Abstract
The present study examined the efficacy of Ocimum basilicum (basil) extract, a natural herb, with antioxidant properties, against testicular toxicity induced by cadmium (Cd), which is one of the most important toxic heavy metals. The intoxicated rats showed significant alterations in the testicular tissue including decreased seminiferous epithelium height and changes in the arrangement of spermatogenic layers. Hypospermatogensis with cytoplasmic vacuolization and pyknotic nuclei were observed. Intertubular hemorrahage and absence of spermatozoa were noted. Decreased cell proliferation was reflected by a decrease in Ki-67 expression, whereas the increase in apoptotic rate was associated with a decrease in the Bcl/Bax ratio. Concomitant treatment with aqueous basil extract led to an improvement in histological, morphometrical and immunohistochemical changes induced by Cd. The beneficial effects of basil extract could be attributed to its antioxidant properties.
Keywords: Apoptosis, Cadmium, Ocimum basilicum, Ki-67, Rats, Testis
Introduction
Spermatogenesis is a well-organized complex process, in which diploid spermatogonia proliferate and differentiate into terminally haploid mature functional sperm. During normal spermatogenesis not all spermatogonia in the testis undergo spermiogenesis to become mature sperm, and most of them are eliminated through spontaneous germ cell apoptosis [1]. Germ cell apoptosis can be induced by various environmental or physiological stresses [2, 3]. Two different mechanisms of cell apoptosis have been described: the extrinsic receptor-mediated pathway and the intrinsic mitochondria-dependent pathway. The extrinsic pathway is initiated by the bending of extracellular death ligands, such as Fas ligand (FasL) to their corresponding cell-surface receptors such as Fas. By contrast, the intrinsic pathway is mainly regulated by Bcl-2 family members including Bax, Bak, Bcl-2, and Bcl-xl which positively or negatively regulate mitochondrial outer membrane permeability to promote the release of cytochrome c and other apoptotic molecules [4, 5].
Epidemiological studies have shown correlation between heavy metals concentrations in the body and human health. The body absorbs these toxic substances which are distributed into body systems and lead to different diseases. Cadmium (Cd) is one of the most important heavy metals and shows high toxicity to different biological systems. Occupational exposure to Cd, such as working with Cd-containing pigments, plastic, glass, metal alloys and electrode material in nickel-cadmium batteries, and non-occupational exposure, such as food, water and cigarette smoke induces uptake of Cd from the environment into the body through pulmonary and enteral pathways [6]. Cd accumulates mostly in the liver, kidney, and spleen [7]. Cd is considered as a highly polluting material and its toxic effects in liver, kidney, vascular system and reproduction in humans and animals [8]. Eybl and Kotyzová [9] reported that Cd causes damage to tissues and potentially leads to carcinogenesis. Cd stimulates the production of reactive oxygen species (ROS) in association with its inhibitory effect on mitochondrial electron transport. As a result, lipids are oxidized resulting in damage to membranes [10]. Long-term exposure to Cd induced testicular toxicity [11] and apoptosis in testicular germ cells of rats [12]. Mice treated with Cd showed decreased testosterone level, increased lipid peroxidation, and caused degeneration of testicular germ cells [13].
The therapeutic use of plants and their extracts may be a promising approach for the treatment of different diseases. Ocimum basilicum (basil) is an annual herb of the Lamiaceae family and is widely cultivated in different regions of the world. O. basilicum is widely used in folk medicine to treat a wide range of diseases and has numerous pharmacological activities. Many studies have reported that basil leaf extracts have potent antioxidant, anti-aging, anticancer, antiviral, and antimicrobial properties [14-16]. Sethi et al. [17] reported that the leaves of O. sanctum possess good antioxidant and antistress potentials in experimental animals. Consumption of basil or basil oil has been associated with a reduction in total cholesterol, low-density lipoprotein and triglyceride levels [18]. Supplementation with O. sanctum leaf extract reduced the severity of hydropericardium, hepatitis, myocarditis accompanied with hemorrhages, lung edema, lymphocytic depletion in lymphoid organs and focal interstitial nephritis [19]. Ocimum leaf extracts were found to protect the liver from heavy metals [20] and prevent isoproterenol-induced myocardial necrosis in rats [21]. Basil or basil oil is useful in the prevention and treatment of cardiovascular disease [22]. Sakr et al. [23] reported that O. basilicum extract improved hepatotoxicity and apoptosis induced by CCl4 in rats. In the present study, we examined the effect of O. basilicum extract on Cd-induced testicular alterations in albino rats.
Materials and Methods
Cadmium
Cadmium chloride was obtained from Raheja Centre, Mumbia, India, packed under license of Belami Fine Chemicals, Ltd (Mumbai, India). Before use, Cd was dissolved in distilled water and administered orally at a dose level of 30 mg/kg b.w. 5 days/wk for 8 weeks [24].
Ocimum extract
Fresh leaves of O. basilicum were collected from a garden within the Faculty of Science, Menoufia University, Shebin El-kom, Egypt. The leaves were rinsed with clean water to remove any foreign matter. Leaves were dried in the shade and ground to a fine powder using a laboratory mixer. One hundred grams of leaf powder was refluxed with 750 ml of double distilled water for 1 hour and concentrated using a rotary evaporator. The extract was stored at -20℃ until used for experiments. The aqueous extract was used at a dose level of 20 mg/kg O. basilicum [25].
Animals
Male albino Wistar rats weighting 140±6 g were kept in an animal house under constant temperature conditions (24±2℃) for at least 1 week before and through the experimental work, being maintained on a standard diet composed of 20% casein, 15% corn oil, 55% corn starch, 5% salt mixture, and 5% vitamins. Water was available ad-libitum. All the experiments were done in compliance with the guide for the care and use of laboratory animals. Animals were divided into 4 groups (n=10 each) as follows:
Group 1, Rats were fed on the standard diet and served as a control group; group 2, Rats were treated with oral aqueous O. basilicum extract at a dose level of 20 mg/kg 5 days/wk for 8 weeks; group 3, Rats were treated with oral administration of Cd at a dose level of level of 30 mg/kg b.w. 5 days/wk for 8 weeks [24]; group 4, Rats were treated with Cd (30 mg/kg b.w) followed by oral administration of aqueous O. basilicum extract (20 mg/kg) 5 days/wk for 8 weeks.
Histological study
Animals were dissected and their testes were removed. For histological preparations, the testes were fixed in Bouin's fluid, dehydrated, cleared and embedded in paraffin wax. Five-micrometer thick sections were prepared and stained with Ehrlich's haematoxylin and eosin [26]. The mean thickness of the tunica albuginea and that of the basal lamina of the seminiferous tubule was measured using an ocular micrometer. The diameter of seminiferous tubules and height of the germinal epithelium were measured in the normal spermatogenic cells on the inner surface of the basement membrane through the most advanced cell types lining the lumen of the tubules.
Immunohistochemical study
For immunohistochemical localization of Ki-67, Bax, and Bcl-2, fixed wax sections were stained using the avidin-biotin peroxidase method. Formalin-fixed paraffin-embedded tissue sections were deparaffinized, endogenous peroxidase activity was blocked with H2O2 in methanol and the sections were heated in 0.01 mol/l citrate buffer in a microwave pressure cooker for 20 minutes. The slides were allowed to cool to room temperature, and nonspecific binding was blocked with normal horse serum for 20 minutes at room temperature. The MIB-1 monoclonal antibody was used for detection of nuclear Ki-67, a marker of proliferating cells (1:40, code No. M7187, Dako, Cambridge, UK). Anti-Bcl-2 and anti-Bax (Dako) monoclonal antibodies were used for detection of bcl-2 and bax, respectively. Counterstaining was performed using Mayer's hematoxylin (Cat. No. 94585, BioGenex, Menarini Diagnostics, Antony, France). For evaluation of each marker, the percentage of positively stained cells in the total number of cells under ×40 magnification was calculated. Assessment for Bax, Bcl-2, and Ki-67 was performed according to the following semi-quantitative scale: (-), negative; (+), equivocally positive; (++), weakly positive; (+++), positive; and (++++), strongly positive [27].
Statistical analysis
The results were expressed as mean±SD of different groups. The differences between the mean values were evaluated by ANOVA followed by Student's t-test using Minitab 12 computer program (Minitab Inc., State Collage, PA, USA).
Results
Change in body and testis weights
Exposure of albino rats to Cd led to a significant decrease in the body weight of animals compared to the control (P<0.05). A significant increase in body weight was observed in animals treated with Cd+O. basilicum. Animals given ocimum alone did not show differences in body weight compared to the controls. Similarly, testis weights decreased in animals treated with Cd and increased in those treated with Cd+O. basilicum (Table 1).
Table 1.
Change in body and testes weight in different animal groups

*Significant at P<0.05.
Histological results
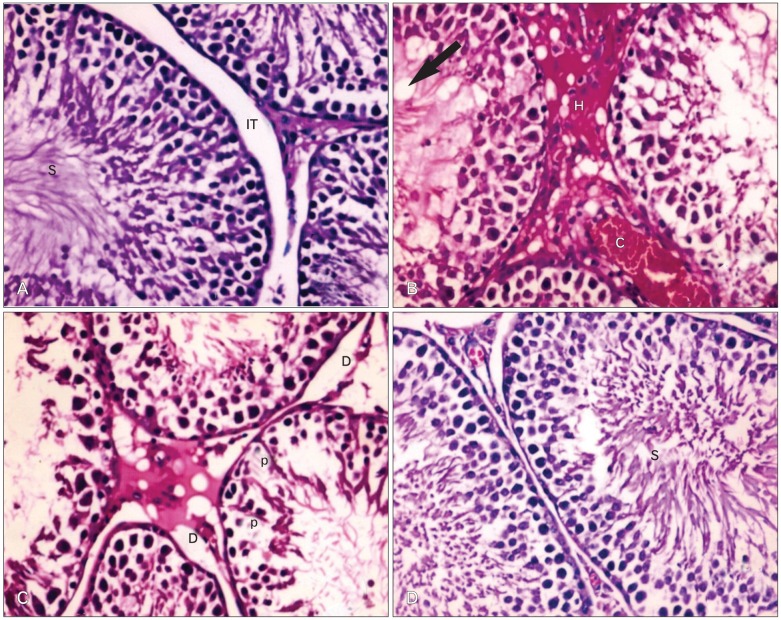
Examination of the testis of control rats showed the typical features of normal seminiferous tubules, spermatogenic cells, intertubular connective tissue and spermatozoa (Fig. 1A). Animals receiving Ocimum extract showed normal histological structure of the testes. Treatment with Cd resulted in intertubular hemorrahage and disruption of the arrangement of spermatogenic layers and appearance of vacuoles (Fig. 1B). The seminiferous tubules appeared to contain few spermatogenic cells. The spermatogonia showed cytoplasmic vacuolization with pyknotic nuclei and sperms were scattered in the lumen of the tubules (Fig. 1C). Compared to testis sections of animals exposed to Cd alone, testis sections of animals treated with Cd and Ocimum extract showed less prominent histopathological alterations (Fig. 1D).
Fig. 1.
(A) Section in testis of a control rat showing normal seminiferous tubules. S, sperms; IT, interstitial tissue. (B) Section in testis of a rat treated with Cd showing intertubular hemorrhage (H) and large vacuoles (arrow). (C) Section in testis of a rat treated with Cd showing degenerated germ cells with pyknotic nuclei (p) and degenerated interstitial tissue (D). (D) Section in testis of a rat treated with Cd and Ocimum showing increase of spermatogenic cells (A-D, ×400).
Morphometric results
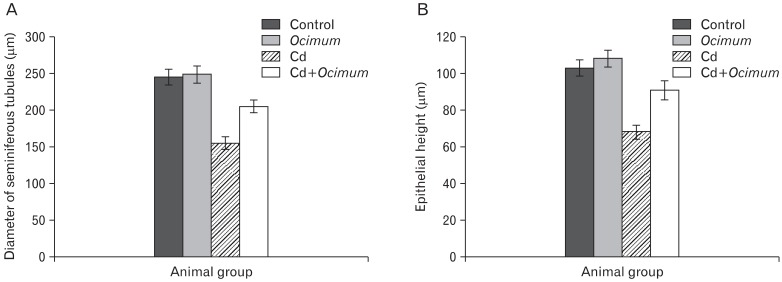
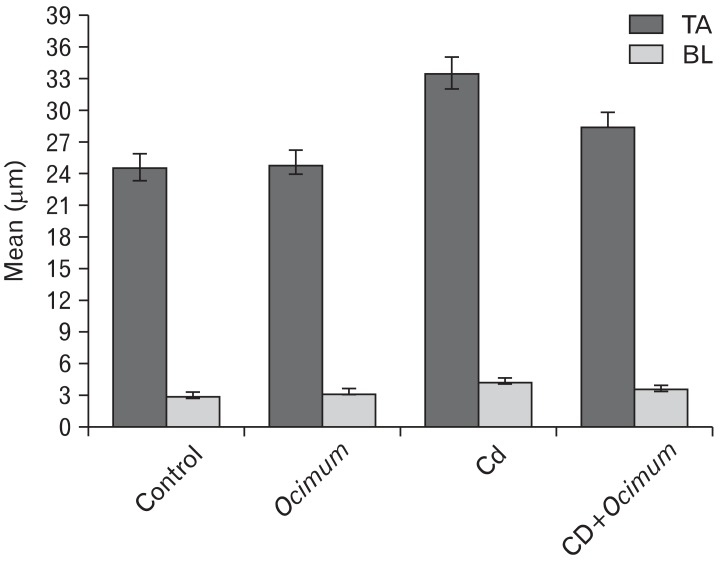
Treatment of rats with Cd for 8 weeks caused atrophy of the seminiferous tubules. The diameter of the seminiferous tubules was significantly smaller (155±8.5 µm) in Cd treated animals than in controls (245±10.5 µm) (Fig. 2A). The germ cell height of seminiferous tubules was decreased in compared to that of the control. The mean epithelial height was 103±4.4 µm and 68±3.8 µm in the controls and Cd groups, respectively (Fig. 2B). Compared with Cd-treated animals, those treated with Cd and Ocimum extract showed an improvement in the mean tubular diameter and germ cell height. In animals receiving Ocimum extract the diameter and height of seminiferous tubules were normal. The mean thickness of the tunica albuginea and basal lamina of seminiferous tubules was significantly increased in animals treated with Cd in comparison with controls, whereas the thickness of these layers was significantly decreased in animals treated with Cd+ Ocimum (Fig. 3).
Fig. 2.
Change in diameter of seminiferous tubules (A) and germ epithelial height (B) of testes of different groups.
Fig. 3.
Change in tunica albogenia (TA) and basal lamina (BL) in testes of different animal groups.
Immunohistochemical results
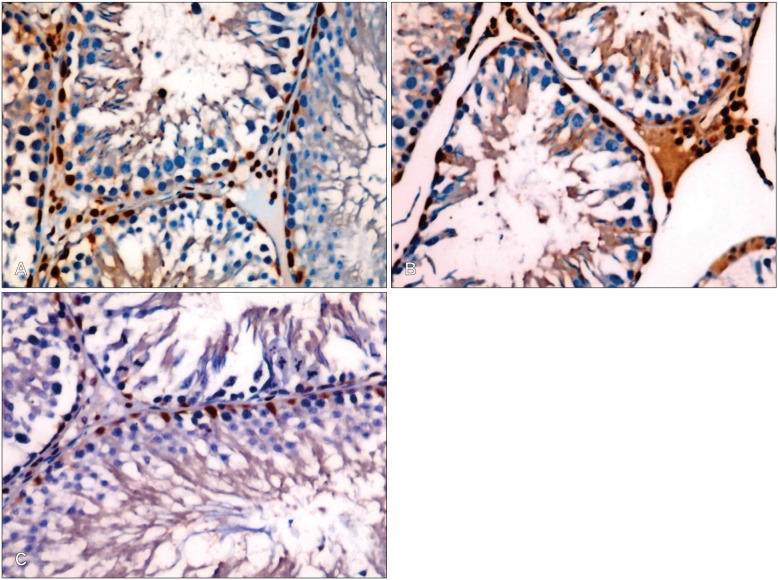
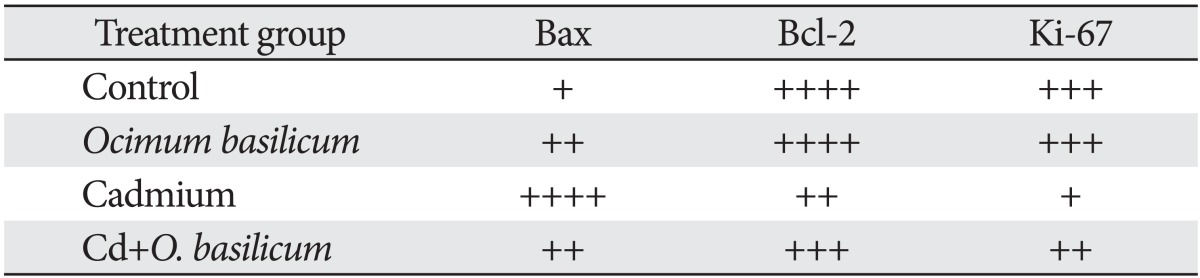
Table 2 shows the expression of Bax and Bcl-2 in the testes of the different experimental groups. Testicular tissue obtained from Cd-treated rats showed weak expression of Bcl-2 in comparison with the control group (Fig. 4A, B). Treatment of animals with Cd+Ocimum extract upregulated the expression of Bcl-2 (Fig. 4C). Increase expression of Bax was observed in animals treated with Cd (Fig. 5A). Bax expression decreased in rats treated with Cd+Ocimum (Fig. 5B). The Bcl/Bax ratio decreased in animals receiving Cd (Table 2). In the seminiferous epithelium Ki-67 is expressed in the nuclei of spermatogonia. We observed an increase in expression of Ki-67 in control rats (Fig. 6A), whereas animals treated with Cd showed decreased expression of Ki-67 (Fig. 6B). Animals treated with Cd+Ocimum extract showed an increase in the expression of Ki-67 (Fig. 6C).
Table 2.
Expression of Bax, bcl-2, and Ki-67 in testes of different animal groups
+, equivocally positive; ++, weakly positive; +++, positive; ++++, strongly positive.
Fig. 4.
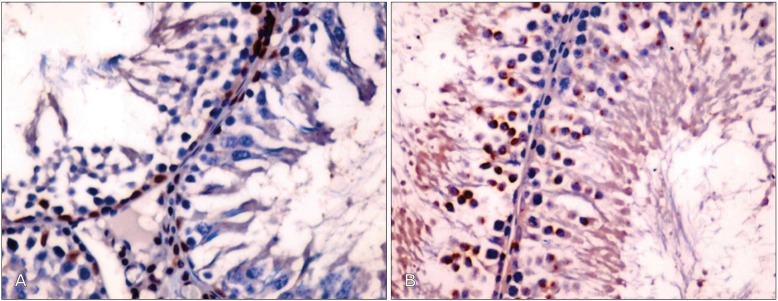
(A) Weak expression of Bcl-2 in germ cells of a rat treated with Cd. (B) An increase in expression of Bcl-2 after treatment with Cd+Ocimum (A and B, ×400).
Fig. 5.
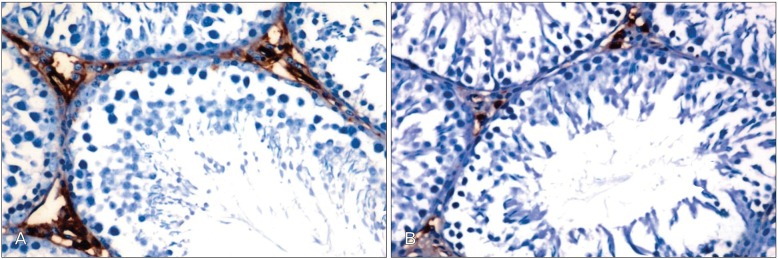
(A) Section in testis of a rat treated with Cd showing increase of Bax expression in Leydig cells. (B) Section in testis of a rat treated with Cd+Ocimum showing decrease of Bax expression (A and B, ×400).
Fig. 6.
(A) Section in testis of a control rat showing marked expression of Ki-67. (B) Section in testis of a rat treated with Cd showing decrease of Ki-67 expression (C). An increase in expression of Ki-67 after treatment with Cd+Ocimum (A-C, ×400).
Discussion
Cd is one of the main environmental and occupational pollutants in industrialized countries. Exposure to Cd is associated with serious health hazards. The present results showed that exposure of rats to Cd resulted in significant decreases in body and testis weights. Histological analysis revealed intertubular hemorrhage, and degeneration of spermatogenic cells and interstitial tissue. A reduction in seminiferous tubule diameter and germinal epithelial height was observed. Moreover, the mean thickness of the tunica albuginea and basal lamina of the seminiferous tubules increased significantly in animals treated with Cd in comparison with controls. Similarly, de Souza et al. [28] reported that high doses of Cd significantly reduced testis and epididymis weight, gonado-somatic index and the length of the seminiferous tubules in rats. Scanning electron microscopy examination of the testes showed a compact and fibrous appearance of the interstitial tissue and absence of fenestrae. Burukoğlu and Bayçu [29] reported that Cd impairs reproductive capacity by causing severe testicular degeneration, seminiferous tubule damage and necrosis in rats. Testis weights decreased in rats exposed to Cd. Blanco et al. [30] attributed this effect to the necrotic and degenerative changes induced by Cd. Ige et al. [31] reported that CdSO4 induced alterations in testicular weight, sperm count, sperm motility, and sperm morphology in rats.
The present study showed that Cd induced testicular apoptosis as indicated by an increase in Bax and decrease in Bcl-2 in germ cells. In agreement with this result, Al-Azemi et al. [27] reported that Cd-treatment upregulates Bax and downregulates Bcl-2 expression in the testis of rats. Zhang et al. [32] found that Cd has obvious adverse effects on the proliferation of piglet Sertoli cells,resulting in DNA damage, cell apoptosis, and aberrant morphology. Induction of apoptosis by exposure to Cd has been reported by other investigators [33, 34]. Ki-67 is a marker of cell proliferation. It is expressed in the nuclear matrix of cells during the late G1-, S-, G2- and M phases of the cell cycle, it peaks in the G2- and early M-phases [35]. The expression of Ki-67 decreased in the testes of animals treated with Cd. Similarly, Falana et al. [36] reported that lead caused testicular alterations in the testes of rats and decreased the expression of Ki-67. Di-(n-butyl) phthalate exposure delays germ cell development in both the fetal and postnatal life of rats, and reduces Ki-67 expression [37].
Testicular oxidative stress induced by different pollutants leads to male infertility. Cd is an inducer of oxidative stress [38, 39]. Cd may increase oxidative stress by binding to the sulfhydryl groups of proteins and by depleting glutathione [40]. Oxidative stress may promote alteration in DNA repair mechanisms and induction of cell proliferation [41]. Sen Gupta et al. [42] reported that the levels of the testicular antioxidants enzymes, superoxide dismutase, catalase and glutathione peroxidase are greatly diminished upon Cd exposure. Cd may induce germ cell apoptosis by enhancing the generation of ROS-like superoxide ion, hydroxyl radicals and hydrogen peroxide, oxidative stress can result in peroxidation, mitochondrial dysfunction or DNA damage in germ cells and oxidative damage in Leydig cells [43]. In the present study, Cd inhibited spermatogenesis and induced apoptosis. This may be attributed to an enhancement of ROS production in rats exposed to Cd.
In the present work, we showed that O. basilicum extract protects the testis from Cd toxicity as indicated by the restoration of the histological structure and increase in the number of germ cell layers. Moreover, testicular apoptosis decreased as indicated by a decrease of Bax and increase in Bcl-2 in germ cells. In accordance with these results, Khaki et al. [44] reported that O. basilicum extract protected rats from testicular damage and reduced apoptosis after exposure to an electromagnetic field. Asuquo et al. [45] found that O. gratissimum extract improved the testicular histopathological alterations in diabetic rats. The protective properties of O. basilicum have been extensively investigated. Sakr et al. [23] reported that treating animals with CCl4 and aqueous leaf extracts of O. basilica led to an improvement, in both histopathological and biochemical alterations induced by CCl4. Furthermore, apoptosis was reduced in hepatic cells. Sharma et al. [20] reported that O. basilicum has a nephroprotective effect against mercury toxicity. Basil or basil oil can be used in prevention and treatment of cardiovascular disease [22] and in prevention of isoproterenol induced myocardial necrosis [21]. Aqueous leaf extracts of O. basilicum protect rats against paracetamol-induced hepatotoxicity [46].
The leaves of O. basilicum are a rich source of flavonoids which possess various biological properties related to antioxidant mechanisms [47]. Caffeic acid is another component in the leaf of the O. basilicum that has antioxidant, anti-inflammatory, and cancer chemopreventive activities [48]. Another constituent of O. basilicum is A p-coumaric acid possess radical scavenging and antioxidant activity at high concentration [49]. Dasgupta et al. [50] reported that O. basilicum increased the activity of xenobiotic metabolizing phase I and phase II enzymes, promotingantioxidant-enzyme responses by significantly increasing activities of the hepatic glutathione reductase, superoxide dismutase, and catalase, increasing glutathione content and decreasing lipid peroxidation and lactate dehydrogenase activity in the liver of mice. The extract also showed significant anti lipid peroxidation effects in vitro, in addition to exhibiting significant activity in scavenging superoxide radical and nitric oxide radicals, indicating their potent antioxidant effects [51]. The results of the present study indicate that the ameliorative effect of O. basilicum against testicular toxicity of Cd may be attributed to its antioxidant properties.
References
- 1.Brinkworth MH, Weinbauer GF, Schlatt S, Nieschlag E. Identification of male germ cells undergoing apoptosis in adult rats. J Reprod Fertil. 1995;105:25–33. doi: 10.1530/jrf.0.1050025. [DOI] [PubMed] [Google Scholar]
- 2.Furuchi T, Masuko K, Nishimune Y, Obinata M, Matsui Y. Inhibition of testicular germ cell apoptosis and differentiation in mice misexpressing Bcl-2 in spermatogonia. Development. 1996;122:1703–1709. doi: 10.1242/dev.122.6.1703. [DOI] [PubMed] [Google Scholar]
- 3.Richburg JH. The relevance of spontaneous- and chemically-induced alterations in testicular germ cell apoptosis to toxicology. Toxicol Lett. 2000;112-113:79–86. doi: 10.1016/s0378-4274(99)00253-2. [DOI] [PubMed] [Google Scholar]
- 4.Print CG, Loveland KL. Germ cell suicide: new insights into apoptosis during spermatogenesis. Bioessays. 2000;22:423–430. doi: 10.1002/(SICI)1521-1878(200005)22:5<423::AID-BIES4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Park HH, Lo YC, Lin SC, Wang L, Yang JK, Wu H. The death domain superfamily in intracellular signaling of apoptosis and inflammation. Annu Rev Immunol. 2007;25:561–586. doi: 10.1146/annurev.immunol.25.022106.141656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waisberg M, Joseph P, Hale B, Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology. 2003;192:95–117. doi: 10.1016/s0300-483x(03)00305-6. [DOI] [PubMed] [Google Scholar]
- 7.Toman R, Golian J, Šiška B, Massányi P, Lukáč N, Adamkovičová M. Cadmium and selenium in animal tissues and their interactions after an experimental administration to rats. Slovak J Anim Sci. 2009;42(Suppl 1):115–118. [Google Scholar]
- 8.Jarup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238:201–208. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Eybl V, Kotyzová D. Protective effect of manganese in cadmium-induced hepatic oxidative damage, changes in cadmium distribution and trace elements level in mice. Interdiscip Toxicol. 2010;3:68–72. doi: 10.2478/v10102-010-0013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galazyn-Sidorczuk M, Brzóska MM, Jurczuk M, Moniuszko-Jakoniuk J. Oxidative damage to proteins and DNA in rats exposed to cadmium and/or ethanol. Chem Biol Interact. 2009;180:31–38. doi: 10.1016/j.cbi.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Nava-Hernández MP, Hauad-Marroquín LA, Bassol-Mayagoitia S, García-Arenas G, Mercado-Hernández R, Echávarri-Guzmán MA, Cerda-Flores RM. Lead-, cadmium-, and arsenic-induced DNA damage in rat germinal cells. DNA Cell Biol. 2009;28:241–248. doi: 10.1089/dna.2009.0860. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Soh J. Cadmium-induced apoptosis is mediated by the translocation of AIF to the nucleus in rat testes. Toxicol Lett. 2009;188:45–51. doi: 10.1016/j.toxlet.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Marettová E, Maretta M, Legáth J. Changes in the peritubular tissue of rat testis after cadmium treatment. Biol Trace Elem Res. 2010;134:288–295. doi: 10.1007/s12011-009-8473-z. [DOI] [PubMed] [Google Scholar]
- 14.Manosroi J, Dhumtanom P, Manosroi A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006;235:114–120. doi: 10.1016/j.canlet.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 15.de Almeida I, Alviano DS, Vieira DP, Alves PB, Blank AF, Lopes AH, Alviano CS, Rosa Mdo S. Antigiardial activity of Ocimum basilicum essential oil. Parasitol Res. 2007;101:443–452. doi: 10.1007/s00436-007-0502-2. [DOI] [PubMed] [Google Scholar]
- 16.Akujobi CO, Anyanwu BN, Onyeze GO, Ibekwe VI. Antibacterial activities and preliminary phytochemical screening of four medical plants. J Appl Sci. 2004;7:4328–4338. [Google Scholar]
- 17.Sethi J, Sood S, Seth S, Talwar A. Protective effect of Tulsi (Ocimum sanctum) on lipid peroxidation in stress induced by anemic hypoxia in rabbits. Indian J Physiol Pharmacol. 2003;47:115–119. [PubMed] [Google Scholar]
- 18.Harnafi H, Aziz M, Amrani S. Sweet basil (Ocimum basilicum L.) improves lipid metabolism in hypercholesterolemic rats. E Spen Eur E J Clin Nutr Metab. 2009;4:181–186. [Google Scholar]
- 19.Batra M, Gupta RP. Effects of Ocimum sanctum leaf on pathology and immune response in chickens experimentally infected with hydropericardium syndrome. Indian J Vet Pathol. 2006;30:1–4. [Google Scholar]
- 20.Sharma MK, Kumar M, Kumar A. Ocimum sanctum aqueous leaf extract provides protection against mercury induced toxicity in Swiss albino mice. Indian J Exp Biol. 2002;40:1079–1082. [PubMed] [Google Scholar]
- 21.Sood S, Narang D, Dinda AK, Maulik SK. Chronic oral administration of Ocimum sanctum Linn. augments cardiac endogenous antioxidants and prevents isoproterenol-induced myocardial necrosis in rats. J Pharm Pharmacol. 2005;57:127–133. doi: 10.1211/0022357055146. [DOI] [PubMed] [Google Scholar]
- 22.Rupert T. Cooking with fresh herbs and spices can boost immune systems, reduce cholesterol, and help protect against cancer. Med J Aust. 2009;361:264–270. [Google Scholar]
- 23.Sakr SA, El-Abd SF, Osman M, Kandil AM, Helmy MS. Ameliorative effect of aqueous leave extract of Ocimum basilicum on Ccl4-induced hepatotoxicity and apoptosis in albino rats. J Am Sci. 2011;7:116–127. [Google Scholar]
- 24.Ohta H, Yamauchi Y, Nakakita M, Tanaka H, Asami S, Seki Y, Yoshikawa H. Relationship between renal dysfunction and bone metabolism disorder in male rats after long-term oral quantitative cadmium administration. Ind Health. 2000;38:339–355. doi: 10.2486/indhealth.38.339. [DOI] [PubMed] [Google Scholar]
- 25.Offiah VN, Chikwendu UA. Antidiarrhoeal effects of Ocimum gratissimum leaf extract in experimental animals. J Ethnopharmacol. 1999;68:327–330. doi: 10.1016/s0378-8741(99)00100-2. [DOI] [PubMed] [Google Scholar]
- 26.Lillie RD, Fulmer HM. Histopathological technique and practical histochemistry. 4th ed. New York: McGraw Hill; 1976. [Google Scholar]
- 27.Al-Azemi M, Omu FE, Kehinde EO, Anim JT, Oriowo MA, Omu AE. Lithium protects against toxic effects of cadmium in the rat testes. J Assist Reprod Genet. 2010;27:469–476. doi: 10.1007/s10815-010-9426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Souza Predes F, Diamante MA, Dolder H. Testis response to low doses of cadmium in Wistar rats. Int J Exp Pathol. 2010;91:125–131. doi: 10.1111/j.1365-2613.2009.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burukoğlu D, Bayçu C. Protective effects of zinc on testes of cadmium-treated rats. Bull Environ Contam Toxicol. 2008;81:521–524. doi: 10.1007/s00128-007-9211-x. [DOI] [PubMed] [Google Scholar]
- 30.Blanco A, Moyano R, Vivo J, Flores-Acuña R, Molina A, Blanco C, Agüera E, Monterde JG. Quantitative changes in the testicular structure in mice exposed to low doses of cadmium. Environ Toxicol Pharmacol. 2007;23:96–101. doi: 10.1016/j.etap.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Ige SF, Olaleye SB, Akhigbe RE, Akanbi TA, Oyekunle OA, Udoh UA. Testicular toxicity and sperm quality following cadmium exposure in rats: ameliorative potentials of Allium cepa. J Hum Reprod Sci. 2012;5:37–42. doi: 10.4103/0974-1208.97798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang M, He Z, Wen L, Wu J, Yuan L, Lu Y, Guo C, Zhu L, Deng S, Yuan H. Cadmium suppresses the proliferation of piglet Sertoli cells and causes their DNA damage, cell apoptosis and aberrant ultrastructure. Reprod Biol Endocrinol. 2010;8:97. doi: 10.1186/1477-7827-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou T, Zhou G, Song W, Eguchi N, Lu W, Lundin E, Jin T, Nordberg G. Cadmium-induced apoptosis and changes in expression of p53, c-jun and MT-I genes in testes and ventral prostate of rats. Toxicology. 1999;142:1–13. doi: 10.1016/s0300-483x(99)00115-8. [DOI] [PubMed] [Google Scholar]
- 34.Xu G, Zhou G, Jin T, Zhou T, Hammarström S, Bergh A, Nordberg G. Apoptosis and p53 gene expression in male reproductive tissues of cadmium exposed rats. Biometals. 1999;12:131–139. doi: 10.1023/a:1009273711068. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki K, Murakami T, Kawasaki M, Takahashi M. The cell cycle associated change of the Ki-67 reactive nuclear antigen expression. J Cell Physiol. 1987;133:579–584. doi: 10.1002/jcp.1041330321. [DOI] [PubMed] [Google Scholar]
- 36.Falana BA, Ogundele OM, Duru FI, Oshinubi AA, Falode DT. Role of Se+Zn in regeneration (Ki-67) following Pb toxicity (p53and cad) in the germinal epithelium of adult Wistar rats. Pak J Biol Sci. 2013;16:67–73. doi: 10.3923/pjbs.2013.67.73. [DOI] [PubMed] [Google Scholar]
- 37.Ferrara D, Hallmark N, Scott H, Brown R, McKinnell C, Mahood IK, Sharpe RM. Acute and long-term effects of in utero exposure of rats to di(n-butyl) phthalate on testicular germ cell development and proliferation. Endocrinology. 2006;147:5352–5362. doi: 10.1210/en.2006-0527. [DOI] [PubMed] [Google Scholar]
- 38.Tremellen K. Oxidative stress and male infertility: a clinical perspective. Hum Reprod Update. 2008;14:243–258. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 39.Turner TT, Lysiak JJ. Oxidative stress: a common factor in testicular dysfunction. J Androl. 2008;29:488–498. doi: 10.2164/jandrol.108.005132. [DOI] [PubMed] [Google Scholar]
- 40.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 41.Beyersmann D, Hartwig A. Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch Toxicol. 2008;82:493–512. doi: 10.1007/s00204-008-0313-y. [DOI] [PubMed] [Google Scholar]
- 42.Sen Gupta R, Sen Gupta E, Dhakal BK, Thakur AR, Ahnn J. Vitamin C and vitamin E protect the rat testes from cadmium-induced reactive oxygen species. Mol Cells. 2004;17:132–139. [PubMed] [Google Scholar]
- 43.Szuster-Ciesielska A, Stachura A, Slotwińska M, Kamińska T, Sniezko R, Paduch R, Abramczyk D, Filar J, Kandefer-Szerszeń M. The inhibitory effect of zinc on cadmium-induced cell apoptosis and reactive oxygen species (ROS) production in cell cultures. Toxicology. 2000;145:159–171. doi: 10.1016/s0300-483x(00)00144-x. [DOI] [PubMed] [Google Scholar]
- 44.Khaki A, Fathiazad F, Nouri M, Khaki AA. Effect of Ocimum basilicum on apoptosis in testis of rats after exposure to electromagnetic field. Afr J Pharm Pharmacol. 2011;5:1534–1537. [Google Scholar]
- 45.Asuquo OR, Edet AG, Mesembe O, Atanghwo JI. Ethanolic extracts of Vernonia amygdalina and Ocimum gratissimum enhance testicular improvement in diabetic Wistar rats. Internet J Altern Med. 2010;8 http://dx.doi.org/10.5580/89d. [Google Scholar]
- 46.Khuon OS. Pharmacological effect of aqueous leaves extract of Ocimum basilicum against liver toxicity induced by acetaminophen in male rats. Thi-Qar J Agric Res. 2012;1:13–28. [Google Scholar]
- 47.Zhang JW, Li SK, Wu WJ. The main chemical composition and in vitro antifungal activity of the essential oils of Ocimum basilicum Linn. var. pilosum (Willd.) Benth. Molecules. 2009;14:273–278. doi: 10.3390/molecules14010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neradil J, Veselská R, Slanina J. UVC-protective effect of caffeic acid on normal and transformed human skin cells in vitro. Folia Biol (Praha) 2003;49:197–202. [PubMed] [Google Scholar]
- 49.Yeh CT, Yen GC. Effects of phenolic acids on human phenolsulfotransferases in relation to their antioxidant activity. J Agric Food Chem. 2003;51:1474–1479. doi: 10.1021/jf0208132. [DOI] [PubMed] [Google Scholar]
- 50.Dasgupta T, Rao AR, Yadava PK. Chemomodulatory efficacy of basil leaf (Ocimum basilicum) on drug metabolizing and antioxidant enzymes, and on carcinogen-induced skin and forestomach papillomagenesis. Phytomedicine. 2004;11:139–151. doi: 10.1078/0944-7113-00289. [DOI] [PubMed] [Google Scholar]
- 51.Meera R, Devi P, Kameswari B, Madhumitha B, Merlin NJ. Antioxidant and hepatoprotective activities of Ocimum basilicum Linn. and Trigonella foenum-graecum Linn. against H2O2 and CCL4 induced hepatotoxicity in goat liver. Indian J Exp Biol. 2009;47:584–590. [PubMed] [Google Scholar]