Abstract
Recent studies have suggested that nestin facilitates cellular structural remodeling in vasculature-associated cells in response to ischemic injury. The current study was designed to investigate the potential role of post-ischemic nestin expression in parenchymal astrocytes. With this aim, we characterized ischemia-induced nestin expression in the CA1 hippocampal region, an area that undergoes a delayed neuronal death, followed by a lack of neuronal generation after transient forebrain ischemia. Virtually all of the nestin-positive cells in the ischemic CA1 hippocampus were reactive astrocytes. However, induction of nestin expression did not correlate simply with astrogliosis, but rather showed characteristic time- and strata-dependent expression patterns. Nestin induction in astrocytes of the pyramidal cell layer was rapid and transient, while a long-lasting induction of nestin was observed in astrocytes located in the CA1 dendritic subfields, such as the stratum oriens and radiatum, until at least day 28 after ischemia. There was no detectable expression in the stratum lacunosum moleculare despite the evident astroglial reaction. Almost all of the nestin-positive cells also expressed a transcription factor for neural/glial progenitors, i.e., Sox-2 or Sox-9, and some cells were also positive for Ki-67. However, all of the nestin-positive astrocytes expressed the calcium-binding protein S100β, which is known to be expressed in a distinct, post-mitotic astrocyte population. Thus, our data indicate that in the ischemic CA1 hippocampus, nestin expression was induced in astroglia that were becoming reactive, but not in a progenitor/stem cell population, suggesting that nestin may allow for the structural remodeling of these cells in response to ischemic injury.
Keywords: Nestin, Transient forebrain ischemia, Reactive astrocytes, Hippocampus
Introduction
Nestin is a type VI intermediate filament protein that was originally identified in the multipotent stem/progenitor cells of the central nervous system [1-3]. However, nestin is also expressed in putative stem/progenitor cells in a variety of non-neuronal tissues, including striated muscle, cardiac muscle, skin, liver, pancreas, and kidney, and is generally considered a marker of stem cells [4-8].
Radial glia are considered multipotent progenitors that give rise to both neurons and glia in the neocortex [9-13]. Nestin expression has been demonstrated in radial glia in the embryonic brain, but mature astrocytes do not show nestin expression [14]. The downregulation of nestin expression in astrocytes parallels the increase of glial fibrillary acidic protein (GFAP) in differentiating astrocytes. In the adult brain, nestin is expressed only in astroglial neural stem cells in 2 neurogenic regions, where neurogenesis is believed to continue after birth: the subventricular zone of the lateral ventricle and the subgranular zone (SGZ) of the hippocampus [15, 16].
However, re-expression of downregulated nestin has been found to occur in reactive astrocytes following several neuropathological disorders, including brain ischemia [17-21]. Furthermore, Pforte et al. [22] demonstrated that many reactive astrocytes in the CA1 hippocampal region express nestin following transient forebrain ischemia. There is increasing evidence that parenchymal astrocytes may be intrinsically different from SGZ astrocytes that function as adult neural stem cells [23, 24]. In addition, neuron production in the ischemic hippocampus is limited to the dentate gyrus and does not occur in the CA1 region [25-28]. Thus, it can be speculated that the significance of nestin induction for SGZ astrocytes is different from its significance for astrocytes found in the CA1 hippocampal region.
In the present study, we characterized nestin expression induced in the CA1 hippocampal region, an area that undergoes delayed neuronal death, followed by a lack of neuronal generation after transient forebrain ischemia. Our results clearly indicate that distinct temporal patterns of nestin induction occur in the different hippocampal layers, i.e., in the pyramidal cell layer and the CA1 dendritic subfields such as the strata oriens and radiatum. Thus, we focused our attention on the temporal expression pattern of nestin in reactive astrocytes in these areas by quantifying the number of nestin-positive astrocytes in the subfields of the CA1 hippocampal region during a 4-week survival period after ischemia. In addition, double-labeling techniques using various cell type-specific markers were employed to characterize these nestin-positive astrocytes.
Materials and Methods
Animal preparation
All surgical interventions and presurgical and postsurgical animal care were provided in accordance with the Laboratory Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the Guidelines and Policies for Rodent Survival Surgery provided by the Institutional Animal Care and Use Committee in the College of Medicine, The Catholic University of Korea.
Adult male Sprague Dawley rats (250-300 g) were used in this study. Transient forebrain ischemia was induced by the 4-vessel occlusion and reperfusion method described by Pulsinelli and Brierley [29] with minor modifications [30]. Briefly, the vertebral arteries were electrocauterized and cut to stop circulation in these vessels. After 24 hours, both common carotid arteries were occluded for 10 minutes with miniature aneurismal clips. Only those animals showing completely flat electroencephalograms after vascular occlusion were classified as ischemic and used in the study. Body temperatures (measured rectally) were maintained at 37.5±0.3℃ with a heating lamp during and after ischemia. Sham-operated rats, with cauterized vertebral arteries and ligatures placed around the carotid arteries, were used as controls. No animal convulsed or died following reperfusion or sham operation. Animals were allowed to live for 3, 7, 14, 21, or 28 days after reperfusion. At each time point following reperfusion, animals (n=11/time points for ischemic group, n=3/time points for sham operation group) were deeply anesthetized with 16.9% urethane (10 ml/kg) and killed by transcardial perfusion with a fixative containing 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). For localization of the ischemia-damaged area, tissue sections were stained with Cresyl violet for histological studies.
Immunohistochemistry and double labeling
Free-floating sections (25-µm thick) were processed for double-immunofluorescence histochemistry. After blocking with 10% normal goat serum for 1 hour, the sections were incubated with overnight at 4℃ with a mix of monoclonal mouse anti-nestin antibody (1:500, Serotec, Oxford, UK) and one of following antibodies: polyclonal rabbit anti-GFAP (1:1,500, Millipore Corp., Temecula, CA, USA), polyclonal rabbit anti-Sox-9 (1:100, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), polyclonal rabbit anti-Ki-67 (1:1,000, Novocastra Laboratories Ltd., Newcastle-upon-Tyne, UK), polyclonal rabbit anti-S100β (1:1,000, Dako, Glostrup, Denmark) or monoclonal mouse anti-Sox-2 (1:400, Abcam, Cambridge, UK). In addition, the sections were incubated at 4℃ overnight with a mix of polyclonal rabbit anti-S100β (1:400, Dako) and monoclonal mouse anti-GFAP (1:500, Millipore Corp.). Antibody staining was visualized with the following secondary antibodies: Cy3-conjugated anti-mouse (1:2,000, Jackson ImmunoResearch, West Grove, PA, USA), Cy3-conjugated anti-rabbit (1:2,000, Jackson ImmunoResearch), and Alexa Fluor 488 goat anti-mouse (1:300, Molecular Probes, Eugene, OR, USA). The specificity of immunoreactivity was confirmed by the absence of immunohistochemical reaction in sections from which primary or secondary antibodies were omitted. Counterstaining of cell nuclei was carried out with DAPI (4',6-diamidino-2'-phenyindole, 1:1,000, Roche, Mannheim, Germany) for 10 min. Slides were viewed with a confocal microscope (LSM 700, Carl Zeiss Co. Ltd., Oberkochen, Germany). Images were converted to the TIFF format, and contrast levels were adjusted using Adobe Photoshop v. 7.0 (Adobe System, San Jose, CA, USA).
Quantification analysis
To count the number of GFAP-labeled cells and GFAP/nestin double-labeled cells in the area that covered the pyramidal cell layer and the stratum radiatum of the CA1 area, 5 coronal sections per animal, cut at 100-µm intervals, were obtained from the invariable region between bregma levels -2.5 and -4.1 mm dorsal [31]. Only process-bearing cells showing their nucleus in the plane of the section were recorded. For CA1 pyramidal cell measurements, 3 areas from the middle third of the CA1 subfield were chosen along 200 µm of the pyramidal band in each hemisphere and were captured at 200× magnification using a confocal laser microscope. The labeled cells in the stratum radiatum were counted in 2 areas (22,500 µm2 per field) in each hemisphere. The average value for the labeled cells in each field was calculated for each animal before the means and standard errors were determined for the total number of animals (n=5). Comparisons between control group and different experimental groups were assessed by the Bonferroni test. Statistical significance was accepted for P<0.05.
Results
Spatiotemporal induction patterns of nestin in GFAP-positive astrocytes in the hippocampal CA1 region after transient forebrain ischemia
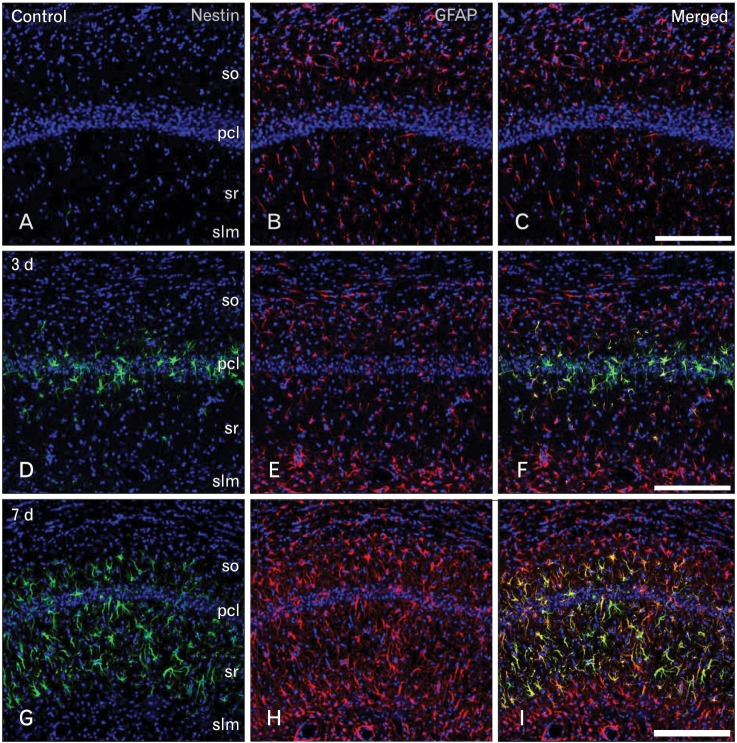
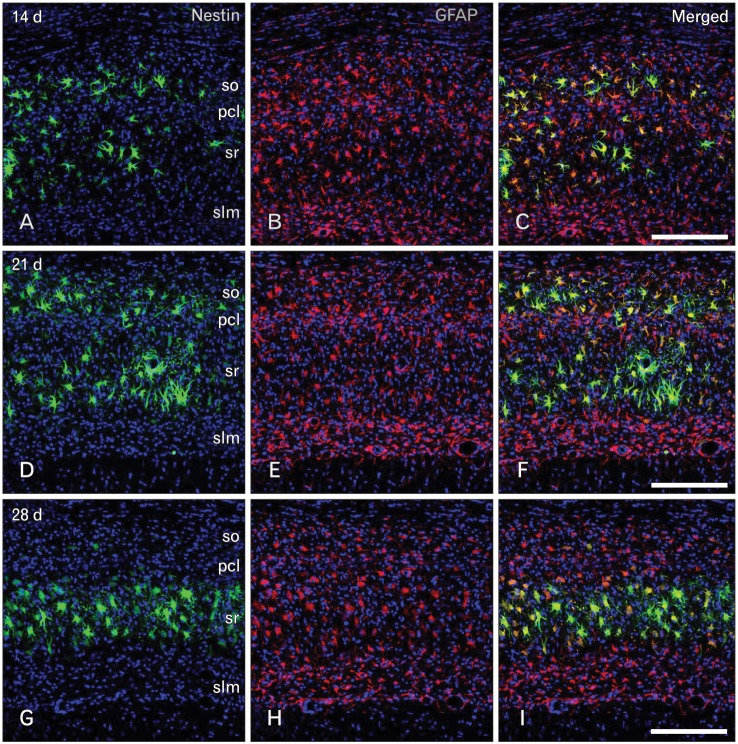
Routine histological staining with cresyl violet showed that characteristic neuronal cell loss in the CA1 region was observed in the hippocampus following 10 minutes of ischemia (data not shown). To investigate the relationship between nestin induction and astroglial responses in the ischemic hippocampus, we performed double-labeling with nestin and GFAP. In sham-operated rats, no significant immunoreactivity for nestin was detected in the CA1 hippocampal region, which contained GFAP-positive thin glial processes (Fig. 1A-C). At 3 days of reperfusion, a rather uniform upregulation of GFAP immunoreactivity was observed in all laminae of the CA1 region (Fig. 1E, F). By contrast, intense nestin induction appeared in reactive astrocytes in the pyramidal cell layer, but significant induction of nestin was not observed in other laminae (Fig. 1D). At 7 days after ischemia, GFAP immunoreactivity was markedly increased in all strata of the CA1 region (Fig. 1H, I). At this time point, increased nestin expression was evident in the strata radiatum and oriens, in addition to the pyramidal cell layer, while no specific expression was observed in the stratum lacunosum moleculare (Fig. 1G). At 14 days after ischemia, there was a further increase in GFAP immunoreactivity in all strata of the CA1 region (Fig. 2B, C). By contrast, nestin expression was localized in the dendritic layer of the CA1 region, the strata radiatum, and oriens, while its conspicuous reduction was observed in the CA1 pyramidal cell layer (Fig. 2A). In addition, no specific staining was observed in the stratum lacunosum moleculare. At 21 days (Fig. 2E, F) and 28 days (Fig. 2H, I) after ischemia, the labeling patterns of GFAP staining remained unchanged compared with those at day 14; however, its increased immunoreactivity was clearly evident in the stratum lacunosum moleculare, where GFAP-positive astrocytes displayed a less hypertrophic appearance than those in the stratum radiatum. Nestin expression was still observed in the strata radiatum and oriens of the CA1 hippocampus at 21 days after ischemia (Fig. 2D), but it had decreased to control levels in the stratum oriens and remained only in the stratum radiatum at 28 days (Fig. 2G).
Fig. 1.
Spatiotemporal relationship between nestin and glial fibrillary acidic protein (GFAP) in the hippocampal CA1 region in the early phase following transient forebrain ischemia. (A-C) No specific staining for nestin was detected in the hippocampal CA1 region of sham-operated rats, while GFAP-positive astrocytes showed thin glial processes. pcl, pyramidal cell layer; slm, stratum lacunosum moleculare; so, stratum oriens; sr, stratum radiatum. (D-F) At day 3 after ischemia, significant induction of nestin expression was detected in the CA1 pyramidal cell layer, while a rather uniform upregulation of GFAP immunoreactivity was observed in all laminae of the CA1 region. (G-I) At 7 days, GFAP immunoreactivity was markedly increased in all strata of the CA1 region, while nestin expression was more pronounced in the CA1 dendritic layers and absent in the stratum lacunosum moleculare. Scale bars in (C, F, I)=200 µm (A-I).
Fig. 2.
Spatiotemporal relationship between nestin and glial fibrillary acidic protein (GFAP) in the hippocampal CA1 region during the later phase following transient forebrain ischemia. (A-C) At 14 days after ischemia, GFAP-positive astrocytes were distributed in all strata of the hippocampal CA1 region, while nestin expression was localized in the CA1 dendritic layers including the strata radiatum (sr) and oriens (so), but was rare in the pyramidal cell layer (pcl) and in the stratum lacunosum moleculare (slm). Over the chronic interval of 21 days (D-F) and 28 days (G-I), the labeling intensity and patterns of GFAP staining were similar to those at day 14. Nestin expression was still observed in the CA1 dendritic layers at 21 days after ischemia, while it remained only in the stratum radiatum at 28 days. Note the absence of nestin expression in the stratum lacunosum moleculare. Scale bars in (C, F, I)=200 µm (A-I).
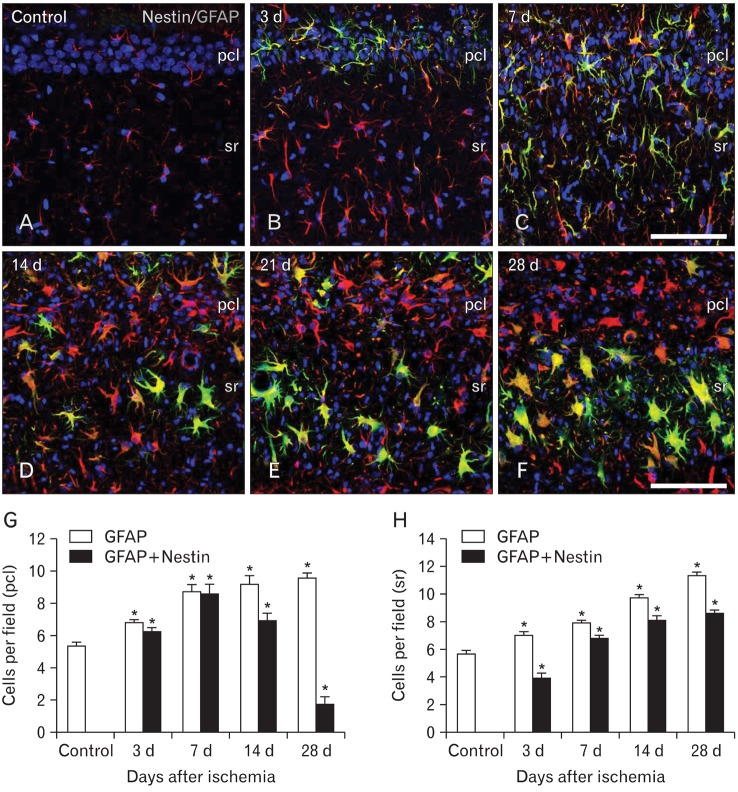
Inspection of immunolabeled sections at higher magnification revealed that nestin expression showed spatiotemporal differences in 2 strata of the CA1 hippocampal region, the pyramidal cell layer and the stratum radiatum (Fig. 3A-F). Induced nestin expression appeared in the pyramidal cell layer by 3 days (Fig. 3B), which extended into the stratum radiatum by 7 days (Fig. 3C). By 14 days after ischemia, nestin expression in the pyramidal cell layer had decreased, but was still observed in the stratum radiatum (Fig. 3D). At 21 (Fig. 3E) and 28 days (Fig. 3F) after ischemia, nestin-positive astrocytes occupied the same area, the stratum radiatum, but labeled cells displayed a more hypertrophic appearance compared with those at day 14.
Fig. 3.
Spatiotemporal distribution of nestin-positive astrocytes in the CA1 hippocampus of control (A) and ischemic rats (B-F). Nestin expression appeared in the pyramidal cell layer (pcl) by 3 days (B), and extended into the stratum radiatum (sr) by 7 days (C). By 14 days after ischemia (D), nestin expression was more pronounced in the stratum radiatum, and further increased in this stratum at 21 days (E) and 28 days (F). Note that nestin-positive astrocytes were rarely observed in the pyramidal cell layer by 14 days, despite the evident astroglial reaction. (G, H) Temporal profile of glial fibrillary acidic protein (GFAP) single-labeled cells and nestin/GFAP double-labeled cells in the pyramidal cell layer (G) and stratum radiatum (H) of the CA1 region after transient forebrain ischemia. The data are expressed as the mean±SD; *P<0.01 from the Bonferroni test with each time point compared to sham-operated controls. Scale bars in (C, F)=100 µm (A-F).
Quantification analysis of time- and lamina-dependent expression of nestin in reactive astrocytes in the ischemic hippocampal CA1 region
To determine the spatiotemporal profile of nestin-positive cells and their relationship with reactive astrocytes in the sublaminae of CA1, we quantified the number of nestin/GFAP double-labeled cells among all GFAP-positive cells in both the pyramidal cell layer and the stratum radiatum. The absolute number of GFAP-positive cells in the pyramidal cell layer was significantly increased in the hippocampus of post-ischemic rats compared to the number in sham-operated rats (Fig. 3G). The number of nestin/GFAP double-positive cells was also increased in the pyramidal cell layer at 3 and 7 days after ischemia; the number subsequently declined, but it remained significantly elevated above sham levels at 28 days. Thus, the increase in GFAP-positive cells observed between 7 and 28 days after ischemia appeared to be in direct contrast to the decrease in nestin/GFAP double-labeled cells that occurred over this time. More than 90% of all GFAP-positive astrocytes expressed nestin at 3-7 days after ischemia, but this proportion declined thereafter, and at 28 days, only 18% of GFAP-positive astrocytes expressed nestin. In contrast, the number of both GFAP-positive and nestin/GFAP double-positive cells in the stratum radiatum was significantly increased after 28 days in the ischemic rats compared to levels in the sham-operated rats (Fig. 3H). Thus, nestin expression in astrocytes of the CA1 dendritic layers increased progressively until 14-21 days after ischemia, when the highest level of induction was observed (83-85% of GFAP-positive cells). This pattern persisted throughout the study period; 76% of all of GFAP-positive cells expressed nestin 28 days after ischemia.
Characterization of nestin-positive astrocytes in the ischemic hippocampal CA1 region
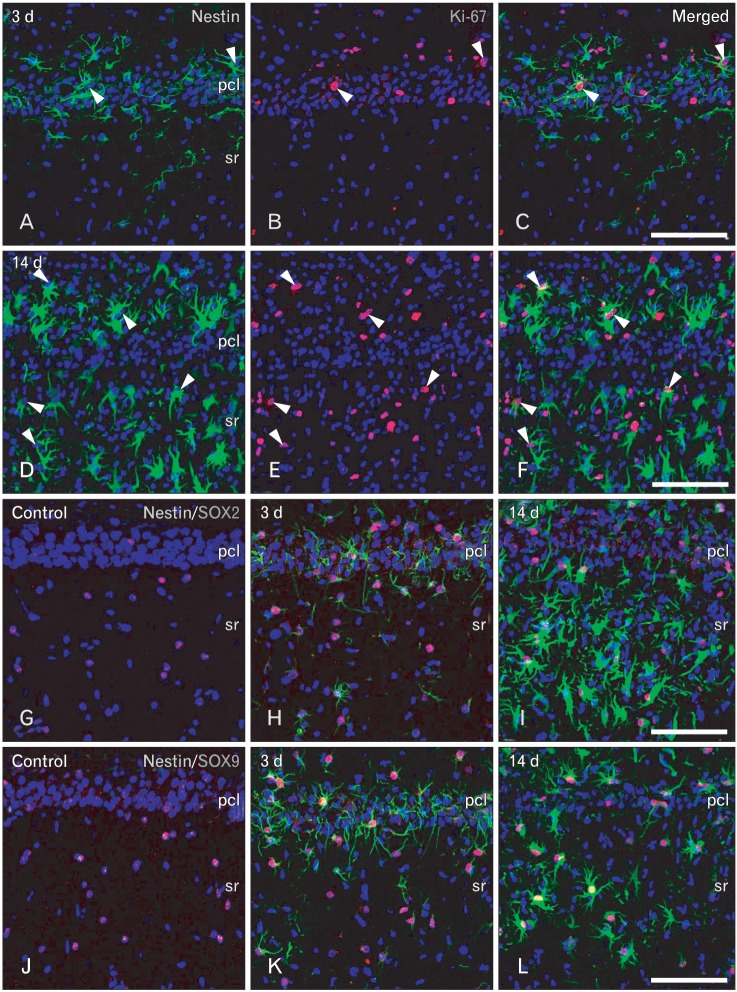
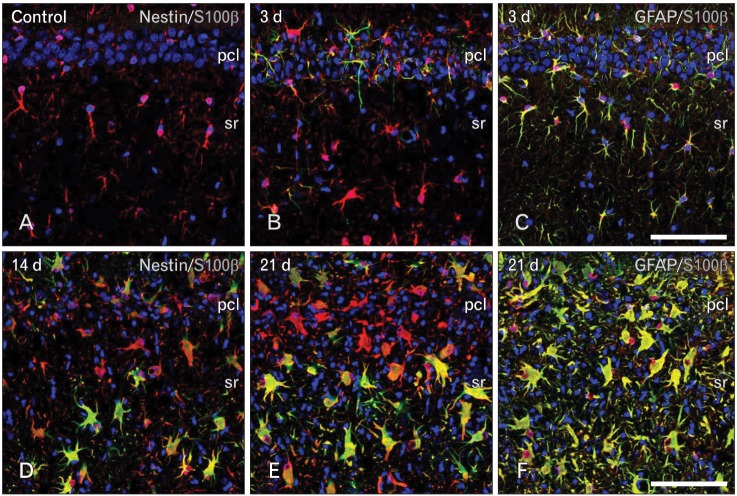
For the phenotype characterization of nestin-positive astrocytes in the ischemic CA1 hippocampal region, we performed double-labeling with nestin and proliferation marker Ki-67. Consistent with previous data [32], only some nestin-positive cells in the CA1 hippocampus were positive for Ki-67 (Fig. 4A-F). We then characterized these nestin-positive cells by double-labeling using nestin and either Sox-2 or Sox-9, transcription factors for neural/glial progenitors [33-35]. All of the nuclei of nestin-positive cells were immunoreactive for Sox-2 or Sox-9 over 3-28 days following ischemic injury (Fig. 4G-F). However, some cells expressing Sox-2 or Sox-9 were not immunoreactive for nestin in the hippocampal CA1 region of control and ischemic rats, corresponding to GFAP-positive, nestin-negative astrocytes (data not shown). We then clarified whether nestin-positive astrocytes expressed the calcium-binding protein, S100β, which is known to be expressed in a distinct post-mitotic astrocyte population [15, 36, 37]. Nearly all of the nestin-positive astrocytes co-expressed S100β, corresponding to a subpopulation of the S100β-positive cells at all time points examined (Fig. 5A, B, D, E). Considering that virtually all of the S100β-positive cells in the ischemic CA1 region were GFAP-positive astrocytes (Fig. 5C, F), nestin-negative S100β-positive cells may be considered as reactive astrocytes.
Fig. 4.
Characterization of nestin-positive cells in the CA1 hippocampal region after transient forebrain ischemia. (A-F) Double-labeling with nestin and Ki-67 showed that some nestin-positive cells exhibited Ki-67-labeled nuclei (arrowheads) on days 3 (A-C) and 14 (D-F) after ischemia. pcl, pyramidal cell layer; sr, stratum radiatum. (G-L) Double-labeling with nestin and the transcription factors Sox-2 (G-I) or Sox-9 (J-L). Note that almost all nestin-positive cells expressed Sox-2- or Sox-9-labeled nuclei at days 3 (H, K) and 14 (I, L) after ischemia. Also note that Sox-2- or Sox-9-positive nuclei were also observed in the control hippocampus (G, J), where no nestin expression was observed. Scale bars in (C, F, I, L)=100 µm (A-L).
Fig. 5.
Relationship between S100β and nestin (A, B, D, E) or glial fibrillary acidic protein (GFAP) (C, F) in the CA1 hippocampal region after transient forebrain ischemia. All of the nestin-positive cells expressed S100β, corresponding to only a small fraction of all of the S100β-positive cells at all time points examined. Note that virtually all of the S100β-positive cells in the ischemic CA1 region were GFAP-positive astrocytes. Scale bars in (C, F)=100 µm (A-F).
Discussion
The present study provides a detailed characterization of the time course and cellular localization of nestin expression in the hippocampal CA1 region, where neuronal death has been observed after a 4-vessel occlusion insult [29]. As previously demonstrated [38, 39], transient ischemia induced the upregulation of GFAP immunoreactivity within 3 days in all strata of the CA1 hippocampal region, and this was sustained until at least day 28 after reperfusion. Nestin induction in the ischemic CA1 hippocampus was colocalized entirely with GFAP, indicating that virtually all of the nestin-positive cells were indeed GFAP-positive astrocytes. However, these cells corresponded to only a small fraction of all of the GFAP-positive astrocytes within the hippocampal CA1 subfields.
Our most important finding is that nestin and GFAP are not upregulated in parallel in reactive astrocytes of the post-ischemic CA1 region. GFAP-positive astrocytes showed an even distribution throughout all strata of the CA1 region, but nestin induction in these astrocytes showed distinct time- and strata-dependent expression patterns. Nestin induction in astrocytes of the pyramidal cell layer was rapid and transient, despite the astroglial reaction being clearly observed up to day 28. In contrast, a long-lasting induction of nestin was observed in astrocytes of the CA1 dendritic subfields, such as the strata oriens and radiatum, until at least day 28. This temporal pattern seems to correlate with the time point of astrogliosis following ischemic injury [38-40], with nestin induction apparently occurring during the initial stage of astroglial activation and preceding astroglial hypertrophy after ischemic injury. Thus, our results suggest that nestin induction may play a role in the induction of astrogliosis.
However, increased GFAP immunoreactivity in the ischemic hippocampus was not necessarily followed by nestin induction, indicating that nestin expression is not in itself predictive of astroglial reaction. Very little nestin expression was detected in the stratum lacunosum moleculare, which contains pre-terminal and terminal branches of the apical dendrites of pyramidal neurons of CA1 [41], despite the evident astroglial reaction in this area (characterized by intense GFAP immunoreactivity). Given that this stratum receives its primary inputs from entorhinal cortical neurons [41], which are resistant to ischemia, it seems that nestin might be induced in reactive astrocytes only in areas that have undergone irreversible neuronal damage. In this regard, a prolonged and persistent induction of nestin in the CA1 dendritic subfield overlaps with the period during which there is the ongoing degeneration of dendrites and terminals of neurons resulting from the ischemic insults to these layers.
Nestin is involved in cell division, structural integrity and mobility of cells, and tissue stability [42-45]. Thus, nestin induction in reactive astrocytes plays a role in the transformation of normal resting astrocytes to reactive astrocytes in response to injury. Interestingly, astrocytes in the stratum lacunosum moleculare displayed a less hypertrophic appearance than those in other strata, and the persistent and prominent hypertrophy of astrocytes is confined to regions of ischemic neuronal necrosis [19, 46]. Thus, it seems that the pronounced changes in astrocyte morphology seen during reactive astrocytosis are accompanied by nestin induction. Furthermore, nestin expression is induced in vasculature-associated cells, which appear to undergo dynamic structural changes in response to ischemic injury [47]. Taken together, our data suggest that nestin expression may be induced in astroglia becoming reactive, and it may allow for the structural remodeling of these cells in response to ischemic injury.
Nestin is widely used as a marker of neuroepithelial stem/progenitor cells. Several studies have demonstrated that newly born neurons appear in the degenerated CA1 region following forebrain ischemia [48-50], and that a subpopulation of reactive astrocytes within the injury site may act as adult neural stem cells [51]. In the current study, almost all nestin-positive cells also expressed a transcription factor found in neural and glial progenitor cells, i.e., Sox-2 or Sox-9 [33-35]. In this sense, nestin-positive astrocytes in the CA1 hippocampal region after ischemic injury may correspond to adult neural stem cells. However, there is increasing evidence that parenchymal astrocytes may be intrinsically different from SGZ astrocytes that function as adult neural stem cells [23, 24]. Additionally, all of the nestin-positive astrocytes in the current study expressed the calcium-binding protein S100β, which is known to be expressed in a distinct post-mitotic astrocyte population [15, 36, 37]. Thus, it seems likely that nestin-positive cells in the CA1 hippocampal region are indeed parenchymal astrocytes rather than adult neural stem cells.
In summary, our data in a rat model of transient forebrain ischemia demonstrate that 1) nestin induction in astrocytes of the pyramidal cell layer is rapid and transient, while a long-lasting induction of nestin occurs in astrocytes located in the CA1 dendritic subfields; 2) nestin expression is not induced in the stratum lacunosum moleculare, despite the evident astroglial reaction; and 3) nestin-positive astrocytes also expressed S100β, a marker of post-mitotic astrocytes. These findings indicate that nestin induction in CA1 astrocytes shows characteristic time- and strata-dependent expression patterns and is apparently related to the severity of neuronal damage in the various hippocampal layers. Thus, nestin expression induced in astroglia becoming reactive may allow for the structural remodeling of these cells in response to ischemic injury.
Acknowledgements
This study was supported by the Mid-career Researcher Program through the National Research Foundation of Korea (NRF) grant funded by the MEST (no. 2011-0028319).
References
- 1.Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 3.Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 4.Sejersen T, Lendahl U. Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci. 1993;106(Pt 4):1291–1300. doi: 10.1242/jcs.106.4.1291. [DOI] [PubMed] [Google Scholar]
- 5.Kachinsky AM, Dominov JA, Miller JB. Intermediate filaments in cardiac myogenesis: nestin in the developing mouse heart. J Histochem Cytochem. 1995;43:843–847. doi: 10.1177/43.8.7542682. [DOI] [PubMed] [Google Scholar]
- 6.Toma JG, Akhavan M, Fernandes KJ, Barnabé-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 7.Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Müller B, Vallejo M, Thomas MK, Habener JF. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50:521–533. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]
- 8.Kim SY, Lee SH, Kim BM, Kim EH, Min BH, Bendayan M, Park IS. Activation of nestin-positive duct stem (NPDS) cells in pancreas upon neogenic motivation and possible cytodifferentiation into insulin-secreting cells from NPDS cells. Dev Dyn. 2004;230:1–11. doi: 10.1002/dvdy.20012. [DOI] [PubMed] [Google Scholar]
- 9.Barres BA. A new role for glia: generation of neurons. Cell. 1999;97:667–670. doi: 10.1016/s0092-8674(00)80777-1. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez-Buylla A, García-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 11.Tamamaki N, Nakamura K, Okamoto K, Kaneko T. Radial glia is a progenitor of neocortical neurons in the developing cerebral cortex. Neurosci Res. 2001;41:51–60. doi: 10.1016/s0168-0102(01)00259-0. [DOI] [PubMed] [Google Scholar]
- 12.Campbell K, Götz M. Radial glia: multi-purpose cells for vertebrate brain development. Trends Neurosci. 2002;25:235–238. doi: 10.1016/s0166-2236(02)02156-2. [DOI] [PubMed] [Google Scholar]
- 13.Mori T, Buffo A, Götz M. The novel roles of glial cells revisited: the contribution of radial glia and astrocytes to neurogenesis. Curr Top Dev Biol. 2005;69:67–99. doi: 10.1016/S0070-2153(05)69004-7. [DOI] [PubMed] [Google Scholar]
- 14.Gilyarov AV. Nestin in central nervous system cells. Neurosci Behav Physiol. 2008;38:165–169. doi: 10.1007/s11055-008-0025-z. [DOI] [PubMed] [Google Scholar]
- 15.Ehninger D, Kempermann G. Neurogenesis in the adult hippocampus. Cell Tissue Res. 2008;331:243–250. doi: 10.1007/s00441-007-0478-3. [DOI] [PubMed] [Google Scholar]
- 16.von Bohlen und Halbach O. Immunohistological markers for proliferative events, gliogenesis, and neurogenesis within the adult hippocampus. Cell Tissue Res. 2011;345:1–19. doi: 10.1007/s00441-011-1196-4. [DOI] [PubMed] [Google Scholar]
- 17.Rosen GD, Sherman GF, Galaburda AM. Radial glia in the neocortex of adult rats: effects of neonatal brain injury. Brain Res Dev Brain Res. 1994;82:127–135. doi: 10.1016/0165-3806(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 18.Lin RC, Matesic DF, Marvin M, McKay RD, Brüstle O. Re-expression of the intermediate filament nestin in reactive astrocytes. Neurobiol Dis. 1995;2:79–85. doi: 10.1006/nbdi.1995.0008. [DOI] [PubMed] [Google Scholar]
- 19.Duggal N, Schmidt-Kastner R, Hakim AM. Nestin expression in reactive astrocytes following focal cerebral ischemia in rats. Brain Res. 1997;768:1–9. doi: 10.1016/s0006-8993(97)00588-x. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Chopp M. Temporal profile of nestin expression after focal cerebral ischemia in adult rat. Brain Res. 1999;838:1–10. doi: 10.1016/s0006-8993(99)01502-4. [DOI] [PubMed] [Google Scholar]
- 21.Krum JM, Rosenstein JM. Transient coexpression of nestin, GFAP, and vascular endothelial growth factor in mature reactive astroglia following neural grafting or brain wounds. Exp Neurol. 1999;160:348–360. doi: 10.1006/exnr.1999.7222. [DOI] [PubMed] [Google Scholar]
- 22.Pforte C, Henrich-Noack P, Baldauf K, Reymann KG. Increase in proliferation and gliogenesis but decrease of early neurogenesis in the rat forebrain shortly after transient global ischemia. Neuroscience. 2005;136:1133–1146. doi: 10.1016/j.neuroscience.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 23.Lim DA, Huang YC, Alvarez-Buylla A. The adult neural stem cell niche: lessons for future neural cell replacement strategies. Neurosurg Clin N Am. 2007;18:81–92. doi: 10.1016/j.nec.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Ihrie RA, Alvarez-Buylla A. Cells in the astroglial lineage are neural stem cells. Cell Tissue Res. 2008;331:179–191. doi: 10.1007/s00441-007-0461-z. [DOI] [PubMed] [Google Scholar]
- 25.Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- 26.Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 27.Smith ML, Auer RN, Siesjö BK. The density and distribution of ischemic brain injury in the rat following 2-10 min of forebrain ischemia. Acta Neuropathol. 1984;64:319–332. doi: 10.1007/BF00690397. [DOI] [PubMed] [Google Scholar]
- 28.Tonchev AB, Yamashima T. Differential neurogenic potential of progenitor cells in dentate gyrus and CA1 sector of the postischemic adult monkey hippocampus. Exp Neurol. 2006;198:101–113. doi: 10.1016/j.expneurol.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 29.Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–272. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- 30.Lee MY, Shin SL, Choi YS, Kim EJ, Cha JH, Chun MH, Lee SB, Kim SY. Transient upregulation of osteopontin mRNA in hippocampus and striatum following global forebrain ischemia in rats. Neurosci Lett. 1999;271:81–84. doi: 10.1016/s0304-3940(99)00526-1. [DOI] [PubMed] [Google Scholar]
- 31.Paxinos G, Tork I, Tecott LH, Valentino KL. Altas of the developing rat brain. San Diego: Academic Press; 2008. [Google Scholar]
- 32.Liu J, Bartels M, Lu A, Sharp FR. Microglia/macrophages proliferate in striatum and neocortex but not in hippocampus after brief global ischemia that produces ischemic tolerance in gerbil brain. J Cereb Blood Flow Metab. 2001;21:361–373. doi: 10.1097/00004647-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Stolt CC, Lommes P, Sock E, Chaboissier MC, Schedl A, Wegner M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17:1677–1689. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu MS, Chang CF, Yang CC, Bau YC, Ho LL, Hung SC. Signalling pathway in the induction of neurite outgrowth in human mesenchymal stem cells. Cell Signal. 2006;18:519–530. doi: 10.1016/j.cellsig.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 35.Domowicz MS, Henry JG, Wadlington N, Navarro A, Kraig RP, Schwartz NB. Astrocyte precursor response to embryonic brain injury. Brain Res. 2011;1389:35–49. doi: 10.1016/j.brainres.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seri B, García-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Filippov V, Kronenberg G, Pivneva T, Reuter K, Steiner B, Wang LP, Yamaguchi M, Kettenmann H, Kempermann G. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. 2003;23:373–382. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka H, Araki M, Masuzawa T. Reaction of astrocytes in the gerbil hippocampus following transient ischemia: immunohistochemical observations with antibodies against glial fibrillary acidic protein, glutamine synthetase, and S-100 protein. Exp Neurol. 1992;116:264–274. doi: 10.1016/0014-4886(92)90006-c. [DOI] [PubMed] [Google Scholar]
- 39.Ordy JM, Wengenack TM, Bialobok P, Coleman PD, Rodier P, Baggs RB, Dunlap WP, Kates B. Selective vulnerability and early progression of hippocampal CA1 pyramidal cell degeneration and GFAP-positive astrocyte reactivity in the rat four-vessel occlusion model of transient global ischemia. Exp Neurol. 1993;119:128–139. doi: 10.1006/exnr.1993.1014. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt-Kastner R, Szymas J. Immunohistochemistry of glial fibrillary acidic protein, vimentin and S-100 protein for study of astrocytes in hippocampus of rat. J Chem Neuroanat. 1990;3:179–192. [PubMed] [Google Scholar]
- 41.Witter MP, Amaral DG. Hippocampal formation. In: Paxinos G, editor. The Rat Nervous System. 3rd ed. San Diego: Elsevier Academic Press; 2004. pp. 635–704. [Google Scholar]
- 42.Herrmann H, Aebi U. Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr Opin Cell Biol. 2000;12:79–90. doi: 10.1016/s0955-0674(99)00060-5. [DOI] [PubMed] [Google Scholar]
- 43.Wang N, Stamenović D. Contribution of intermediate filaments to cell stiffness, stiffening, and growth. Am J Physiol Cell Physiol. 2000;279:C188–C194. doi: 10.1152/ajpcell.2000.279.1.C188. [DOI] [PubMed] [Google Scholar]
- 44.Chen LW, Zhang JP, Kwok-Yan Shum D, Chan YS. Localization of nerve growth factor, neurotrophin-3, and glial cell line-derived neurotrophic factor in nestin-expressing reactive astrocytes in the caudate-putamen of 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine-treated C57/Bl mice. J Comp Neurol. 2006;497:898–909. doi: 10.1002/cne.21014. [DOI] [PubMed] [Google Scholar]
- 45.Calderone A. Nestin+ cells and healing the infarcted heart. Am J Physiol Heart Circ Physiol. 2012;302:H1–H9. doi: 10.1152/ajpheart.00716.2011. [DOI] [PubMed] [Google Scholar]
- 46.Petito CK, Morgello S, Felix JC, Lesser ML. The two patterns of reactive astrocytosis in postischemic rat brain. J Cereb Blood Flow Metab. 1990;10:850–859. doi: 10.1038/jcbfm.1990.141. [DOI] [PubMed] [Google Scholar]
- 47.Shin YJ, Kim HL, Park JM, Cho JM, Kim SY, Lee MY. Characterization of nestin expression and vessel association in the ischemic core following focal cerebral ischemia in rats. Cell Tissue Res. 2013;351:383–395. doi: 10.1007/s00441-012-1538-x. [DOI] [PubMed] [Google Scholar]
- 48.Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt W, Reymann KG. Proliferating cells differentiate into neurons in the hippocampal CA1 region of gerbils after global cerebral ischemia. Neurosci Lett. 2002;334:153–156. doi: 10.1016/s0304-3940(02)01072-8. [DOI] [PubMed] [Google Scholar]
- 50.Salazar-Colocho P, Lanciego JL, Del Rio J, Frechilla D. Ischemia induces cell proliferation and neurogenesis in the gerbil hippocampus in response to neuronal death. Neurosci Res. 2008;61:27–37. doi: 10.1016/j.neures.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 51.Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]