Abstract
The present experiment evaluated the effects of naltrexone, a non-selective opioid receptor antagonist, on post-abstinence alcohol drinking in C57BL/6NCRL and DBA/2J male mice. Home cage 2-bottle (alcohol vs. water) free-choice procedures were employed. During the pre-abstinence period, alcohol intake was much lower for the DBA/2J mice relative to the C57BL/6NCRL mice, and this strain difference was observed for groups receiving either 3% or 10% alcohol concentrations. The four-day abstinence period effectively reduced alcohol intakes (i.e., a negative alcohol deprivation effect, negative ADE) in both groups of DBA/2J mice, but had no effect on alcohol intakes in either group of C57BL/6NCRL mice. Both groups trained with 3% alcohol received the second four-day abstinence period, where the effects of acute administration of either naltrexone or saline on post-abstinence alcohol drinking were assessed. Naltrexone was more effective in reducing post-abstinence drinking of 3% alcohol in the DBA/2J mice than in the C57BL/6NCRL mice. In the DBA/2J mice, naltrexone further reduced, relative to saline-injected controls, the low levels of post-abstinence alcohol intake. Thus, the low baseline levels of alcohol drinking in DBA/2J mice were further diminished by the four-day abstinence period (negative ADE), and this suppressed post-abstinence level of alcohol drinking was still further reduced by acute administration of naltrexone. The results indicate that naltrexone is effective in reducing further the low levels of alcohol drinking induced by the negative ADE.
Keywords: Alcohol, C57BL/6NCRL, DBA/2J, Naltrexone, Negative ADE
1. Introduction
Naltrexone, a non-selective opioid receptor antagonist, reduces alcohol drinking in rats and in mice (Coonfield et al., 2002; Davidson and Amit, 1997; Egli, 2005; Froehlich et al., 1990; Goodwin et al., 2001; Grahame et al., 2000; Heyser et al., 1997; Hubbell and Reid, 1988; Le et al., 1993; Middaugh et al., 1999a, 1999b; Phillips et al., 1997; Salimov et al., 1995a, 1995b; Volpicelli et al., 1986). Naltrexone’s effects are particularly well-documented in high alcohol drinking strains of rats (Davidson and Amit, 1997; Dhaher et al., 2012; Heyser et al., 2003; Hill and Kiefer, 1997; Holter and Spanagel, 1999; Juarez and Eliana, 2007; Myers and Lankford, 1996) and in high alcohol drinking strains of mice, notably C57BL/6 and related sub-strains (Escher and Mittleman, 2006; Grahame et al., 2000; Kamdar et al., 2007; Kim et al., 2004; Phillips et al., 1997). While it is clear that naltrexone reliably reduces high levels of alcohol drinking, its effects on lower levels of alcohol drinking have not been reported. For example, there are no published reports of naltrexone’s effects on alcohol drinking in DBA/2J mice, an inbred mouse strain that exhibits little tendency to drink alcohol.
The present experiment investigated the effects of acute administration of naltrexone on alcohol drinking in two inbred strains of mice (C57BL/6NCRL and DBA/2J) known to differ in their tendencies to drink alcohol. Of primary interest are the effects of acute naltrexone administration on alcohol drinking following a period of alcohol abstinence. Relapse-like elevation of post-abstinence alcohol drinking (i.e., positive alcohol deprivation effect, or positive ADE) has been reported in rats and in mice (Holstein et al., 2011; Khisti et al., 2006; McKinzie et al., 1998; Melendez et al., 2006; Rodd-Henricks et al., 2000a, 2000b; Rodd et al., 2004; Salimov et al., 1993; Salimov and Salimova, 1993a, 1993b; Sinclair, 1972; Sinclair, 1979; Sinclair and Li, 1989; Sinclair and Senter, 1967; Sparta et al., 2009; Tambour et al., 2008; Wolstenholme et al., 2011). Although far less extensively studied, several reports indicate that in rodents, when alcohol drinking levels are low, the alcohol abstinence period may induce even less post-abstinence alcohol drinking than pre-abstinence baseline levels i.e., negative alcohol deprivation effect, or negative ADE (DiBattista, 1991; Melendez et al., 2006; Salimov et al., 1995a; Sinclair and Bender, 1978; Sinclair and Sheaff, 1973; Sinclair and Tiihonen, 1988). While investigators have reported that acute administration of naltrexone suppressed post-abstinence relapse-like alcohol drinking, subsequently reducing the magnitude of the positive ADE (Heyser et al., 2003; Holter and Spanagel, 1999; Kuzmin et al., 2006; Oslin et al., 2003; Pellicano and Sadile, 2006; Sinclair, 1998; Stromberg et al., 2001), there are no published studies evaluating the effects of naltrexone administration on negative ADE.
Thus, we evaluated the effects of an acute subcutaneous injection of a single 10.0 mg/kg dose of naltrexone on post-abstinence alcohol drinking in groups of C57BL/6NCRL and DBA/2J male mice trained to drink a 3% alcohol solution. Separate groups of each strain were also trained to drink a 10% alcohol solution, but these groups were not evaluated for the effects of an injection of naltrexone. Of particular interest was the effect of the abstinence period on further reducing the low levels of drinking of 3% alcohol in DBA/2J mice (i.e., negative ADE) and the effectiveness of naltrexone in reducing still further the lower levels of abstinence-induced suppression of alcohol drinking in these DBA/2J mice. The 10.0 mg/kg naltrexone dose was employed based on reports that an acute injection of this dose reliably reduced alcohol consumption (Critcher et al., 1983; Davidson and Amit, 1997; Gardell et al., 1996; Reid et al., 1996) and, in addition, significantly suppressed post-abstinence positive ADE as well (Holter and Spanagel, 1999). Moreover, a single acute injection of naltrexone has been reported to more reliably reduce alcohol drinking than either repeated or chronic administration of naltrexone (Holter and Spanagel, 1999; Iso and Brush, 1991).
2. Materials and methods
2.1. Animals
The subjects were 48 male C57BL/6NCRL mice obtained from Charles River Laboratories (Kingston, NY, USA) and 48 male DBA/2J mice obtained from Jackson Laboratories (Bar Harbor, ME, USA). Mice were approximately 60 days old at the beginning of the experiment. During the 12-day pre-abstinence period, the mean body weights of the C57BL/6NCRL mice receiving access to 3% alcohol or 10% alcohol were 25.8 g and 26.0 g, respectively, and the mean body weights of the DBA/2J mice receiving access to 3% alcohol or 10% alcohol were 21.7 g and 21.8 g, respectively. All mice were acclimated to the colony room for one week prior to the beginning of the study. The 96 mice were housed in pairs of the same strain, in 48 clear plastic shoe box cages, in a light controlled room maintained on a 12 hour light/dark cycle (lights on at 0600 hours). They were provided with unrestricted access to food (PMI 5012 rat chow) and tap water. All experimental procedures were performed in accordance with the guidelines of the Institutional Care and Use Committee of the National Institute on Drug Abuse, National Institute of Health and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources Commission on Life Sciences. Guide for the Care and Use of Laboratory Animals, 1996) and approved by the IACUC at Rutgers University.
2.2. Housing apparatus
Mice were housed two per cage in each of the 48 plastic shoebox cages, providing each mouse a proximal cage-mate. Each shoebox cage contained a ¼-in thick clear plastic barrier that divided the shoebox cage lengthwise into two compartments of approximately equal size. The plastic barrier was the height of the shoebox cage and was drilled with 20 circular holes, of one quarter-inch diameter each, to allow for ventilation between the two compartments. One mouse was housed on each side of the plastic barrier. This housing apparatus allowed the evaluation of alcohol drinking of individual mice without housing each mouse in isolation, a condition which substantially elevates alcohol drinking in mice (Pohorecky, 1991).
2.3. Drugs
Bulk alcohol (95%) was obtained from Rutgers University Chemical Stores. Alcohol was diluted in tap water to produce the alcohol concentrations (volume to volume, vol./vol.) employed in the study. Naltrexone hydrochloride was purchased from Merck Laboratories and was dissolved in 0.9% saline to a volume equivalent to 1.0 ml/kg. All drug doses refer to the total salt.
2.4. Alcohol Provision and Deprivation Procedure
All mice were provided with continuous access in the home cage to two sippers, one containing alcohol and the other containing tap water. Fluids were contained in clear glass vials equipped with a rubber stopper and a stainless steel ball valve sipper. Sipper tube position (left vs. right) was randomized across days. All mice and drinking tubes were weighed daily at approximately 1000 hours, after which drinking tubes were emptied and refilled with fresh solutions.
All subjects in the 10% alcohol groups (24 C57BL/6NCRL mice and 24 DBA/2J mice) were run prior to any of the subjects in the 3% alcohol groups and received alcohol concentrations of 2%, 4%, 6%, 8%, and 10% during daily drinking sessions 1, 2, 3, 4, and 5, respectively. Thereafter, all subjects in the 10% alcohol groups received the 10% alcohol concentration during all remaining days of the experiment (except for the first abstinence period) (see upper panel of Fig. 1). All subjects in the 3% alcohol groups (24 C57BL/6NCRL mice and 24 DBA/2J mice) received the 3% alcohol concentration during all days of the experiment (except for the first and second abstinence periods) (see lower panel of Fig. 1).
Fig. 1.
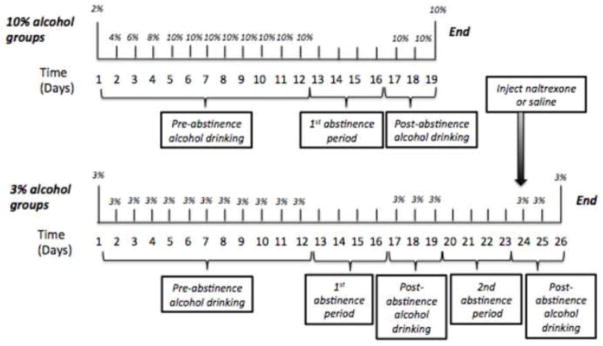
Experimental time-line showing % alcohol available as a function of Time (Experimental Days). Upper Panel: For both of the 10% alcohol groups (C57BL/6NCRL and DBA/2J) the duration of the experiment was 19 days, and included one 4-day abstinence period (Days 13–16). Lower Panel: For both of the 3% alcohol groups (C57BL/6NCRL and DBA/2J) the duration of the experiment was 26 days, and included two 4-day abstinence periods (Days 13–16 and Days 20–23). A subcutaneous injection of either Naltrexone (10.0 mg/kg) or 0.9% Saline was given one-hour prior to the first post-abstinence drinking session (Day 24) following the second 4-day abstinence period.
All 96 subjects received the first abstinence period, which began after the completion of the 12th daily alcohol drinking session. The first abstinence period consisted of 4 consecutive days (13–16) without alcohol, when all mice were provided with access to two drinking tubes containing only tap water. After the first abstinence period, all subjects received the first post-abstinence session (day 17), during which all mice received access to the alcohol concentration employed during the pre-abstinence period (3% or 10%), while the other drinking tube contained tap water. Procedures during days 18 and 19 were identical to those for day 17.
Only the 48 mice in the 3% alcohol groups received the second abstinence period, which consisted of 4 consecutive days (20–23) without alcohol, when all mice were provided with access to two drinking tubes containing only tap water. Upon observing the significant negative ADE in the DBA/2J mice receiving 10% alcohol, we then ran the 3% alcohol procedures in both strains to determine if we would replicate the negative ADE in the DBA/2J mice at the lower concentration. When we did observe the reliable negative ADE in the DBA/2J receiving 3% alcohol following Abstinence 1, we investigated the possibility that naltrexone would further reduce the low levels of drinking of 3% alcohol following Abstinence 2. After the second abstinence period, the mice in the 3% group were randomly assigned to either the naltrexone or saline group. For both strains, these groups differed in mean ethanol intake, ethanol preference, and bodyweight by less than 10%. Approximately 1 hour prior to the first post-abstinence session (day 24), mice in the naltrexone group received 10mg/kg of naltrexone hydrochloride via subcutaneous injection, with an injection volume of 1.0mg/ml, while the mice in the saline group received a subcutaneous injection of 0.9% isotonic saline. On Day 24, all mice received access to the 3% alcohol concentration employed during the pre-abstinence period, while the other drinking tube contained tap water.
2.5. Alcohol drinking measures
For each subject, for each daily drinking session, alcohol fluid consumed (g) and water fluid consumed (g) and bodyweight (kg) were measured, then alcohol intake (grams of alcohol consumed per kg of bodyweight) and percent (%) alcohol preference [(grams of alcohol fluid consumed divided by grams of alcohol fluid consumed + grams of water fluid consumed) x 100] were derived.
2.6. Abstinence Ratio Calculation
The post-abstinence ratio for each subject was calculated as: B / (A + B), where A = mean of the last 5 sessions of the pre-abstinence period, and B = session 1 of the post-abstinence period. A post-abstinence ratio > 0.50 indicates that post-abstinence alcohol drinking was elevated relative to the pre-abstinence baseline level of alcohol drinking (i.e., abstinence-induced “rebound” or “relapse-like” effect or positive alcohol deprivation effect (positive ADE)), while a post-abstinence ratio < 0.50 indicates that post-abstinence alcohol drinking was reduced relative to the pre-abstinence baseline level of alcohol drinking (i.e., negative alcohol deprivation effect or negative ADE). The mean post-abstinence ratio for a subject provides a single number that reflects changes in that subject’s alcohol drinking induced by the abstinence period, and does so while allowing all subjects, regardless of their level of alcohol drinking, to contribute equally to the group mean post-abstinence ratio. Moreover, the post-abstinence ratio provides a measure of change for a subject relative to that subject’s baseline, and therefore, allows comparisons of changes in alcohol drinking between strains and/or groups of subjects that differ markedly in their baseline levels of alcohol drinking. To evaluate within-subject changes in alcohol drinking across the abstinence period, the post-abstinence ratio for each subject was compared to that subject’s pre-abstinence ratio, which was calculated as: A / (A + B), where A = mean of the last 5 sessions of the pre-abstinence period, and B = session 1 of the post-abstinence period.
2.7. Statistics
Analyses of group differences in mean alcohol intake and mean % alcohol preference were based on the means of the last 5 daily alcohol drinking sessions, prior to the first abstinence period (days 8–12). Main effects of mouse strain drinker (C57BL/6NCRL vs. DBA/2J) and main effects of alcohol concentration (3% vs. 10%) and interaction effects between mouse strain and alcohol concentration were assessed by a 2 x 2 two-way univariate Analysis of Variance (ANOVA) using General Linear Model (Systat Statistical Software, Richmond, CA, USA), with an alpha level of 0.05. Mean group differences between strains (C57BL/6NCRL vs. DBA/2J) in post-abstinence ratios following the first abstinence period were assessed by one-way ANOVA. The effect of the abstinence period in producing changes in alcohol intake or changes in percent alcohol preference was evaluated by comparing the pre-abstinence ratio to the post-abstinence ratio using one-way ANOVA. Effects of the abstinence period in producing elevated (positive ADE) or suppressed (negative ADE) mean post-abstinence ratios were assessed by repeated-measures one-way ANOVA. Mean differences between strains (C57BL/6NCRL vs. DBA/2J) in post-abstinence ratios following the naltrexone injection or saline injection of the second abstinence period were assessed by one-way ANOVA. The effects of the naltrexone injection or the effects of the saline injection, following the second abstinence period, relative to the effects of the non-injection, following the first abstinence period, were assessed using one-way repeated-measures ANOVA.
3. Results
3.1. Pre-abstinence levels of alcohol drinking
3.1.1. Pre-abstinence alcohol intake (g/kg)
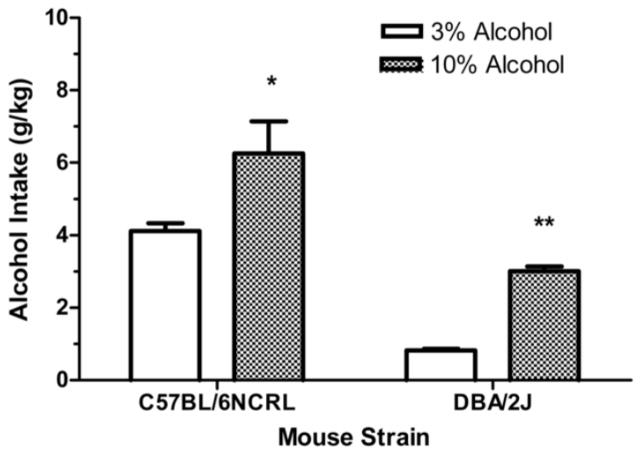
For both the C57BL/6NCRL mice and the DBA/2J mice, the groups trained with the 10% alcohol concentration yielded higher mean daily g/kg alcohol intakes than did the groups trained with the 3% alcohol concentration (Fig. 2), and this is consistent with previous studies comparing daily intakes of these alcohol concentrations in these inbred strain of mice (Yoneyama et al., 2008). A 2 x 2 factorial ANOVA revealed a significant main effect of strain, (F (1, 92) = 51.11, p < 0.01), a significant main effect of concentration, (F (1, 92) = 22.25, p < 0.01), and no significant interaction effect between strain and concentration, (F < 1). Separate one-way ANOVAs revealed that the 10% alcohol concentration induced significantly more alcohol intake than did the 3% alcohol concentration, in the C57BL/6NCRL mice (p < 0.05) and in the DBA/2J mice (p < 0.01).
Fig. 2. Pre-abstinence alcohol intake (g/kg).
Mean daily g/kg alcohol intake (grams of alcohol consumed per kilogram of bodyweight) during the last 5 sessions preceding the first abstinence period for the C57BL/6NCRL mice trained with the 3% alcohol concentration (n=24) and the C57BL/6NCRL mice trained with the 10% alcohol concentration (n=24) and for the DBA/2J mice trained with the 3% alcohol concentration (n=24) and the DBA/2J mice trained with the 10% alcohol concentration (n=24). The vertical bars represent the standard error of the mean (SEM). The single asterisk (*) indicates that the groups differed at the 0.05 level of significance. The double asterisk (**) indicates that the groups differed at the 0.01 level of significance.
3.1.2. Pre-abstinence alcohol preference (%)
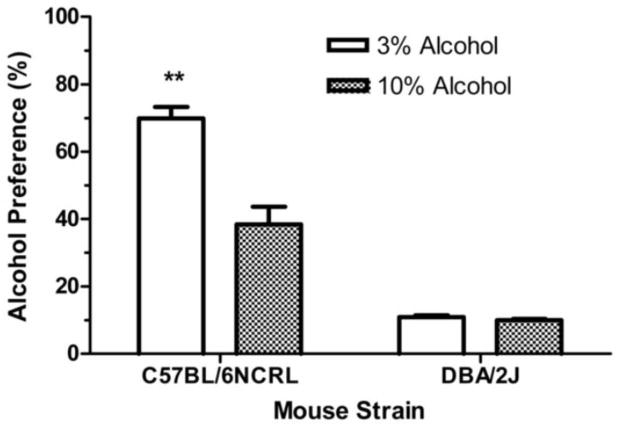
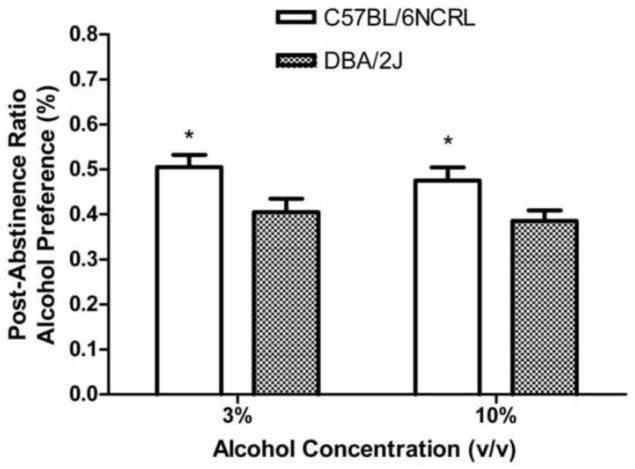
The 2 x 2 factorial ANOVA (Fig. 3) revealed a significant main effect of strain, (F (1, 92) = 192.38, p < 0.01), a significant main effect of concentration, (F (1, 92) = 26.44, p < 0.01), and a significant interaction effect between strain and concentration, (F (1, 92) = 23.73, p < 0.01). One-way ANOVA revealed that the 3% alcohol concentration induced significantly higher alcohol preference than did the 10% alcohol concentration, in the C57BL/6NCRL mice (p < 0.01) but there was no effect of alcohol concentration in the DBA/2J mice (p > 0.20).
Fig. 3. Pre-abstinence alcohol preference (%).
Mean daily percent alcohol preference [(grams of alcohol fluid consumed divided by grams of alcohol fluid consumed + grams of water fluid consumed) x 100] during the last 5 sessions preceding the first abstinence period for the C57BL/6NCRL mice trained with the 3% alcohol concentration (n=24) and the C57BL/6NCRL mice trained with the 10% alcohol concentration (n=24) and for the DBA/2J mice trained with the 3% alcohol concentration (n=24) and the DBA/2J mice trained with the 10% alcohol concentration (n=24). The vertical bars represent the standard error of the mean (SEM). The double asterisk (**) indicates that the groups differed at the 0.01 level of significance.
3.2. Post-abstinence alcohol drinking
3.2.1. Post-abstinence alcohol intake (g/kg)
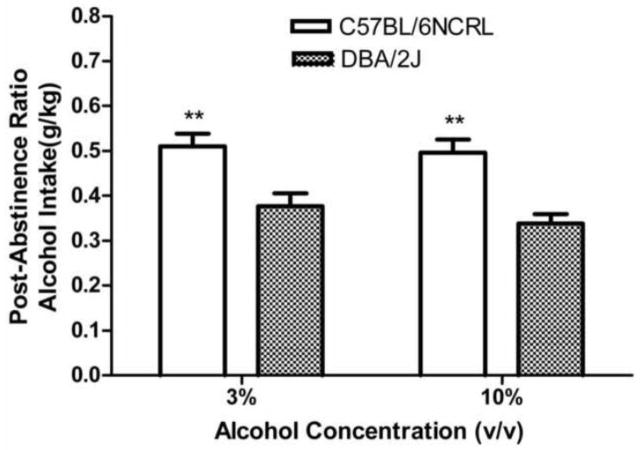
The effect of the first abstinence period (where no injections were given) was to reduce post-abstinence alcohol intake of DBA/2J mice, but not of C57BL/6NCRL mice (Fig. 4). For both DBA/2J groups, the post-abstinence ratio was significantly lower than the pre-abstinence ratio (F (1,23) = 18.18, p < 0.001) and (F (1,23) = 62.58, p< 0.001) for the groups trained with 3% and 10% alcohol concentrations, respectively. This indicates that for both DBA/2J groups the effect of the abstinence period was to reduce alcohol intake (i.e., negative ADE). In contrast, for both C57BL/6NCRL groups, the post-abstinence ratio did not differ significantly from the pre-abstinence ratio (F’s < 1). For the groups trained with the 3% alcohol concentration, one-way ANOVA revealed that the post-abstinence ratio evaluating the alcohol intake measure for the C57BL/6NCRL group was significantly higher than the post-abstinence ratio for the DBA/2J group, (F (1,46) = 10.82, p < 0.01). For the groups trained with the 10% alcohol concentration, one-way ANOVA revealed that the post-abstinence ratio evaluating the alcohol intake measure for the C57BL/6NCRL group was also significantly higher than for the DBA/2J group, (F (1,46) = 19.44, p < 0.01).
Fig. 4. Strain differences in post-abstinence alcohol intake (g/kg).
Mean post-abstinence ratios based on g/kg alcohol intake (grams of alcohol consumed per kilogram of bodyweight) for the first abstinence period (no injection) for the C57BL/6NCRL mice trained with the 3% alcohol concentration (n=24) and the C57BL/6NCRL mice trained with the 10% alcohol concentration (n=24) and for the DBA/2J mice trained with the 3% alcohol concentration (n=24) and the DBA/2J mice trained with the 10% alcohol concentration (n=24). The post-abstinence ratio for each subject was calculated as [(session 1 of the post-abstinence period) / (session 1 of the post-abstinence period + mean of the last 5 sessions of the pre-abstinence period)]. The vertical bars represent the standard error of the mean (SEM). The double asterisk (**) indicates that the groups differed at the 0.01 level of significance.
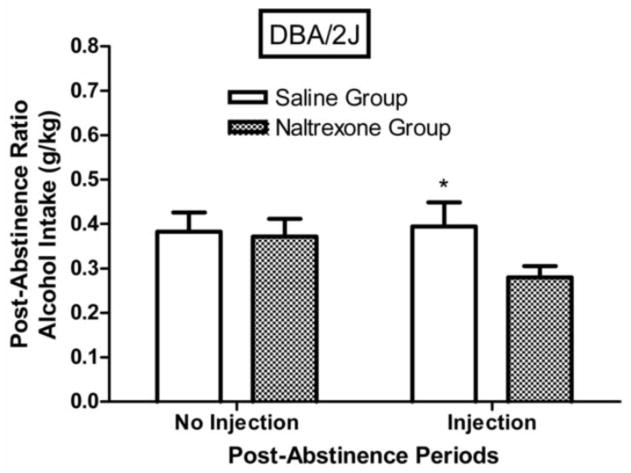
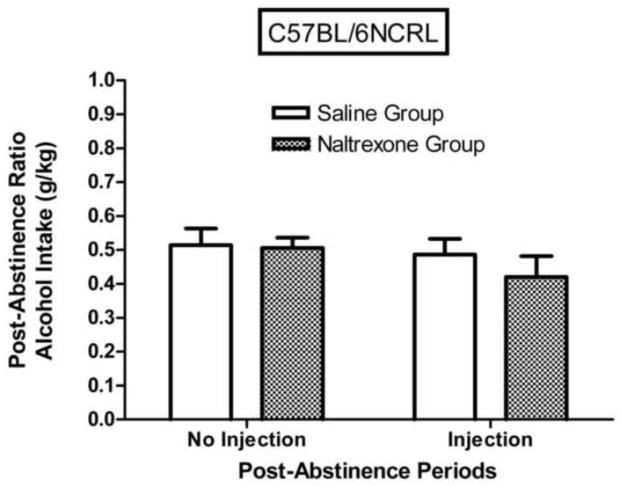
The effect of the second abstinence period (where naltrexone or saline injections were given) was to reduce post-abstinence alcohol intake of DBA/2J mice (Fig. 6), but not of C57BL/6NCRL mice (Fig. 7). For the DBA/2J injection groups combined, the post-abstinence ratio was significantly lower than the pre-abstinence ratio (F (1,23) = 26.42, p < 0.001), indicating that the effect of the second abstinence period was to reduce alcohol intake (i.e., negative ADE), but for the C57BL/6NCRL injection groups combined, the post-abstinence ratio did not differ significantly from the pre-abstinence ratio (F (1,23) = 1.48, p > 0.20).
Fig. 6. Naltrexone effects on post-abstinence alcohol intake (g/kg) in DBA/2J mice.
Mean post-abstinence ratios based on g/kg alcohol intake (grams of alcohol consumed per kilogram of bodyweight) for the first abstinence period (no injection) and for the second abstinence period (injection) for the DBA/2J mice trained with the 3% alcohol concentration (n=24). The injection given prior to the first session following the second abstinence period was either naltrexone (n=12) or saline (n=12). The post-abstinence ratio for each subject was calculated as [(session 1 of the post-abstinence period) / (session 1 of the post-abstinence period + mean of the last 5 sessions of the pre-abstinence period)]. The vertical bars represent the standard error of the mean (SEM). The single asterisk (*) indicates that the groups differed at the 0.05 level of significance.
Fig. 7. Naltrexone effects on post-abstinence alcohol intake (g/kg) in C57BL/6NCRL mice.
Mean post-abstinence ratios based on g/kg alcohol intake (grams of alcohol consumed per kilogram of bodyweight) for the first abstinence period (no injection) and for the second abstinence period (injection) for the C57BL/6NCRL mice trained with the 3% alcohol concentration (n=24). The injection given prior to the first session following the second abstinence period was either naltrexone (n=12) or saline (n=12). The post-abstinence ratio for each subject was calculated as [(session 1 of the post-abstinence period) / (session 1 of the post-abstinence period + mean of the last 5 sessions of the pre-abstinence period)]. The vertical bars represent the standard error of the mean (SEM).
3.2.2. Post-abstinence alcohol preference (%)
The effect of the first abstinence period (where no injections were given) was to reduce post-abstinence alcohol preference of DBA/2J mice, but not of C57BL/6NCRL mice (Fig. 5). For both DBA/2J groups, the post-abstinence ratio was significantly lower than the pre-abstinence ratio (F (1,23) = 10.32, p < 0.01) and (F (1,23) = 24.68, p< 0.001) for the groups trained with 3% and 10% alcohol concentrations, respectively. This indicates that for both DBA/2J groups the effect of the abstinence period was to reduce alcohol preference (i.e., negative ADE), but for both C57BL/6NCRL groups, the post-abstinence ratio did not differ significantly from the pre-abstinence ratio (F’s < 1). For the groups trained with the 3% alcohol concentration, one-way ANOVA revealed that the post-abstinence ratio for the % alcohol preference measure for the C57BL/6NCRL group was significantly higher than for the DBA/2J group, (F (1,46) = 6.03, p < 0.02). For the groups trained with the 10% alcohol concentration, one-way ANOVA revealed that the post-abstinence ratio for the % alcohol preference measure for the C57BL/6NCRL group was also significantly higher than for the DBA/2J group, (F (1,46) = 5.82, p < 0.02).
Fig. 5. Strain differences in post-abstinence alcohol preference (%).
Mean post-abstinence ratios based on % alcohol preference [(grams of alcohol fluid consumed divided by grams of alcohol fluid consumed + grams of water fluid consumed) x 100] for the first abstinence period (no injection) for the C57BL/6NCRL mice trained with the 3% alcohol concentration (n=24) and the C57BL/6NCRL mice trained with the 10% alcohol concentration (n=24) and for the DBA/2J mice trained with the 3% alcohol concentration (n=24) and the DBA/2J mice trained with the 10% alcohol concentration (n=24). The post-abstinence ratio for each subject was calculated as [(session 1 of the post-abstinence period) / (session 1 of the post-abstinence period + mean of the last 5 sessions of the pre-abstinence period)]. The vertical bars represent the standard error of the mean (SEM). The single asterisk (*) indicates that the groups differed at the 0.05 level of significance.
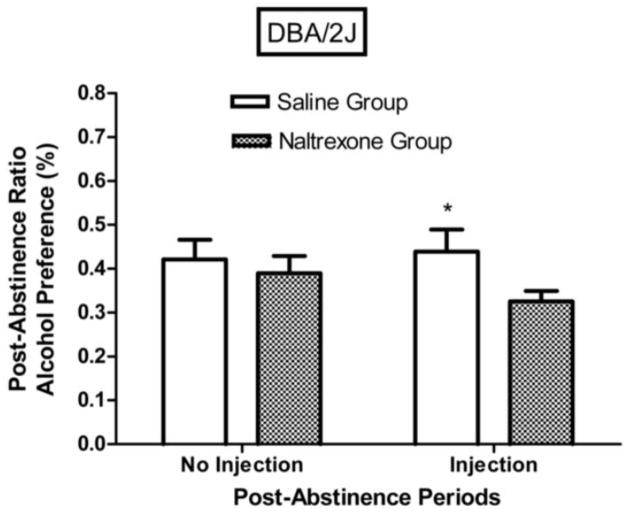
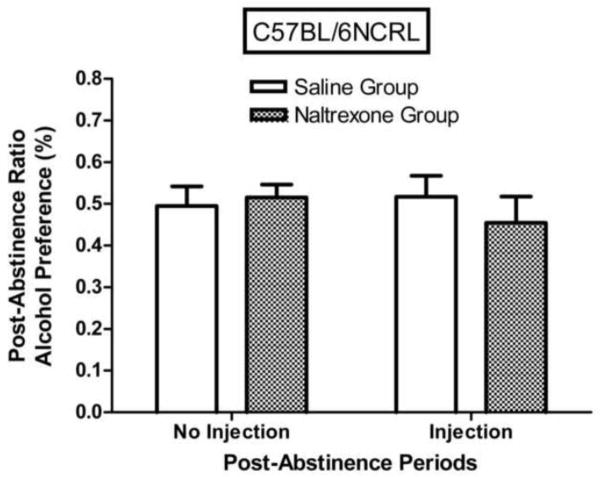
The effect of the second abstinence period (where injections were given) was to reduce post-abstinence alcohol preference of DBA/2J mice (Fig. 8), but not of C57BL/6NCRL mice (Fig. 9). For the DBA/2J injection groups combined, the post-abstinence ratio was significantly lower than the pre-abstinence ratio (F (1,23) = 15.85, p < 0.001), indicating that the effect of the second abstinence period was to reduce alcohol intake (i.e., negative ADE), but for the C57BL/6NCRL injection groups combined, the post-abstinence ratio did not differ significantly from the pre-abstinence ratio (F < 1).
Fig. 8. Naltrexone effects on post-abstinence alcohol preference (%) in DBA/2J mice.
Mean post-abstinence ratios based on % alcohol preference [(grams of alcohol fluid consumed divided by grams of alcohol fluid consumed + grams of water fluid consumed) x 100] for the first abstinence period (no injection) and for the second abstinence period (injection) for the DBA/2J mice trained with the 3% alcohol concentration (n=24). The injection given prior to the first session following the second abstinence period was either naltrexone (n=12) or saline (n=12). The post-abstinence ratio for each subject was calculated as [(session 1 of the post-abstinence period) / (session 1 of the post-abstinence period + mean of the last 5 sessions of the pre-abstinence period)]. The vertical bars represent the standard error of the mean (SEM). The single asterisk (*) indicates that the groups differed at the 0.05 level of significance.
Fig. 9. Naltrexone effects on post-abstinence alcohol preference (%) in C57BL/6NCRL mice.
Mean post-abstinence ratios based on % alcohol preference [(grams of alcohol fluid consumed divided by grams of alcohol fluid consumed + grams of water fluid consumed) x 100] for the first abstinence period (no injection) and for the second abstinence period (injection) for the C57BL/6NCRL mice trained with the 3% alcohol concentration (n=24). The injection given prior to the first session following the second abstinence period was either naltrexone (n=12) or saline (n=12). The post-abstinence ratio for each subject was calculated as [(session 1 of the post-abstinence period) / (session 1 of the post-abstinence period + mean of the last 5 sessions of the pre-abstinence period)]. The vertical bars represent the standard error of the mean (SEM).
3.3. Naltrexone’s effects on post-abstinence alcohol drinking
3.3.1. Naltrexone’s effects on post-abstinence alcohol intake (g/kg)
One-way repeated-measures ANOVA comparing mean pre-abstinence ratios to mean post-abstinence ratios for the DBA/2J mice revealed that naltrexone injection significantly reduced g/kg alcohol intake (F (1,11) = 73.98, p < 0.01), indicating that naltrexone injection induced a significant negative ADE. On the other hand, the effect of the saline injection on alcohol intake of the DBA/2J mice was not significant (F (1,11) = 3.77, p > 0.05). One-way repeated-measures ANOVA comparing mean pre-abstinence ratios to mean post-abstinence ratios for the C57BL/6NCRL mice revealed that naltrexone injection did not significantly reduce g/kg alcohol intake (F (1,11) = 1.68, p > 0.20), indicating that naltrexone injection did not induce a significant negative ADE. The effect of the saline injection on alcohol intake of the C57BL/6NCRL mice was not significant, F < 1.
One-way repeated measures ANOVA (Fig. 6) for the DBA/2J mice revealed that the post-abstinence ratio for the alcohol intake measure was significantly lower following naltrexone injection relative to following saline injection, (F (1,22) = 3.66, p < 0.05). On the other hand, one-way repeated measures ANOVA (Fig. 7) for the C57BL/6NCRL mice revealed that the post-abstinence ratio for the alcohol intake measure was not significantly lower following naltrexone injection relative to following the saline injection, F < 1. The post-abstinence ratios for the alcohol intake measures for the naltrexone injection groups were 0.28 ± 0.04 and 0.42 ± 0.06 for the DBA/2J and C57BL/6NCRL mice, respectively (see Figs. 6 and 7). One-way ANOVA revealed that this difference was significant, (F (1, 22) = 4.42, p < 0.05). On the other hand, for the saline injection groups, the post-abstinence ratios for the alcohol intake measures were 0.40 ± 0.04 and 0.49 ± 0.06 for the DBA/2J and C57BL/6NCRL mice, respectively (see Figs. 6 and 7). One-way ANOVA revealed that this difference was not significant, (F (1, 22) = 1.62, p > 0.20).
3.3.2. Naltrexone’s effects on post-abstinence alcohol preference (%)
One-way repeated-measures ANOVA comparing mean pre-abstinence ratios to mean post-abstinence ratios for the DBA/2J mice revealed that naltrexone injection significantly reduced alcohol preference (F (1,11) = 52.70, p < 0.001), indicating that naltrexone injection induced a significant negative ADE. On the other hand, the effect of the saline injection on alcohol preference of the DBA/2J mice was not significant, F < 1. One-way repeated-measures ANOVA comparing mean pre-abstinence ratios to mean post-abstinence ratios for the C57BL/6NCRL mice revealed that naltrexone injection did not significantly reduce alcohol preference, F < 1, indicating that naltrexone injection did not induce a significant negative ADE. The effect of the saline injection on alcohol preference of the C57BL/6NCRL mice was also not significant, F < 1.
One-way repeated measures ANOVA (Fig. 8) for the DBA/2J mice revealed that the post-abstinence ratio for the alcohol preference measure was significantly lower following naltrexone injection relative to following saline injection (F (1,22) = 4.22, p < 0.03). On the other hand, one-way repeated measures ANOVA (Fig. 9) for the C57BL/6NCRL mice revealed that the post-abstinence ratio for the alcohol preference measure was not significantly lower following the naltrexone injection relative to following the saline injection, F < 1. The post-abstinence ratios for the naltrexone injection groups were 0.33 ± 0.04 and 0.45 ± 0.06, for the DBA/2J and C57BL/6NCRL mice, respectively (see Figs. 8 and 9). One-way ANOVA revealed that this difference was significant (F (1, 22) = 3.66, p < 0.05). On the other hand, for the saline injection groups, the abstinence ratios for the alcohol preference measures were 0.44 ± 0.04 and 0.52 ± 0.05, for the DBA/2J and C57BL/6NCRL mice, respectively (see Figs. 8 and 9). One-way ANOVA revealed that this difference was not significant (F (1, 22) = 1.18, p > 0.20).
4. Discussion
The results indicate that the DBA/2J mice provided low pre-abstinence levels of alcohol intake and alcohol preference which were each further reduced by the 4-day abstinence period, providing evidence of negative ADE (negative alcohol deprivation effect). The C57BL/6NCRL mice provided higher pre-abstinence levels of alcohol intake and alcohol preference and provided no evidence of negative ADE. These effects of the abstinence period on alcohol drinking and preference are consistent with the literature, which indicates that negative ADE may be observed in post-abstinence alcohol drinking when low levels of alcohol drinking are observed during the pre-abstinence period (DiBattista, 1991; Melendez et al., 2006; Salimov et al., 1995a; Sinclair and Bender, 1978; Sinclair and Sheaff, 1973; Sinclair and Tiihonen, 1988).
Naltrexone injection was more effective in reducing post-abstinence alcohol intake and post-abstinence alcohol preference in DBA/2J mice than was saline injection, and neither of these injection effects were observed in C57BL/6NCRL mice. The finding that naltrexone’s effect may depend on baseline alcohol intake is consistent with the finding that naltrexone reduced alcohol drinking in rats with low alcohol preference but increased alcohol drinking in rats with high alcohol preference (Iso and Brush, 1991). Moreover, in alcohol-preferring C57BL/6J mice, naltrexone has been reported to increase or to have no effect on alcohol drinking (Phillips et al., 1997). Thus, the failure to observe an effect of naltrexone on alcohol drinking in the high-alcohol drinking C57BL/6NCRL mice is not unprecedented.
In the present study, in saline injected DBA/2J mice, post-abstinence alcohol intake and post-abstinence alcohol preference were both significantly reduced relative to pre-abstinence levels, providing evidence that negative ADE was induced by the second four-day abstinence period. The finding that the naltrexone injected DBA/2J mice provided even more dramatic reductions in these post-abstinence alcohol drinking measures indicates that naltrexone’s effect was to further reduce alcohol intake and alcohol preference beyond the levels of suppression attributable to negative ADE. These results provide the first report demonstrating that naltrexone significantly reduced low levels of alcohol drinking and serve to extend the range of the documented effectiveness of naltrexone in suppressing alcohol drinking.
The C57BL/6NCRL mice provided higher pre-abstinence levels of alcohol intake and alcohol preference than did the DBA/2J mice, and did so with either the 3% or the 10% alcohol concentrations. These between-strain differences are consistent with those reported in previous studies that compared mean daily alcohol intakes of 3% and 10% alcohol solutions (Belknap et al., 1978; Belknap et al., 1993; Fuller, 1964; Rodgers, 1966; Yoneyama et al., 2008). For example, Yoneyama et al. (2008) compared voluntary consumption of 3% alcohol in 22 inbred mouse strains and reported that C57BL/6J mice consumed approximately 4.2 g/kg per day of 3% alcohol, while DBA/2J mice consumed approximately 1.0 g/kg per day. Khisti et al. (2006) reported that C57BL/6J mice drank approximately 4.8 g/kg alcohol per day when provided with access to 10% alcohol for 18 hrs/day, while Yoneyama et al. (2008) reported that DBA/2J mice consumed approximately 2.0 g/kg alcohol per day when provided with 10% alcohol for 24 hrs/day. It should be noted that training with the 3% and 10% concentrations of alcohol yielded mean daily percent alcohol preference scores of less than 12%, and this is consistent with reports of previous investigators who have also reported low levels of alcohol preference in DBA/2J mice trained with alcohol concentrations of 10% or lower (Fuller, 1964; Yoneyama et al., 2008).
Alcohol drinking by the C57BL/6NCRL mice was not significantly altered by the first four-day abstinence period (i.e., no evidence of positive ADE or negative ADE). In high alcohol drinking strains of mice, including C57BL/6NCRL mice, the abstinence period has typically been reported to induce positive ADE or relapse-like elevations in alcohol intake (Khisti et al., 2006; Salimov et al., 1993; Salimov et al., 1995b; Salimov and Salimova, 1993a, 1993b); however, in the present study, the C57BL/6NCRL mice showed no evidence of positive ADE. While studies reporting positive ADE in mice typically employed more extended training with higher concentrations of alcohol, Khisti et al. (2006) had noted that 14 days of training with 10% alcohol was sufficient to obtain stable alcohol intake and, following a 4-day abstinence period, they found consistent increases of 50% to 100% in ethanol intake and ethanol preference, providing evidence of positive ADE in C57BL/6NCRL mice. While we also found that 12 daily sessions of training with 10% alcohol was sufficient to induce stable alcohol intake, we found no evidence of positive ADE following a 4-day abstinence period. It should be noted that the mice in the Khisti et al. (2006) study were isolation-housed, while the mice in the present study were housed in proximal pairs, on separate sides of a barrier strip. There is evidence that isolation housing triggers stress-induced alcohol drinking (Araujo et al., 2005; Jones et al., 1990; Lopez et al., 2011; McCool and Chappell, 2009; Päivärinta, 1990; Pohorecky, 1991) which may contribute to the development of positive ADE reported by other investigators. These data, therefore, suggest that the effects of isolation housing are not necessary to observe the suppressing effects of naltrexone on alcohol drinking in studies of ADE. With respect to the effects of stressors on alcohol drinking, it should be noted that to reduce novelty-induced stress, the mice in the present study were not assessed for locomotor activity or open-field behavior, nor were they subjected to blood draws to obtain samples for the purpose of assaying blood alcohol concentrations.
In the C57BL/6NCRL mice, the administration of naltrexone following the second four-day abstinence period did not significantly alter either alcohol intake or alcohol preference relative to the saline injected controls or relative to post-abstinence alcohol drinking following the first four-day abstinence period. Thus, naltrexone was less effective in reducing alcohol drinking in the C57BL/6NCRL mice relative to the DBA/2J mice, even though the pre-abstinence and post-abstinence levels of alcohol drinking and alcohol preference were much higher in the C57BL/6NCRL mice as compared to the DBA/2J mice.
For the C57BL/6NCRL mice, the group trained with the 3% alcohol concentration yielded significantly higher mean daily percent alcohol preference than did the group trained with the 10% alcohol concentration. This finding is discrepant with reports that the 10% alcohol concentration induced elevated alcohol preference relative to the 3% alcohol concentration in C57BL/6 mice (Belknap et al., 1993; Fuller, 1964; McClearn and Rodgers, 1959; Rodgers, 1966; Yoneyama et al., 2008). For example, Yoneyama et al. (2008) found that C57BL/6J mice showed greater percent alcohol preference (79%) of the 10% alcohol concentration, as compared to the percent alcohol preference (55%) of the 3% alcohol concentration. This discrepancy in results may be due to differences between studies in the procedures employed. For example, the present study employed a between-groups design, where each subject was tested with only one alcohol concentration, whereas, previous studies have employed repeated-measures assessments, where each subject was tested with an ascending sequence of several different alcohol concentrations.
These results may be relevant for the clinical use of naltrexone for the treatment of alcoholism because it extends the range of alcohol drinking levels upon which naltrexone therapy may be effectively employed. While it is well established that naltrexone is effective in reducing high levels of alcohol drinking (Davidson and Amit, 1997; Froehlich et al., 1990; Grahame et al., 2000; Heyser et al., 1997; Holter and Spanagel, 1999; Hubbell and Reid, 1988; Volpicelli et al., 1986), our results show for the first time that naltrexone has a significant effect on low levels of alcohol drinking, reducing further the suppressed levels of alcohol drinking following a period of abstinence (i.e., negative ADE). This suggests that an abstinent recovering alcoholic experiencing a lapse, who consumes a small amount of alcohol, may be effectively treated with naltrexone. Our data reveal that the administration of naltrexone will reduce even low levels of post-abstinence alcohol drinking, thereby reducing the chances of regressing into higher levels of alcohol drinking.
The evidence in the literature that naltrexone reduces post-abstinence relapse-like elevation of alcohol drinking may be taken to indicate that naltrexone’s effect is to induce alcohol drinking levels that more closely approximate pre-abstinence baseline levels. This interpretation, that naltrexone facilitates the recovery of pre-abstinence baseline levels of alcohol drinking, is not supported by the present data. Our results show that following administration of naltrexone alcohol drinking further deviated from pre-abstinence baseline levels, indicating that the effect of naltrexone is to reduce alcohol drinking, across a broad range of alcohol drinking levels, rather than to mitigate deviations of alcohol drinking from baseline levels.
5. Conclusion
In DBA/2J mice, the initial four-day abstinence period reduced alcohol intake and alcohol preference, relative to pre-abstinence baseline levels, providing evidence of a negative alcohol deprivation effect (i.e., negative ADE); however, in contrast, the initial four-day abstinence period had no effect on either alcohol drinking measures in C57BL/6NCRL mice. Following a second four-day abstinence period, an acute injection of naltrexone reduced post-abstinence drinking of 3% alcohol in DBA/2J mice more than in C57BL/6NCRL mice. In addition, naltrexone was effective in further reducing the low levels of post-abstinence alcohol drinking in DBA/2J mice. These data also provide the first evaluation of naltrexone’s effects on the negative ADE and indicate that naltrexone’s effect on post-abstinence alcohol drinking in DBA/2J mice was to further suppress alcohol intake and alcohol preference.
Highlights.
We evaluated the effects of acute naltrexone on post-abstinence alcohol intake.
In DBA/2J mice, abstinence reduced alcohol intake below pre-abstinence levels.
This effect (negative ADE) was not observed in C57BL/6NCRL mice.
Naltrexone further amplified post-abstinence negative ADE in DBA/2J mice.
Naltrexone was less effective in reducing alcohol intake in C57BL/6NCRL mice.
Acknowledgments
We thank Kris Szalc and Nikyta Sharma for their technical assistance. This research was supported by NIH/NIDA grants DA 013471 and DA 020555, and funds from the Center of Alcohol Studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araujo NP, Camarini R, Souza-Formigoni MLO, Carvalho RC, Abílio VC, Silva RH, et al. The importance of housing conditions on behavioral sensitization and tolerance to ethanol. Pharmacol Biochem Behav. 2005;82:40–5. doi: 10.1016/j.pbb.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Coleman RR, Foster K. Alcohol consumption and sensory threshold differences between C57BL/6J and DBA/2J mice. Physiol Psychol. 1978;6:71–4. [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–10. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Coonfield DL, Hill KG, Kaczmarek HJ, Ferraro FM, Kiefer SW. Low doses of naltrexone reduce palatability and consumption of ethanol in outbred rats. Alcohol. 2002;26:43–7. doi: 10.1016/s0741-8329(01)00180-x. [DOI] [PubMed] [Google Scholar]
- Critcher E, Lin C, Patel J, Myers R. Attenuation of alcohol drinking in tetrahydroisoquinoline-treated rats by morphine and naltrexone. Pharmacol Biochem Behav. 1983;18:225–9. doi: 10.1016/0091-3057(83)90367-2. [DOI] [PubMed] [Google Scholar]
- Davidson D, Amit Z. Naltrexone blocks acquisition of voluntary ethanol intake in rats. Alcohol Clin Exp Res. 1997;21:677–83. [PubMed] [Google Scholar]
- Dhaher R, Toalston JE, Hauser SR, Bell RL, McKinzie DL, McBride WJ, et al. Effects of naltrexone and LY255582 on ethanol maintenance, seeking, and relapse responding by alcohol-preferring (P) rats. Alcohol. 2012;46:17–27. doi: 10.1016/j.alcohol.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBattista D. Examination of the negative alcohol-deprivation effect in the golden hamster (Mesocricetus auratus) Alcohol. 1991;8:337–43. doi: 10.1016/0741-8329(91)90528-5. [DOI] [PubMed] [Google Scholar]
- Egli M. Can experimental paradigms and animal models be used to discover clinically effective medications for alcoholism? Addict Biol. 2005;10:309–19. doi: 10.1080/13556210500314550. [DOI] [PubMed] [Google Scholar]
- Escher T, Mittleman G. Schedule-induced alcohol drinking: non-selective effects of acamprosate and naltrexone. Addict Biol. 2006;11:55–63. doi: 10.1111/j.1369-1600.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- Froehlich J, Harts J, Lumeng L, Li TK. Naloxone attenuates voluntary ethanol intake in rats selectively bred for high ethanol preference. Pharmacol Biochem Behav. 1990;35:385–90. doi: 10.1016/0091-3057(90)90174-g. [DOI] [PubMed] [Google Scholar]
- Fuller JL. Measurement of alcohol preference in genetic experiments. J Comp Physiol Psychol. 1964;57:85. doi: 10.1037/h0043100. [DOI] [PubMed] [Google Scholar]
- Gardell LR, Hubbell CL, Reid LD. Naltrexone persistently reduces rats’ intake of a palatable alcoholic beverage. Alcohol Clin Exp Res. 1996;20:584–8. doi: 10.1111/j.1530-0277.1996.tb01097.x. [DOI] [PubMed] [Google Scholar]
- Goodwin FL, Campisi M, Babinska I, Amit Z. Effects of naltrexone on the intake of ethanol and flavored solutions in rats. Alcohol. 2001;25:9–19. doi: 10.1016/s0741-8329(01)00163-x. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Mosemiller AK, Low MJ, Froehlich JC. Naltrexone and alcohol drinking in mice lacking β-endorphin by site-directed mutagenesis. Pharmacol Biochem Behav. 2000;67:759–66. doi: 10.1016/s0091-3057(00)00411-1. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Moc K, Koob GF. Effects of naltrexone alone and in combination with acamprosate on the alcohol deprivation effect in rats. Neuropsychopharmacology. 2003;28:1463–71. doi: 10.1038/sj.npp.1300175. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Koob GF. Increased Ethanol Self-Administration after a Period of Imposed Ethanol Deprivation in Rats Trained in a Limited Access Paradigm. Alcohol Clin Exp Res. 1997;21:784–91. [PubMed] [Google Scholar]
- Hill KG, Kiefer SW. Naltrexone treatment increases the aversiveness of alcohol for outbred rats. Alcohol Clin Exp Res. 1997;21:637–41. [PubMed] [Google Scholar]
- Holstein SE, Spanos M, Hodge CW. Adolescent C57BL/6J Mice Show Elevated Alcohol Intake, but Reduced Taste Aversion, as Compared to Adult Mice: A Potential Behavioral Mechanism for Binge Drinking. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holter SM, Spanagel R. Effects of opiate antagonist treatment on the alcohol deprivation effect in long-term ethanol-experienced rats. Psychopharmacology (Berl) 1999;145:360–9. doi: 10.1007/s002130051069. [DOI] [PubMed] [Google Scholar]
- Hubbell CL, Reid LD. Opioids modulate rats’ intakes of alcoholic beverages, Opioids, Bulimia, and Alcohol Abuse and Alcoholism. Springer-Verlag; New York: 1988. [Google Scholar]
- Institute of Laboratory Animal Resources Commission on Life Sciences. Guide for the Care and Use of Laboratory Animals. National Research Council; 1996. [Google Scholar]
- Iso H, Brush FR. Opposite effects of naltrexone on ETOH intake by Syracuse high and low avoidance rats. Alcohol. 1991;8:443–8. doi: 10.1016/s0741-8329(91)90085-b. [DOI] [PubMed] [Google Scholar]
- Jones G, Marsden C, Robbins TW. Increased sensitivity to amphetamine and reward-related stimuli following social isolation in rats: possible disruption of dopamine-dependent mechanisms of the nucleus accumbens. Psychopharmacology (Berl) 1990;102:364–72. doi: 10.1007/BF02244105. [DOI] [PubMed] [Google Scholar]
- Juarez J, Eliana BDT. Alcohol consumption is enhanced after naltrexone treatment. Alcohol Clin Exp Res. 2007;31:260–4. doi: 10.1111/j.1530-0277.2006.00313.x. [DOI] [PubMed] [Google Scholar]
- Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS. Acute effects of naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology (Berl) 2007;192:207–17. doi: 10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Wolstenholme J, Shelton KL, Miles MF. Characterization of the ethanol-deprivation effect in substrains of C57BL/6 mice. Alcohol. 2006;40:119–26. doi: 10.1016/j.alcohol.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG, Han BD, Park JM, Kim MJ, Stromberg MF. Effect of the combination of naltrexone and acamprosate on alcohol intake in mice. Psychiatry Clin Neurosci. 2004;58:30–6. doi: 10.1111/j.1440-1819.2004.01189.x. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Kreek MJ, Bakalkin G, Liljequist S. The nociceptin/orphanin FQ receptor agonist Ro 64-6198 reduces alcohol self-administration and prevents relapse-like alcohol drinking. Neuropsychopharmacology. 2006;32:902–10. doi: 10.1038/sj.npp.1301169. [DOI] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Quan B, Chow S. The effects of selective blockade of delta and mu opiate receptors on ethanol consumption by C57BL/6 mice in a restricted access paradigm. Brain Res. 1993;630:330–2. doi: 10.1016/0006-8993(93)90672-a. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Doremus-Fitzwater TL, Becker HC. Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol. 2011;45:355–64. doi: 10.1016/j.alcohol.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClearn G, Rodgers D. Differences in alcohol preference among inbred strains of mice. Q J Stud Alcohol. 1959;20:691–5. [Google Scholar]
- McCool BA, Chappell AM. Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcohol Clin Exp Res. 2009;33:273–82. doi: 10.1111/j.1530-0277.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Murphy JM, Li TK, Lumeng L, McBride WJ. Development of alcohol drinking behavior in rat lines selectively bred for divergent alcohol preference. Alcohol Clin Exp Res. 1998;22:1584–90. [PubMed] [Google Scholar]
- Melendez RI, Middaugh LD, Kalivas PW. Development of an Alcohol Deprivation and Escalation Effect in C57BL/6J Mice. Alcohol Clin Exp Res. 2006;30:2017–25. doi: 10.1111/j.1530-0277.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Bandy ALE, McGroarty KK. Ethanol Consumption by C57BL/6 Mice: Influence of Gender and Procedural Variables. Alcohol. 1999a;17:175–83. doi: 10.1016/s0741-8329(98)00055-x. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Cuison ER, Jr, Groseclose CH. Naltrexone effects on ethanol reward and discrimination in C57BL/6 mice. Alcohol Clin Exp Res. 1999b;23:456–64. [PubMed] [Google Scholar]
- Myers R, Lankford MF. Suppression of alcohol preference in high alcohol drinking rats: Efficacy of amperozide versus naltrexone. Neuropsychopharmacology. 1996;14:139–49. doi: 10.1016/0893-133X(95)00081-N. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, et al. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–52. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Päivärinta P. Social isolation increases the stimulatory effect of ethanol on locomotor activity. Pharmacol Biochem Behav. 1990;36:401–3. doi: 10.1016/0091-3057(90)90422-e. [DOI] [PubMed] [Google Scholar]
- Pellicano MP, Sadile AG. Differential alcohol drinking behaviour and dependence in the Naples low-and high-excitability rat lines. Behav Brain Res. 2006;171:199–206. doi: 10.1016/j.bbr.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Wenger CD, Dorow JD. Naltrexone effects on ethanol drinking acquisition and on established ethanol consumption in C57BL/6J mice. Alcohol Clin Exp Res. 1997;21:691–702. [PubMed] [Google Scholar]
- Pohorecky LA. Stress and alcohol interaction: an update of human research. Alcohol Clin Exp Res. 1991;15:438–59. doi: 10.1111/j.1530-0277.1991.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Reid LD, Gardell LR, Chattopadhyay S, Hubbell CL. Periodic naltrexone and propensity to take alcoholic beverage. Alcohol Clin Exp Res. 1996;20:1329–34. doi: 10.1111/j.1530-0277.1996.tb01130.x. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Murphy JM, McBride WJ, Lumeng L, Li TK. The Expression of an Alcohol Deprivation Effect in the High–Alcohol-Drinking Replicate Rat Lines Is Dependent On Repeated Deprivations. Alcohol Clin Exp Res. 2000a;24:747–53. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Shaikh SR, Murphy JM, McBride WJ, Lumeng L, et al. Alcohol deprivation effect is prolonged in the alcohol preferring (P) rat after repeated deprivations. Alcohol Clin Exp Res. 2000b;24:8–16. [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJK, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav. 2004;79:439–50. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Rodgers DA. Factors underlying differences in alcohol preference among inbred strains of mice. Psychosom Med. 1966;28:498–513. [Google Scholar]
- Salimov R, Salimova N, Klodt P, Maisky A. Interaction between alcohol deprivation and morphine withdrawal in mice. Drug Alcohol Depend. 1993;34:59–66. doi: 10.1016/0376-8716(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Salimov R, Salimova N, Ratkin A, Shvets L, Maisky A. Genetic control of alcohol deprivation effect in congenic mice. Alcohol. 1995a;12:469–74. doi: 10.1016/0741-8329(95)00033-n. [DOI] [PubMed] [Google Scholar]
- Salimov R, Salimova N, Shvets L, Shvets N. Explorative and drinking behavior after prolonged access to alcohol and following chronic piracetam administration in mice. Alcohol. 1995b;12:485–9. doi: 10.1016/0741-8329(95)00003-8. [DOI] [PubMed] [Google Scholar]
- Salimov RM, Salimova NB. The alcohol-deprivation effect in hybrid mice. Drug Alcohol Depend. 1993a;32:187–91. doi: 10.1016/0376-8716(93)80012-4. [DOI] [PubMed] [Google Scholar]
- Salimov RM, Salimova NB. L-glutamate abolishes differential responses to alcohol deprivation in mice. Alcohol. 1993b;10:251–7. doi: 10.1016/0741-8329(93)90001-5. [DOI] [PubMed] [Google Scholar]
- Sinclair J. The alcohol-deprivation effect: Influence of various factors. Q J Stud Alcohol. 1972;33:769–82. [PubMed] [Google Scholar]
- Sinclair J. Alcohol-deprivation effect in rats genetically selected for their ethanol preference. Pharmacol Biochem Behav. 1979;10:597–602. doi: 10.1016/0091-3057(79)90239-9. [DOI] [PubMed] [Google Scholar]
- Sinclair J, Bender DO. Compensatory behaviors: Suggestion for a common basis from deficits in hamsters. Life Sci. 1978;22:1407–12. doi: 10.1016/0024-3205(78)90634-3. [DOI] [PubMed] [Google Scholar]
- Sinclair J, Li TK. Long and short alcohol deprivation: effects on AA and P alcohol-preferring rats. Alcohol. 1989;6:505–9. doi: 10.1016/0741-8329(89)90059-1. [DOI] [PubMed] [Google Scholar]
- Sinclair J, Senter R. Increased Preference for Ethanol in Rats Following Alcohol Deprivation. Psychon Sci. 1967;8:11–2. [Google Scholar]
- Sinclair J, Sheaff B. A negative alcohol-deprivation effect in hamsters. Q J Stud Alcohol. 1973;34:71–7. [PubMed] [Google Scholar]
- Sinclair J, Tiihonen K. Lack of alcohol-deprivation effect in AA rats. Alcohol. 1988;5:85–7. doi: 10.1016/0741-8329(88)90048-1. [DOI] [PubMed] [Google Scholar]
- Sinclair JD. From optimal complexity to naltrexone extinction of alcoholism. In: Hoffman RR, Sherrick MF, Warm JS, editors. Viewing psychology as a whole: The integrative science of William N Dember. American Psychological Association; Washington, DC: 1998. pp. 491–508. [Google Scholar]
- Sparta DR, Ferraro FM, III, Fee JR, Knapp DJ, Breese GR, Thiele TE. The Alcohol Deprivation Effect in C57BL/6J Mice is Observed Using Operant Self-Administration Procedures and is Modulated by CRF-1 Receptor Signaling. Alcohol Clin Exp Res. 2009;33:31–42. doi: 10.1111/j.1530-0277.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg MF, Mackler SA, Volpicelli JR, O’Brien CP. Effect of acamprosate and naltrexone, alone or in combination, on ethanol consumption. Alcohol. 2001;23:109–16. doi: 10.1016/s0741-8329(00)00137-3. [DOI] [PubMed] [Google Scholar]
- Tambour S, Brown LL, Crabbe JC. Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcohol Clin Exp Res. 2008;32:2100–6. doi: 10.1111/j.1530-0277.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Davis MA, Olgin JE. Naltrexone blocks the post-shock increase of ethanol consumption. Life Sci. 1986;38:841–7. doi: 10.1016/0024-3205(86)90601-6. [DOI] [PubMed] [Google Scholar]
- Wolstenholme JT, Warner JA, Capparuccini MI, Archer KJ, Shelton KL, Miles MF. Genomic analysis of individual differences in ethanol drinking: evidence for non-genetic factors in C57BL/6 mice. PLoS ONE. 2011;6:e21100. doi: 10.1371/journal.pone.0021100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–60. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]