Abstract
Background
Several researchers have determined the tumor length to be an important prognostic indictor of esophageal cancer (EC). However, controversy exists concerning the optimal cut-off points for tumor length to predict overall survival. The aim of this study was to determine the prognostic value of tumor length and propose the optimum cut-off point for tumor length in predicting survival difference in elderly patients with esophageal squamous cell carcinoma (ESCC).
Methods
From January 2001 to December 2009, a retrospective analysis of 132 consecutive patients older than 70 years with ESCC was conducted. A receiver-operating characteristic (ROC) curve for survival prediction was plotted to verify the optimum cut-off point for tumor length. Univariate and multivariate analyses were performed to evaluate prognostic parameters for survival.
Results
A ROC curve for survival prediction was plotted to verify the optimum cut-off point for tumor length, which was 4.0 cm. Patients with tumor length ≤4.0 cm had significantly better 5-year survival rate than patients with a tumor length >4.0 cm (60.7% versus 31.6%, P = 0.007). Multivariate analyses showed that tumor length (>4.0 cm versus ≤4.0 cm, P = 0.036), differentiation (poor versus well/moderate, P = 0.032), N staging (N1-3 versus N0, P = 0.018), and T grade (T3-4 versus T1-2, P = 0.002) were independent prognostic factors.
Conclusion
Tumor length is a predictive factor for long-term survival in elderly patients with ESCC, especially in T3-4 grade or nodal-negative patients. We conclude that 4.0 cm may be the optimum cut-off point for tumor length in predicting survival in elderly patients with ESCC.
Keywords: Esophageal cancer, esophagectomy, prognostic factor, squamous cell carcinoma, survival, tumor length
Introduction
Esophageal cancer (EC) is the eighth most common type of cancer worldwide. It is endemic in many parts of the world, particularly in developing nations, and accounts for more than 200,000 deaths every year in China (1). As a result of worldwide increases in the elderly population, there has been a concomitant increase in the number of EC patients (2). Therefore, assessing the prognostic factors in elderly patients with EC will become increasingly important.
Tumor length is still a controversial prognostic factor in EC patients. Several researchers have determined tumor length to be an important prognostic indictor of EC after surgery (3-5). However, there have been few studies regarding tumor length in elderly EC patients (6,7). Furthermore, controversy exists concerning the optimal cut-off points for tumor length to predict overall survival (4,5,8). Different study sizes, different histological types, variable inclusion criteria, and, most importantly, unreliable statistical methods used to determine the cut-off points have contributed to this controversy (4,5,8-10).
We have chosen to study esophageal squamous cell carcinoma (ESCC) as it is the most common type of EC in China, in contrast to the predominance of adenocarcinoma in the Western world. Thus, the aim of this study was to determine the prognostic value of tumor length and propose the optimum cut-off point for tumor length in predicting survival difference in elderly patients with ESCC.
Patients and methods
Patients
From January 2001 to December 2009, a retrospective analysis was conducted of 132 consecutive patients older than 70 years with ESCC who underwent curative esophagectomy in the Department of Thoracic Surgery, Zhejiang Cancer Hospital, Hangzhou, China. All of the patients included in the analysis fit the following criteria: 1) ESCC confirmed by histopathology; 2) older than 70 years; 3) curative esophagectomy with R0 resection (en bloc resection with margins histologically free of disease); 4) at least six lymph nodes were examined for pathological diagnosis; and 5) surgery was neither preceded nor followed by adjuvant chemotherapy and/or radiotherapy.
All of the above patients were followed up by posting letters or by telephone interviews. The last follow-up was 30 November 2011. All subjects gave written informed consent to the study protocol, which was approved by the Ethical Committees of Zhejiang Cancer Hospital, Hangzhou, China.
Surgery
All patients were treated with radical resection. The standard surgical approach consisted of a limited thoracotomy on the right side and intrathoracic gastric reconstruction (the Ivor Lewis procedure) for lesions at the middle/lower third of the esophagus. Upper third lesions were treated by cervical anastomosis (the McKeown procedure). In our institute, two types of lymphadenectomy were carried out as a standard procedure for ESCC. The majority of patients underwent two-field (thoracoabdominal) lymphadenectomy. In this cohort of patients, thoracoabdominal lymphadenectomy was performed, including the subcarinal, paraesophageal, pulmonary ligament, diaphragmatic and paracardial lymph nodes, as well as those located along the lesser gastric curvature, the origin of the left gastric artery, the celiac trunk, the common hepatic artery, and the splenic artery. Three-field (cervical-thoracoabdominal) lymphadenectomy was performed only if the cervical lymph nodes were thought to be abnormal upon preoperative evaluation.
Pathological analysis
The fresh specimen was routinely dissected and measured by surgeons immediately after resection of the tumor. The length of each tumor was measured with a hand-held ruler. Then the specimens were sent for pathology examination after preservation in 10% formalin. Differentiation, depth of tumor invasion (T grades), and nodal involvement (N stagings) were recorded according to the results of pathology reports. In addition, pathologists also described the tumor length (measured on the 10% formalin fixed specimens). In this study, the surgeons' measure was used. All patients were restaged according to the seventh edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (11).
Statistical analysis
Statistical evaluation was conducted with SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). The mean values are presented as the means ± standard deviations (SD). Independent t test was used to compare groups of continuous, normally distributed variables. The Pearson chi-square test was used to determine the significance of differences for dichotomous variables. A receiver-operating characteristic (ROC) curve for survival prediction (survival versus death) was plotted to verify the optimum cut-off point for tumor length. The area under curve (AUC) was used as an estimation of diagnostic accuracy. The Youden index (sensitivity + specificity – 1) was used to identify the tumor length threshold values corresponding to the value of the ROC curve farthest from the identity line. This index corresponds to the optimal cut-off, defined as the value with the highest average of sensitivity and specificity.
The overall cumulative probability of survival was calculated by the Kaplan–Meier method, and the difference was assessed by the log-rank test. Univariate and multivariate analyses of Cox regression proportional hazard model were performed to evaluate the prognostic parameters for survival. Hazard ratios (HRs) with 95% confidence intervals (CIs) were used to quantify the strength of the association between predictors and survival. A P value less than 0.05 was considered to be statistically significant.
Results
Patient characteristics
Among the 132 patients, 11 (8.3%) were women, and 121 (91.7%) were men (Table I). The mean age was 73.7 ± 2.6 years, with an age range from 70 to 85 years.
Table I.
Baseline characteristics of 132 elderly patients with ESCC.
| Cases (n, %) | |
|---|---|
| Age (mean ± SD, years) | 73.6 ± 2.6 |
| Gender | |
| Female | 11 (8.3) |
| Male | 121 (91.7) |
| Tumor length (mean ± SD, cm) | 4.59 ± 1.74 |
| Tumor location | |
| Upper | 6 (4.5) |
| Middle | 55 (41.7) |
| Lower | 71 (53.8) |
| Differentiation | |
| Well | 17 (12.9) |
| Moderate | 81 (61.3) |
| Poor | 34 (25.8) |
| T grade | |
| T1 | 19 (14.4) |
| T2 | 16 (12.1) |
| T3 | 89 (67.4) |
| T4 | 8 (6.1) |
| N staging | |
| N0 | 58 (43.9) |
| N1 | 42 (31.8) |
| N2 | 18 (13.7) |
| N3 | 14 (10.6) |
| TLN (mean ± SD, nodes) | 22.7 ± 9.7 |
| MLN (mean ± SD, nodes) | 2.2 ± 3.7 |
ESCC = esophageal squamous cell carcinoma; MLN = metastatic lymph nodes; TLN = total lymph nodes.
Analysis of tumor length
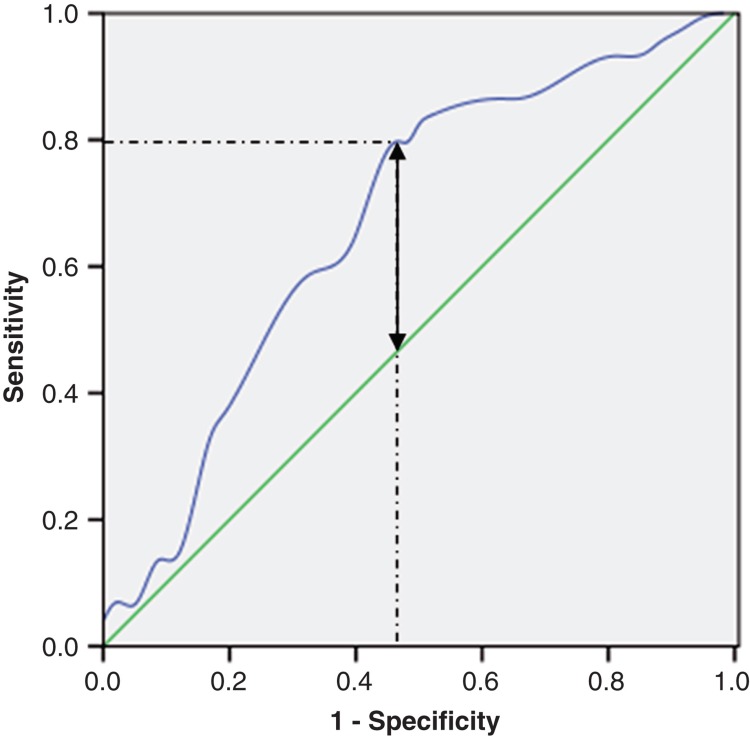
The tumor length in these 132 patients ranged from 0.8 to 9.2 cm, mean 4.59 ± 1.74 cm. A ROC curve for survival prediction was plotted to verify the optimum cut-off point for tumor length, which was 4.0 cm (Figure 1). Then, patients were divided into two groups for survival analysis (patients with tumors ≤4.0 cm in length and patients with tumors >4.0 cm in length). Tumors >4.0 cm in length had a 66.0% chance of being T3-4, whereas tumors ≤4.0 cm in length had a 65.7% chance of being T1-2 (P = 0.001) (Table II).
Figure 1.
A ROC curve plots the sensitivity on the y-axis against 1 minus the specificity on the x-axis. A diagonal line at 45 degrees, known as the line of chance, would result from a test which allocated subjects randomly. Each point on the ROC curve corresponds to a value of tumor length. In general, a good cut-off point is one which produces both a large sensitivity and a large specificity. This can be interpreted as choosing the point on the ROC curve with the largest vertical distance from the line of chance (two-way arrow). The AUC for tumor length was 67.1% with a sensitivity of 79.7% and a specificity of 53.4% (1 – 46.6%) by Youden index (dotted lines). The threshold value corresponding to the tumor length was 4.0 cm.
Table II.
Characteristics of patients with tumor length more or less than 4.0 cm.
| Tumor length (n, %) |
P value | ||
|---|---|---|---|
| ≤4.0 cm | >4.0 cm | ||
| Age (years) | 0.020 | ||
| ≤75 | 47 (48.5) | 50 (51.5) | |
| >75 | 9 (25.7) | 26 (74.3) | |
| Gender | 0.832 | ||
| Female | 5 (45.5) | 6 (54.5) | |
| Male | 51 (42.1) | 70 (57.9) | |
| Differentiation | 0.864 | ||
| Well/Moderate | 42 (42.9) | 56 (57.1) | |
| Poor | 14 (41.2) | 20 (58.8) | |
| Tumor location | 0.454 | ||
| Upper/Middle | 28 (45.9) | 33 (55.1) | |
| Lower | 28 (39.4) | 43 (60.6) | |
| N staging | 0.119 | ||
| N0 | 29 (50.0) | 29 (50.0) | |
| N1-3 | 27 (36.5) | 47 (63.5) | |
| T grade | 0.001 | ||
| T1-2 | 23 (65.7) | 12 (34.3) | |
| T3-4 | 33 (34.0) | 64 (66.0) | |
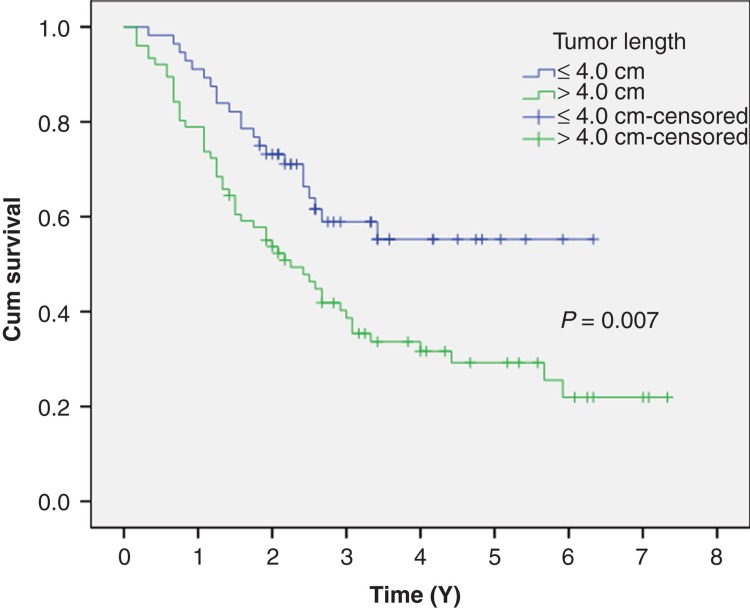
The 5-year overall survival was 43.9% in our study. Patients with tumor length ≤4.0 cm had significantly better 5-year survival rate than patients with a tumor length >4.0 cm (60.7% versus 31.6%, P = 0.007) (Figure 2).
Figure 2.
Patients with tumor length ≤4.0 cm had a significantly better 5-year survival rate than patients with a tumor length >4.0 cm (60.7% versus 31.6%, P = 0.007).
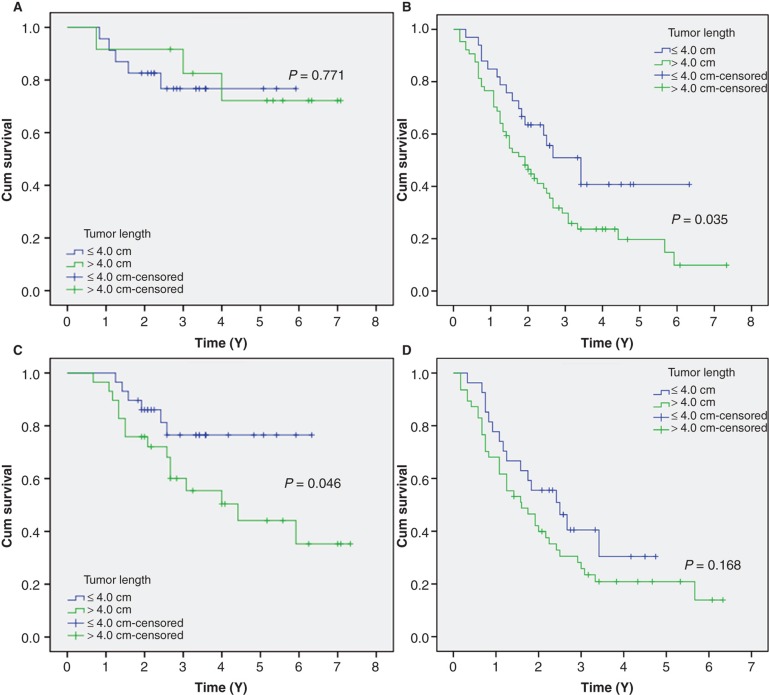
In the group of T1-2 disease, the 5-year survival of patients with tumor length ≤4.0 cm was similar in patients with tumor length >4.0 cm (78.3% versus 75.0%, P = 0.771) (Figure 3A). In the T3-4 group, however, the 5-year survival of patients with tumor length ≤4.0 cm was better than that of patients with tumor length >4.0 cm (48.5% versus 23.4%, P = 0.035) (Figure 3B).
Figure 3.
Kaplan–Meier survival curves stratified by tumor length in (A) T1-2 patients, (B) T3-4 patients, (C) N0 patients, and (D) N1-3 patients.
The 5-year survival in patients with tumor length ≤4.0 cm was better than patients with a tumor length >4.0 cm in N0 staging (79.3% versus 48.3%, P = 0.046) (Figure 3C). However, no significant differences in 5-year survival were found between the patients with tumor length ≤4.0 cm and >4.0 cm in N1-3 staging (40.7% versus 21.3%, P = 0.168) (Figure 3D).
Analyses of prognostic factors
Univariate analyses showed that vessel involvement (P = 0.037), differentiation (P = 0.027), perineural invasion (P = 0.009), tumor length (P = 0.007), N staging (P = 0.000), and T grade (P = 0.000) were predictive of survival. Then multivariate analyses were performed with the Cox proportional hazards model. In that model, we demonstrated that tumor length (P = 0.036), differentiation (P = 0.032), N staging (P = 0.018), and T grade (P = 0.002) were independent prognostic factors (Table III).
Table III.
Univariate and multivariate analyses in elderly patients with ESCC.
| Cases (n, %) | Survival(%) | Univariate analysis HR (95% CI) | P value | Multivariate analyses HR (95% CI) | P value | |
|---|---|---|---|---|---|---|
| Age (years) | 0.780 | 0.322 | ||||
| ≤75 | 97 (73.5) | 45.4 | 1.000 | 1.000 | ||
| >75 | 35 (26.5) | 40.0 | 1.075 (0.647–1.785) | 0.743 (0.413–1.337) | ||
| Gender | 0.420 | 0.175 | ||||
| Female | 11 (8.3) | 54.5 | 1.000 | 1.000 | ||
| Male | 121 (91.7) | 43.0 | 1.453 (0.586–3.606) | 1.989 (0.737–5.372) | ||
| Tumor location | 0.642 | 0.422 | ||||
| Upper/Middle | 61 (46.2) | 42.6 | 1.000 | 1.000 | ||
| Lower | 71 (53.8) | 45.1 | 1.115 (0.706–1.761) | 1.231 (0.741–2.045) | ||
| TLN (nodes) | 0.288 | 0.176 | ||||
| ≤15 | 32 (24.2) | 37.5 | 1.000 | 1.000 | ||
| >15 | 100 (75.8) | 46.0 | 0.756 (0.452–1.266) | 0.672 (0.378–1.194) | ||
| Vessel involvement | 0.037 | 0.194 | ||||
| No | 100 (75.8) | 46.0 | 1.000 | 1.000 | ||
| Yes | 32 (24.2) | 37.5 | 1.733 (1.032–2.911) | 1.456 (0.825–2.569) | ||
| Perineural invasion | 0.009 | 0.281 | ||||
| No | 113 (85.6) | 47.8 | 1.000 | 1.000 | ||
| Yes | 19 (14.4) | 21.1 | 2.130 (1.205–3.766) | 1.432 (0.745–2.752) | ||
| Differentiation | 0.027 | 0.032 | ||||
| Well/Moderate | 98 (74.2) | 49.0 | 1.000 | 1.000 | ||
| Poor | 34 (25.8) | 29.4 | 1.715 (1.053–2.794) | 1.774 (1.049–3.000) | ||
| Tumor length (cm) | 0.007 | 0.036 | ||||
| ≤4 | 56 (42.4) | 60.7 | 1.000 | 1.000 | ||
| >4 | 76 (57.6) | 31.6 | 1.963 (1.191–3.237) | 1.769 (1.038–3.016) | ||
| N staging | 0.000 | 0.018 | ||||
| N0 | 58 (43.9) | 63.8 | 1.000 | 1.000 | ||
| N1-3 | 74 (56.1) | 28.4 | 3.059 (1.838–5.091) | 1.949 (1.119–3.395) | ||
| T grade | 0.000 | 0.002 | ||||
| T1-2 | 35 (26.5) | 77.1 | 1.000 | 1.000 | ||
| T3-4 | 97 (73.5) | 32.0 | 4.450 (2.129–9.298) | 3.342 (1.538–7.261) |
CI = confidence interval; ESCC = esophageal squamous cell carcinoma; HR = hazard ratio; TLN = total lymph nodes.
Discussion
The aging of the population and a longer life expectancy have led to more elderly patients with cancers being referred for treatment. For many of them, in particular for EC, surgery remains the mainstay of treatment. There is no established cut-off to define a patient as ‘elderly' in relation to surgery, but most studies available so far set the age limit at 70 (12,13). In our study, we determined the prognostic value of tumor length in ESCC patients older than 70 years. It was found that tumor length is a predictive factor for long-term survival in elderly patients with ESCC, especially in T3-4 grade or nodal-negative patients. We conclude that 4.0 cm may be the optimum cut-off point for tumor length in predicting survival in elderly patients with ESCC.
Before 1987, the AJCC staging system used tumor length (T1, <5 cm; T2, >5 cm; and T3, evidence of extraesophageal spread) to predict patient prognosis (14). However, at the 1987 AJCC annual meeting, the current TNM staging system was adopted, in which tumor length is not a staging criterion (15,16). The recent edition of the AJCC TNM staging system was published in 2009. In this newly published staging system for EC, adenocarcinoma and ESCC are regarded as two different entities and should be staged separately. It also proposes depth of tumor invasion, number of metastatic lymph nodes, histologic type, and tumor location as independent staging factors for EC. However, it does not emphasize the prognostic role of tumor length in EC (11).
Tumor length is still a controversial prognostic factor in EC patients. Tachibana et al. (9) evaluated 129 patients with ESCC and indicated that tumor length was related to survival but was not an independent prognostic factor on multivariate analysis. Bollschweiler et al. (10) and Igaki et al. (17) demonstrated similar results. However, several researchers have determined tumor length to be an important prognostic indictor of EC after surgery (3-5). Eloubeidi et al. (8) analyzed the outcomes of 10,441 EC patients from the National Cancer Institute Surveillance, Epidemiology, and End Results database. Their results demonstrated that tumor length was an important prognostic factor of overall survival for patients with EC. However, there have been few studies regarding tumor length in elderly patients. In our study, we determined that the tumor length (≤4.0 cm versus >4.0 cm) is a predictive factor for long-term survival in elderly patients with ESCC.
Many studies have shown that tumor length of 3.0 cm was the optimum cut-off point for survival prediction in EC (4,5,9,10). Eloubeidi et al. (8) suggested a cut-off point of tumor length in EC between 3.0 cm and 4.0 cm. In our study, however, a ROC curve for survival prediction was plotted to verify the optimum cut-off point for tumor length, which was 4.0 cm (Figure 1). Our results showed that tumors >4.0 cm in length had a 66.0% chance of being T3-4, whereas tumors ≤4.0 cm in length had a 65.7% chance of being T1-2 (P = 0.001). Bhutani et al. (18) showed that tumors ≥5 cm in length had an 89% chance of being T3 or greater, whereas tumors <5 cm in length had a 92% chance of being T1 or T2. Our study also showed that patients with tumor length ≤4.0 cm had a significantly better 5-year survival than patients with tumor length >4.0 cm (60.7% versus 31.6%, P = 0.007).
It has been widely agreed upon that lymph node status, depth of tumor invasion, and overall TNM stage are strong, independent prognostic factors in EC (19,20). It may well be that the influence of tumor length on the subgroup with different T grades and N stagings is important for the understanding of its role in overall survival in elderly patients with EC. In our study, the esophageal tumor length had a significant impact on survival of N0 patients (P = 0.046). Our finding is quite consistent with the previous studies showing that tumor length had a greater prognostic value for localized EC than for cancer with metastases (5,8), but is contrary to the result of Khan et al. (21), who suggested that tumor length is not a prognostic factor for EC patients with N0 staging (P = 0.861). In our study, tumor length was also a prognostic factor after controlling the factor of T grades. Its predictive value was significant for T3-4 lesions between patients with tumor length ≤4.0 cm and >4.0 cm (P = 0.035). From the database of 132 elderly patients with ESCC who underwent surgery, our results clearly demonstrate that tumor length can serve as an independent predictor of long-term survival for elderly patients with ESCC, especially in T3-4 grade or nodal-negative patients.
The question of how many lymph nodes should be dissected has been a point of debate in previous studies. Rizk et al. (22) reported that the prognosis of patients after esophagectomy worsens significantly when four or more lymph nodes have metastases, irrespective of T stage. A consensus conference of experts meeting in 1995 suggested that accurate pathological staging of EC required resection of at least 15 lymph nodes (23). Greenstein et al. (24) and Yang et al. (25) recommended 18 nodes as the minimum number of resectable lymph nodes. However, it is proposed by International Union Against Cancer (UICC) and AJCC that at least six lymph nodes should be removed during resection of EC (26,27). Accordingly, we excluded patients who had fewer than six lymph nodes dissected (range: 6–61 nodes).
Potential limitations of the present study include the relatively small number of patients, the use of a retrospective analysis, and the short duration of the mean follow-up duration. In addition, because the study used data from a single institution but with different pathologists and different surgeons, there may have been a lack of uniformity in measurement methods. In our study, tumor length was measured immediately after resection by surgeons. However, pathologists also described the tumor length (measured on the 10% formalin-fixed specimens) in their pathology reports. Thus, there were some differences between these two measures. Furthermore, due to the limited number of elderly patients with ESCC, our analysis may suffer from type I or type II errors. The results of the study should therefore be regarded with caution. Larger prospective studies will need to be performed to confirm these preliminary results and determine the optimum cut-off point.
Conclusion
In summary, our study showed that tumor length is a predictive factor for long-term survival in elderly patients with ESCC, especially in T3-4 grade or nodal-negative patients. We conclude that 4.0 cm may be the optimum cut-off point for tumor length in predicting survival in elderly patients with ESCC. Larger prospective studies will need to be performed to confirm these preliminary results and determine the optimum cut-off point.
Acknowledgements
The authors would like to thank Dr Lu Chen for data collection.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Rahamim JS, Murphy GJ, Awan Y, Junemann-Ramirez M. The effect of age on the outcome of surgical treatment for carcinoma of the oesophagus and gastric cardia. Eur J Cardiothorac Surg. 2003;23:805–10. doi: 10.1016/s1010-7940(03)00034-4. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths EA, Brummell Z, Gorthi G, Pritchard SA, Welch IM. Tumor length as a prognostic factor in esophageal malignancy: univariate and multivariate survival analyses. J Surg Oncol. 2006;93:258–67. doi: 10.1002/jso.20449. [DOI] [PubMed] [Google Scholar]
- 4.Wang BY, Goan YG, Hsu PK, Hsu WH, Wu YC. Tumor length as a prognostic factor in esophageal squamous cell carcinoma. Ann Thorac Surg. 2011;91:887–93. doi: 10.1016/j.athoracsur.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Yendamuri S, Swisher SG, Correa AM, Hofstetter W, Ajani JA, Francis A, et al. Esophageal tumor length is independently associated with long-term survival. Cancer. 2009;115:508–16. doi: 10.1002/cncr.24062. [DOI] [PubMed] [Google Scholar]
- 6.Tougeron D, Hamidou H, Scotté M, Di Fiore F, Antonietti M, Paillot B, et al. Esophageal cancer in the elderly: an analysis of the factors associated with treatment decisions and outcomes. BMC Cancer. 2010;10:510. doi: 10.1186/1471-2407-10-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang W, Igaki H, Tachimori Y, Sato H, Daiko H, Kato H. Three-field lymph node dissection for esophageal cancer in elderly patients over 70 years of age. Ann Thorac Surg. 2001;72:867–71. doi: 10.1016/s0003-4975(01)02896-x. [DOI] [PubMed] [Google Scholar]
- 8.Eloubeidi MA, Desmond R, Arguedas MR, Reed CE, Wilcox CM. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer. 2002;95:1434–43. doi: 10.1002/cncr.10868. [DOI] [PubMed] [Google Scholar]
- 9.Tachibana M, Kinugasa S, Dhar DK, Kotoh T, Shibakita M, Ohno S, et al. Prognostic factors after extended esophagectomy for squamous cell carcinoma of the thoracic esophagus. J Surg Oncol. 1999;72:88–93. doi: 10.1002/(sici)1096-9098(199910)72:2<88::aid-jso9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Bollschweiler E, Baldus SE, Schröder W, Schneider PM, Hölscher H. Staging of esophageal carcinoma: length of tumor and number of involved regional lymph nodes. Are these independent prognostic factors? J Surg Oncol. 2006;94:355–63. doi: 10.1002/jso.20569. [DOI] [PubMed] [Google Scholar]
- 11.Rice TW, Rusch VW, Ishwaran H, Blackstone EH. Worldwide Esophageal Cancer Collaboration. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Staging Manuals. Cancer. 2010;116:3763–73. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 12.Poon R, Law SY, Chu KM, Branicki GJ, Wong J. Esophagectomy for carcinoma of the esophagus in the elderly. Ann Surg. 1998;227:357–64. doi: 10.1097/00000658-199803000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruol A, Portale G, Zaninotto G, Cagol M, Cavallin F, Castoro C, et al. Results of esophagectomy for esophageal cancer in elderly patients: age has little influence on outcome and survival. J Thorac Cardiovasc Surg. 2007;133:1186–92. doi: 10.1016/j.jtcvs.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 14.Thompson WM. Esophageal cancer. Int J Radiat Oncol Biol Phys. 1983;9:1533–65. doi: 10.1016/0360-3016(83)90329-2. [DOI] [PubMed] [Google Scholar]
- 15.Sobin LH, Hermanek P, Hutter RV. TNM classification of malignant tumors: a comparison between the new (1987) and the old editions. Cancer. 1988;61:2310–14. doi: 10.1002/1097-0142(19880601)61:11<2310::aid-cncr2820611127>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 16.Lizuka T, Isono K, Kakegawa T, Watanabe H. Parameters linked to ten-year survival in Japan of resected esophageal carcinoma. Japanese Committee for Registration of Esophageal Carcinoma Cases. Chest. 1989;96:1005–11. doi: 10.1378/chest.96.5.1005. [DOI] [PubMed] [Google Scholar]
- 17.Igaki H, Kato H, Tachimori Y, Sato H, Daiko H, Nakanishi Y. Prognostic evaluation for squamous cell carcinomas of the lower thoracic esophagus treated with three-field lymph node dissection. Eur J Cardiothorac Surg. 2001;19:887–93. doi: 10.1016/s1010-7940(01)00701-1. [DOI] [PubMed] [Google Scholar]
- 18.Bhutani MS, Barde CJ, Markert RJ, Gopalswamy N. Length of esophageal cancer and degree of luminal stenosis during upper endoscopy predict T stage by endoscopic ultrasound. Endoscopy. 2002;34:461–3. doi: 10.1055/s-2002-31996. [DOI] [PubMed] [Google Scholar]
- 19.Peyre CG, Hagen JA, DeMeester SR, Altorki NK, Ancona E, Griffin SM, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg. 2008;248:549–56. doi: 10.1097/SLA.0b013e318188c474. [DOI] [PubMed] [Google Scholar]
- 20.Wijnhoven BP, Tran KT, Esterman A, Watson DI, Tilanus HW. An evaluation of prognostic factors and tumor staging of resected carcinoma of the esophagus. Ann Surg. 2007;245:717–25. doi: 10.1097/01.sla.0000251703.35919.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan OA, Alexiou C, Soomro I, Duffy JP, Morgan WE, Beggs FD. Pathological determinants of survival in node-negative oesophageal cancer. Br J Surg. 2004;91:1586–91. doi: 10.1002/bjs.4778. [DOI] [PubMed] [Google Scholar]
- 22.Rizk N, Venkatraman E, Park B, Flores R, Bains M, Rusch V. The prognostic importance of the number of involved lymph nodes in esophageal cancer: implications for revisions of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg. 2006;132:1374–81. doi: 10.1016/j.jtcvs.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 23.Fumagalli U. Resective surgery for cancer of the thoracic esophagus. Results of a Consensus Conference held at the VIth World Congress of the International Society for Diseases of the Esophagus. Dis Esophagus. 1996;9:30–8. [Google Scholar]
- 24.Greenstein AJ, Litle VR, Swanson SJ, Divino CM, Packer S, Wisnivesky JP. Effect of the number of lymph nodes sampled on postoperative survival of lymph node-negative esophageal cancer. Cancer. 2008;112:1239–46. doi: 10.1002/cncr.23309. [DOI] [PubMed] [Google Scholar]
- 25.Yang HX, Xu Y, Fu JH, Wang JY, Lin P, Rong TH. An evaluation of the number of lymph nodes examined and survival for node-negative esophageal carcinoma: data from China. Ann Surg Oncol. 2010;17:1901–11. doi: 10.1245/s10434-010-0948-9. [DOI] [PubMed] [Google Scholar]
- 26.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, et al. American Joint Committee on Cancer: AJCC cancer staging manual. 6th ed. New York: Springer; 2002. [Google Scholar]
- 27.Sorbin LH, Wittkind CL. TNM classification of malignant tumors. 6th ed. New York: Wiley & Sons; 2002. [Google Scholar]