Abstract
After stroke, the pattern of brain activation during performance of a motor task is related to outcome. Here, we compare this relationship in the early (10–14 days) and late (at least 3 months) phases after first-ever stroke. A negative linear relationship between task-related brain activation, as measured by functional magnetic resonance imaging, and outcome is seen in several identical primary and nonprimary motor regions that is independent of time after stroke. In other words, patients with poorer outcome scores recruit more widely within motor-related regions in both the early or late poststroke phase. However, in contralesional middle intraparietal sulcus, contralesional cerebellum, and ipsilesional rostral premotor cortex, this relationship is seen only in the early poststroke phase. Thus, patients with poorer outcome scores recruit these areas in only the early and not the late poststroke phase. These results suggest that there are differences in the cerebral implementation of action in patients with poor outcome that are dependent on the time since stroke. Thus, in those patients with the most to gain from rehabilitation, different therapeutic approaches may be required at different stages after stroke.
Functional imaging techniques are useful for studying changes in cerebral reorganization after focal brain damage. However, care needs to be taken in interpreting the results, because they may be influenced by several factors including time after stroke1 and outcome.2 Recent studies suggest that there is a negative correlation between the size of activation in brain regions involved in performance of a motor task and outcome in the late (at least 3 months after onset of symptoms) phase after first-ever stroke.2,3 In other words, patients with poorer outcome continue to recruit more parts of the primary and nonprimary motor system than patients with better outcome. This has been interpreted as the functional engagement of alternative parallel motor output pathways in those patients with significant damage to the fast direct connections between primary motor cortex and spinal cord motor neurons.4 The same negative correlation also has been observed in similar motor-related brain regions in the early (10–14 days after onset of symptoms) poststroke phase.1 We hypothesized that recruitment of alternative parallel motor output pathways can occur immediately after damage to the corticomotoneuronal pathway. We therefore expected to see a significant negative correlation between brain activation and outcome in identical nonprimary motor regions in both phases after stroke. However, a difference between the correlation analyses would indicate differences in the cerebral implementation of a motor task at early and late stages after stroke. Thus, we studied motor activation patterns in several patients in either of the two phases after first-ever stroke and formally compared the correlation between brain activation and outcome for the early and late groups.
Subjects and Methods
Subjects
All patients were right-handed5 and had suffered from first-ever stroke with weakness of at least wrist and finger extensors and hand interossei (to ≤4+ on the Medical Research Council scale) lasting at least 48 hours. Exclusion criteria consisted of (1) carotid artery occlusion or stenosis greater than or equal to 70%; (2) language or cognitive deficits sufficient to impair cooperation in the study; (3) inability to perform the motor task because of complete paralysis of handgrip. Full written consent was obtained from all subjects in accordance with the Declaration of Helsinki. The study was approved by the Joint Ethics Committee of the Institute of Neurology, University College London and National Hospital for Neurology and Neurosurgery, University College London Hospitals NHS Trust, London.
Behavioral Evaluation
Patients were evaluated at the time of scanning using nine different outcome measures (Table 1). A principal component analysis was performed on these scores to obtain a single representative outcome score for each patient as previously described.1,2
Table 1. Range of Outcome Scores within Early and Late Poststroke Groups.
| Early Poststroke Group |
Late Poststroke Group |
|||||
|---|---|---|---|---|---|---|
| Outcome Score (min-max reference range) | Min | Max | Mean (SD) |
Min | Max | Mean (SD) |
| Barthel (0–20) | 11 | 19 | 15.5 (3.6) | 9 | 20 | 17.9 (3.8) |
| Rankin (5–0) | 4 | 2 | 3.1 (1.0) | 4 | 1 | 2.1 (1.2) |
| Orpington prognostic scale (6.8–1.2) | 3.6 | 2 | 2.6 (0.6) | 4 | 1.6 | 2.2 (0.7) |
| Action Research Arm Test (0–57) | 13 | 56 | 43.6 (16.7) | 10 | 57 | 43.6 (18.2) |
| Grip Strength (0–100% unaffected side) | 3.2 | 91.8 | 55.6 (30.2) | 17.8 | 106.9 | 73.1 (27.8) |
| Motricity Index–upper limb (1–100) | 62 | 93 | 70.0 (30.1) | 62 | 100 | 83.4 (18.1) |
| Motricity Index–lower limb (1–100) | 40 | 92 | 77.2 (18.8) | 59 | 100 | 87.2 (18.4) |
| Nine-Hole Peg Test (0–100% unaffected side) | 0 | 90.3 | 34.8 (33.2) | 0 | 107.3 | 54.0 (38.2) |
| 10-meter walk (m/sec) | 0 | 1.95 | 0.48 (0.69) | 0 | 2.3 | 0.93 (0.64) |
Motor Paradigm
During a continuous scanning session, subjects performed paced isometric dynamic handgrips with their impaired hand in 20 seconds blocks alternating with 20 seconds of rest. Target forces and rates of handgrip were constant within each 20-second block but were varied between blocks in a randomized counterbalanced order. Target forces were set between 10% and 60% of each patient’s own maximum grip strength during maximum voluntary contraction (MVC) and were indicated by a horizontal bar on the screen. The required rate of handgrip was indicated visually by a cross displayed at the bottom of the screen for 0.3 seconds at 40% of each patient’s own maximum rate. A moving vertical column represented the force applied during handgrip. Subjects were specifically asked to attend to this continuous feedback. Before scanning, subjects were pretrained until comfortable with the task. Mirror movements were assessed for as previously described.1,2
Data Acquisition
A Siemens VISION system (Siemens, Erlangen, Germany), operating at 2T, was used to acquire both T1-weighted anatomical images (1 × 1 × 1.5mm voxels) and T2*-weighted magnetic resonance imaging transverse echo planar images (64 × 64 3 × 3mm2 pixels, TE = 40 milliseconds) with blood oxygenation level dependent contrast. The site of cerebral infarction was determined from the T1-weighted anatomical images. Each echo planar image comprised 48 1.8mm thick contiguous axial slices taken every 3mm, positioned to cover the whole cerebrum, with an effective repetition time (TR) of 3.65 seconds per volume. The first six volumes were discarded to allow for T1 equilibration effects.
Image Analysis
Imaging data were analyzed using Statistical Parametric Mapping (SPM99; Wellcome Department of Imaging Neuroscience, http://www.fil.ion.ucl.ac.uk/spm/) implemented in Matlab5 (The Mathworks, Natick, MA).6,7 All scans were preprocessed as previously described.1,2 Images from patients with left-sided lesions were flipped about the midsagittal plane, so that all patients were assumed to have right hemispheric lesions.
In the first stage of statistical analysis, a single-subject fixed effects model was used. All handgrips were defined as a single-event type and modeled as delta functions.8 The data for the second stage of analysis comprised the pooled parameter estimates for the main effects of handgrip across all subjects. Contrast images for all patients were entered into the model as two separate groups. Relative outcome scores for each patient were entered into the model, also as two separate covariates, one for each group. The parameter estimates for each covariate resulting from the least mean squares fit of the model to the data were calculated.
Regions in which there was a correlation between task-related brain activation and relative outcome scores were identified by the linear contrast of the covariate representing each group’s outcome scores. To determine brain regions in which there were similar correlations, we performed a conjunction analysis between the correlation analyses for the early and late groups. Conjunction analysis relies on the conjoint testing of multiple null hypotheses,9 in this case that there is no correlation between task-related signal change and outcome in either the early or late groups.
To identify brain regions in which the correlation between task-related signal change and outcome differed significantly between the two groups, we performed an F-test across the two contrasts of interest (ie, early correlation vs late correlation, and late correlation vs early correlation). The coefficients of correlation between task-related brain activation and outcome for each group were calculated for significant voxels to determine the direction of the difference.
All resulting SPM{t}s and SPM{F}s were thresholded at p value less than 0.05, corrected for multiple comparisons across the whole brain.
Anatomical identification was carefully performed by superimposing the maxima of activation foci both on the Montreal Neurological Institute (MNI) brain and on the normalized structural images of each subject, and labeling with the aid of the atlas of Duvernoy.10
Results
Clinical Data
The early group comprised eight patients (range, 29–71 years; mean, 52.8 years). Five had right-sided and three had left-sided infarcts. Three patients had infarcts isolated to the capsular region, one patient had an infarct in the corona radiata, three patients had pontine infarcts, and one patient had an infarct in the striatocapsular region with extension to the insular cortex.
The late group comprised 20 stroke patients (range, 28–72 years; mean, 53.2 years). Fourteen patients had right-sided and six left-sided infarcts. Eight patients had infarcts involving the internal capsule, four had pontine infarcts, and six had infarcts in the striatocapsular region with extension to the insular cortex. In addition, one patient suffered from a thalamic hemorrhage and one from hemorrhagic infarction of the posterior cerebral hemisphere. No patient in either group had lesions involving the hand representation of primary motor cortex. Clinical details of these patients have been published previously.1,2
Although there were slight differences in the mean outcome scores for each group, the range of impairment and disability was similar across the two groups (Table 1; Fig). The first principal component of all the outcome scores provided a single relative outcome score for each patient and accounted for 80.1% of the variance in the entire data set.
Fig.
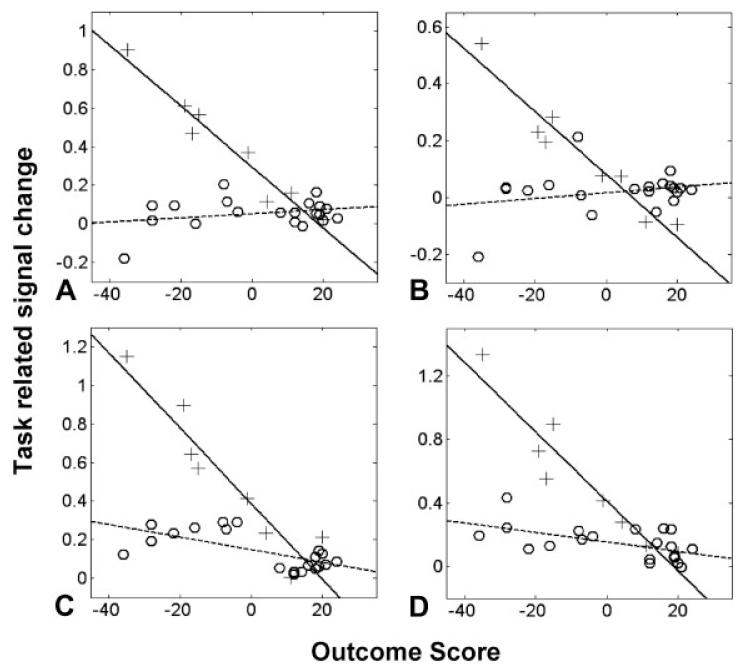
Plots of task-related signal change against relative outcome scores. A higher outcome score represents better outcome. Each plus sign represents one patient in the early poststroke group, and each circle represents one patient in the late poststroke group. (A) Contralesional intraparietal sulcus (x = −36, y = −62, z = 50). (B) Contralesional intraparietal sulcus (x = −32, y = −64, z = 36). (C) Ipsilesional rostral premotor cortex (x = 36, y = 0, z = 62). (D) Contralesional cerebellum (lobule VI) (x = −34, y = −62, z = −20). Correlation coefficients are given in Table 3.
All patients were able to perform the task adequately. No patient displayed mirror movements. A 100mm visual analog scale was used to assess the perceived effortfulness of the task (where 0 = no effort and 100 = maximum effort). There was no correlation between the ratings for effort and the overall outcome scores for all patients (r2 = 0.12; p = not significant). Furthermore, there was no significant difference in the perceived effortfulness of the task between each group (t = 0.51; p = not significant).
Imaging Results
The conjunction analysis identified several brain regions in which there was a negative correlation between task-related signal change and outcome common to both the early and late phases (Table 2). There were no regions in which a positive correlation was seen in both groups.
Table 2. Similarities between Correlation Analyses.
| Talairach Coordinates in MNI Space |
Early Poststroke Correlation Analysis |
Late Poststroke Correlation Analysis |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | Side | x | y | z | Z-value | r | p | r | p |
| Negative correlation | |||||||||
| Primary motor cortex | I | 26 | −32 | 68 | 4.98 | −0.73 | 0.04 | −0.75 | <0.01 |
| I | 42 | −14 | 36 | 4.93 | −0.7 | 0.05 | −0.8 | <0.01 | |
| C | −32 | −30 | 56 | 5.08 | −0.77 | 0.02 | −0.72 | <0.01 | |
| C | −34 | −22 | 50 | 4.91 | −0.75 | 0.03 | −0.77 | <0.01 | |
| Postcentral gyrus | C | −32 | −32 | 50 | 5.17 | −0.89 | <0.01 | −0.77 | <0.01 |
| Inferior postcentral sulcus | C | −58 | −18 | 26 | 4.94 | −0.78 | 0.02 | −0.7 | <0.01 |
| Superior postcentral sulcus | I | 18 | −42 | 70 | 4.97 | −0.72 | 0.04 | −0.74 | <0.01 |
| Premotor cortex | I | 24 | −18 | 68 | 5.63 | −0.9 | <0.01 | −0.72 | <0.01 |
| C | −20 | −16 | 68 | 5.18 | −0.78 | 0.02 | −0.76 | <0.01 | |
| C | −28 | −12 | 50 | 5.58 | −0.85 | <0.01 | −0.74 | <0.01 | |
| C | −32 | −8 | 62 | 5.06 | −0.75 | 0.03 | −0.73 | <0.01 | |
| Supplementary motor area | I | 2 | 2 | 66 | 5.52 | −0.8 | 0.01 | −0.7 | <0.01 |
| Presupplementary motor area | I | 2 | 26 | 60 | 5.56 | −0.8 | 0.02 | −0.76 | <0.01 |
| C | 0 | 22 | 38 | 5.09 | −0.69 | 0.05 | −0.82 | <0.01 | |
| Caudal cingulate sulcus | C | −8 | −8 | 54 | 5.16 | −0.77 | 0.02 | −0.72 | <0.01 |
| Prefrontal cortex | I | 42 | 24 | 30 | 5.05 | −0.84 | <0.01 | −0.68 | <0.01 |
| Posterior superior temporal sulcus | I | 62 | −46 | 14 | 5.04 | −0.9 | <0.01 | −0.71 | <0.01 |
| Cerebellum (VI) | I | 26 | −62 | −20 | 5.24 | −0.74 | 0.03 | −0.79 | <0.01 |
| Cerebellum (VIIB) | C | −12 | −66 | −40 | 5.58 | −0.83 | 0.01 | −0.75 | <0.01 |
| Positive correlation none |
|||||||||
Coordinates represent voxels significant at p < 0.05, corrected for multiple comparisons across whole-brain volume. The correlation coefficient (r) and corresponding p value for each correlation analysis are also given.
I = ipsilesional; C = contralesional.
In directly comparing the two groups, the F-test identified three brain regions in which the correlation between task-related brain activation and outcome was dissimilar in the early and late phases (Table 3). These were contralesional intraparietal sulcus (both superficial and deep parts of middle intraparietal sulcus), contralesional cerebellum (lobule VI), and ipsilesional rostral premotor cortex. In each of these areas, the correlation coefficient was significantly more negative in the early than the late group (see Fig).
Table 3. Differences between Correlation Analyses.
| Talai rach Coordinates in MNI Space |
Early Poststroke Correlation Analysis |
Late Poststroke Correlation Analysis |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | Side | x | y | z | Z-value | r | p | r | p |
| Intraparietal sulcus | C | −36 | −62 | 50 | 5.85 | −0.97 | <0.01 | 0.26 | ns |
| C | −32 | −64 | 36 | 4.98 | −0.96 | <0.01 | 0.26 | ns | |
| Premotor cortex | I | 36 | 0 | 62 | 5.02 | −0.94 | <0.01 | −0.6 | <0.01 |
| Cerebellum (VI) | C | −34 | −74 | −18 | 5.18 | −0.95 | <0.01 | −0.56 | 0.01 |
Coordinates represent voxels significant at p < 0.05, corrected for multiple comparisons across whole-brain volume. The correlation coefficient (r) and corresponding p value for each correlation analysis are also given.
C = contralesional; ns = not significant; I = ipsilesional.
These results were obtained assuming that all patients had right-sided lesions with left-hand weakness (ie, images from patients with left-sided lesions were flipped about the midsagittal line). This approach often is used when studying the motor system of stroke patients with different lesion locations. It is an appropriate approximation when considering regions such as sensorimotor cortex, premotor cortex, and cerebellum, because these are parts of the motor system that are lateralized depending on the hand used. However, left and right parietal regions may have different functions in the normal human brain independent of the hand used.11 In view of our result in intraparietal sulcus, an identical post hoc analysis was conducted using unflipped images. The F-test did not show any significant areas at the chosen threshold (p < 0.05, corrected for whole-brain comparison), demonstrating that the differences between the two groups are lateralized for the side of the lesion.
Discussion
We previously have demonstrated a negative correlation between task-related signal change and outcome in a variety of primary and nonprimary motor regions in both the early1 and late2 phases after stroke. We now have formally compared these results and have demonstrated that patients with poorer outcome recruit more widely within several identical motor-related regions whether in the early or late poststroke phase. However, in other brain regions there is an interaction between task-related signal change, outcome, and time from stroke. In making these comparisons, it is important to remember that the absolute parameters of the motor task were adjusted at each scanning session in a way that equated task effort across patients. Thus, differences in brain activation patterns between patients are unlikely to be caused by differences in perceived task difficulty.
Similarities between the Early and Late Poststroke Groups
A negative correlation between brain activation and outcome is present in several identical brain regions irrespective of time after stroke. These include sensorimotor cortex, premotor cortex, supplementary motor area, cingulate motor areas, and cerebellum. These regions are thought to participate in motor loops which are independent of those involving primary motor cortex, with their own projections to spinal cord motor neurons.4 We previously have interpreted this negative correlation as recruitment of alternative motor output pathways in those patients with significant damage to the direct projections from primary motor cortex to spinal cord motor neurons. Our current results suggest that these pathways are available to participate in the generation of motor output as soon as 10 to 14 days after focal brain damage, rather than being slowly recruited over time. Lesion-induced reorganization may occur much more quickly, as suggested by immediate changes in cortical representations after repetitive transcranial magnetic stimulation.12
Differences between the Early and Late Poststroke Groups
We were interested to investigate whether there are different ways of generating the same motor output at different stages after a stroke. However, a direct comparison of average brain activation patterns at early and late stages after stroke is confounded by the fact that each group will contain patients at different stages of recovery. These differences in outcome are correlated with task-related activation in several brain regions in both early and late poststroke patients.1,2 Direct statistical comparison of the correlation analyses from each group is a novel way to overcome this problem. Our analysis demonstrated a stronger negative correlation between task-related activation and outcome in the early compared with the late poststroke phase in contralesional middle intraparietal sulcus, contralesional superior cerebellum (lobule VI), and ipsilesional dorsolateral premotor cortex.
Posterior and middle parts of intraparietal sulcus are activated by tasks requiring increased visuomotor attention.13-16 Furthermore, work in both primates and humans suggests that rostral premotor regions are involved in higher order aspects of motor control,17 and more attentionally demanding tasks are known to increase activation in this region.18 Unlike caudal premotor cortex, rostral premotor cortex has no direct connections with spinal cord or with primary motor cortex in primates19,20 but is interconnected with prefrontal cortex, parietal cortex, and the reticular formation.21-23 Our findings support the idea that patients with greater deficits use a network of brain regions involved in visuomotor attention in the early but not late post-stroke phase.
Increased brain activation also was seen in superior contralesional cerebellum in those with poorer outcomes, but only in the early group. It is likely that early after hemiparetic stroke there is a greater discrepancy between predicted and actual consequences of an action in those with greater impairment. In the late phase, the discrepancy may be less because the degree of sensory feedback is now closer to that “expected.” In normal subjects, this error is signaled in the ipsilateral cerebellum very close to the region preferentially activated by patients with a greater deficit in the early compared with the late poststroke phase.24 There are known anatomical connections between cerebellum and posterior parietal cortex in primates,25 and it is possible that detection of an error signal may be modulated by attention. Certainly, the superior cerebellum is activated during attentionally demanding tasks, even in the absence of a motor response.26
Conclusions
Stroke patients with greater deficit appear to engage attentional networks more in the early compared with late poststroke phase. Attention may no longer be a useful tool for optimizing motor performance in the late poststroke phase. Alternatively, increasing the degree to which a motor task is attended to by chronic stroke patients might facilitate performance by enhancing detection of a discrepancy between predicted and actual consequences of an action. These questions will need to be addressed empirically in the clinical setting.
Acknowledgments
N.S.W. and R.S.J.F. are supported by the Wellcome Trust (037830/Z/95/C/JRS/KM/JAT). N.S.W. was previously supported by the Stroke Association during part of this work. M.M.B’s Chair in Stroke Medicine is supported by the Reta Lila Weston Trust for Medical Research. A.J.T. holds the Garfield Weston Chair of Clinical Neurology and Neurological Rehabilitation.
We thank P. Aston and E. Featherstone for the design and programming involved in creating the handgrip manipulandum. We also thank the staff of the Acute Brain Injury Unit and Neurorehabilitation Unit at the National Hospital for Neurology and Neurosurgery for their assistance.
References
- 1.Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003;126:1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansen-Berg H, Rushworth MF, Bogdanovic MD, et al. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci USA. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strick PL. Anatomical organization of multiple motor areas in the frontal lobe: implications for recovery of function. Adv Neurol. 1988;47:293–312. [PubMed] [Google Scholar]
- 5.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 6.Friston KJ, Holmes AP, Worsley KJ, et al. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 7.Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited—again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- 8.Friston KJ, Fletcher P, Josephs O, et al. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- 9.Price CJ, Friston KJ. Cognitive conjunction: a new approach to brain activation experiments. Neuroimage. 1997;5:261–270. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- 10.Duvernoy HM. The Human Brain: surface, blood supply, and three-dimensional anatomy. Springer-Verlag; New York: 1991. [Google Scholar]
- 11.Rushworth MF, Krams M, Passingham RE. The attentional role of the left parietal cortex: the distinct lateralization and localization of motor attention in the human brain. J Cogn Neurosci. 2001;13:698–710. doi: 10.1162/089892901750363244. [DOI] [PubMed] [Google Scholar]
- 12.Lee L, Siebner HR, Rowe JB, et al. Acute remapping within the motor system induced by low-frequency repetitive transcranial magnetic stimulation. J Neurosci. 2003;23:5308–5318. doi: 10.1523/JNEUROSCI.23-12-05308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gitelman DR, Nobre AC, Parrish TB, et al. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122:1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- 14.Nobre AC, Sebestyen GN, Gitelman DR, et al. Functional localization of the system for visuospatial attention using positron emission tomography. Brain. 1997;120:515–533. doi: 10.1093/brain/120.3.515. [DOI] [PubMed] [Google Scholar]
- 15.Nobre AC, Gitelman DR, Dias EC, Mesulam MM. Covert visual spatial orienting and saccades: overlapping neural systems. Neuroimage. 2000;11:210–216. doi: 10.1006/nimg.2000.0539. [DOI] [PubMed] [Google Scholar]
- 16.Shikata E, Hamzei F, Glauche V, et al. Functional properties and interaction of the anterior and posterior intraparietal areas in humans. Eur J Neurosci. 2003;17:1105–1110. doi: 10.1046/j.1460-9568.2003.02540.x. [DOI] [PubMed] [Google Scholar]
- 17.Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 18.Simon SR, Meunier M, Piettre P, et al. Spatial attention and memory versus motor preparation: premotor cortex involvement as revealed by fMRI. J Neurophysiol. 2002;88:2047–2057. doi: 10.1152/jn.2002.88.4.2047. [DOI] [PubMed] [Google Scholar]
- 19.Muakkassa KF, Strick PL. Frontal lobe inputs to primate motor cortex: evidence for four somatotopically organised “premotor” areas. Brain Res. 1979;177:176–182. doi: 10.1016/0006-8993(79)90928-4. [DOI] [PubMed] [Google Scholar]
- 20.Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu MT, Preston JB, Strick PL. Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. J Comp Neurol. 1994;341:375–392. doi: 10.1002/cne.903410308. [DOI] [PubMed] [Google Scholar]
- 22.Keizer K, Kuypers HG. Distribution of corticospinal neurons with collaterals to the lower brain stem reticular formation in monkey (Macaca fascicularis) Exp Brain Res. 1989;74:311–318. doi: 10.1007/BF00248864. [DOI] [PubMed] [Google Scholar]
- 23.Marconi B, Genovesio A, Battaglia-Mayer A, et al. Eye-hand coordination during reaching. I. Anatomical relationships between parietal and frontal cortex. Cereb Cortex. 2001;11:513–527. doi: 10.1093/cercor/11.6.513. [DOI] [PubMed] [Google Scholar]
- 24.Blakemore SJ, Frith CD, Wolpert DM. The cerebellum is involved in predicting the sensory consequences of action. Neuroreport. 2001;12:1879–1884. doi: 10.1097/00001756-200107030-00023. [DOI] [PubMed] [Google Scholar]
- 25.Clower DM, West RA, Lynch JC, et al. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci. 2001;21:6283–6291. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen G, Buxton RB, Wong EC, Courchesne E. Attentional activation of the cerebellum independent of motor involvement. Science. 1997;275:1940–1943. doi: 10.1126/science.275.5308.1940. [DOI] [PubMed] [Google Scholar]