Abstract
Stroke is the leading cause of long-term disability worldwide and a condition for which there is no universally accepted treatment. The development of new effective therapeutic strategies relies on a better understanding of the mechanisms underlying recovery of function. Noninvasive techniques to study brain function, including functional magnetic resonance imaging, positron emission tomography, transcranial magnetic stimulation, electroencephalography, and magnetoencephalography, led to recent studies that identified some of these operating mechanisms, resulting in the formulation of novel approaches to motor rehabilitation.
Stroke is the leading cause of disability worldwide. The value of specific rehabilitation therapies aimed at assisting adaptation to impairment is now well recognized, but therapeutic strategies designed to restore function by minimizing impairment are by comparison poorly developed. This review considers the advances made toward understanding how cerebral reorganization following focal damage is related to functional recovery, and how these insights might be translated into clinical benefits for patients.
THE BRAIN AS A PLASTIC STRUCTURE
The term plasticity is often used when mechanisms of recovery after focal brain injury are considered. More than 50 years ago, Hebb1 postulated that increments in synaptic efficacy occur during learning when firing of one neuron repeatedly produces firing in another neuron to which it is connected, leading to the notion of plasticity as a behavioral adaptation (ie, learning) that is associated with a change of function at the level of the synapse. Expressed in a systems framework, the term plasticity may refer to changes in brain networks that carry behavioral implications over time. The cortex with its myriad synaptic connections is the ideal site for plasticity to take place.2 Plastic changes can occur at the cortical level in a number of ways. First, it has been repeatedly demonstrated that enriched environments and skill learning in adult animals are associated with growth of dendrites, increases in dendritic spines, and synaptogenesis.3 Second, long-term potentiation and long-term depression are mechanisms of changing synaptic efficacy in hippocampus4 and neocortex under certain conditions.5 Indeed, motor skill learning in animal models is accompanied by changes in the strength of connections within primary motor cortex.6 Furthermore, there is evidence that these mechanisms may operate in human motor learning as well.7,8 Third, cortical maps are maintained at least in part by γ-aminobutyric acid and can be altered intentionally by pharmacologic manipulations9 and unintentionally by lesions. The link between change in brain structure and change in behavior is firmly established.
Work in animal models has unequivocally demonstrated that focal damage in adult brains renders widespread cortical regions more able to change structure and function in response to afferent signals in a way previously seen in the developing brain. Activity-driven changes in these regions may be enhanced by experimental manipulations10 or pharmacologic interventions11 and correlate with functional recovery. These findings are clearly very exciting to clinicians. It is hypothesized that similar injury-induced changes occur in the human brain, and that manipulation of these processes may provide a means of promoting recovery. Techniques like functional magnetic resonance imaging and positron emission tomography, which allow measurement of task-related brain activation with excellent spatial resolution; transcranial magnetic stimulation (TMS), a safe, noninvasive way to excite or inhibit the human cortex with high temporal resolution; and magnetoencephalography and electroencephalography, with even greater temporal resolution, allow the study of these changes.
HOW DOES THE HUMAN BRAIN RESPOND TO FOCAL INJURY?
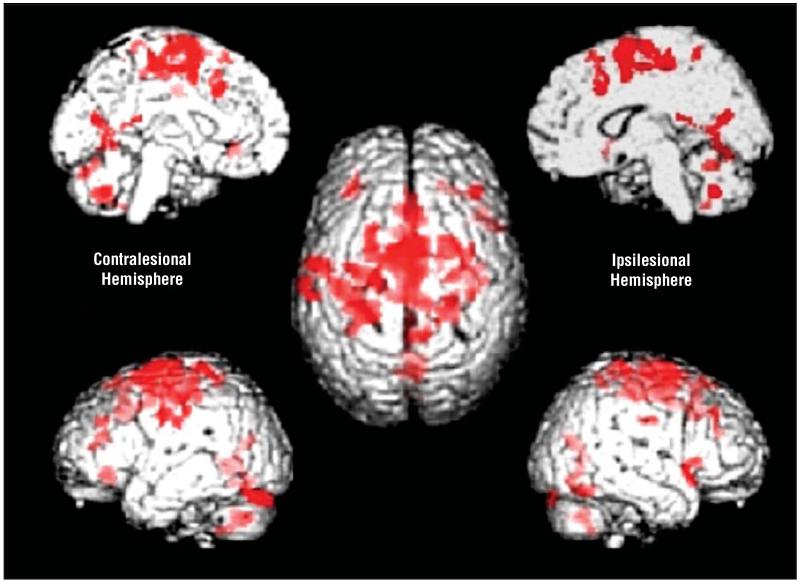
After focal brain injury resulting in motor deficits, the degree of damage to corticospinal tract correlates well with motor recovery. He et al12 proposed that interruption of the projections from primary motor cortex (M1) to spinal cord motor neurons would lead to increased recruitment of secondary motor areas such as dorsolateral premotor cortex (PMd) and supplementary motor area. Recent studies in monkeys have demonstrated that secondary motor areas have direct projections to spinal cord motor neurons, although they are less numerous and less excitatory than those from M1. Thus, although they may contribute to recovery, it is unlikely that they will completely substitute for projections from M1. This view is supported by a recent functional imaging study performed in patients with chronic stroke that demonstrated a negative linear correlation between outcome and task-related brain activation in a number of secondary motor areas such as PMd, supplementary motor area, and cingulate motor areas13 (Figure 1). Patients with no residual impairment have relatively normal activation maps compared with controls, while patients with more marked impairment recruit larger portions of secondary motor areas. But do these regions contribute to recovery? Disruption of ipsilesional PMd14 and contralesional PMd15 by TMS increases motor reaction times in patients with chronic stroke but not controls. Furthermore, TMS to ipsilesional PMd was disruptive in patients with little impairment,14 while TMS to contralesional PMd was more disruptive in patients with greater motor impairment,15 suggesting functionally relevant recruitment of contralesional PMd in those with greatest need. In addition, it seems that some secondary motor areas may take on new functions after functional recovery. Ipsilesional PMd in particular seems to behave as an “executive” motor region similar to M1, with task-related activation increasing linearly as a function of increasing force of hand grip in those with incomplete recovery but not in controls.16 Evidence is thus emerging that supports the functional relevance of secondary motor area recruitment. Outcome may be limited in some patients by the degree of damage to direct corticospinal projections, but recruitment and adaptation of surviving secondary motor areas in both hemispheres may help patients to achieve the best results.14,15 Although these results are described in patients with chronic stroke, recruitment of secondary motor areas occurs in those with greater deficit in the early as well as chronic phase after stroke.16
Figure 1.
Brain regions (shown in red) in which there is a negative linear correlation between increases in BOLD (blood oxygen level–dependent) signal during hand grip and outcome in a group of patients with chronic stroke. The center brain is shown from above (left hemisphere on the left), and then clockwise from top left, left medial surface, right medial surface, right lateral surface, and left lateral surface.
The primary motor cortex (M1) is divided into anterior (Brodmann area [BA] 4a) and posterior (BA4p) segments. A negative correlation between size of activation and outcome has been reported in ipsilesional ventral BA4a and BA4p, and in contralesional BA4p but not BA4a.13 It seems clear that an intact ipsilesional M1 contributes significantly to functional recovery,17 but the role of contralesional M1 is less clear. Despite the fact that contralesional M1 is recruited by patients with chronic stroke with less than complete recovery, its disruption by TMS does not appear to impair performance of motor tasks with the paretic hand in patients with various degrees of recovery.17 More significantly, it is possible that activity in contralesional M1 influences negatively recovery in some patients by contributing to abnormal interhemispheric interactions during voluntary movement of the paretic hand.18 The role of contralesional M1 after stroke clearly requires further investigation.
In the chronic setting, it appears that the damaged brain will utilize surviving structures and networks that can generate some form of motor signal to spinal cord motor neurons. In addition, some areas take on a new role in motor performance. What such studies do not tell us is how this reorganized state evolved. Detailed longitudinal functional magnetic resonance imaging studies of similar patients indicate an initial overactivation in many primary and secondary motor regions followed by a focusing toward a normal activation pattern that parallels recovery.16 Such changes are reminiscent of those observed in the normal brain during motor skill learning. In brains with lesions, it is likely that surviving elements of highly preserved neural systems such as those subserving motor skill learning will be engaged to maximize functional motor recovery. The degree to which mechanisms underlying cerebral reorganization are successful is likely to depend on the functional integrity of the remaining areas. The chronicity of the stroke may also be important, as early lesion-induced cortical hyperexcitability seems to facilitate cortical plasticity. Advances have been made, but a clearer understanding of the mechanisms underlying cerebral reorganization will be required to develop more effective therapeutic strategies.
POSSIBLE STRATEGIES TO ENHANCE THE HUMAN BRAIN’S RESPONSE TO INJURY
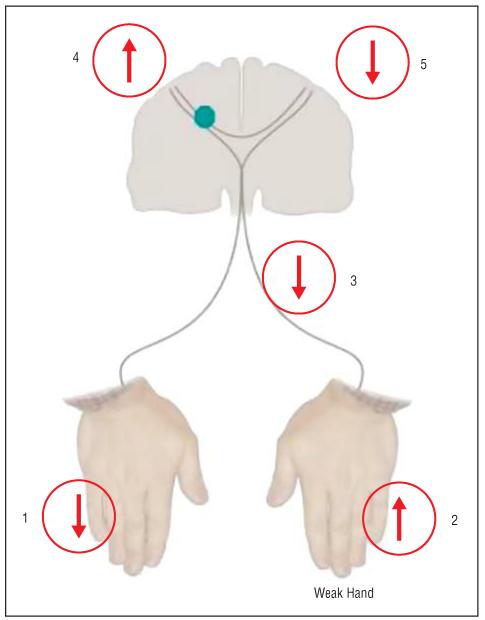
It is clear that functionally relevant adaptive changes take place in the human brain after focal injury. But what drives these changes? Can we modulate them? The lesson from animal models is that manipulation of environmental, behavioral, and pharmacologic contexts can influence cerebral reorganization and consequently the process of recovery of function. How can these lessons be translated into benefit for patients? From our knowledge of how the brain responds to focal injury and how this relates to recovery, we can generate hypothesis-driven approaches to neurorehabilitation. For example, motor performance of a paretic hand could theoretically be influenced by a number of different operational strategies (Figure 2):
Reduction of somatosensory input from the intact hand, as in cutaneous anesthesia, leads to performance improvements in the nonanesthesized hand in healthy volunteers.19 In patients with chronic stroke, cutaneous anesthesia of the intact hand results in behavioral gains in the paretic hand that outlast briefly the duration of the anesthesia20 (indicated by 1 in Figure 2). These findings are consistent with the proposed beneficial influence of immobilization of the intact hand (which reduces somatosensory input from the immobilized limb) in patients with chronic stroke undergoing constraintinduced movement therapy.
Increase in somatosensory input from the paretic hand, eg, by using somatosensory stimulation, may improve motor function21 (indicated by 2 in Figure 2). Motor training of the paretic hand as administered during rehabilitative treatments also increases somatosensory input and results in well-documented behavioral gains.
Anesthesia of a body part proximal to the paretic hand (upper arm, 3 in Figure 2) may become another option to benefit hand motor function.22 In this case, anesthesia of regions of the brachial plexus that innervate the affected upper limb, but not the affected hand, in patients with chronic stroke results in training-dependent improvements in motor function of the paretic hand, a finding consistent with the view that the cortical representation of the paretic hand extended over the nearby deafferented upper arm representation.
Plasticity within the affected motor cortex may be enhanced (4 in Figure 2). Enhancement of the ability of peri-infarct and nonprimary motor regions of the affected hemisphere to respond to motor training or other neurorehabilitative interventions may be important. Cortical stimulation can modify activity in the motor cortex in animals23 and modulates cortical plasticity in humans. For example, TMS synchronously applied to a human motor cortex engaged in a motor training task enhances use-dependent plasticity in the contralateral hand.24 Overall, these findings suggest that noninvasive cortical stimulation could represent an adjuvant to motor training in efforts to recover lost function after cortical lesions like stroke. Consistent with this view, a recent study showed that noninvasive cortical stimulation can enhance motor function in patients with chronic stroke (Friedhelm Hummel, MD, Pablo Celnik, MD, Pascal Giraux, MD, PhD, Agnes Floel, MD, Wan-Hsun Wu, PhD, Christian Gerloff, MD, and L.G.C., unpublished data, 2004.
Activity within the intact motor cortex may be down-regulated (5 in Figure 2). In addition to local effects under the stimulated location, cortical stimulation applied to one site can induce distant effects on cortical function and behavior.25 For example, TMS applied to one motor cortex elicits activation changes in positron emission tomographic scans in the opposite motor cortex. Low-frequency repetitive TMS applied to one motor cortex down-regulates motor cortical excitability in the homonymous motor representation in the opposite hemisphere26 consistent with the concept of a physiologic balance of reciprocal inhibitory projections between both hemispheres. Recent studies showed that this balance is disturbed in patients with cortical lesions such as stroke in the process of generation of a voluntary movement by the paretic hand. Specifically, some of these patients show an abnormally high interhemispheric inhibitory drive from M1 in the intact hemisphere to M1 in the affected hemisphere,18 a finding that is more prominent in more impaired individuals. Therefore, it is possible that one way to enhance motor function in the paretic hand is the down-regulation of activity in the ipsilateral, intact motor cortex (with the purpose of reducing abnormal inhibition from the intact to the affected hemisphere), a hypothesis under investigation. A previous study indeed showed that 1-Hz TMS applied to one motor cortex in healthy individuals results in improvements in motor performance in the ipsilateral hand.27
Pharmacological interventions may enhance recovery processes acting on adrenergic and dopaminergic neurotransmission. In addition to the previously described behavioral and physiological interventions, recovery processes can be substantially influenced by pharmacologic strategies that influence adrenergic and dopaminergic neurotransmission.28
Figure 2.
Diagram showing possible operational strategies to influence hand function (see “Possible Strategies to Enhance the Human Brain’s Response to Injury” section for details).
It is likely that neuroimaging (functional magnetic resonance imaging, positron emission tomography) and electrophysiologic (TMS, electroencephalography, magnetoencephalography) techniques will enhance our understanding of the mechanisms underlying the beneficial effects of particular interventions. Recent studies have shown increased task-related activation in affected hemispheres (eg, in M1 or PMd) and reduced activation in unaffected hemispheres after a period of treatment,29-31 but it remains to be determined whether these results relate to the mechanisms or only the consequences of the rehabilitative process. Further experiments that test the effects of interventions on particular aspects of brain function, eg, use-dependent plasticity,7 in different patient groups may help unravel the underlying mechanisms. Such an approach could allow treatments to be targeted at suitable patients. Furthermore, the timing of an intervention may also be important. For example, modulating attention toward a motor task may be more or less beneficial depending on the chronicity of the stroke.32
In summary, recent studies have started to unveil the mechanisms underlying human cortical plasticity and its relationship to recovery of motor function after focal brain lesions. On the basis of this increased understanding, novel interventional strategies are being tested that raise hope for the development of new treatments for this condition.
Contributor Information
Dr Nick S. Ward, Wellcome Department of Imaging Neuroscience, Institute of Neurology, University College London, London, England.
Dr Leonardo G. Cohen, Human Cortical Physiology Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Md.
REFERENCES
- 1.Hebb DO. Organization of Behavior. John Wiley & Sons; New York, NY: 1949. [Google Scholar]
- 2.Donoghue JP. Plasticity of adult sensorimotor representations. Curr Opin Neurobiol. 1995;5:749–754. doi: 10.1016/0959-4388(95)80102-2. [DOI] [PubMed] [Google Scholar]
- 3.Ivanco TL, Greenough WT. Physiological consequences of morphologically detectable synaptic plasticity: potential uses for examining recovery following damage. Neuropharmacology. 2000;39:765–776. doi: 10.1016/s0028-3908(00)00004-6. [DOI] [PubMed] [Google Scholar]
- 4.Collingridge GL, Bliss TV. Memories of NMDA receptors and LTP. Trends Neurosci. 1995;18:54–56. [PubMed] [Google Scholar]
- 5.Hess G, Donoghue JP. Long-term potentiation and long-term depression of horizontal connections in rat motor cortex. Acta Neurobiol Exp (Warsz) 1996;56:397–405. doi: 10.55782/ane-1996-1143. [DOI] [PubMed] [Google Scholar]
- 6.Rioult-Pedotti M-S, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- 7.Butefisch CM, Davis BC, Wise SP, et al. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci U S A. 2000;97:3661–3665. doi: 10.1073/pnas.050350297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziemann U, Iliac TV, Pauli C, Meintzschel F, Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci. 2004;24:1666–1672. doi: 10.1523/JNEUROSCI.5016-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- 10.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 11.Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- 12.He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci. 1993;13:952, 980. doi: 10.1523/JNEUROSCI.13-03-00952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003;126:1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of human premotor cortex after stroke recovery. Brain. 2004;127:747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- 15.Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Werhahn KJ, Conforto AB, Kadom N, Hallett M, Cohen LG. Contribution of the ipsilateral motor cortex to recovery after chronic stroke. Ann Neurol. 2003;54:464–472. doi: 10.1002/ana.10686. [DOI] [PubMed] [Google Scholar]
- 18.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 19.Werhahn KJ, Mortensen J, Van Boven RW, Zeuner KE, Cohen LG. Enhanced tactile spatial acuity and cortical processing during acute hand deafferentation. Nat Neurosci. 2002;5:936–938. doi: 10.1038/nn917. [DOI] [PubMed] [Google Scholar]
- 20.Floel A, Nagorsen U, Werhahn KJ, et al. Influence of somatosensory input on motor function in patients with chronic stroke. Ann Neurol. 2004;56:206–212. doi: 10.1002/ana.20170. [DOI] [PubMed] [Google Scholar]
- 21.Conforto AB, Kaelin-Lang A, Cohen LG. Increase in hand muscle strength of stroke patients after somatosensory stimulation. Ann Neurol. 2002;51:122–125. doi: 10.1002/ana.10070. [DOI] [PubMed] [Google Scholar]
- 22.Muellbacher W, Richards C, Ziemann U, et al. Improving hand function in chronic stroke. Arch Neurol. 2002;59:1278–1282. doi: 10.1001/archneur.59.8.1278. [DOI] [PubMed] [Google Scholar]
- 23.Nudo RJ, Jenkins WM, Merzenich MM. Repetitive microstimulation alters the cortical representation of movements in adult rats. Somatosens Mot Res. 1990;7:463–483. doi: 10.3109/08990229009144720. [DOI] [PubMed] [Google Scholar]
- 24.Butefisch C, Khurana V, Kopylev L, Cohen LG. Enhancing encoding of a motor memory in the primary motor cortex by cortical stimulation. J Neurophysiol. 2004;91:2110–2116. doi: 10.1152/jn.01038.2003. [DOI] [PubMed] [Google Scholar]
- 25.Siebner HR, Peller M, Willoch F, et al. Lasting cortical activation after repetitive TMS of the motor cortex: a glucose metabolic study. Neurology. 2000;54:956–963. doi: 10.1212/wnl.54.4.956. [DOI] [PubMed] [Google Scholar]
- 26.Schambra HM, Sawaki L, Cohen LG. Modulation of excitability of human motor cortex (M1) by 1 Hz transcranial magnetic stimulation of the contralateral M1. Clin Neurophysiol. 2003;114:130–133. doi: 10.1016/s1388-2457(02)00342-5. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi M, Hutchinson S, Theoret H, Schlaug G, Pascual-Leone A. Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology. 2004;62:91–98. doi: 10.1212/wnl.62.1.91. [DOI] [PubMed] [Google Scholar]
- 28.Butefisch CM, Davis BC, Sawaki L, et al. Modulation of use-dependent plasticity by d-amphetamine. Ann Neurol. 2002;51:59–68. doi: 10.1002/ana.10056. [DOI] [PubMed] [Google Scholar]
- 29.Pariente J, Loubinoux I, Carel C, et al. Fluoxetine modulates motor performance and cerebral activation of patients recovering from stroke. Ann Neurol. 2001;50:718–729. doi: 10.1002/ana.1257. [DOI] [PubMed] [Google Scholar]
- 30.Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125:2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- 31.Schaechter JD, Kraft E, Hilliard TS, et al. Motor recovery and cortical reorganization after constraint-induced movement therapy in stroke patients: a preliminary study. Neurorehabil Neural Repair. 2002;16:326–338. doi: 10.1177/154596830201600403. [DOI] [PubMed] [Google Scholar]
- 32.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. The influence of time after stroke on brain activations during a motor task. Ann Neurol. 2004;55:829–834. doi: 10.1002/ana.20099. [DOI] [PMC free article] [PubMed] [Google Scholar]