Abstract
The emergence of novel viral diseases is driven by socioeconomic, demographic and environmental changes. These include land use changes such as deforestation, agricultural expansion and habitat degradation. However, the links between land use change and disease emergence are poorly understood and likely complex. In this review, we propose two hypotheses for the mechanisms by which land use change can lead to viral emergence: 1) by perturbing disease dynamics in multi-host disease systems via impacts on cross-species transmission rates (the ‘perturbation’ hypothesis); and 2) by allowing exposure of novel hosts to a rich pool of pathogen diversity (the ‘pathogen pool’ hypothesis). We discuss ways that these two hypotheses might be tested using a combination of ecological and virological approaches, and how this may provide novel control and prevention strategies.
Introduction
Emerging infectious diseases (EIDs), and in particular emerging viruses, are a key threat to global public health, to livestock, wildlife and to ecosystem functioning [1,2]. Some EIDs threaten public health through pandemics with large-scale mortality (e.g., HIV/AIDS). Others cause smaller outbreaks with high fatality rates or lack effective therapies and vaccines (e.g., Ebola virus, rabies, multi-drug resistant TB) [3,4]. As a group, EIDs and re-emerging diseases cause millions of deaths each year, and some single outbreak events (e.g., SARS) have cost the global economy tens of billions of dollars [5]. The World Economic Forum considers EIDs as “major” risks, comprising significant likelihood of occurrence and significant economic threat over the next 10 years, comparable in scale to unsustainable population growth [6,7]. Predicting and preventing the emergence of novel diseases with pandemic potential is therefore a global public health priority [8].
Yet, despite these impacts and perceived importance, our understanding of what causes diseases to emerge is rudimentary. The underlying causes tend to be changes in socioeconomic factors (e.g., increased travel and trade), demography (e.g., population expansion), agriculture (e.g., intensification of livestock production), medical science (e.g., increased antibiotic use) and to the environment (e.g. land use change, deforestation) [2,9,10]. It is thought that these ‘drivers’ of emergence foster conditions for pathogens to expand host range, and adapt to new niches, and that understanding how they affect the process of disease emergence could have use in predicting and combating EID threats [8,11].
The ecology of disease emergence
Human cases of new diseases stimulate intense research. Once reservoir-to-human transmission has occurred for a new EID, and led to human illness or mortality, significant efforts are often made to identify the reservoirs of the causative agent, or its capacity to spread once in the human population. These may have significant broad value for preventing future outbreaks or reducing pandemic threats. Studies that analyze how networks of contact, travel and trade, for example, have been used to predict pandemic spread of new EIDs, and to propose quarantine measures or therapeutic stockpiles to interrupt it [12-14]. These studies, however, are all focused on the later stages of the disease emergence process. There has been much less attention given to the preconditions requisite for epidemics to commence [15].
One key limitation to a fundamental understanding of the process of disease emergence is that new EIDs are caused by previously unknown pathogens, of unknown ecology, in unknown hosts. Another barrier to progress is that this is a fundamentally ecological problem that requires large-scale field studies and interdisciplinary collaboration among the ecological and medical sciences. The process of emergence also likely involves complexity that is often not brought into epidemiological analyses (e.g. the dynamics of seasonally fluctuating wildlife reservoir populations), and they require long-term field and lab commitment [16]. For example, long term studies of Lyme disease ecology have revealed the importance of synchronous tree masting [17], reservoir population changes with habitat fragmentation [18], and loss of predators [19] in the emergence and impact of that disease. Similarly, understanding the relative role of fruit bat population biology and livestock intensification in the emergence of Nipah virus required multi-year collaboration among ecologists, mathematical modelers, virologists, wildlife biologists and veterinary pathologists [16,20]. That said, there are some broad patterns that suggest fruitful avenues of research.
Disease emergence and land use change
Human activity has altered ecosystems on a global scale [21,22]. Changes include deforestation, expansion of agriculture, pollution, eutrophication, depletion of marine fisheries and increased nitrogen fixation [21,22]. Anthropogenic influence on landscapes has increased most rapidly in the last century with global population growth [23]. These changes have led to perturbation of biotic systems (e.g., biodiversity loss and biological invasions) the environment (e.g., water supply, climate), with subsequent direct and indirect impacts on human and wildlife populations [22]. Some impacts are positive (e.g. increased wealth in many regions), but many are negative (e.g. increased risk of drought, famine, emerging diseases).
These changes seem to be particularly important for zoonotic diseases, which account for ∼60% of all EIDs [24,25]. Around 1/5 of EID events since 1940 [unpubl. data, updated from 11] and an even higher proportion of zoonotic diseases, have been associated with land use changes, such as agricultural conversion, deforestation and activities associated with the extractive industries (e.g., mining, logging). These statistics support suggestions that increasing interaction among humans, domestic animals and wildlife following land use change is a significant contributor to disease emergence [26,27].
However, despite the relative frequency with which land use change has been associated with disease emergence events [11,18,26,28], land use change/disease emergence hypotheses tend to be vague or case-specific and currently lack a general theoretical foundation. This limits our ability to derive testable hypotheses and implement tailored management strategies to reduce the risks.
Two hypotheses for disease emergence due to land use change
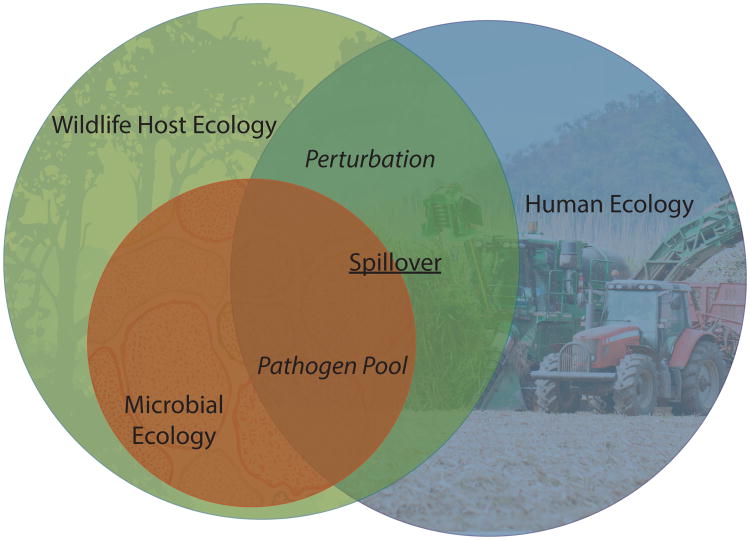
Current conceptual models tend to focus on two main mechanisms for disease emergence under land use change (Figure 1): 1) Land use change perturbs disease dynamics in multi-host disease systems by disrupting the cross-species transmission rate (hereafter the ‘perturbation’ hypothesis); and 2) Land use change allows exposure of novel hosts to a rich pool of pathogen diversity, influencing the cross-species transmission rate (hereafter the ‘pathogen pool’ hypothesis). These are not exclusive processes, and may be confounded when considering the mechanisms of disease emergence in dynamic landscapes. This is because human ecology – the presence, distribution and behavior of people - is the common denominator for both. Untangling the two hypotheses to better understand disease emergence and develop control and prevention strategies requires careful consideration of this dynamic coupled natural-human system.
Figure 1.
Conceptual model of how land use change drives the emergence of infectious diseases in people. Land use change is a complex, dynamic process that underpins many of the novel zoonoses identified in humans during the last few decades. While the ultimate goal of public health is to identify and prevent transmission of these pathogens to people (‘spillover’), our mechanistic understanding of what drives them to emerge is poor. We propose two hypotheses which are probably not mutually exclusive. In the ‘pathogen pool’ hypothesis, anthropogenic activities in previously pristine environments bring people into contact with a large reservoir of microbial diversity in wildlife for which humans are naïve. In the ‘perturbation’ hypothesis, land use changes alter the dynamics of pathogen transmission among wildlife, and promote cross-species transmission.
Analyzing land use change and disease emergence
A key limitation to studies of how disease emergence is driven by land use change is, of course, our significant lack of knowledge of the diversity of pathogens present in wildlife in a region, of the ecology of these pathogens, and their impact on different hosts (including should they emerge into people) [8,29]. Unusual or infrequent pathogen transmission between species (“spillover”) is the defining characteristic of a zoonosis. Conceptual models place the factors influencing the force of infection from animals to humans into three categories: 1) the prevalence of infection in the animal reservoir, 2) the rate at which humans come into contact with these animals, and 3) the probability that humans become infected when contact occurs [15]. These components interact and are each influenced by diverse properties of natural and human systems, with additional factors associated with pathogen modes of transmission and evolutionary constraints (e.g., phylogeny) [15,30,31].
Under land use change, human ecology directly drives the contact rate among humans and reservoir hosts (e.g., how and when contact with wildlife occurs) and can influence the likelihood of infection given contact (e.g., the type of contact, such as butchering vs cohabitation). Additionally, the human impact on the landscape may simultaneously influence the prevalence of infection in animal reservoirs by perturbing the abundance and distribution of different animal reservoirs [32]. Thus, the interaction of human ecology with biodiversity is fundamentally important to zoonotic disease emergence due to land use change.
Links between biodiversity, disease risk and land use change
Most of the early theoretical models of disease dynamics have concerned single-host single-pathogen systems [33,34]. More recent studies have begun to use a community ecology perspective to understand multi-host disease systems [15,35-40]. Some of these have demonstrated correlations between host diversity and disease risk, both positive and negative [18]. But what are the mechanisms?
Under the ‘perturbation’ hypothesis, biodiversity has been related to both an increase (via the “amplification effect”) and a decrease (via the “dilution effect” or other functionally similar means [35]) of the intrinsic risk of cross-species transmission. There is currently mixed support for which of these outcomes is generally more common or likely from ecosystem perturbation [32,39,41-43]. The underlying mechanisms involve the change in host species richness, abundance, quality or contact rate, which governs cross-species transmission rates via their effects on pathogen prevalence and the number of infectious individuals [35,39,42]. In order to better understand the way land use change affects risk of disease emergence, understanding the relationships between multiple hosts and multiple pathogens is critical.
Under the ‘pathogen pool’ hypothesis, land use change may foster exposure of hosts (humans and associated species, e.g. livestock, pests) to a pool of microbes harbored by wildlife for which they have no prior exposure. This increases the risk of novel cross-species transmission events. This can be distinguished from the ‘perturbation’ hypothesis because the mechanism focuses on novel contact between novel host-pathogen groups, and not necessarily on perturbing the community ecology of pathogens in reservoirs. Under the ‘pathogen pool’ hypothesis, risk of disease emergence should correlate with the factors that drive contact between novel host-pathogen pairs. This should include aspects of human ecology (our abundance, distribution, behavior) that dictate contact with reservoirs in landscape [44,45], as well as the baseline microbial diversity in reservoirs, referred to in earlier studies as the ‘zoonotic pool’ [46].
Several studies have thus proposed that areas of higher biodiversity (e.g., the tropics) should confer greater risk of zoonotic disease emergence under land use change [11,47]. The assumption is that pathogen diversity is a function of host diversity, such that human activities in highly biodiverse regions result in novel exposure to a more diverse pool of pathogens and an elevated risk of ‘spillover’. However, relationships between host and pathogen biodiversity are often unclear or lack consistent empirical support across taxa [42,48-54]. Thus, testing this hypothesis requires better characterization of viral diversity in wildlife to determine predictable relationships between host and pathogen biodiversity, should they exist. It also requires a better understanding of the factors that drive the microbial diversity within landscapes.
Future perspectives – human ecology and pathogenic landscape?
Despite significant global resources spent on pandemic prevention, new zoonoses, and in particular viral zoonoses, continue to emerge in the human population [55]. Their impact is high, even in the absence of significant mortality (e.g. SARS) and analyses of global and historical trends suggest their emergence is accelerating, even after accounting for reporting bias [11]. The increasing number of zoonotic diseases spilling over from a range of wild animal species is of particular concern. Clearly the global changes promoting novel disease emergence are currently outstripping our potential to leverage fundamental knowledge to predict and prevent pandemics. At the same time, the underlying environmental and socioeconomic drivers continue to accelerate in impact, compounding this problem.
On average, studies suggest that protecting biodiversity or limiting human influence in landscapes should reduce the risk of zoonotic disease emergence [18]. Yet, given our poor understanding of the specific mechanisms involved, there is currently little uptake of this as a disease management option. Elucidating the dominant mechanisms of disease emergence in dynamic landscapes thus remains a critical priority in infectious disease research, and ultimately pandemic prevention.
In this review, we have identified two key hypotheses that could be a priority for future research and that will begin to integrate an understanding of human ecology with host-pathogen community ecology. The long-term result may be a strategy to identify high-risk regions, populations and perhaps even occupations that play the largest role in disease emergence due to land use change. Designing ways to alter land management plans in these regions, or limit exposure for populations at risk may limit exposure to and/or minimize the consequences of EIDs. Given the high economic and health costs of EIDs, even small gains in risk reduction via novel, tailored strategies could be a highly cost effective way to manage EID risk [56].
Emerging viral zoonoses are a critical threat to public health and are driven by socioeconomic and environmental changes
Our understanding of how environmental changes, in particular land use change causes viruses to emerge is rudimentary
We propose two hypotheses on how land use change causes disease emergence
These are: the ‘perturbation’ hypothesis, and the ‘pathogen pool’ hypothesis
We discuss how these could be tested, using a combination of virological and community ecology studies
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Daszak P, Tabor G, Kilpatrick A, Epstein J, Plowright R. Conservation medicine and a new agenda for emerging diseases. Annals of the New York Academy of Sciences. 2004:1–11. doi: 10.1196/annals.1307.001. [DOI] [PubMed] [Google Scholar]
- 2.Smolinski MS, Hamburg MA, Lederberg J. Committee on Emerging Microbial Threats to Health in the 21st Century: Microbial threats to health: Emergence, detection, and response. Washington D.C: The National Academies Press; 2003. [PubMed] [Google Scholar]
- 3.Salmón-Mulanovich G, Vásquez A, Albújar C, Guevara C, Laguna-Torres A, Salazar M, Zamalloa H, Cáceres M, Gómez-Benavides J, Pacheco V, et al. Human rabies and rabies in vampire and nonvampire bat species, southeastern Peru, 2007. Emerging Infectious Diseases. 2009;15 doi: 10.3201/eid1508.081522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilpin CM, Simpson G, Vincent S, O'Brien TP, Knight TA, Globan M, Coulter C, Konstantinos A. Evidence of primary transmission of multidrug-resistant tuberculosis in the Western Province of Papua New Guinea. Medical Journal of Australia. 2008;188:148–152. doi: 10.5694/j.1326-5377.2008.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee JW, McKibbin WJ. Globalization and disease: The case of SARS. In: Brooking Institution, editor. Brookings discussion papers in international economics. 2004. [Google Scholar]
- 6.WEF. Global Risks 2010: A Global Risk Network Report. World Economic Forum report; Geneva, Switzerland. 2010. [Google Scholar]
- 7.WEF. Global Risks 2012: A Global Risk Network Report; World Economic Forum report; Geneva, Switzerland. 2012. [Google Scholar]
- 8.Morse SS, Mazet JAK, Woolhouse M, Parrish CR, Carroll D, Karesh WB, Zambrana-Torellio C, Lipkin WI, Daszak P. Prediction and prevention of the next pandemic zoonosis. Lancet. 2012;380:1956–1965. doi: 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daszak P, Cunningham AA, Hyatt AD. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Tropica. 2001;78:103–116. doi: 10.1016/s0001-706x(00)00179-0. [DOI] [PubMed] [Google Scholar]
- 10.Weiss RA, McMichael AJ. Social and environmental risk factors in the emergence of infectious diseases. Nature Medicine. 2004;10:S70–S76. doi: 10.1038/nm1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–994. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halloran ME, Ferguson NM, Eubank S, Longini IM, Cummings DAT, Lewis B, Xu SF, Fraser C, Vullikanti A, Germann TC, et al. Modeling targeted layered containment of an influenza pandemic in the United States. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4639–4644. doi: 10.1073/pnas.0706849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson NM, Cummings DAT, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–452. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilpatrick AM, Chmura AA, Gibbons DW, Fleischer RC, Marra PP, Daszak P. Predicting the global spread of H5N1 avian influenza. Proceedings of the National Academy of Sciences of the United States of America; 2006. pp. 19368–19373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd-Smith JO, George D, Pepin KM, Pitzer VE, Pulliam JRC, Dobson AP, Hudson PJ, Grenfell BT. Epidemic dynamics at the human-animal interface. Science. 2009;326:1362–1367. doi: 10.1126/science.1177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daszak P, Zambrana-Torrelio C, Bogich TL, Fernandez M, Epstein JH, Murray KA, Hamilton H. Interdisciplinary approaches to understanding disease emergence: The past, present, and future drivers of Nipah virus emergence. Proceedings of the National Academy of Sciences; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostfeld RS, Jones CG, Wolff JO. Of mice and mast. Bioscience. 1996;46:323–330. [Google Scholar]
- 18.Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Holt RD, Hudson P, Jolles A, Jones KE, Mitchell CE, et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468:647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levi T, Kilpatrick AM, Mangle M, Wilmers CC. Deer, predators, and the emergence of Lyme disease. Proceedings of the National Academy of Sciences of the United States of America; 2012. pp. 10942–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pulliam JRC, Epstein JH, Dushoff J, Rahman SA, Bunning M, Jamaluddin AA, Hyatt AD, Field HE, Dobson AP, Daszak P, et al. Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. Journal of the Royal Society Interface. 2012;9:89–101. doi: 10.1098/rsif.2011.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of Earth's ecosystems. Science. 1997;277:494. [Google Scholar]
- 22.Hassan R, Scholes R, Ash N. Ecosystems and Human Wellbeing. Vol. 1. Washington; Millenium Ecosystem Assessment: 2005. [Google Scholar]
- 23.Cohen JE. Human population: The next half century. Science. 2003;302:1172–1175. doi: 10.1126/science.1088665. [DOI] [PubMed] [Google Scholar]
- 24.Woolhouse MEJ, Gowtage-Sequeria S. Host range and emerging and re-emerging pathogens. Emerging Infectious Diseases. 2005;11 doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor LH, Latham SM, Woolhouse MEJ. Risk factors for human disease emergence. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patz JA, Daszak P, Tabor GM, Aguirre AA, Pearl M, Epstein J, Wolfe ND, Kilpatrick AM, Foufopoulos J, Molyneux D, et al. Unhealthy landscapes: Policy recommendations on land use change and infectious disease emergence. Environmental Health Perspectives. 2004;112:1092–1098. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daszak P, Cunningham A, Hyatt A. Emerging infectious diseases of wildlife - threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- 28.Burnside WR, Brown JH, Burger O, Hamilton MJ, Moses M, Bettencourt LMA. Human macroecology: linking pattern and process in big-picture human ecology. Biological Reviews. 2012;87:194–208. doi: 10.1111/j.1469-185X.2011.00192.x. [DOI] [PubMed] [Google Scholar]
- 29.Woolhouse M, Scott F, Hudson Z, Howey R, Chase-Topping M. Human viruses: discovery and emergence. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367:2864–2871. doi: 10.1098/rstb.2011.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Streicker DG, Turmelle AS, Vonhof MJ, Kuzmin IV, McCracken GF, Rupprecht CE. Host Phylogeny Constrains Cross-Species Emergence and Establishment of Rabies Virus in Bats. Science. 2010;329:676–679. doi: 10.1126/science.1188836. [DOI] [PubMed] [Google Scholar]
- 31.Parrish CR, Holmes EC, Morens DM, Park EC, Burke DS, Calisher CH, Laughlin CA, Saif LJ, Daszak P. Cross-Species Virus Transmission and the Emergence of New Epidemic Diseases. Microbiology and Molecular Biology Reviews. 2008;72:457–470. doi: 10.1128/MMBR.00004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brearley G, Rhodes J, Bradley A, Baxter G, Seabrook L, Lunney D, Liu Y, McAlpine C. Wildlife disease prevalence in human-modified landscapes. Biological Reviews. 2012 n/a-n/a. [Google Scholar]
- 33.Anderson RM, May RM. Population biology of infectious-diseases .1. Nature. 1979;280:361–367. doi: 10.1038/280361a0. [DOI] [PubMed] [Google Scholar]
- 34.May RM, Anderson RM. Population biology of infectious diseases 2. Nature. 1979;280:455–461. doi: 10.1038/280455a0. [DOI] [PubMed] [Google Scholar]
- 35.Roche B, Dobson AP, Guégan JF, Rohani P. Linking community and disease ecology: the impact of biodiversity on pathogen transmission. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367:2807–2813. doi: 10.1098/rstb.2011.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Power Alison G, Mitchell Charles E. Pathogen Spillover in Disease Epidemics. The American Naturalist. 2004;164:S79–S89. doi: 10.1086/424610. [DOI] [PubMed] [Google Scholar]
- 37.Thomas MB, Arthurs SP, Watson EL. Trophic and Guild Interactions and the Influence of Multiple Species on Disease Trophic and Guild in Biological Interactions Control. In: Brodeur J, Boivin G, editors. Progress in Biological Control. Vol. 3 Springer; Netherlands: 2006. pp. 101–122. [Google Scholar]
- 38.Roche B, Guégan JF. Ecosystem dynamics, biological diversity and emerging infectious diseases. Comptes Rendus Biologies. 2011;334:385–392. doi: 10.1016/j.crvi.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vourc'h G, Plantard O, Morand S. How Does Biodiversity Influence the Ecology of Infectious Disease? In: Morand S, Beaudeau F, Cabaret J, editors. New Frontiers of Molecular Epidemiology of Infectious Diseases. Springer; Netherlands: 2012. pp. 291–309. [Google Scholar]
- 40.Wilcox B, Gubler D. Disease ecology and the global emergence of zoonotic pathogens. Environmental Health and Preventive Medicine. 2005;10:263–272. doi: 10.1007/BF02897701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Randolph SE, Dobson ADM. Pangloss revisited: a critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology. 2012:1–17. doi: 10.1017/S0031182012000200. FirstView. [DOI] [PubMed] [Google Scholar]
- 42.Ostfeld RS, Keesing F. Effects of Host Diversity on Infectious Disease. Annual Review of Ecology, Evolution, and Systematics. 2012;43:157–182. [Google Scholar]
- 43.Friggens M, Beier P. Anthropogenic disturbance and the risk of flea-borne disease transmission. Oecologia. 2010;164:809–820. doi: 10.1007/s00442-010-1747-5. [DOI] [PubMed] [Google Scholar]
- 44.Hausermann H, Tschakert P, Smithwick E, Ferring D, Amankwah R, Klutse E, Hagarty J, Kromel L. Contours of Risk: Spatializing Human Behaviors to Understand Disease Dynamics in Changing Landscapes. EcoHealth. 2012;9:251–255. doi: 10.1007/s10393-012-0780-8. [DOI] [PubMed] [Google Scholar]
- 45.Vazquez-Prokopec GM, Stoddard ST, Paz-Soldan V, Morrison AC, Elder JP, Kochel TJ, Scott TW, Kitron U. Usefulness of commercially available GPS data-loggers for tracking human movement and exposure to dengue virus. International Journal of Health Geographics. 2009;8 doi: 10.1186/1476-072X-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morse SS. Factors in the emergence of infectious disease. Emerging Infectious Diseases. 1995;1:7–15. doi: 10.3201/eid0101.950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolfe ND, Daszak P, Kilpatrick AM, Burke DS. Bushmeat hunting, deforestation and prediction of zoonotic emergence. Emerging Infectious Diseases. 2005;11:1822–1827. doi: 10.3201/eid1112.040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vacher C, Vile D, Helion E, Piou D, Desprez-Loustau ML. Distribution of parasitic fungal species richness: influence of climate versus host species diversity. Diversity and Distributions. 2008;14:786–798. [Google Scholar]
- 49.Bordes F, Guégan JF, Morand S. Microparasite species richness in rodents is higher at lower latitudes and is associated with reduced litter size. Oikos. 2011;120:1889–1896. [Google Scholar]
- 50.Dobson A, Lafferty KD, Kuris AM, Hechinger RF, Jetz W. Homage to Linnaeus: How many parasites? How many hosts? Proceedings of the National Academy of Sciences. 2008;105:11482–11489. doi: 10.1073/pnas.0803232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindenfors P, Nunn CL, Jones KE, Cunningham AA, Sechrest W, Gittleman JL. Parasite species richness in carnivores: effects of host body mass, latitude, geographical range and population density. Global Ecology and Biogeography. 2007;16:496–509. [Google Scholar]
- 52.Guernier V, Guégan JF. May Rapoport's Rule Apply to Human Associated Pathogens? EcoHealth. 2009;6:509–521. doi: 10.1007/s10393-010-0290-5. [DOI] [PubMed] [Google Scholar]
- 53.Guernier V, Hochberg ME, Guégan JF. Ecology Drives the Worldwide Distribution of Human Diseases. PLoS Biology. 2004;2:e141. doi: 10.1371/journal.pbio.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunn RR, Davies TJ, Harris NC, Gavin MC. Global drivers of human pathogen richness and prevalence. Proceedings of the Royal Society B-Biological Sciences. 2010;277:2587–2595. doi: 10.1098/rspb.2010.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karesh WB, Dobson A, Lloyd-Smith J, Loh E, Lubroth J, Dixon MA, Bennett M, Aldrich S, Thomas J, Heymann D. The ecology of zoonoses: Their natural and unnatural histories. Lancet. 2012;380:1936–1945. doi: 10.1016/S0140-6736(12)61678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murray KA, Skerratt LF, Speare R, Ritchie S, Smout F, Hedlefs R, Lee J. Cooling off health security hot spots: Getting on top of it down under. Environment International. 2012;48:56–64. doi: 10.1016/j.envint.2012.06.015. [DOI] [PubMed] [Google Scholar]
Annotated refs
- **.Jones KE, Patel N, Levy M, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–994. doi: 10.1038/nature06536. This paper shows that analysis of the underlying causes of disease emergence can be used to predict where on the planet the next emerging disease is most likely to originate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crowl TA, Crist TO, Parmenter RR, Belovsky G, Lugo AE. The spread of invasive species and infectious disease as drivers of ecosystem change. Frontiers in Ecology and the Environment. 2008;6:238–246. [Google Scholar]
- 3.Daszak P, Tabor G, Kilpatrick A, Epstein J, Plowright R. Conservation medicine and a new agenda for emerging diseases. Annals of the New York Academy of Sciences. 2004:1–11. doi: 10.1196/annals.1307.001. [DOI] [PubMed] [Google Scholar]
- 4*.Smolinski MS, Hamburg MA, Lederberg J. Microbial threats to health: Emergence, detection, and response. Washington D.C.: The National Academies Press; 2003. Committee on Emerging Microbial Threats to Health in the 21st Century. This book is a superb reference for the current view of emerging diseases. It is a follow up to the IOM Forum on Microbial Threats 1992 report, and has a far more global view, with new information on environmental and ecological drivers of disease emergence. [PubMed] [Google Scholar]
- 5.Merson MH, O'Malley J, Serwadda D, Apisuk C. HIV prevention 1 - The history and challenge of HIV prevention. Lancet. 2008;372:475–488. doi: 10.1016/S0140-6736(08)60884-3. [DOI] [PubMed] [Google Scholar]
- 6.Georges AJ, Leroy EM, Renaut AA, Benissan CT, Nabias RJ, Ngoc MT, Obiang PI, Lepage JP, Bertherat EJ, Benoni DD, et al. Ebola hemorrhagic fever outbreaks in Gabon, 1994-1997: epidemiologic and health control issues. J Infect Dis. 1999;179(Suppl 1):S65–75. doi: 10.1086/514290. [DOI] [PubMed] [Google Scholar]
- 7.Salmón-Mulanovich G, Vásquez A, Albújar C, Guevara C, Laguna-Torres A, Salazar M, Zamalloa H, Cáceres M, Gómez-Benavides J, Pacheco V, et al. Human rabies and rabies in vampire and nonvampire bat species, southeastern Peru, 2007. Emerging Infectious Diseases. 2009;15 doi: 10.3201/eid1508.081522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilpin CM, Simpson G, Vincent S, O'Brien TP, Knight TA, Globan M, Coulter C, Konstantinos A. Evidence of primary transmission of multidrug-resistant tuberculosis in the Western Province of Papua New Guinea. Medical Journal of Australia. 2008;188:148–152. doi: 10.5694/j.1326-5377.2008.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 9.Treasury. The economic impact of Severe Acute Respiratory Syndrome (SARS). Economic Roundup WINTER 2003. The Treasury, Australian Government. 2003. [Google Scholar]
- 10.Lee JW, McKibbin WJ. Globalization and disease: The case of SARS. In: Brooking Institution, editor. Brookings discussion papers in international economics. [Google Scholar]
- 11.WEF. Global Risks 2010: A Global Risk Network Report. World Economic Forum report; Geneva, Switzerland. p. 2010. [Google Scholar]
- 12.Pike J, Bogich T, Finnoff D, Daszak P. Economic optimization of a global strategy to reduce the pandemic threat. In prep. 2012 doi: 10.1073/pnas.1412661112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morse SS. Examining the origins of emerging viruses. In: Morse SS, editor. Emerging Viruses. Oxford University Press; 1993. pp. 10–28. [Google Scholar]
- 14.Daszak P, Cunningham AA, Hyatt AD. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Tropica. 2001;78:103–116. doi: 10.1016/s0001-706x(00)00179-0. [DOI] [PubMed] [Google Scholar]
- 15.Weiss RA, McMichael AJ. Social and environmental risk factors in the emergence of infectious diseases. Nature Medicine. 2004;10:S70–S76. doi: 10.1038/nm1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morse SS, Mazet JAK, Woolhouse M, Parrish CR, Carroll D, Karesh WB, Zambrana-Torellio C, Lipkin WI, Daszak P. Prediction and prevention of the next pandemic zoonosis. Lancet. 2012;380:1956–1965. doi: 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–994. doi: 10.1038/nature06536. This is the first paper to analyze prior emerging disease events, correct for reporting bias, and statistically prove that they are increasing in frequency, that zoonoses are becoming more predominant, and that it is possible to use these data to identify where the next unknown EID is most likely to originate (EID ‘hotspots’). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halloran ME, Ferguson NM, Eubank S, Longini IM, Cummings DAT, Lewis B, Xu SF, Fraser C, Vullikanti A, Germann TC, et al. Modeling targeted layered containment of an influenza pandemic in the United States. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4639–4644. doi: 10.1073/pnas.0706849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19*.Ferguson NM, Cummings DAT, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–452. doi: 10.1038/nature04795. This paper uses state-of-the-art mathematical modeling, tagged to data from real influenza outbreaks to estimate, for the first time, the number of doses of antivirals required to be in place, in the point of origin to prevent a pandemic. This is a bold effort to demonstrate one strategy for pandemic prevention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilpatrick AM, Chmura AA, Gibbons DW, Fleischer RC, Marra PP, Daszak P. Predicting the global spread of H5N1 avian influenza. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19368–19373. doi: 10.1073/pnas.0609227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilpatrick AM, Gluzberg Y, Burgett J, Daszak P. A quantitative risk assessment of the pathways by which West Nile virus could reach Hawaii. Ecohealth. 2004;1:205–209. [Google Scholar]
- 22.Ostfeld RS, Jones CG, Wolff JO. Of mice and mast. Bioscience. 1996;46:323–330. [Google Scholar]
- 23.Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Holt RD, Hudson P, Jolles A, Jones KE, Mitchell CE, et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468:647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levi T, Kilpatrick AM, Mangle M, Wilmers CC. Deer, predators, and the emergence of Lyme disease. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:10942–10947. doi: 10.1073/pnas.1204536109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25**.Pulliam JRC, Epstein JH, Dushoff J, Rahman SA, Bunning M, Jamaluddin AA, Hyatt AD, Field HE, Dobson AP, Daszak P, et al. Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. Journal of the Royal Society Interface. 2012;9:89–101. doi: 10.1098/rsif.2011.0223. This paper analyzes environmental and agricultural trends in the years before the emergence of Nipah virus in Malaysia. It demonstrates that agricultural intensification, coupled with repeated introduction of a bat-borne virus provided the perfect conditions for Nipah virus to emerge in that place, at that time. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26**.Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of Earth's ecosystems. Science. 1997;277:494. This paper was one of the first to clearly lay out the many ways that human activities have altered the Earth's biological systems to the point that it has now become an essentially human habitat. [Google Scholar]
- 27.Hassan R, Scholes R, Ash N. Ecosystems and Human Wellbeing. Vol. 1. Washington: Millenium Ecosystem Assessment; 2005. [Google Scholar]
- 28.Cohen JE. Human population: The next half century. Science. 2003;302:1172–1175. doi: 10.1126/science.1088665. [DOI] [PubMed] [Google Scholar]
- 29.Woolhouse MEJ, Gowtage-Sequeria S. Host range and emerging and re-emerging pathogens. Emerging Infectious Diseases. 2005;11 doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30**.Taylor LH, Latham SM, Woolhouse MEJ. Risk factors for human disease emergence. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. This paper represents one of the first attempts to systematically analyze the patterns of disease emergence in people. It has been cited extensively for the finding that around 60% of all human pathogens are zoonotic in origin (come from non-human animals), but around 70% of emerging pathogens are zoonotic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patz JA, Daszak P, Tabor GM, Aguirre AA, Pearl M, Epstein J, Wolfe ND, Kilpatrick AM, Foufopoulos J, Molyneux D, et al. Unhealthy landscapes: Policy recommendations on land use change and infectious disease emergence. Environmental Health Perspectives. 2004;112:1092–1098. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daszak P, Cunningham A, Hyatt A. Emerging infectious diseases of wildlife - threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- 33.Burnside WR, Brown JH, Burger O, Hamilton MJ, Moses M, Bettencourt LMA. Human macroecology: linking pattern and process in big-picture human ecology. Biological Reviews. 2012;87:194–208. doi: 10.1111/j.1469-185X.2011.00192.x. [DOI] [PubMed] [Google Scholar]
- 34.Lloyd-Smith JO, George D, Pepin KM, Pitzer VE, Pulliam JRC, Dobson AP, Hudson PJ, Grenfell BT. Epidemic dynamics at the human-animal interface. Science. 2009;326:1362–1367. doi: 10.1126/science.1177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Streicker DG, Turmelle AS, Vonhof MJ, Kuzmin IV, McCracken GF, Rupprecht CE. Host Phylogeny Constrains Cross-Species Emergence and Establishment of Rabies Virus in Bats. Science. 2010;329:676–679. doi: 10.1126/science.1188836. [DOI] [PubMed] [Google Scholar]
- 36.Parrish CR, Holmes EC, Morens DM, Park EC, Burke DS, Calisher CH, Laughlin CA, Saif LJ, Daszak P. Cross-Species Virus Transmission and the Emergence of New Epidemic Diseases. Microbiology and Molecular Biology Reviews. 2008;72:457–470. doi: 10.1128/MMBR.00004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson RM, May RM. Population biology of infectious-diseases .1. Nature. 1979;280:361–367. doi: 10.1038/280361a0. [DOI] [PubMed] [Google Scholar]
- 38.May RM, Anderson RM. Population biology of infectious diseases 2. Nature. 1979;280:455–461. doi: 10.1038/280455a0. [DOI] [PubMed] [Google Scholar]
- 39.Roche B, Dobson AP, Guégan JF, Rohani P. Linking community and disease ecology: the impact of biodiversity on pathogen transmission. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367:2807–2813. doi: 10.1098/rstb.2011.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Power Alison G, Mitchell Charles E. Pathogen Spillover in Disease Epidemics. The American Naturalist. 2004;164:S79–S89. doi: 10.1086/424610. [DOI] [PubMed] [Google Scholar]
- 41.Thomas MB, Arthurs SP, Watson EL. Trophic and Guild Interactions and the Influence of Multiple Species on Disease. In: Trophic and Guild in Biological Interactions Control. In: Brodeur J, Boivin G, editors. Progress in Biological Control. Vol. 3 Springer; Netherlands: 2006. pp. 101–122. [Google Scholar]
- 42.Roche B, Guégan JF. Ecosystem dynamics, biological diversity and emerging infectious diseases. Comptes Rendus Biologies. 2011;334:385–392. doi: 10.1016/j.crvi.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vourc'h G, Plantard O, Morand S. How Does Biodiversity Influence the Ecology of Infectious Disease? In: Morand S, Beaudeau S, Cabaret J, editors. New Frontiers of Molecular Epidemiology of Infectious Diseases. Springer; Netherlands: 2012. pp. 291–309. [Google Scholar]
- 44.Wilcox B, Gubler D. Disease ecology and the global emergence of zoonotic pathogens. Environmental Health and Preventive Medicine. 2005;10:263–272. doi: 10.1007/BF02897701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dobson A, Foufopoulos J. Emerging infectious pathogens of wildlife. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2001;356:1001–1012. doi: 10.1098/rstb.2001.0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Randolph SE, Dobson ADM. Pangloss revisited: a critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology. 2012:1, 17. doi: 10.1017/S0031182012000200. FirstView. [DOI] [PubMed] [Google Scholar]
- 47.Hausermann H, Tschakert P, Smithwick E, Ferring D, Amankwah R, Klutse E, Hagarty J, Kromel L. Contours of Risk: Spatializing Human Behaviors to Understand Disease Dynamics in Changing Landscapes. EcoHealth. 2012;9:251–255. doi: 10.1007/s10393-012-0780-8. [DOI] [PubMed] [Google Scholar]
- 48.Vazquez-Prokopec GM, Stoddard ST, Paz-Soldan V, Morrison AC, Elder JP, Kochel TJ, Scott TW, Kitron U. Usefulness of commercially available GPS data-loggers for tracking human movement and exposure to dengue virus. International Journal of Health Geographics. 2009;8 doi: 10.1186/1476-072X-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morse SS. Factors in the emergence of infectious disease. Emerging Infectious Diseases. 1995;1:7–15. doi: 10.3201/eid0101.950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolfe ND, Daszak P, Kilpatrick AM, Burke DS. Bushmeat hunting, deforestation and prediction of zoonotic emergence. Emerging Infectious Diseases. 2005;11:1822–1827. doi: 10.3201/eid1112.040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vacher C, Vile D, Helion E, Piou D, Desprez-Loustau ML. Distribution of parasitic fungal species richness: influence of climate versus host species diversity. Diversity and Distributions. 2008;14:786–798. [Google Scholar]
- 52.Bordes F, Guégan JF, Morand S. Microparasite species richness in rodents is higher at lower latitudes and is associated with reduced litter size. Oikos. 2011;120:1889–1896. [Google Scholar]
- 53.Dobson A, Lafferty KD, Kuris AM, Hechinger RF, Jetz W. Homage to Linnaeus: How many parasites? How many hosts? Proceedings of the National Academy of Sciences. 2008;105:11482–11489. doi: 10.1073/pnas.0803232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindenfors P, Nunn CL, Jones KE, Cunningham AA, Sechrest W, Gittleman JL. Parasite species richness in carnivores: effects of host body mass, latitude, geographical range and population density. Global Ecology and Biogeography. 2007;16:496–509. [Google Scholar]
- 55.Guernier V, Guégan JF. May Rapoport's Rule Apply to Human Associated Pathogens? EcoHealth. 2009;6:509–521. doi: 10.1007/s10393-010-0290-5. [DOI] [PubMed] [Google Scholar]
- 56.Guernier V, Hochberg ME, Guégan JF. Ecology Drives the Worldwide Distribution of Human Diseases. PLoS Biology. 2004;2:e141. doi: 10.1371/journal.pbio.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karesh WB, Dobson A, Lloyd-Smith J, Loh E, Lubroth J, Dixon MA, Bennett M, Aldrich S, Thomas J, Heymann D. The ecology of zoonoses: Their natural and unnatural histories. Lancet. 2012;380:1936–1945. doi: 10.1016/S0140-6736(12)61678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murray KA, Skerratt LF, Speare R, Ritchie S, Smout F, Hedlefs R, Lee J. Cooling off health security hot spots: Getting on top of it down under. Environment International. 2012;48:56–64. doi: 10.1016/j.envint.2012.06.015. [DOI] [PubMed] [Google Scholar]