Abstract
Electron spin resonance spectroscopy using the spin trap 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) was employed to detect the formation of hydroxyl radicals (OH.) by phagocytosing polymorphonuclear leukocytes (PMN). An electron spin resonance signal with the identical g value and splitting characteristics of the DMPO/OH). adduct was detected on incubation of normal PMN with opsonized zymosan. Adduct formation was strongly inhibited by superoxide dismutase and by the OH. scavenger mannitol, but catalase had little or no effect. (DMPO/OH). was not formed by PMN from a patient with chronic granulomatous disease; in contrast, adduct formation by PMN which lack myeloperoxidase was greater than normal. These findings are discussed in relation to the formation of OH. by PMN.
Full text
PDF
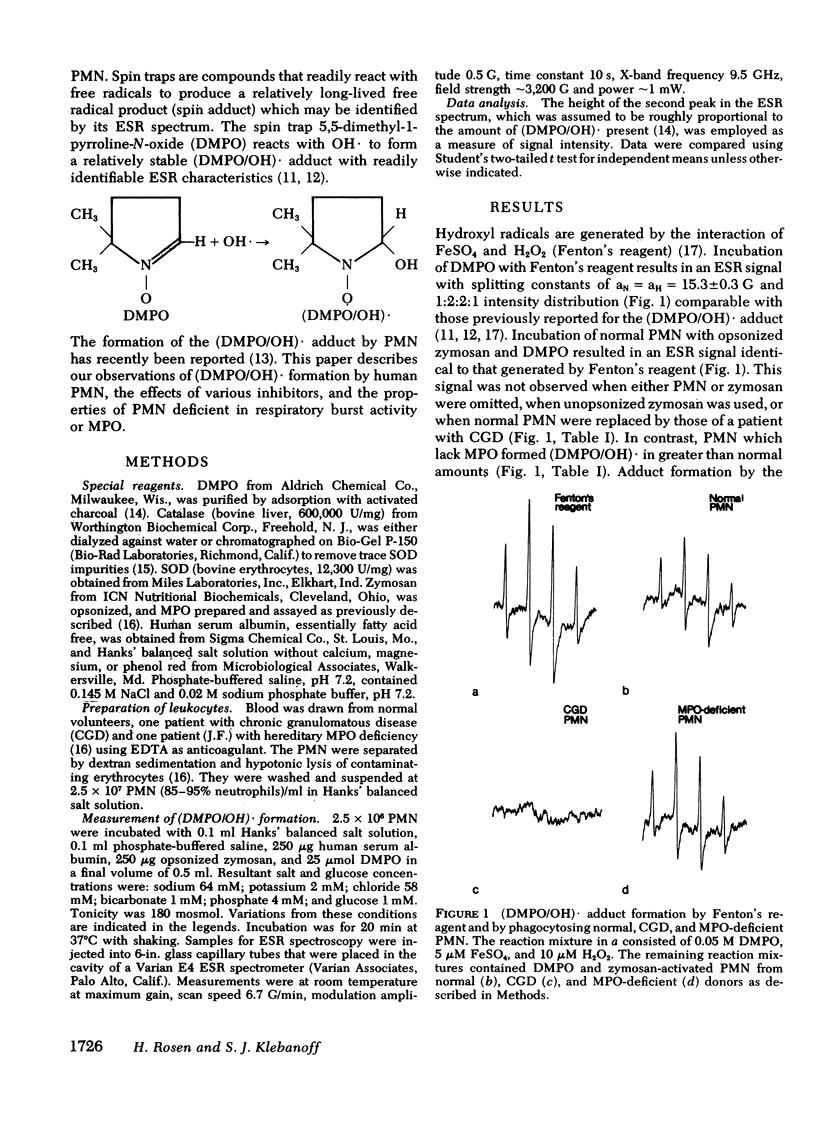



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Buettner G. R., Oberley L. W. Considerations in the spin trapping of superoxide and hydroxyl radical in aqueous systems using 5,5-dimethyl-1-pyrroline-1-oxide. Biochem Biophys Res Commun. 1978 Jul 14;83(1):69–74. doi: 10.1016/0006-291x(78)90398-4. [DOI] [PubMed] [Google Scholar]
- Green M. R., Hill H. A., Okolow-Zubkowska M. J., Segal A. W. The production of hydroxyl and superoxide radicals by stimulated human neutrophils- measurements by EPR spectroscopy. FEBS Lett. 1979 Apr 1;100(1):23–26. doi: 10.1016/0014-5793(79)81123-0. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Superoxide dismutase: a contaminant of bovine catalase. Biochem J. 1973 Oct;135(2):379–381. doi: 10.1042/bj1350379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem. 1975 Nov 25;250(22):8812–8817. [PubMed] [Google Scholar]
- Klebanoff S. J., Rosen H. Ethylene formation by polymorphonuclear leukocytes. Role of myeloperoxidase. J Exp Med. 1978 Aug 1;148(2):490–506. doi: 10.1084/jem.148.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- Tauber A. I., Babior B. M. Evidence for hydroxyl radical production by human neutrophils. J Clin Invest. 1977 Aug;60(2):374–379. doi: 10.1172/JCI108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Rustagi P. K., LoBuglio A. F. Human granulocyte generation of hydroxyl radical. J Exp Med. 1978 Feb 1;147(2):316–323. doi: 10.1084/jem.147.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]


