Abstract
Membrane potential (Vm), the voltage across the plasma membrane, arises because of the presence of different ion channels/transporters with specific ion selectivity and permeability. Vm is a key biophysical signal in non-excitable cells, modulating important cellular activities, such as proliferation and differentiation. Therefore, the multiplicities of various ion channels/transporters expressed on different cells are finely tuned in order to regulate the Vm. It is well-established that cancer cells possess distinct bioelectrical properties. Notably, electrophysiological analyses in many cancer cell types have revealed a depolarized Vm that favors cell proliferation. Ion channels/transporters control cell volume and migration, and emerging data also suggest that the level of Vm has functional roles in cancer cell migration. In addition, hyperpolarization is necessary for stem cell differentiation. For example, both osteogenesis and adipogenesis are hindered in human mesenchymal stem cells (hMSCs) under depolarizing conditions. Therefore, in the context of cancer, membrane depolarization might be important for the emergence and maintenance of cancer stem cells (CSCs), giving rise to sustained tumor growth. This review aims to provide a broad understanding of the Vm as a bioelectrical signal in cancer cells by examining several key types of ion channels that contribute to its regulation. The mechanisms by which Vm regulates cancer cell proliferation, migration, and differentiation will be discussed. In the long term, Vm might be a valuable clinical marker for tumor detection with prognostic value, and could even be artificially modified in order to inhibit tumor growth and metastasis.
Keywords: cancer, cell cycle, differentiation, ion channel, membrane potential, migration, proliferation, stem cell
Introduction
The presence of various ion channels and transporters at the plasma membrane provides different permeability to distinct ions, such as Na+, K+, Ca2+, and Cl−. Due to the unequal distribution of these ions, a voltage difference exists between the cytoplasm and the extracellular environment, which is known as the membrane potential (Vm). Vm is expressed relative to the extracellular environment. A cell is depolarized when the Vm is relatively less negative, whereas a hyperpolarized cell possesses a more negative Vm. Vm changes because of alterations in the conductance of one or more types of ion. The Goldman–Hodgkin–Katz equation shows that the Vm depends on the permeability (P) and both the intracellular and extracellular concentrations of major ions (Goldman, 1943; Hodgkin and Katz, 1949):
where R is the ideal gas constant, T the temperature, and F the Faraday constant. In addition, intercellular communications (e.g., gap junction connections) are also able to influence Vm (Hulser and Lauterwasser, 1982; Levin, 2007a). In excitable cells, such as neurons and muscle fibers (Nakajima and Horn, 1967; Bean, 2007), changes in Vm underlie the action potential (AP) waveform. APs fire in response to a depolarization that exceeds a threshold value. Fine-tuning of APs is tightly regulated by the activities of several key ion channels and transporters, including voltage-gated Na+ channels (VGSCs), voltage-gated K+ channels (Kv), and the Na+/K+-ATPase (Caldwell and Keynes, 1957; Hille, 1992).
Emerging evidence suggests that the Vm also plays important functional roles in non-excitable cells. In the late 1960's, while studying mitotic activities in sarcoma cells, Clarence D. Cone Jr. reported that Vm underwent hyperpolarization before entering M phase, and suggested that the level of Vm correlated with cell cycle progression (Cone, 1969). He subsequently showed that membrane hyperpolarization reversibly blocked DNA synthesis and mitosis (Cone, 1970). He later generalized existing data at that time and postulated that the Vm level was correlated with the level of differentiation. For example, terminally differentiated cells (e.g., fibroblasts and epithelium) possess hyperpolarized Vm (Cone, 1971). Since then, changes in Vm, representing the long-term, slowly changing bioelectric gradient in non-excitable cells (Lobikin et al., 2012), have been shown to control critical cell functions including proliferation, migration, and differentiation (Binggeli and Weinstein, 1986; Schwab et al., 2007; Blackiston et al., 2009; Sundelacruz et al., 2009). Recently, studies have also demonstrated that Vm is able to, directly or indirectly, control wound healing (Nuccitelli, 2003a,b; McCaig et al., 2009), left-right patterning (Adams et al., 2006), development (Nuccitelli, 2003a; Adams, 2008), and regeneration (Levin, 2007b, 2009). Therefore, given the increasing evidence showing that ion channels/transporters functionally participate in cancer progression (Kunzelmann, 2005; Fiske et al., 2006; Stuhmer et al., 2006; Prevarskaya et al., 2010; Becchetti, 2011; Brackenbury, 2012), it is not surprising that Vm has been implicated in cancer development, since Vm is itself determined by the combined activities of ion channels/transporters at the cell membrane. This article aims to summarize current understanding of the Vm as a bioelectric regulator in cancer, and examines the therapeutic potential of Vm for tumor detection and treatment.
Cancer cells possess depolarized Vm
Cone's theory proposing the general correlation between proliferation and Vm (Cone, 1971) was supported by several previous studies which demonstrated significant Vm depolarization during malignant transformation of normal cells (Tokuoka and Morioka, 1957; Johnstone, 1959). Direct in vitro and in vivo comparisons of Vm levels between normal and cancerous breast cells (Marino et al., 1994), hepatocytes and hepatocellular carcinoma cells (Binggeli and Cameron, 1980; Stevenson et al., 1989), normal and neoplastic adrenocortical tissues (Lymangrover et al., 1975), normal embryonic fibroblasts and fibrosarcoma (Binggeli and Weinstein, 1985), benign and cancerous skin cells (Melczer and Kiss, 1957; Woodrough et al., 1975), and between normal and cancerous ovarian tissue (Redmann et al., 1972) showed that cancer cells tended to be more depolarized than their normal counterparts. In addition, the intracellular Na+ level is markedly higher in tumors compared to non-cancerous tissues, whereas the K+ level remains more stable (Smith et al., 1978; Cameron et al., 1980; Sparks et al., 1983). A similar scenario occurs in fast proliferating Chinese hamster ovary (CHO) and 3T3 cells (Cone and Tongier, 1973). Thus, an increased intracellular Na+ concentration could be a determinant of a depolarized phenotype in rapidly cycling cancer cells.
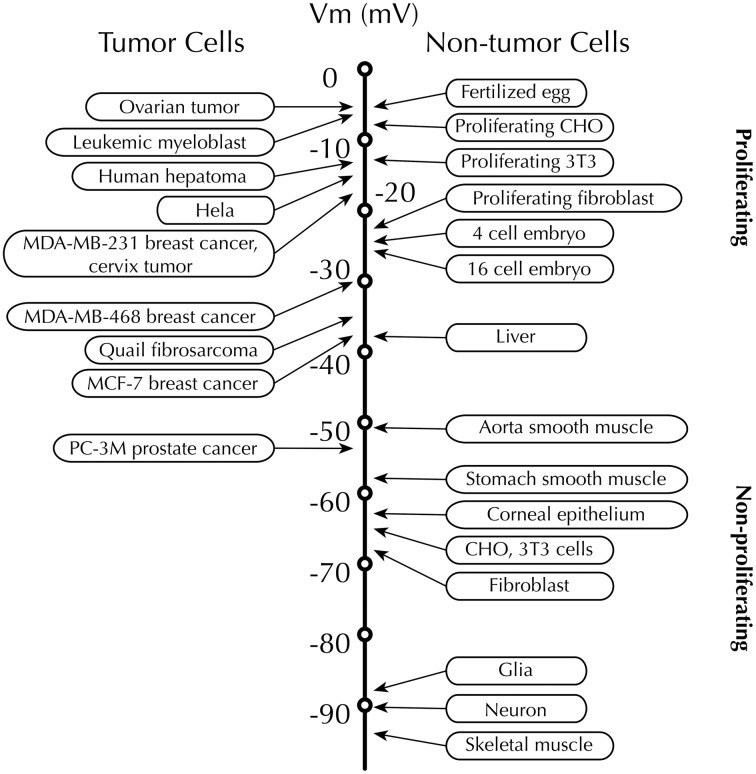
Recordings from rodent and human tissues have revealed that proliferative cells, especially rapidly proliferating tumor cells, displayed depolarized Vm, whereas non-proliferating, terminally differentiated somatic cells, such as muscle cells and neurons, are characterized by their hyperpolarized Vm (Figure 1) [reviewed in Binggeli and Weinstein (1986)]. Given these findings, is Vm merely an epiphenomenon, which only indicates the outcome of the activities of various ion channels and transporters, or is it is actually a functional instructor that is capable of promoting tumorigenesis? A similar question had been posed 50 years ago soon after Cone revealed the relationship between mitotic activity and Vm level (Cone and Tongier, 1971). For example, depolarization can initiate mitosis in CHO cells and mouse spleen lymphocytes (Cone and Tongier, 1971; Kiefer et al., 1980). By contrast, hyperpolarized Vm immediately precedes mitotic arrest (Cone and Tongier, 1973). More recently, in vivo evidence shows that membrane depolarization itself, regardless of the types of ions and ion channel/transporter proteins, is able to bring cancerous transformation (i.e., increased proliferation, change in morphology and abnormal angiogenesis) in Xenopus laevis embryos (Lobikin et al., 2012).
Figure 1.
Membrane potential (Vm) scale. Rapidly proliferating cancer cells possess depolarized Vm, while the Vm of quiescent cells is generally more negative. Proliferative somatic cells are also depolarized, suggesting that Vm is functionally instructive in cell development (Levin, 2007b). Scale adapted from Binggeli and Weinstein (1986), with additional data from Fraser et al. (2005); Mycielska et al. (2005); Yang et al. (2012).
Hanahan and Weinberg proposed 10 hallmarks of cancer, including sustaining proliferative signaling, activating invasion and metastasis, and angiogenesis (Hanahan and Weinberg, 2011). The following sections review the prevailing evidence that implicates Vm in several of these processes.
Vm and cancer cell proliferation
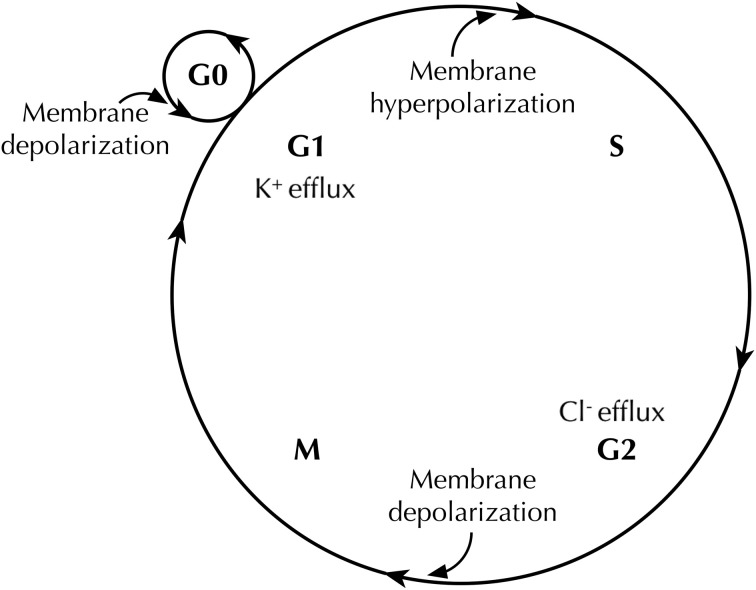
In general, in both highly proliferative tumor and non-tumor cells, depolarization is believed to serve as a signal that could initiate mitosis and DNA synthesis (Orr et al., 1972; Binggeli and Weinstein, 1986). Artificially altering Vm by modulating the extracellular ionic constitution or applying the Na+/K+-ATPase inhibitor ouabain revealed interesting results: First, hyperpolarizing CHO cells to −45 mV started to induce mitotic arrest and cell division was fully blocked at −75 mV. The cell cycle was resumed by depolarizing the cells to −10 mV (Cone, 1971). Secondly, quiescent (G0) mature chick spinal cord neurons showed mitotic activity after depolarization (Cone and Cone, 1976) (Figure 2). Recently, artificial control of Vm was accomplished in Xenopus laevis embryos by expressing glycine-gated Cl− channels and applying the activator ivermectin. Depolarization (caused by lowering the Cl− concentration in the extracellular medium, which caused Cl− efflux) was found to be directly responsible for malignant proliferation. This proliferation was ion and ion channel non-specific, because (1) the phenotype caused by depolarization could be rescued by expressing a hyperpolarizing channel gene, and (2) the malignant phenotype could be induced or suppressed simply by adjusting extracellular Cl− concentration, as predicted by Goldman–Hodgkin–Katz equation (Lobikin et al., 2012). Therefore, the depolarized Vm frequently found in cancerous cell types could be regarded as a “sustaining proliferative signal” that instructs cells to rapidly advance in the cell cycle.
Figure 2.
Membrane potential (Vm) changes during the cell cycle. Vm undergoes hyperpolarization at G1/S border, by virtue of K+ efflux through various K+ channels. Before cells enter M phase, increased Cl− efflux accompanies Vm depolarization. Quiescent cells at G0 stage show mitotic activities after Vm depolarization (Cone and Cone, 1976).
An additional layer of complexity in this model is that the Vm fluctuates during cell cycle progression, and follows a multi-step and rhythmic pattern (Wonderlin and Strobl, 1996; Blackiston et al., 2009) (Figure 2). A number of studies suggest that membrane hyperpolarization at the G1/S checkpoint is generally required for S phase initiation. For example, depolarizing the cell membrane halts G1/S progression in glia (Canady et al., 1990), Schwann cells (Wilson and Chiu, 1993), lymphocytes (Price et al., 1989; Freedman et al., 1992; Wang et al., 1992), V79 Chinese hamster lung cells (Sachs et al., 1974), C1300 mouse neuroblastoma cells (Boonstra et al., 1981), and MCF-7 human breast cancer cells (Wonderlin et al., 1995). The Vm then appears to remain relatively hyperpolarized through S phase in some cell types (Sachs et al., 1974; Boonstra et al., 1981; Strobl et al., 1995; Wonderlin et al., 1995), but is more depolarized in others (Arcangeli et al., 1995; Macfarlane and Sontheimer, 2000). The G2/M transition exhibits a depolarized Vm (Sachs et al., 1974; Boonstra et al., 1981; Blackiston et al., 2009), although it is not known whether or not this depolarization is a prerequisite for progression. In fact, the exact Vm thresholds for driving progression appear to depend heavily on cell type, the state of differentiation, and the density of cell monolayer in culture (Cone and Tongier, 1973; Blackiston et al., 2009).
Importantly, the fluctuation of Vm levels across the cell cycle does not necessarily contradict the observation that depolarized Vm could be a hallmark of cancer cells. The mean Vm values in cancer cells are consistently depolarized relative to most normal somatic cell types (Figure 1). For example, MCF-7 cells arrested at G1 phase have a Vm of −9 mV and hyperpolarize to ~−30 mV in the S phase (Wonderlin et al., 1995). Both these values are more depolarized than normal breast cells, e.g., the mean Vm of unsynchronized MCF-10A cells is between −40 and −58 mV (Marino et al., 1994; Wonderlin et al., 1995; Fraser et al., 2005).
Evidence suggests that the fluctuation in K+ concentration plays a significant contribution to changes in Vm during the cell cycle. For example, in neuroblastoma and Ehrlich ascites cells, there is a transient decrease in K+ efflux before entering the G2 phase, a relatively high level of K+ efflux during the M phase (Mills and Tupper, 1976; Boonstra et al., 1981). Given the diversity of K+ channel types (Hille, 1992; Miller, 2000; Wang, 2004), their relative contributions to the Vm and Vm-dependent cell cycle progression is probably context-dependent and highly complex. For example, inhibition of cell proliferation with K+ channel inhibitors does not correlate with changes in the Vm in rat C6 glioma cells (Rouzaire-Dubois et al., 2000). In addition, the Vm is likely to be determined by the collective activities of a variety of ions/channels/transporters, which may exhibit reciprocal interactions and form a large and complex network responsible for Vm regulation and its downstream effects.
Ion channel-dependent regulation of proliferation and Vm
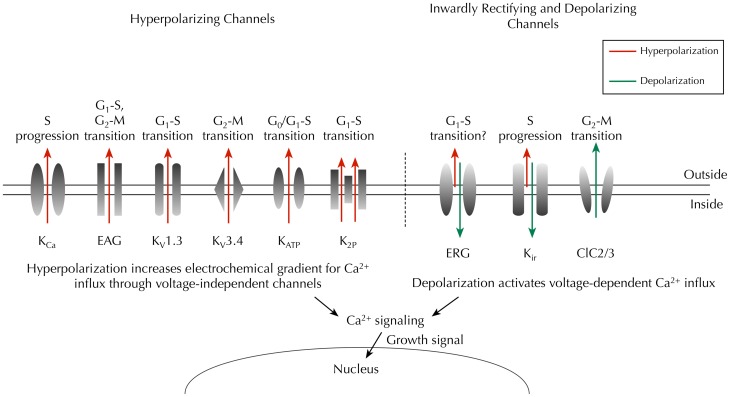
Numerous studies have shown that pharmacological or genetic block of Kv channels reduces proliferation of cancer cells (e.g., Fraser et al., 2000; Ouadid-Ahidouch et al., 2000; Abdul and Hoosein, 2002; Chang et al., 2003; Menendez et al., 2010). Increasing evidence suggests that Ether à go-go (EAG) K+ channels may serve as biomarkers for cancer (Ouadid-Ahidouch et al., 2001; Farias et al., 2004; Pardo et al., 2005; Hemmerlein et al., 2006; Ousingsawat et al., 2007; Ortiz et al., 2011; Rodriguez-Rasgado et al., 2012). Inhibition of EAG channel expression reduces proliferation in several cancer cell lines, whereas implantation of CHO cells over-expressing EAG channels in mice induces tumors (Pardo et al., 1999). In synchronized SH-SY5Y cells, human IEAG is reduced to less than 5% in G1 phase, compared to unsynchronized controls, suggesting that the activity of EAG channels is cell cycle-dependent (Meyer and Heinemann, 1998). Indeed, in MCF-7 cells, inhibiting EAG channels with astemizole increases the proportion of cells in G1 phase and reduces the proportion in S phase (Borowiec et al., 2007). In contrast, activation of hEAG channels is responsible for hyperpolarization at late G1 before the cells enter the S phase (Ouadid-Ahidouch et al., 2001). Interestingly, the hyperpolarization is accompanied by increased Ca2+-activated K+ (KCa) channel currents (Ouadid-Ahidouch et al., 2001), which might result from the elevated intracellular Ca2+ due to the increased electrochemical gradient (Figure 3) (Nilius and Wohlrab, 1992; Ouadid-Ahidouch and Ahidouch, 2008).
Figure 3.
Key ion channels that regulate Vm and cell cycle progression in cancer. Hyperpolarizing channels (outward IK, red) would increase the driving force for Ca2+ influx through voltage-independent channels, whereas inwardly rectifying K+ channels (predominantly inward IK, green) and chloride channels (outward Cl−, green) would depolarize the Vm, thus enabling activation of voltage-dependent Ca2+ influx (Schwab et al., 2012). Time- and domain-dependent Ca2+ signaling is then proposed to activate pathways that promote cell cycle progression and proliferation. Abbreviations: KCa, Ca2+-activated K+ channel; EAG, ether à go-go channel; Kv, voltage-gated K+ channel; KATP, ATP-sensitive K+ channel; K2P, two-pore domain K+ channel; ERG, EAG-related gene K+ channel; Kir, classic inward-rectifier K+ channel; ClC2/3, chloride 2/3 channel.
When KCa channels were found in Friend murine erythroleukemia cells, they were thought to be one of the main controllers of the Vm (Arcangeli et al., 1987). KCa channels have been found since in glioma (Liu et al., 2002), prostate cancer (Gessner et al., 2005), breast cancer (Haren et al., 2010), and the CD133+ subpopulation of SH-SY5Y cells (Park et al., 2010). Inhibiting KCa channels with iberiotoxin arrests D54-MG glioma cells in S phase, and leads to apoptosis (Weaver et al., 2004). Thus, the functional contribution of KCa channels to cell cycle regulation appears to be distinct from Kv channels. In addition, in MCF-7 cells, inhibition of ATP-sensitive K+ (KATP) channels reversibly arrests cells in the G0/G1 phase (Woodfork et al., 1995). The two-pore domain K+ channel, TREK1, increases proliferation of PC-3 and LNCaP prostate cancer cells (Voloshyna et al., 2008). In CHO cells, overexpression of TREK1 increases the number of cells in S phase, and reduces the number of cells at G0/G1 phase (Voloshyna et al., 2008).
Human EAG-related gene (HERG) K+ channels are strongly inwardly rectifying and conduct K+ influx when the voltage is more negative than the K+ equilibrium potential (Trudeau et al., 1995; Smith et al., 1996). HERG channels are expressed at early developmental stages in the neural crest, central nervous system, dorsal root ganglion (DRG) and skeletal muscle, and are replaced by classic inward rectifier K+ current (IKir) later in development (Arcangeli et al., 1997; Crociani et al., 2000). HERG channels are upregulated in a number of cancers (Arcangeli, 2005). Moreover, IHERG increases tumor cell proliferation (Bianchi et al., 1998; Wang et al., 2002). The activity of IHERG itself is cell cycle dependent (Arcangeli et al., 1995), suggesting a complex relationship between IHERG, Vm, and proliferation. Additional inward rectifier K+ (Kir) channels have been reported in various cancer cell types, and are required for proliferation, including Kir2.2 (Lee et al., 2010), Kir3.1, and Kir3.4 (Plummer et al., 2004; Takanami et al., 2004; Plummer et al., 2005; Wagner et al., 2010). In contrast, overexpression Kir4.1 in glioma cells hyperpolarizes the Vm and increases the number of cells in quiescent G0/G1, reducing the proportion in G2/M phase (Higashimori and Sontheimer, 2007). Thus, different Kir channels may play opposing roles in regulation of Vm/proliferation, as a result of their heterogeneous voltage dependence (Figure 3). Cl− conductance also appears to be linked to the cell cycle and regulate proliferation. For example, in D54-MG cells, Cl− efflux through the outward rectifying ClC3 Cl− channel is significantly increased during M phase (Habela et al., 2008). In addition, the ClC2 channel is expressed in M phase in transfected NRK-49F rat kidney fibroblast cells (Zheng et al., 2002).
The mechanisms underlying ion channel-dependent proliferation of cancer cells have been reviewed in detail elsewhere (Wang, 2004; Ouadid-Ahidouch and Ahidouch, 2008; Prevarskaya et al., 2010). These include possible non-conducting, direct interactions between ion channels and other pro-proliferative signaling mechanisms. For example, coexpression of HERG and tumor necrosis factor receptor 1 (TNFR1) has been found at the cell membrane of SKBR3 and SH-SY5Y cell lines, and HERG appears to recruit TNFR1 to the membrane, therefore enhancing TNF-α-induced cancer cell proliferation (Wang et al., 2002). Alternatively, ion channel-mediated Vm hyperpolarization would increase the electrochemical gradient for Ca2+ and therefore elevate the intracellular Ca2+ concentration through voltage-independent Ca2+ channels, such as transient receptor potential (TRP) channels (Nilius and Wohlrab, 1992; Wang, 2004; Ouadid-Ahidouch and Ahidouch, 2008). Ca2+ signaling is functional across the whole cell cycle (Santella et al., 2005). For example, Ca2+ is required for G1 progression and G1/S transition (Hazelton et al., 1979; Choi et al., 2006). In turn, intracellular Ca2+ and calmodulin (CaM) can regulate KCa and EAG channels (Khanna et al., 1999; Ziechner et al., 2006; Ouadid-Ahidouch and Ahidouch, 2008). Thus, there may be a reciprocal, auto-regulatory relationship between ion channel activity, Vm, intracellular Ca2+ signaling, and proliferation.
In summary, a multiplicity of ion channels (predominantly K+-conducting) participates in Vm regulation (both depolarization and hyperpolarization) in cancer cells. In turn, changes in Vm promote transition through cell cycle checkpoints. Changes in Vm are likely to trigger intracellular signaling messengers such as Ca2+ in order to drive sustained proliferation.
Role of Vm in cancer cell migration
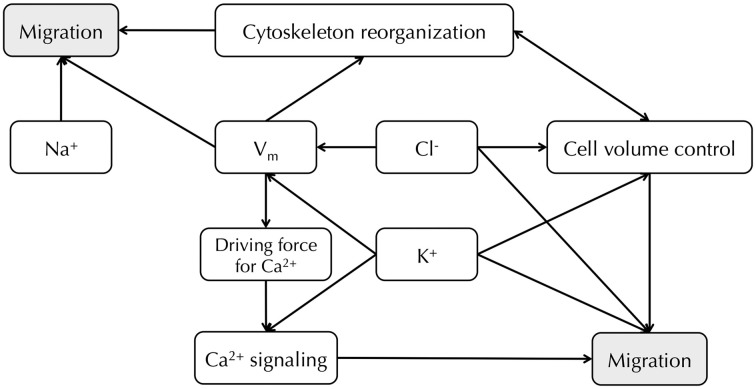
Metastasis involves loss of adhesion at the primary site, increased migration and invasion, circulation through the vascular/lymphatic systems and growth of secondary tumors at distant sites (Gupta and Massague, 2006; Prevarskaya et al., 2010). Among the various steps in the metastatic cascade, it is well-established that cell migration is tightly controlled by the movement of ions and water [Figure 4; reviewed in depth in Schwab et al. (2007, 2012)]. Vm is regarded as an indirect factor that can affect cell migration, whose main regulatory role might be setting up the electrical driving force for Ca2+ (Prevarskaya et al., 2010; Schwab et al., 2012). A hyperpolarized Vm can increase intracellular Ca2+ via TRP channels, whereas membrane depolarization could activate voltage-gated Ca2+ channels (Schwab et al., 2012). Intracellular Ca2+ displays a concentration gradient in migrating cells, with lowest concentration at the leading edge (Brundage et al., 1991). During cell migration, oscillations in Ca2+ concentration are observed within microdomains, such that Ca2+ flickering is highest in the lamellipodia (Wei et al., 2009). These fluctuations play a role in regulating tractional forces (Lee et al., 1999; Ridley et al., 2003), direction sensing, and cytoskeleton reorganization (Pettit and Fay, 1998). Vm may also affect downstream intracellular signaling cascades that could contribute to cell migration in a Ca2+-independent way (Figure 4). For example, in kidney epithelial cells, Vm depolarization induces diphosphorylation of myosin light chain (MLC) without inducing Ca2+ signaling, but instead by activating the Rho-Rho kinase (ROK) pathway (Szaszi et al., 2005). In addition, actin filaments undergo reorganization following Vm depolarization in bovine eye endothelial and epithelial cells (Chifflet et al., 2003, 2004), suggesting a functional role for Vm in cytoskeletal reorganization, although it is not clear whether or not Ca2+ is involved. Furthermore, applied electrical fields, which would impact on Vm, can enhance motility and galvanotaxis (Djamgoz et al., 2001; Levin, 2003, 2009; Schwab et al., 2012).
Figure 4.
Relationship between Na+, K+, Cl− channels and Vm in cancer cell migration. Vm provides the driving force for Ca2+, and downstream Ca2+ signaling leads to cell migration (Schwab et al., 2012). Vm also regulates cytoskeleton reorganization (Chifflet et al., 2003, 2004). Cl− and K+ channels both contribute to Vm regulation and cell volume control (Soroceanu et al., 1999; Sontheimer, 2008; Habela et al., 2009; Schwab et al., 2012). Inhibiting particular Na+, K+, and Cl− channels can reduce cancer cell migration (Sontheimer, 2008; Brackenbury, 2012; Schwab et al., 2012).
A number of Na+, K+, and Cl− channels, that potentially contribute to the Vm, are directly implicated in cancer cell migration. For example, functional VGSCs have been found in a number of cancer types [reviewed in Brackenbury (2012)], and suppressing VGSCs with siRNA or pharmacological agents inhibits migration and invasion (Roger et al., 2003; Fraser et al., 2005; Brackenbury et al., 2007; House et al., 2010; Yang et al., 2012). In several breast carcinoma/melanoma cell lines, KCa2.3, which is responsible for maintaining a hyperpolarized Vm, enhances migration, likely via promotion of intracellular Ca2+ signaling (Potier et al., 2006; Chantome et al., 2009). In addition, KCa3.1 activity causes a local shrinkage at the rear of migrating MDCK-F cells, therefore supporting retraction at this pole during movement (Schwab et al., 2006). In order to maintain electroneutrality, K+ efflux must be accompanied by an anion, and Cl− is the most likely candidate (Schwab et al., 2007, 2012). In agreement with this, Cl− channels, which contribute to the depolarized Vm in glioma cells, enhance migration and invasion by permitting the release of K+, Cl−, and water at the leading edge, resulting in shrinkage and facilitating movement into tortuous extracellular spaces (Soroceanu et al., 1999; Sontheimer, 2008; Habela et al., 2009; Schwab et al., 2012).
In conclusion, a direct role for Vm in regulating cancer cell migration is much less clear than for proliferation. Given the great variety of ion channels and transporters that are involved in the process of cell migration, the concept of the “transportome” has been proposed (Schwab et al., 2012), which implies that rather than individual ion channels or transporters, it is a complex network of ion translocators that directs the migration and invasion of cells (Figure 4). Further work is required to establish to what extent Vm directly impacts on this network.
Vm and the differentiation of cancer stem cells
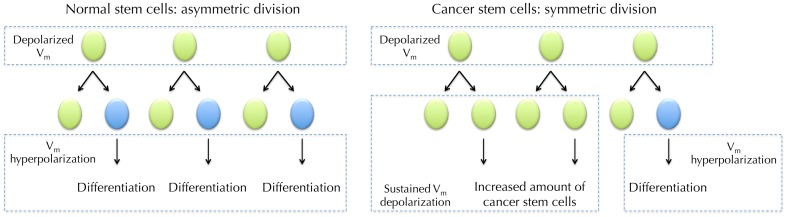
Stem cells and cancer cells share similar properties, such as the ability to differentiate and self-renew, increased membrane transporter activity and the ability to migrate and metastasize (Wicha et al., 2006). The cancer stem cell (CSC) hypothesis contains two key concepts: (1) cancers arise from dysregulated transformation of normal tissue stem cells or progenitor cells, and (2) cellular components that display stem cell properties can lead to cancer progression (Wicha et al., 2006). In contrast to normal, regulated asymmetric division of stem cells during tissue homeostasis, where a stem cell produces one copy of itself and one cell that later differentiates into a mature cell, the dysregulation of transformed CSCs during tumorigenesis involves “symmetric division” in which each malign CSC generates two identical daughter cells (giving rise to either proliferation or differentiation), which significantly expands the malign stem cell reservoir (Figure 5) (Liu et al., 2005).
Figure 5.
Vm in normal stem cell (SC) differentiation and hypothesized role for Vm in cancer stem cells (CSCs). Depolarized Vm is needed during the maintenance of SCs. SC undergoes asymmetric division where it produces one copy of itself and one progeny that later differentiate into mature cells. The maturation requires Vm hyperpolarization (Sundelacruz et al., 2008). However, CSCs frequently undergo symmetric division, in which one CSC divides into two identical CSC progenies (Wicha et al., 2006). Sustained Vm depolarization may help to maintain the increasing CSCs in an undifferentiated state. Proliferation of CSCs then increases cancer malignancy.
A role for Vm in differentiation of normal stem cells has been previously reported. Studies in quail neural crest cells and a subpopulation of SH-SY5Y cells have demonstrated that stem cells exhibit distinct bioelectrical profiles during development (Arcangeli et al., 1997; Biagiotti et al., 2006; Sundelacruz et al., 2009). In particular, a hyperpolarized Vm is required during stem cell maturation (Sundelacruz et al., 2009). For example, Kir-induced Vm hyperpolarization is required during human myoblast fusion (Liu et al., 1998). In a genome-wide microarray analysis of depolarization-regulated genes in postnatal mouse cerebellar granule neurons, among 87 depolarization-responsive genes, 22 are developmentally up-regulated and 26 are developmentally down-regulated (Sato et al., 2005). Remarkably, 18 of the 22 (82%) developmentally up-regulated genes coincide with depolarization down-regulated genes, and 20 of 26 (77%) developmentally down-regulated genes with depolarization up-regulated genes (Sato et al., 2005). Vm hyperpolarization is also a functional determinant of human mesenchymal stem cell (hMSC) differentiation. Pharmacologically-induced Vm depolarization suppresses adipogenic and osteogenic differentiation of hMSCs (Sundelacruz et al., 2008). In addition, depolarization reduces the differentiated phenotype of hMSC-derived cells and improves their ability to transdifferentiate, without fully restoring a stem cell-like genetic profile (Sundelacruz et al., 2013). Taken together, these data suggest that Vm depolarization may maintain cells in an undifferentiated stage at the gene expression level. Therefore, it is not unreasonable to postulate that depolarized Vm may also help maintain a population of undifferentiated CSCs (Figure 5). This possibility would raise additional, related questions: does a more depolarized Vm promote the proliferation of CSCs? Does Vm affect the pattern of symmetric vs. asymmetric division? Further work is required to investigate these possibilities.
Clinical implications
Given that the fluctuation of Vm can functionally regulate tumorigenesis, differentiation, and promote cancer progression, it may serve as a potential marker for tumor detection and treatment, with prognostic value. For example, bioelectrical impedance analysis, which determines tissue electrical properties, has shown promise as a prognostic indicator to monitor cancer progression (Gupta et al., 2004a,b); , and recently, the development of non-invasive, voltage-sensitive optical probes provides a potential approach for in vivo Vm measurement (Adams and Levin, 2012; Chernet and Levin, 2013). Considering the vast array of therapeutic drugs that target ion channels (Sontheimer, 2008; Stuhmer and Pardo, 2010; D'amico et al., 2013; Djamgoz and Onkal, 2013), modulating the Vm of malign tissues by adjusting the activities of varies ion channels/transporters may provide a convenient clinical approach.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Medical Research Council [Fellowship number G1000508(95657)].
References
- Abdul M., Hoosein N. (2002). Expression and activity of potassium ion channels in human prostate cancer. Cancer Lett. 186, 99–105 10.1016/S0304-3835(02)00348-8 [DOI] [PubMed] [Google Scholar]
- Adams D. S. (2008). A new tool for tissue engineers: ions as regulators of morphogenesis during development and regeneration. Tissue Eng. Part A 14, 1461–1468 10.1089/ten.tea.2008.0080 [DOI] [PubMed] [Google Scholar]
- Adams D. S., Levin M. (2012). General principles for measuring resting membrane potential and ion concentration using fluorescent bioelectricity reporters. Cold Spring Harb. Protoc. 2012, 385–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D. S., Robinson K. R., Fukumoto T., Yuan S., Albertson R. C., Yelick P., et al. (2006). Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development 133, 1657–1671 10.1242/dev.02341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangeli A. (2005). Expression and role of hERG channels in cancer cells. Novartis Found. Symp. 266, 225–232 discussion: 232–234. 10.1002/047002142X.ch17 [DOI] [PubMed] [Google Scholar]
- Arcangeli A., Bianchi L., Becchetti A., Faravelli L., Coronnello M., Mini E., et al. (1995). A novel inward-rectifying K+ current with a cell-cycle dependence governs the resting potential of mammalian neuroblastoma cells. J. Physiol. 489(Pt 2), 455–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangeli A., Rosati B., Cherubini A., Crociani O., Fontana L., Ziller C., et al. (1997). HERG- and IRK-like inward rectifier currents are sequentially expressed during neuronal development of neural crest cells and their derivatives. Eur. J. Neurosci. 9, 2596–2604 10.1111/j.1460-9568.1997.tb01689.x [DOI] [PubMed] [Google Scholar]
- Arcangeli A., Wanke E., Olivotto M., Camagni S., Ferroni A. (1987). Three types of ion channels are present on the plasma membrane of Friend erythroleukemia cells. Biochem. Biophys. Res. Commun. 146, 1450–1457 10.1016/0006-291X(87)90812-6 [DOI] [PubMed] [Google Scholar]
- Bean B. P. (2007). The action potential in mammalian central neurons. Nat. Rev. Neurosci. 8, 451–465 10.1038/nrn2148 [DOI] [PubMed] [Google Scholar]
- Becchetti A. (2011). Ion channels and transporters in cancer. 1. Ion channels and cell proliferation in cancer. Am. J. Physiol. Cell Physiol. 301, C255–C265 10.1152/ajpcell.00047.2011 [DOI] [PubMed] [Google Scholar]
- Biagiotti T., D'amico M., Marzi I., Di Gennaro P., Arcangeli A., Wanke E., et al. (2006). Cell renewing in neuroblastoma: electrophysiological and immunocytochemical characterization of stem cells and derivatives. Stem Cells 24, 443–453 10.1634/stemcells.2004-0264 [DOI] [PubMed] [Google Scholar]
- Bianchi L., Wible B., Arcangeli A., Taglialatela M., Morra F., Castaldo P., et al. (1998). herg encodes a K+ current highly conserved in tumors of different histogenesis: a selective advantage for cancer cells? Cancer Res. 58, 815–822 [PubMed] [Google Scholar]
- Binggeli R., Cameron I. L. (1980). Cellular potentials of normal and cancerous fibroblasts and hepatocytes. Cancer Res. 40, 1830–1835 [PubMed] [Google Scholar]
- Binggeli R., Weinstein R. C. (1985). Deficits in elevating membrane potential of rat fibrosarcoma cells after cell contact. Cancer Res. 45, 235–241 [PubMed] [Google Scholar]
- Binggeli R., Weinstein R. C. (1986). Membrane potentials and sodium channels: hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. J. Theor. Biol. 123, 377–401 10.1016/S0022-5193(86)80209-0 [DOI] [PubMed] [Google Scholar]
- Blackiston D. J., McLaughlin K. A., Levin M. (2009). Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle 8, 3519–3528 10.4161/cc.8.21.9888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra J., Mummery C. L., Tertoolen L. G., Van Der Saag P. T., De Laat S. W. (1981). Cation transport and growth regulation in neuroblastoma cells. Modulations of K+ transport and electrical membrane properties during the cell cycle. J. Cell. Physiol. 107, 75–83 10.1002/jcp.1041070110 [DOI] [PubMed] [Google Scholar]
- Borowiec A. S., Hague F., Harir N., Guenin S., Guerineau F., Gouilleux F., et al. (2007). IGF-1 activates hEAG K(+) channels through an Akt-dependent signaling pathway in breast cancer cells: role in cell proliferation. J. Cell. Physiol. 212, 690–701 10.1002/jcp.21065 [DOI] [PubMed] [Google Scholar]
- Brackenbury W. J. (2012). Voltage-gated sodium channels and metastatic disease. Channels (Austin) 6, 352–361 10.4161/chan.21910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury W. J., Chioni A. M., Diss J. K., Djamgoz M. B. (2007). The neonatal splice variant of Nav1.5 potentiates in vitro invasive behaviour of MDA-MB-231 human breast cancer cells. Breast Cancer Res. Treat. 101, 149–160 10.1007/s10549-006-9281-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundage R. A., Fogarty K. E., Tuft R. A., Fay F. S. (1991). Calcium gradients underlying polarization and chemotaxis of eosinophils. Science 254, 703–706 10.1126/science.1948048 [DOI] [PubMed] [Google Scholar]
- Caldwell P. C., Keynes R. D. (1957). The utilization of phosphate bond energy for sodium extrusion from giant axons. J. Physiol. 137, 12P–13P [PubMed] [Google Scholar]
- Cameron I. L., Smith N. K., Pool T. B., Sparks R. L. (1980). Intracellular concentration of sodium and other elements as related to mitogenesis and oncogenesis in vivo. Cancer Res. 40, 1493–1500 [PubMed] [Google Scholar]
- Canady K. S., Ali-Osman F., Rubel E. W. (1990). Extracellular potassium influences DNA and protein syntheses and glial fibrillary acidic protein expression in cultured glial cells. Glia 3, 368–374 10.1002/glia.440030508 [DOI] [PubMed] [Google Scholar]
- Chang K. W., Yuan T. C., Fang K. P., Yang F. S., Liu C. J., Chang C. S., et al. (2003). The increase of voltage-gated potassium channel Kv3.4 mRNA expression in oral squamous cell carcinoma. J. Oral Pathol. Med. 32, 606–611 10.1034/j.1600-0714.2003.00197.x [DOI] [PubMed] [Google Scholar]
- Chantome A., Girault A., Potier M., Collin C., Vaudin P., Pages J. C., et al. (2009). KCa2.3 channel-dependent hyperpolarization increases melanoma cell motility. Exp. Cell Res. 315, 3620–3630 10.1016/j.yexcr.2009.07.021 [DOI] [PubMed] [Google Scholar]
- Chernet B. T., Levin M. (2013). Transmembrane voltage potential is an essential cellular parameter for the detection and control of tumor development in a Xenopus model. Dis. Model. Mech. 6, 595–607 10.1242/dmm.010835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chifflet S., Correa V., Nin V., Justet C., Hernandez J. A. (2004). Effect of membrane potential depolarization on the organization of the actin cytoskeleton of eye epithelia. The role of adherens junctions. Exp. Eye Res. 79, 769–777 10.1016/j.exer.2004.08.031 [DOI] [PubMed] [Google Scholar]
- Chifflet S., Hernandez J. A., Grasso S., Cirillo A. (2003). Nonspecific depolarization of the plasma membrane potential induces cytoskeletal modifications of bovine corneal endothelial cells in culture. Exp. Cell Res. 282, 1–13 10.1006/excr.2002.5664 [DOI] [PubMed] [Google Scholar]
- Choi J., Chiang A., Taulier N., Gros R., Pirani A., Husain M. (2006). A calmodulin-binding site on cyclin E mediates Ca2+-sensitive G1/s transitions in vascular smooth muscle cells. Circ. Res. 98, 1273–1281 10.1161/01.RES.0000223059.19250.91 [DOI] [PubMed] [Google Scholar]
- Cone C. D., Jr. (1969). Electroosmotic interactions accompanying mitosis initation in sarcoma cells in vitro. Trans. N.Y. Acad. Sci. 31, 404–427 10.1111/j.2164-0947.1969.tb02926.x [DOI] [PubMed] [Google Scholar]
- Cone C. D., Jr. (1970). Variation of the transmembrane potential level as a basic mechanism of mitosis control. Oncology 24, 438–470 10.1159/000224545 [DOI] [PubMed] [Google Scholar]
- Cone C. D., Jr. (1971). Unified theory on the basic mechanism of normal mitotic control and oncogenesis. J. Theor. Biol. 30, 151–181 10.1016/0022-5193(71)90042-7 [DOI] [PubMed] [Google Scholar]
- Cone C. D., Jr., Cone C. M. (1976). Induction of mitosis in mature neurons in central nervous system by sustained depolarization. Science 192, 155–158 10.1126/science.56781 [DOI] [PubMed] [Google Scholar]
- Cone C. D., Jr., Tongier M., Jr. (1971). Control of somatic cell mitosis by simulated changes in the transmembrane potential level. Oncology 25, 168–182 10.1159/000224567 [DOI] [PubMed] [Google Scholar]
- Cone C. D., Jr., Tongier M., Jr. (1973). Contact inhibition of division: involvement of the electrical transmembrane potential. J. Cell. Physiol. 82, 373–386 10.1002/jcp.1040820307 [DOI] [PubMed] [Google Scholar]
- Crociani O., Cherubini A., Piccini E., Polvani S., Costa L., Fontana L., et al. (2000). erg gene(s) expression during development of the nervous and muscular system of quail embryos. Mech. Dev. 95, 239–243 10.1016/S0925-4773(00)00335-X [DOI] [PubMed] [Google Scholar]
- D'amico M., Gasparoli L., Arcangeli A. (2013). Potassium channels: novel emerging biomarkers and targets for therapy in cancer. Recent Pat. Anticancer Drug Discov. 8, 53–65 [DOI] [PubMed] [Google Scholar]
- Djamgoz M. B., Onkal R. (2013). Persistent current blockers of voltage-gated sodium channels: a clinical opportunity for controlling metastatic disease. Recent Pat. Anticancer Drug Discov. 8, 66–84 [DOI] [PubMed] [Google Scholar]
- Djamgoz M. B. A., Mycielska M., Madeja Z., Fraser S. P., Korohoda W. (2001). Directional movement of rat prostate cancer cells in direct-current electric field: involvement of voltage gated Na+ channel activity. J. Cell Sci. 114, 2697–2705 [DOI] [PubMed] [Google Scholar]
- Farias L. M., Ocana D. B., Diaz L., Larrea F., Avila-Chavez E., Cadena A., et al. (2004). Ether a go-go potassium channels as human cervical cancer markers. Cancer Res. 64, 6996–7001 10.1158/0008-5472.CAN-04-1204 [DOI] [PubMed] [Google Scholar]
- Fiske J. L., Fomin V. P., Brown M. L., Duncan R. L., Sikes R. A. (2006). Voltage-sensitive ion channels and cancer. Cancer Metastasis Rev. 25, 493–500 10.1007/s10555-006-9017-z [DOI] [PubMed] [Google Scholar]
- Fraser S. P., Diss J. K., Chioni A. M., Mycielska M. E., Pan H., Yamaci R. F., et al. (2005). Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin. Cancer Res. 11, 5381–5389 10.1158/1078-0432.CCR-05-0327 [DOI] [PubMed] [Google Scholar]
- Fraser S. P., Grimes J. A., Djamgoz M. B. (2000). Effects of voltage-gated ion channel modulators on rat prostatic cancer cell proliferation: comparison of strongly and weakly metastatic cell lines. Prostate 44, 61–76 [DOI] [PubMed] [Google Scholar]
- Freedman B. D., Price M. A., Deutsch C. J. (1992). Evidence for voltage modulation of IL-2 production in mitogen-stimulated human peripheral blood lymphocytes. J. Immunol. 149, 3784–3794 [PubMed] [Google Scholar]
- Gessner G., Schonherr K., Soom M., Hansel A., Asim M., Baniahmad A., et al. (2005). BKCa channels activating at resting potential without calcium in LNCaP prostate cancer cells. J. Membr. Biol. 208, 229–240 10.1007/s00232-005-0830-z [DOI] [PubMed] [Google Scholar]
- Goldman D. E. (1943). Potential, impedance, and rectification in membranes. J. Gen. Physiol. 27, 37–60 10.1085/jgp.27.1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D., Lammersfeld C. A., Burrows J. L., Dahlk S. L., Vashi P. G., Grutsch J. F., et al. (2004a). Bioelectrical impedance phase angle in clinical practice: implications for prognosis in advanced colorectal cancer. Am. J. Clin. Nutr. 80, 1634–1638 [DOI] [PubMed] [Google Scholar]
- Gupta D., Lis C. G., Dahlk S. L., Vashi P. G., Grutsch J. F., Lammersfeld C. A. (2004b). Bioelectrical impedance phase angle as a prognostic indicator in advanced pancreatic cancer. Br. J. Nutr. 92, 957–962 10.1079/BJN20041292 [DOI] [PubMed] [Google Scholar]
- Gupta G. P., Massague J. (2006). Cancer metastasis: building a framework. Cell 127, 679–695 10.1016/j.cell.2006.11.001 [DOI] [PubMed] [Google Scholar]
- Habela C. W., Ernest N. J., Swindall A. F., Sontheimer H. (2009). Chloride accumulation drives volume dynamics underlying cell proliferation and migration. J. Neurophysiol. 101, 750–757 10.1152/jn.90840.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habela C. W., Olsen M. L., Sontheimer H. (2008). ClC3 is a critical regulator of the cell cycle in normal and malignant glial cells. J. Neurosci. 28, 9205–9217 10.1523/JNEUROSCI.1897-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Haren N., Khorsi H., Faouzi M., Ahidouch A., Sevestre H., Ouadid-Ahidouch H. (2010). Intermediate conductance Ca2+ activated K+ channels are expressed and functional in breast adenocarcinomas: correlation with tumour grade and metastasis status. Histol. Histopathol. 25, 1247–1255 [DOI] [PubMed] [Google Scholar]
- Hazelton B., Mitchell B., Tupper J. (1979). Calcium, magnesium, and growth control in the WI-38 human fibroblast cell. J. Cell Biol. 83, 487–498 10.1083/jcb.83.2.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerlein B., Weseloh R. M., Mello De Queiroz F., Knotgen H., Sanchez A., Rubio M. E., et al. (2006). Overexpression of Eag1 potassium channels in clinical tumours. Mol. Cancer 5:41 10.1186/1476-4598-5-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashimori H., Sontheimer H. (2007). Role of Kir4.1 channels in growth control of glia. Glia 55, 1668–1679 10.1002/glia.20574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. (1992). Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates [Google Scholar]
- Hodgkin A. L., Katz B. (1949). The effect of sodium ions on the electrical activity of giant axon of the squid. J. Physiol. 108, 37–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- House C. D., Vaske C. J., Schwartz A. M., Obias V., Frank B., Luu T., et al. (2010). Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res. 70, 6957–6967 10.1158/0008-5472.CAN-10-1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulser D. F., Lauterwasser U. (1982). Membrane potential oscillations in homokaryons. An endogenous signal for detecting intercellular communication. Exp. Cell Res. 139, 63–70 10.1016/0014-4827(82)90318-4 [DOI] [PubMed] [Google Scholar]
- Johnstone B. M. (1959). Micro-electrode penetration of ascites tumour cells. Nature 183, 411 10.1038/183411a0 [DOI] [PubMed] [Google Scholar]
- Khanna R., Chang M. C., Joiner W. J., Kaczmarek L. K., Schlichter L. C. (1999). hSK4/hIK1, a calmodulin-binding KCa channel in human T lymphocytes. Roles in proliferation and volume regulation. J. Biol. Chem. 274, 14838–14849 10.1074/jbc.274.21.14838 [DOI] [PubMed] [Google Scholar]
- Kiefer H., Blume A. J., Kaback H. R. (1980). Membrane potential changes during mitogenic stimulation of mouse spleen lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 77, 2200–2204 10.1073/pnas.77.4.2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzelmann K. (2005). Ion channels and cancer. J. Membr. Biol. 205, 159–173 10.1007/s00232-005-0781-4 [DOI] [PubMed] [Google Scholar]
- Lee I., Park C., Kang W. K. (2010). Knockdown of inwardly rectifying potassium channel Kir2.2 suppresses tumorigenesis by inducing reactive oxygen species-mediated cellular senescence. Mol. Cancer Ther. 9, 2951–2959 10.1158/1535-7163.MCT-10-0511 [DOI] [PubMed] [Google Scholar]
- Lee J., Ishihara A., Oxford G., Johnson B., Jacobson K. (1999). Regulation of cell movement is mediated by stretch-activated calcium channels. Nature 400, 382–386 10.1038/22578 [DOI] [PubMed] [Google Scholar]
- Levin M. (2003). Bioelectromagnetics in morphogenesis. Bioelectromagnetics 24, 295–315 10.1002/bem.10104 [DOI] [PubMed] [Google Scholar]
- Levin M. (2007a). Gap junctional communication in morphogenesis. Prog. Biophys. Mol. Biol. 94, 186–206 10.1016/j.pbiomolbio.2007.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. (2007b). Large-scale biophysics: ion flows and regeneration. Trends Cell Biol. 17, 261–270 10.1016/j.tcb.2007.04.007 [DOI] [PubMed] [Google Scholar]
- Levin M. (2009). Bioelectric mechanisms in regeneration: unique aspects and future perspectives. Semin. Cell Dev. Biol. 20, 543–556 10.1016/j.semcdb.2009.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. H., Bijlenga P., Fischer-Lougheed J., Occhiodoro T., Kaelin A., Bader C. R., et al. (1998). Role of an inward rectifier K+ current and of hyperpolarization in human myoblast fusion. J. Physiol. 510(Pt 2), 467–476 10.1111/j.1469-7793.1998.467bk.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Dontu G., Wicha M. S. (2005). Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 7, 86–95 10.1186/bcr1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Chang Y., Reinhart P. H., Sontheimer H., Chang Y. (2002). Cloning and characterization of glioma BK, a novel BK channel isoform highly expressed in human glioma cells. J. Neurosci. 22, 1840–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobikin M., Chernet B., Lobo D., Levin M. (2012). Resting potential, oncogene-induced tumorigenesis, and metastasis: the bioelectric basis of cancer in vivo. Phys. Biol. 9:065002 10.1088/1478-3975/9/6/065002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymangrover J., Pearlmutter A. F., Franco-Saenz R., Saffran M. (1975). Transmembrane potentials and steroidogenesis in normal and neoplastic human adrenocortical tissue. J. Clin. Endocrinol. Metab. 41, 697–706 10.1210/jcem-41-4-697 [DOI] [PubMed] [Google Scholar]
- Macfarlane S. N., Sontheimer H. (2000). Changes in ion channel expression accompany cell cycle progression of spinal cord astrocytes. Glia 30, 39–48 [DOI] [PubMed] [Google Scholar]
- Marino A. A., Morris D. M., Schwalke M. A., Iliev I. G., Rogers S. (1994). Electrical potential measurements in human breast cancer and benign lesions. Tumour Biol. 15, 147–152 10.1159/000217885 [DOI] [PubMed] [Google Scholar]
- McCaig C. D., Song B., Rajnicek A. M. (2009). Electrical dimensions in cell science. J. Cell Sci. 122, 4267–4276 10.1242/jcs.023564 [DOI] [PubMed] [Google Scholar]
- Melczer N., Kiss J. (1957). Electrical method for detection of early cancerous growth of the skin. Nature 179, 1177–1179 10.1038/1791177b0 [DOI] [PubMed] [Google Scholar]
- Menendez S. T., Rodrigo J. P., Allonca E., Garcia-Carracedo D., Alvarez-Alija G., Casado-Zapico S., et al. (2010). Expression and clinical significance of the Kv3.4 potassium channel subunit in the development and progression of head and neck squamous cell carcinomas. J. Pathol. 221, 402–410 [DOI] [PubMed] [Google Scholar]
- Meyer R., Heinemann S. H. (1998). Characterization of an eag-like potassium channel in human neuroblastoma cells. J. Physiol. 508(Pt 1), 49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. (2000). An overview of the potassium channel family. Genome Biol. 1:REVIEWS0004. 10.1186/gb-2000-1-4-reviews0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills B., Tupper J. T. (1976). Cell cycle dependent changes in potassium transport. J. Cell. Physiol. 89, 123–132 10.1002/jcp.1040890112 [DOI] [PubMed] [Google Scholar]
- Mycielska M. E., Palmer C. P., Brackenbury W. J., Djamgoz M. B. (2005). Expression of Na+-dependent citrate transport in a strongly metastatic human prostate cancer PC-3M cell line: regulation by voltage-gated Na+ channel activity. J. Physiol. 563, 393–408 10.1113/jphysiol.2004.079491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A., Horn L. (1967). Electrical activity of single vascular smooth muscle fibers. Am. J. Physiol. 213, 25–30 [DOI] [PubMed] [Google Scholar]
- Nilius B., Wohlrab W. (1992). Potassium channels and regulation of proliferation of human melanoma cells. J. Physiol. 445, 537–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccitelli R. (2003a). Endogenous electric fields in embryos during development, regeneration and wound healing. Radiat. Prot. Dosimetry 106, 375–383 10.1093/oxfordjournals.rpd.a006375 [DOI] [PubMed] [Google Scholar]
- Nuccitelli R. (2003b). A role for endogenous electric fields in wound healing. Curr. Top. Dev. Biol. 58, 1–26 10.1016/S0070-2153(03)58001-2 [DOI] [PubMed] [Google Scholar]
- Orr C. W., Yoshikawa-Fukada M., Ebert J. D. (1972). Potassium: effect on DNA synthesis and multiplication of baby-hamster kidney cells: (cell cycle-membrane potential-synchronization-transformation). Proc. Natl. Acad. Sci. U.S.A. 69, 243–247 10.1073/pnas.69.1.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz C. S., Montante-Montes D., Saqui-Salces M., Hinojosa L. M., Gamboa-Dominguez A., Hernandez-Gallegos E., et al. (2011). Eag1 potassium channels as markers of cervical dysplasia. Oncol. Rep. 26, 1377–1383 [DOI] [PubMed] [Google Scholar]
- Ouadid-Ahidouch H., Ahidouch A. (2008). K+ channel expression in human breast cancer cells: involvement in cell cycle regulation and carcinogenesis. J. Membr. Biol. 221, 1–6 10.1007/s00232-007-9080-6 [DOI] [PubMed] [Google Scholar]
- Ouadid-Ahidouch H., Chaussade F., Roudbaraki M., Slomianny C., Dewailly E., Delcourt P., et al. (2000). KV1.1 K(+) channels identification in human breast carcinoma cells: involvement in cell proliferation. Biochem. Biophys. Res. Commun. 278, 272–277 10.1006/bbrc.2000.3790 [DOI] [PubMed] [Google Scholar]
- Ouadid-Ahidouch H., Le Bourhis X., Roudbaraki M., Toillon R. A., Delcourt P., Prevarskaya N. (2001). Changes in the K+ current-density of MCF-7 cells during progression through the cell cycle: possible involvement of a h-ether.a-gogo K+ channel. Receptors Channels 7, 345–356 [PubMed] [Google Scholar]
- Ousingsawat J., Spitzner M., Puntheeranurak S., Terracciano L., Tornillo L., Bubendorf L., et al. (2007). Expression of voltage-gated potassium channels in human and mouse colonic carcinoma. Clin. Cancer Res. 13, 824–831 10.1158/1078-0432.CCR-06-1940 [DOI] [PubMed] [Google Scholar]
- Pardo L. A., Contreras-Jurado C., Zientkowska M., Alves F., Stuhmer W. (2005). Role of voltage-gated potassium channels in cancer. J. Membr. Biol. 205, 115–124 10.1007/s00232-005-0776-1 [DOI] [PubMed] [Google Scholar]
- Pardo L. A., Del Camino D., Sanchez A., Alves F., Bruggemann A., Beckh S., et al. (1999). Oncogenic potential of EAG K(+) channels. EMBO J. 18, 5540–5547 10.1093/emboj/18.20.5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. H., Park S. J., Chung M. K., Jung K. H., Choi M. R., Kim Y., et al. (2010). High expression of large-conductance Ca2+-activated K+ channel in the CD133+ subpopulation of SH-SY5Y neuroblastoma cells. Biochem. Biophys. Res. Commun. 396, 637–642 10.1016/j.bbrc.2010.04.142 [DOI] [PubMed] [Google Scholar]
- Pettit E. J., Fay F. S. (1998). Cytosolic free calcium and the cytoskeleton in the control of leukocyte chemotaxis. Physiol. Rev. 78, 949–967 [DOI] [PubMed] [Google Scholar]
- Plummer H. K., 3rd., Dhar M. S., Cekanova M., Schuller H. M. (2005). Expression of G-protein inwardly rectifying potassium channels (GIRKs) in lung cancer cell lines. BMC Cancer 5:104 10.1186/1471-2407-5-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer H. K., 3rd., Yu Q., Cakir Y., Schuller H. M. (2004). Expression of inwardly rectifying potassium channels (GIRKs) and beta-adrenergic regulation of breast cancer cell lines. BMC Cancer 4:93 10.1186/1471-2407-4-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier M., Joulin V., Roger S., Besson P., Jourdan M. L., Leguennec J. Y., et al. (2006). Identification of SK3 channel as a new mediator of breast cancer cell migration. Mol. Cancer Ther. 5, 2946–2953 10.1158/1535-7163.MCT-06-0194 [DOI] [PubMed] [Google Scholar]
- Prevarskaya N., Skryma R., Shuba Y. (2010). Ion channels and the hallmarks of cancer. Trends Mol. Med. 16, 107–121 10.1016/j.molmed.2010.01.005 [DOI] [PubMed] [Google Scholar]
- Price M., Lee S. C., Deutsch C. (1989). Charybdotoxin inhibits proliferation and interleukin 2 production in human peripheral blood lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 86, 10171–10175 10.1073/pnas.86.24.10171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmann K., Muller V., Tanneberger S., Kalkoff W. (1972). The membrane potential of primary ovarian tumor cells in vitro and its dependence on the cell cycle. Acta Biol. Med. Ger. 28, 853–856 [PubMed] [Google Scholar]
- Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., et al. (2003). Cell migration: integrating signals from front to back. Science 302, 1704–1709 10.1126/science.1092053 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Rasgado J. A., Acuna-Macias I., Camacho J. (2012). Eag1 channels as potential cancer biomarkers. Sensors (Basel) 12, 5986–5995 10.3390/s120505986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger S., Besson P., Le Guennec J. Y. (2003). Involvement of a novel fast inward sodium current in the invasion capacity of a breast cancer cell line. Biochim. Biophys. Acta 1616, 107–111 10.1016/j.bbamem.2003.07.001 [DOI] [PubMed] [Google Scholar]
- Rouzaire-Dubois B., Milandri J. B., Bostel S., Dubois J. M. (2000). Control of cell proliferation by cell volume alterations in rat C6 glioma cells. Pflugers Arch. 440, 881–888 10.1007/s004240000371 [DOI] [PubMed] [Google Scholar]
- Sachs H. G., Stambrook P. J., Ebert J. D. (1974). Changes in membrane potential during the cell cycle. Exp. Cell Res. 83, 362–366 10.1016/0014-4827(74)90350-4 [DOI] [PubMed] [Google Scholar]
- Santella L., Ercolano E., Nusco G. A. (2005). The cell cycle: a new entry in the field of Ca2+ signaling. Cell. Mol. Life Sci. 62, 2405–2413 10.1007/s00018-005-5083-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Suzuki K., Yamazaki H., Nakanishi S. (2005). A pivotal role of calcineurin signaling in development and maturation of postnatal cerebellar granule cells. Proc. Natl. Acad. Sci. U.S.A. 102, 5874–5879 10.1073/pnas.0501972102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab A., Fabian A., Hanley P. J., Stock C. (2012). Role of ion channels and transporters in cell migration. Physiol. Rev. 92, 1865–1913 10.1152/physrev.00018.2011 [DOI] [PubMed] [Google Scholar]
- Schwab A., Nechyporuk-Zloy V., Fabian A., Stock C. (2007). Cells move when ions and water flow. Pflugers Arch. 453, 421–432 10.1007/s00424-006-0138-6 [DOI] [PubMed] [Google Scholar]
- Schwab A., Wulf A., Schulz C., Kessler W., Nechyporuk-Zloy V., Romer M., et al. (2006). Subcellular distribution of calcium-sensitive potassium channels (IK1) in migrating cells. J. Cell. Physiol. 206, 86–94 10.1002/jcp.20434 [DOI] [PubMed] [Google Scholar]
- Smith N. R., Sparks R. L., Pool T. B., Cameron I. L. (1978). Differences in the intracellular concentration of elements in normal and cancerous liver cells as determined by X-ray microanalysis. Cancer Res. 38, 1952–1959 [PubMed] [Google Scholar]
- Smith P. L., Baukrowitz T., Yellen G. (1996). The inward rectification mechanism of the HERG cardiac potassium channel. Nature 379, 833–836 10.1038/379833a0 [DOI] [PubMed] [Google Scholar]
- Sontheimer H. (2008). An unexpected role for ion channels in brain tumor metastasis. Exp. Biol. Med. 233, 779–791 10.3181/0711-MR-308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroceanu L., Manning T. J., Jr., Sontheimer H. (1999). Modulation of glioma cell migration and invasion using Cl(-) and K(+) ion channel blockers. J. Neurosci. 19, 5942–5954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks R. L., Pool T. B., Smith N. K., Cameron I. L. (1983). Effects of amiloride on tumor growth and intracellular element content of tumor cells in vivo. Cancer Res. 43, 73–77 [PubMed] [Google Scholar]
- Stevenson D., Binggeli R., Weinstein R. C., Keck J. G., Lai M. C., Tong M. J. (1989). Relationship between cell membrane potential and natural killer cell cytolysis in human hepatocellular carcinoma cells. Cancer Res. 49, 4842–4845 [PubMed] [Google Scholar]
- Strobl J. S., Wonderlin W. F., Flynn D. C. (1995). Mitogenic signal transduction in human breast cancer cells. Gen. Pharmacol. 26, 1643–1649 10.1016/0306-3623(95)00062-3 [DOI] [PubMed] [Google Scholar]
- Stuhmer W., Alves F., Hartung F., Zientkowska M., Pardo L. A. (2006). Potassium channels as tumour markers. FEBS Lett. 580, 2850–2852 10.1016/j.febslet.2006.03.062 [DOI] [PubMed] [Google Scholar]
- Stuhmer W., Pardo L. A. (2010). K(+) channels as therapeutic targets in oncology. Future Med. Chem. 2, 745–755 10.4155/fmc.10.24 [DOI] [PubMed] [Google Scholar]
- Sundelacruz S., Levin M., Kaplan D. L. (2008). Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS ONE 3:e3737 10.1371/journal.pone.0003737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelacruz S., Levin M., Kaplan D. L. (2009). Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev. 5, 231–246 10.1007/s12015-009-9080-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelacruz S., Levin M., Kaplan D. L. P. (2013). Depolarization alters phenotype, maintains plasticity of pre-differentiated mesenchymal stem cells. Tissue Eng. Part A [Epub ahead of print]. 10.1089/ten.tea.2012.0425.rev [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaszi K., Sirokmany G., Di Ciano-Oliveira C., Rotstein O. D., Kapus A. (2005). Depolarization induces Rho-Rho kinase-mediated myosin light chain phosphorylation in kidney tubular cells. Am. J. Physiol. Cell Physiol. 289, C673–C685 10.1152/ajpcell.00481.2004 [DOI] [PubMed] [Google Scholar]
- Takanami I., Inoue Y., Gika M. (2004). G-protein inwardly rectifying potassium channel 1 (GIRK 1) gene expression correlates with tumor progression in non-small cell lung cancer. BMC Cancer 4:79 10.1186/1471-2407-4-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuoka S., Morioka H. (1957). The membrane potential of the human cancer and related cells. I. Gan 48, 353–354 [PubMed] [Google Scholar]
- Trudeau M. C., Warmke J. W., Ganetzky B., Robertson G. A. (1995). HERG, a human inward rectifier in the voltage-gated potassium channel family. Science 269, 92–95 10.1126/science.7604285 [DOI] [PubMed] [Google Scholar]
- Voloshyna I., Besana A., Castillo M., Matos T., Weinstein I. B., Mansukhani M., et al. (2008). TREK-1 is a novel molecular target in prostate cancer. Cancer Res. 68, 1197–1203 10.1158/0008-5472.CAN-07-5163 [DOI] [PubMed] [Google Scholar]
- Wagner V., Stadelmeyer E., Riederer M., Regitnig P., Gorischek A., Devaney T., et al. (2010). Cloning and characterisation of GIRK1 variants resulting from alternative RNA editing of the KCNJ3 gene transcript in a human breast cancer cell line. J. Cell. Biochem. 110, 598–608 10.1002/jcb.22564 [DOI] [PubMed] [Google Scholar]
- Wang H., Zhang Y., Cao L., Han H., Wang J., Yang B., et al. (2002). HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res. 62, 4843–4848 [PubMed] [Google Scholar]
- Wang Y. F., Jia H., Walker A. M., Cukierman S. (1992). K-current mediation of prolactin-induced proliferation of malignant (Nb2) lymphocytes. J. Cell. Physiol. 152, 185–189 10.1002/jcp.1041520123 [DOI] [PubMed] [Google Scholar]
- Wang Z. (2004). Roles of K+ channels in regulating tumour cell proliferation and apoptosis. Pflugers Arch. 448, 274–286 10.1007/s00424-004-1258-5 [DOI] [PubMed] [Google Scholar]
- Weaver A. K., Liu X., Sontheimer H. (2004). Role for calcium-activated potassium channels (BK) in growth control of human malignant glioma cells. J. Neurosci. Res. 78, 224–234 10.1002/jnr.20240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C., Wang X., Chen M., Ouyang K., Song L. S., Cheng H. (2009). Calcium flickers steer cell migration. Nature 457, 901–905 10.1038/nature07577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicha M. S., Liu S., Dontu G. (2006). Cancer stem cells: an old idea–a paradigm shift. Cancer Res. 66, 1883–1890 discussion: 1895–1886. 10.1158/0008-5472.CAN-05-3153 [DOI] [PubMed] [Google Scholar]
- Wilson G. F., Chiu S. Y. (1993). Mitogenic factors regulate ion channels in Schwann cells cultured from newborn rat sciatic nerve. J. Physiol. 470, 501–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonderlin W. F., Strobl J. S. (1996). Potassium channels, proliferation and G1 progression. J. Membr. Biol. 154, 91–107 10.1007/s002329900135 [DOI] [PubMed] [Google Scholar]
- Wonderlin W. F., Woodfork K. A., Strobl J. S. (1995). Changes in membrane potential during the progression of MCF-7 human mammary tumor cells through the cell cycle. J. Cell. Physiol. 165, 177–185 10.1002/jcp.1041650121 [DOI] [PubMed] [Google Scholar]
- Woodfork K. A., Wonderlin W. F., Peterson V. A., Strobl J. S. (1995). Inhibition of ATP-sensitive potassium channels causes reversible cell-cycle arrest of human breast cancer cells in tissue culture. J. Cell. Physiol. 162, 163–171 10.1002/jcp.1041620202 [DOI] [PubMed] [Google Scholar]
- Woodrough R. E., Canti G., Watson B. W. (1975). Electrical potential difference between basal cell carcinoma, benign inflammatory lesions and normal tissue. Br. J. Dermatol. 92, 1–7 10.1111/j.1365-2133.1975.tb03026.x [DOI] [PubMed] [Google Scholar]
- Yang M., Kozminski D. J., Wold L. A., Modak R., Calhoun J. D., Isom L. L., et al. (2012). Therapeutic potential for phenytoin: targeting Na(v)1.5 sodium channels to reduce migration and invasion in metastatic breast cancer. Breast Cancer Res. Treat. 134, 603–615 10.1007/s10549-012-2102-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y. J., Furukawa T., Ogura T., Tajimi K., Inagaki N. (2002). M phase-specific expression and phosphorylation-dependent ubiquitination of the ClC-2 channel. J. Biol. Chem. 277, 32268–32273 10.1074/jbc.M202105200 [DOI] [PubMed] [Google Scholar]
- Ziechner U., Schonherr R., Born A. K., Gavrilova-Ruch O., Glaser R. W., Malesevic M., et al. (2006). Inhibition of human ether a go-go potassium channels by Ca2+/calmodulin binding to the cytosolic N- and C-termini. FEBS J. 273, 1074–1086 10.1111/j.1742-4658.2006.05134.x [DOI] [PubMed] [Google Scholar]