bersani F, Foglizzo V, Linari A, Vigna E, Ladanyi M, et al. The muscle-specific microRNA miR-206 blocks human rhabdomyosarcoma growth in xenotransplanted mice by promoting myogenic differentiation. J Clin Invest. 2009;119:2366–2378. doi: 10.1172/JCI38075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan Q, Luo X, Toloubeydokhti T, Chegini N. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol Hum Reprod. 2007;13:797–806. doi: 10.1093/molehr/gam063. [DOI] [PubMed] [Google Scholar]
- 16.Jin W, Reddy MA, Chen Z, Putta S, Lanting L, Kato M, et al. Small RNA sequencing reveals microRNAs that modulate angiotensin II effects in vascular smooth muscle cells. J Biol Chem. 2012;287:15672–15683. doi: 10.1074/jbc.M111.322669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amant F, de la Rey M, Dorfling CM, van der Walt L, Dreyer G, Dreyer L, et al. Pten mutations in uterine sarcomas. Gynecol Oncol. 2002;85:165–169. doi: 10.1006/gyno.2002.6601. [DOI] [PubMed] [Google Scholar]
- 18.Lancaster JM, Risinger JI, Carney ME, Barrett JC, Berchuck A. Mutational analysis of the PTEN gene in human uterine sarcomas. Am J Obstet Gynecol. 2001;184:1051–1053. doi: 10.1067/mob.2001.114508. [DOI] [PubMed] [Google Scholar]
- 19.de Jong RA, Nijman HW, Wijbrandi TF, Reyners AK, Boezen HM, Hollema H. Molecular markers and clinical behavior of uterine carcinosarcomas: focus on the epithelial tumor component. Mod Pathol. 2011;24:1368–1379. doi: 10.1038/modpathol.2011.88. [DOI] [PubMed] [Google Scholar]
- 20.Zhou X, Zhang J, Jia Q, Ren Y, Wang Y, Shi L, et al. Reduction of miR-21 induces glioma cell apoptosis via activating caspase 9 and 3. Oncol Rep. 2010;24:195–201. doi: 10.3892/or_00001020. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, Yu J, Yu S, Lavker RM, Cai L, Liu W, et al. MicroRNA-21 acts as an oncomir through multiple targets in human hepatocellular carcinoma. J Hepatol. 2010;53:98–107. doi: 10.1016/j.jhep.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Torres A, Torres K, Paszkowski T, Radej S, Staskiewicz GJ, Ceccaroni M, et al. Highly increased maspin expression corresponds with up-regulation of miR-21 in endometrial cancer: a preliminary report. Int J Gynecol Cancer. 2011;21:8–14. doi: 10.1097/IGC.0b013e318200050e. [DOI] [PubMed] [Google Scholar]
- 23.Lui WO, Pourmand N, Patterson BK, Fire A. Patterns of known and novel small RNAs in human cervical cancer. Cancer Res. 2007;67:6031–6043. doi: 10.1158/0008-5472.CAN-06-0561. [DOI] [PubMed] [Google Scholar]
- 24.Liu W, Zabirnyk O, Wang H, Shiao YH, Nickerson ML, Khalil S, et al. Mir-23b targets proline oxidase, a novel tumor suppressor protein in renal cancer. Oncogene. 2010;29:4914–4924. doi: 10.1038/onc.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Hao Y, Yang J, Zhou Y, Li J, Yin S, et al. Genome-wide functional screening of miR-23b as a pleiotropic modulator suppressing cancer metastasis. Nat Commun. 2011;2:554. doi: 10.1038/ncomms1555. [DOI] [PubMed] [Google Scholar]
- 26.Liu T, Tang H, Lang Y, Liu M, Li X. MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by targeting prohibitin. Cancer Lett. 2009;273:233–242. doi: 10.1016/j.canlet.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Peng S, Maihle NJ, Huang Y. Pluripotency factors Lin28 and Oct4 identify a sub-population of stem cell-like cells in ovarian cancer. Oncogene. 2010;29:2153–2159. doi: 10.1038/onc.2009.500. [DOI] [PubMed] [Google Scholar]
- 28.Boyerinas B, Park SM, Hau A, Murmann AE, Peter ME. The role of let-7 in cell differentiation and cancer. Endocr Relat Cancer. 2010;17:F19–36. doi: 10.1677/ERC-09-0184. [DOI] [PubMed] [Google Scholar]
- 29.Subramanian S, Lui WO, Lee CH, Espinosa I, Nielsen TO, Heinrich MC, et al. MicroRNA expression signature of human sarcomas. Oncogene. 2008;27:2015–2026. doi: 10.1038/sj.onc.1210836. [DOI] [PubMed] [Google Scholar]
- 30.Gocek E, Wang X, Liu X, Liu CG, Studzinski GP. MicroRNA-32 upregulation by 1,25-dihydroxyvitamin d3 in human myeloid leukemia cells leads to bim targeting and inhibition of arac-induced apoptosis. Cancer Res. 2011;71:6230–6239. doi: 10.1158/0008-5472.CAN-11-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasmussen KD, Simmini S, Abreu-Goodger C, Bartonicek N, Di Giacomo M, Bilbao-Cortes D, et al. The miR-144/451 locus is required for erythroid homeostasis. J Exp Med. 2010;207:1351–1358. doi: 10.1084/jem.20100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karolina DS, Armugam A, Tavintharan S, Wong MT, Lim SC, Sum CF, et al. MicroRNA 144 impairs insulin signaling by inhibiting the expression of insulin receptor substrate 1 in type 2 diabetes mellitus. PLoS One. 2011;6:e22839. doi: 10.1371/journal.pone.0022839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sangokoya C, Telen MJ, Chi JT. MicroRNA miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood. 2010;116:4338–4348. doi: 10.1182/blood-2009-04-214817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Wang X, Zhu H, Zhu C, Wang Y, Pu WT, et al. Synergistic effects of the GATA-4-mediated miR-144/451 cluster in protection against simulated ischemia/reperfusion-induced cardiomyocyte death. J Mol Cell Cardiol. 2010;49:841–850. doi: 10.1016/j.yjmcc.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 199 kb)
The fold changes (RQ)—as generated with DataAssist software—in the miRs’ expression values in the SKUT-1 (a) and MES-SA (b) cell lines, compared to the leiomyosarcoma (n = 8) and mixed epithelial–mesenchymal uterine (n = 11) tumors, respectively. The control uterine tissue samples (n = 12) were used as a reference. (PNG 55 kb)
(PNG 66 kb)


