Abstract
A special focus area of planetary protection is the monitoring, control, and reduction of microbial contaminations that are detected on spacecraft components and hardware during and after assembly. In this study, wild-type spores of Bacillus pumilus SAFR-032 (a persistent spacecraft assembly facility isolate) and the laboratory model organism B. subtilis 168 were used to study the effects of low-pressure plasma, with hydrogen alone and in combination with oxygen and evaporated hydrogen peroxide as a process gas, on spore survival, which was determined by a colony formation assay. Spores of B. pumilus SAFR-032 and B. subtilis 168 were deposited with an aseptic technique onto the surface of stainless steel screws to simulate a spore-contaminated spacecraft hardware component, and were subsequently exposed to different plasmas and hydrogen peroxide conditions in a very high frequency capacitively coupled plasma reactor (VHF-CCP) to reduce the spore burden. Spores of the spacecraft isolate B. pumilus SAFR-032 were significantly more resistant to plasma treatment than spores of B. subtilis 168. The use of low-pressure plasma with an additional treatment of evaporated hydrogen peroxide also led to an enhanced spore inactivation that surpassed either single treatment when applied alone, which indicates the potential application of this method as a fast and suitable way to reduce spore-contaminated spacecraft hardware components for planetary protection purposes. Key Words: Bacillus spores—Contamination—Spacecraft hardware—Plasma sterilization—Planetary protection. Astrobiology 13, 597–606.
1. Introduction
1.1. Contamination control of spacecraft
A major goal of space exploration is the detection of biosignatures and traces of life, both past and present, in extraterrestrial environments, with the penultimate goal of returning such samples back to Earth (NASA, 2005; Horneck et al., 2010). These missions are currently, and have always been, subjected to the international standards of planetary protection, which were established by the Committee of Space Research (COSPAR) in 1967 (Rummel, 2001; Bruckner et al., 2009; COSPAR, 2011). Depending on the specific mission, planetary protection guidelines are required for the cleaning and sterilization of a spacecraft and its components to avoid contamination from terrestrial organisms (Bruckner et al., 2009; Nicholson et al., 2009). Monitoring of microbial diversity of spacecraft-associated environments over the decades has identified a number of spore-forming species (La Duc et al., 2004b) among cultivable microorganisms, which include, for example, Bacillus safensis (Satomi et al., 2006), B. nealsonii (Venkateswaran et al., 2003), B. horneckiae (Vaishampayan et al., 2010), B. odysseyi (La Duc et al., 2004a), Paenibacillus purispatii (Behrendt et al., 2010), P. pasadenensis (Osman et al., 2006), P. phoenicis (Benardini et al., 2011), and P. barengoltzii (Osman et al., 2006). Several of these spore-forming isolates, such as B. pumilus SAFR-032 (spacecraft assembly facility isolate 032), have exhibited elevated resistance to several stressors, for example, UV radiation and hydrogen peroxide (Link et al., 2004; Venkateswaran et al., 2004; Kempf et al., 2005; Horneck et al., 2012; Vaishampayan et al., 2012). In addition to the persistent contamination of spacecraft assembly facilities and spacecrafts by spore-forming microorganisms, the highly resistant nature of spores could enable these organisms to survive sterilization processes and interplanetary transit aboard spacecrafts, and lead to deposition and subsequent contamination of extraterrestrial environments, such as Mars (Wolfson and Craven, 1971; Schuerger et al., 2005; Committee on Preventing the Forward Contamination of Mars, 2006; Nicholson et al., 2009; Kerney and Schuerger, 2011; Horneck et al., 2012; Vaishampayan et al., 2012). Ergo, the control and reduction of bacterial spores is still one of the most difficult problems in sterilization efforts (Venkateswaran et al., 2004; Setlow, 2006, 2007). Complete reduction of bacterial spores is often impossible without altering or damaging the sensitive materials, which may contain contaminants such as spacecraft electronics (Demidov et al., 1995). Elimination of problematic microbes, such as bacterial spores, will require testing novel cleaning and sterilization technologies (Demidov et al., 1995) that are not only compatible with modern spacecraft and spacecraft hardware but will also successfully remove or inactivate the most problematic microbial species isolated in spacecraft assembly facilities. Common test organisms, such as B. subtilis spores, have been used to study the response of bacterial spores to sporicidal treatments due to their high degree of resistance to various physical and chemical treatments, their reproducible inactivation response, and their genetic tractability and lack of pathogenicity (Nicholson et al., 2000; Nicholson and Galeano, 2003; Setlow, 2006; Humphreys, 2011).
1.2. Plasma sterilization: methods and mechanisms
Methods for limiting the contamination of problematic microorganisms on surfaces are accomplished by chemical, physical, mechanical, and thermal processes (e.g., high pressure, high temperature, UV, and gamma irradiation) (Moisan et al., 2001; Benedikt et al., 2008a; Kong et al., 2009; Heinlin et al., 2011). The majority of such sterilization methods may induce some level of damage to the material, or they are limiting in their ability to completely sterilize (Benedikt et al., 2008b; Rauscher et al., 2009, 2010; Heinlin et al., 2011; De Geyter and Morent, 2012). These processes also have the disadvantages of high cost, difficulty of application, deposition of residues on surfaces, changing properties of the materials, and increasing resistance of microbes to the process (Rutala and Weber, 2001). Plasma sterilization is emerging as an alternative to commonly used sterilization techniques due to many advantages: cost-effective, fast, efficient, safe in terms of thermal, chemical, or irradiation damage to spacecraft materials (Höller et al., 1993; Moisan et al., 2002; Benedikt et al., 2008b; Kong et al., 2009; Ehlbeck et al., 2011). Plasma sterilization methods are distinguished by the use of gas or a gas mixture that is partially excited by passing through an electromagnetic field. Generally, a plasma consists of different biocidal agents: chemically reactive species, ions, and (V)UV photons. Depending on the gas or gas mixture used, the plasma is capable of being tuned to provide more chemically active species or more (V)UV radiation (Moisan et al., 2001, 2002; Benedikt et al., 2008b; Stapelmann et al., 2008; Ehlbeck et al., 2011; Heinlin et al., 2011). Synergistic effects, such as chemical sputtering, take place at low ion energies and in the presence of radical flux densities (Benedikt et al., 2008a; Raballand et al., 2008), or the combination of UV radiation and heat, among others, plays a major role in plasma sterilization efficacy (Moisan et al., 2002; Rauscher et al., 2010; Heinlin et al., 2011). Although the biocidal mechanisms are not yet fully understood, the following cellular inactivation mechanisms may be listed: (i) UV radiation [including radiation from the vacuum UV (VUV) range, below 200 nm] that is capable of damaging nucleic acids (DNA, RNA) and proteins; (ii) the diffusion of highly reactive nitrogen and oxygen species, such as OH radicals, within the microorganisms, which leads to local damage that is most likely an effect of oxidation to cellular components, such as the membrane, proteins, RNA, and DNA; and (iii) lysis of the microbial cells as a result of the rupture of their membranes due to the electrostatic force exerted on them by accumulation of charged particles coming from the plasma (Yasuda et al., 2010; reviewed in Moisan et al., 2002; Moreau et al., 2008 and references therein). A considerable number of studies have been performed, mainly with bacterial spores of the biological model systems of B. subtilis, Escherichia coli, and Geobacillus stearothermophilus, to investigate the effectiveness of different plasma sources (for examples see Hury et al., 1998; Benedikt et al., 2008b; Schuerger et al., 2008; Hong et al., 2009; Morris et al., 2009; Klämpfl et al., 2012).
Because spores are highly resistant to a variety of physical, physicochemical, or thermal treatments (reviewed in Nicholson et al., 2000; Setlow, 2006) that may damage heat-sensitive devices due to extended treatment times to achieve spore reduction and result in an increase in costs, it is desirable to operate at the lowest possible temperature and pressure, and for the shortest period of time. Presented here is a technical demonstration of low-pressure hydrogen plasma, with and without the utilization of hydrogen peroxide as a process gas for the spore inactivation of B. pumilus SAFR-032 and B. subtilis 168 on stainless steel screws.
2. Material and Methods
2.1. Bacillus spp. spores, sporulation and purification
Spores of wild-type B. pumilus strain SAFR-032 (Link et al., 2004) and B. subtilis strain 168 (DSM 402) (Moeller et al., 2006) were obtained by cultivation under vigorous aeration in 2× liquid Schaeffer's sporulation medium under optimal conditions (Schaeffer et al., 1965). Spores were purified and stored as previously described (Nicholson and Setlow, 1990; Link et al., 2004; Moeller et al., 2010, 2012a, 2012b). Spore preparations were free (>99%) of growing cells, germinated spores, and cell debris as determined by phase-contrast microscopy.
2.2. Sample preparation
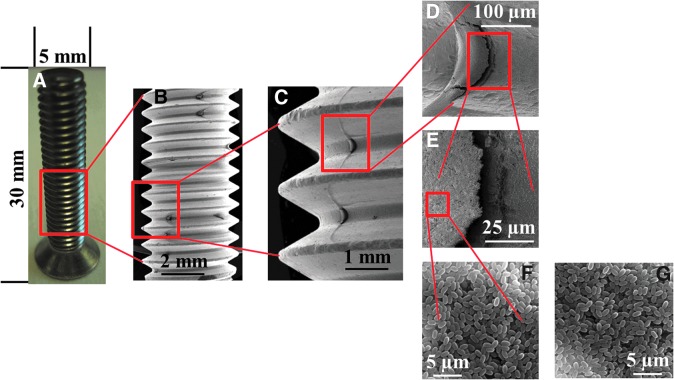
In our study, stainless steel screws were chosen to simulate spore-contaminated spacecraft hardware. Spore suspensions of the two Bacillus spp. strains were prepared in sterile distilled water to a set final concentration, such that a 20 μL aliquot contained 5×108 spores. Stainless steel screws [cross recessed flat head machine screws, steel type H; DIN965-M6x30-A2; 30 mm in length, 3 mm in diameter, screw head with 11 mm in diameter and a thickness of 5 mm; Aug. Hülden GmbH & Co. KG, Cologne (Köln), Germany] were autoclaved (121°C, 30 min) prior to use. Spore-contaminated screw samples, for plasma sterilization, were prepared by applying 20 μL aliquots of spores, dropwise, onto a marked position of the screw (Fig. 1) so that they spread out to a defined area of approximately 5×5 mm, resulting in arrangements of multiple-layer samples each with a thickness of ∼25 spore layers. A single set of spore samples was comprised of three identical repetitions with the same spore concentration. Spore samples were air-dried under ambient laboratory conditions (20±2°C, 40±5% relative humidity).
FIG. 1.
Photograph (A) and scanning electron micrograph images (B–G) of a stainless steel screw contaminated with spores of B. pumilus strain SAFR-032 (B–F) and B. subtilis strain 168 (G). Scale bar: 2 mm (B), 1 mm (C), 100 μm (D), 25 μm (E), and 5 μm (F and G). Color images available online at www.liebertonline.com/ast
2.3. Scanning electron microscopy of spore-contaminated stainless steel screws
For scanning electron microscopy, spore-contaminated stainless steel screws were coated with a thin gold layer (of approximately 10 nm), placed in a scanning electron microscope [Jeol JSM-6510, Eching/Munich (München), Germany], and investigated with an accelerating voltage of 10 kV in high vacuum mode.
2.4. Spore exposure to different plasmas
Spore inactivation via plasma treatments was performed at the Institute for Electrical Engineering and Plasma Technology (AEPT), Ruhr University Bochum, Bochum, Germany (www.aept.ruhr-uni-bochum.de). Triplicate samples of air-dried spore layers (each sample also in triplicate replications with ∼5×108 spores) were exposed simultaneously to different gas mixtures (hydrogen, oxygen; with a total gas flow of 20 sccm), a pressure range of 5–25 Pa, 100–400 W of power, and a hydrogen peroxide concentration of either 30% or 60%, which acted as a process gas. The spore-contaminated screws were treated in a capacitively coupled plasma reactor operated at very high frequency (VHF-CCP). The discharge chamber is composed of the high-performance polymer PEEK (polyether ether ketone) and shaped like a drawer (Fig. 2). The drawer has an inner size of 32×22×6.5 cm, leading to a volume of 4.5 cubic liters. Six flanges with Suprasil2 quartz glass, one at the front, one at the back, and two at each side, allow optical emission spectroscopy of the plasma process for determining the UV dose (data shown in Table 1). A grounded electrode was placed on top of the drawer. A rotary vane pumping system (Trivac D65B with a capacity of 65 m3/h [Oerlikon Leybold Vacuum, Cologne (Köln), Germany]) was used to evacuate the chamber. To control and monitor the pressure, two heated absolute capacitance manometers [(1) Baratron 627B, pressure range of 0.05–100 Pa, (2) 627D, pressure range of 100–1000 Pa, MKS Instruments, Munich (München), Germany] and a butterfly valve [VAT butterfly valve control system Series 612, VAT Germany, Grasbrunn/Munich (München), Germany] were attached to the VHF-CCP. Four mass flow controllers [MKS MFC Type 1179, MKS Instruments, Munich (München), Germany] were used to deliver constant gas flow of hydrogen or oxygen (20 sccm maximum). A fiber optic temperature monitoring system (LUXTRON I652 with a Fluoroptic STF probe, LumaSense Technologies, Frankfurt, Germany) was used to determine gas temperature for different discharge conditions (Table 1).
FIG. 2.
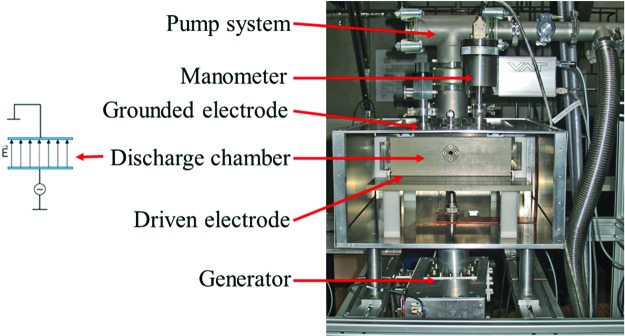
Sketch of the VHF-CCP used for sterilization experiments to measure inactivation kinetics of spores of B. pumilus strain SAFR-032 and B. subtilis strain 168 on stainless steel screws. On the left, a sketch of the grounded and the driven electrode is given, showing the electrical field. Color images available online at www.liebertonline.com/ast
Table 1.
Temperature and UV Dose Rates of the Respective Plasma Treatment
| |
|
|
|
|
UV dose rates [in J/(m2×s)] |
|||
|---|---|---|---|---|---|---|---|---|
| Plasma (flux in sccm) | Power (in W) | Pressure (in Pa) | Hydrogen peroxide concentration (in %)a | Temperature increase (in °C)bafter 60/300 s | UVC (200–280 nm) | UVB (280–320 nm) | UVA (320–400 nm) | Total UV (200–400 nm) |
| H2 (20 sccm) | 100 | 5 | n.a. | 40±3/49±5 | 4.1 | 0.7 | 0.7 | 5.4 |
| H2 (20 sccm) | 400 | 5 | n.a. | 66±5/104±5 | 6.8 | 1.3 | 1.7 | 9.7 |
| H2 (20 sccm) | 400 | 25 | n.a. | 77±5/88±5 | 2.7 | 0.4 | 0.5 | 3.6 |
| H2/O2 (10:10 sccm) | 400 | 10 | n.a. | 77±5/110±5 | 4.2 | 2.0 | 1.8 | 8.0 |
| H2 (20 sccm) | 400 | 5 | 30 | 40±3/49±5 | 6.8 | 1.3 | 1.7 | 9.8 |
Four milliliters of 90°C heated and evaporated hydrogen peroxide in two concentrations (30% and 60%) were used for the spore inactivation. n.a.=not applied.
Starting temperature at time (0 s) was 20±2°C.
2.5. Optical emission spectroscopy for determining UV dose
For determining the UV dose of the applied plasma conditions, an absolutely and relatively calibrated grating spectrometer QE65000 (Ocean Optics, Ostfildern, Germany), attached to the Suprasil2 window at the front of the discharge chamber, was used. The spectrometer measures in the spectral range of λ=200–975 nm, with a spectral resolution of 1.3 nm. The measured intensity is obtained in photons/(cm3×nm×s). To achieve the UV dose, the energy of the photons is calculated by using the following equation: E=h×f, with energy (E), Planck's constant (h), and the frequency of the photons (f), given by f=c/λ, with the speed of light (c), and wavelength (λ). To obtain the surface UV dose in J/(m2×s), the volume of the chamber needs to be subtracted by the surface of the chamber. The results of the calculated UV dose for the applied plasma conditions are given in Table 1.
2.6. Survival assay
With the ignition of plasma, hydrogen peroxide is decomposed into hydrogen, oxygen, −OH, and other reactive oxygen species, so that there is no hydrogen peroxide left on the surface. To avoid hydrogen peroxide residue on the surfaces of samples treated solely with hydrogen peroxide, the chamber was pumped down to at least 5 Pa (or lower if required). To recover the spores from the stainless steel screws after respective plasma/hydrogen peroxide exposure, as well as from the control samples, air-dried spore layers were covered with a 10% aqueous polyvinyl alcohol solution (PVA); and after subsequent air-drying, the spore-PVA layer was stripped off as previously described (Horneck et al., 2001; Moeller et al., 2007, 2009) and resuspended in 1 mL of sterile distilled water, resulting in >95% recovery of the spores. This procedure does not affect spore viability (Horneck et al., 2001). Spore reduction rates were determined by a standard colony-formation assay; spore sample dilutions in distilled water were used to determine colony-forming ability, in terms of colony-forming units (CFU), after incubation overnight at 37°C on nutrient broth agar plates (Difco, Detroit, USA) as described previously (Horneck et al., 2001; Moeller et al., 2007, 2009, 2010, 2012a).
2.7. Numerical and statistical analysis
The spore-surviving fraction was determined from the quotient N/N0, with N=the number of CFU of the plasma/hydrogen peroxide–treated sample and N0 that of the non-exposed controls. Spore inactivation was plotted as a function of time with respect to the plasma and/or hydrogen peroxide treatment. To determine the curve parameters, the following relationship was used: ln N/N0=−SIC×T+n, where SIC is the inactivation constant, T the treatment time, and n the extrapolation number. The SIC was determined from the slope of the dose-effect curves as described by Moeller et al. (2007, 2009, 2010). All data is expressed as averages±standard deviations of at least triplicate experiments. The results were compared statistically by using the Student t test, values were analyzed in multigroup pairwise combinations, and differences with P values of ≤0.05 were considered statistically significant (Moeller et al., 2007, 2009, 2010, 2012b).
3. Results
To determine the kinetics of spore reduction, after single and combined exposure to low-pressure plasma and hydrogen peroxide, stainless steel screws, with air-dried spores of two Bacillus species, were used (B. pumilus SAFR-032 and B. subtilis 168).
3.1. Effects of low-pressure plasma
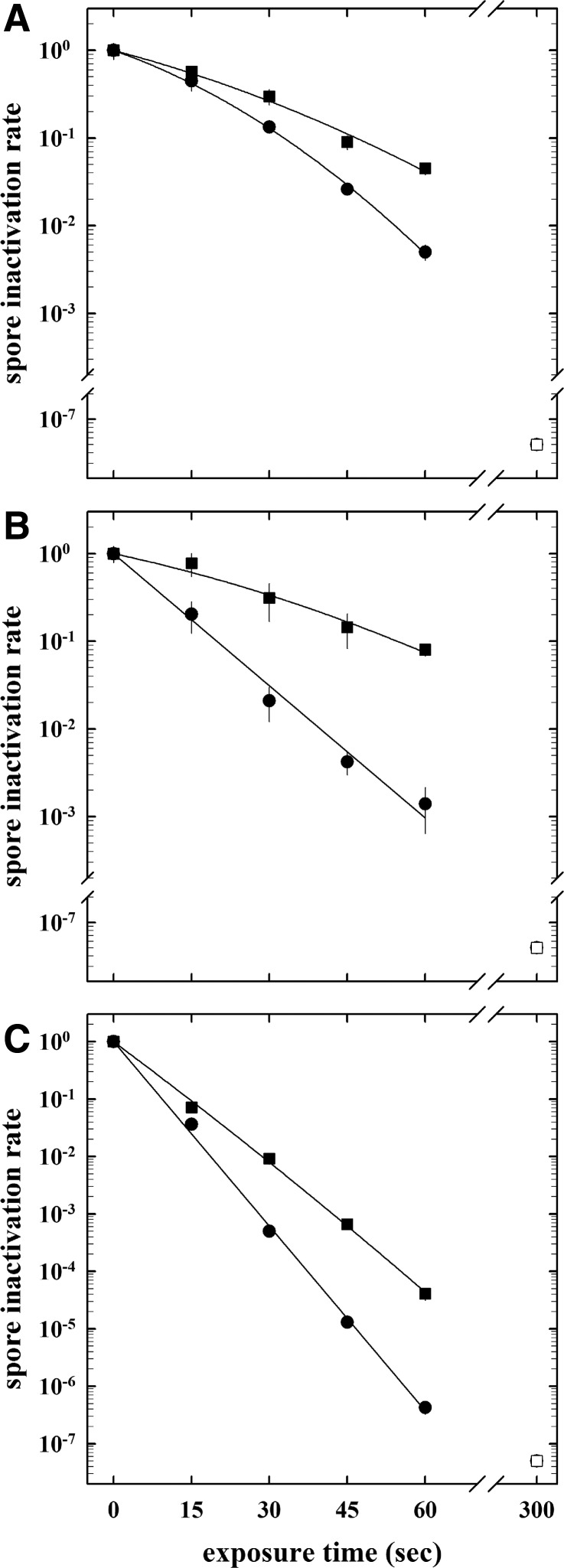
The plasma exposure parameters were as follows: power of 100 and 400 W; pressure of 5, 10, and 25 Pa; gas mixture of hydrogen and/or oxygen (20 sccm in total); exposure times of 15, 30, 45, 60, and 300 s. To determine the effects of the different plasma parameters on spore reduction, spore survivability curves were determined at different time points, inactivation kinetics were plotted, LD90 values and spore inactivation constants (SICs) were calculated and compared by using the Student t test (Table 2). In a direct comparison, spores of spacecraft assembly facility (SAF) isolate B. pumilus strain SAFR-032 were significantly more resistant to all tested plasma treatments than those of the laboratory model system B. subtilis strain 168 (Table 2). Independent of the genotype, plasma treatment, or experimental conditions, no viable (colony-forming) spores were detected after a 5 min plasma treatment, indicating a sufficient treatment time for a complete multilayer spore reduction on a complex surface (Fig. 1). To study the influence of different parameters, various ranges of power, plasma, and pressure compositions have been tested. In Fig. 3A, a representative graph of the spore survival is shown as a function of time of the respective plasma (low-pressure hydrogen plasma, 20 sccm, 400 W, 5 Pa). These survival values were used as the basis for data comparison with data from other plasma exposures; spore reduction was affected by a different experimental parameter, with power (400 W instead of 100 W) as the major contributor, whereas pressure (5 Pa instead of 25 Pa) and plasma composition (H2/O2 plasma compared to H2 plasma) had only minor effects on the spore reduction (Table 2).
Table 2.
Inactivation Characteristics of B. pumilus SAFR-032 and B. subtilis 168 Spores after Plasma Treatment with and without Hydrogen Peroxide as Process Gas
| |
|
|
|
LD90 value (in s)b |
Spore inactivation constant (SIC)c |
||
|---|---|---|---|---|---|---|---|
| Plasma (flux in sccm) | Power (in W) | Pressure (in Pa) | Hydrogen peroxide concentration (in %)a | Bacillus pumilus SAFR-032 | Bacillus subtilis 168 | Bacillus pumilus SAFR-032 | Bacillus subtilis 168 |
| H2 (20 sccm) | 100 | 5 | n.a. | 184.1±12.3* | 73.6±8.5 | (1.2±0.1)×10−2* | (3.1±0.3)×10−2 |
| H2 (20 sccm) | 400 | 5 | n.a. | 40.3±3.0* | 19.7±2.3 | (5.5±0.4)×10−2* | (9.8±1.0)×10−2 |
| H2 (20 sccm) | 400 | 25 | n.a. | 44.5±4.7* | 29.4±3.6 | (5.0±0.4)×10−2* | (7.3±0.6)×10−2 |
| H2/O2 (10:10 sccm) | 400 | 10 | n.a. | 38.7±3.8* | 23.6±2.9 | (6.9±0.8)×10−2* | (1.1±0.2)×10−1 |
| H2 (20 sccm) | 400 | 5 | 30 | 13.6±1.5* | 9.2±0.8 | (1.6±0.2)×10−1* | (2.5±0.3)×10−1 |
| n.a. | n.a. | n.a. | 30 | 55.1±6.7* | 19.5±2.1 | (4.2±0.5)×10−2* | (1.1±0.2)×10−1 |
| n.a. | n.a. | n.a. | 60 | 16.7±1.8* | 11.3±1.2 | (1.2±0.2)×10−1* | (1.7±0.2)×10−1 |
Four milliliters of 90°C heated and evaporated hydrogen peroxide in two concentrations (30% and 60%) were used for the spore inactivation. n.a.=not applied.
Data are expressed as averages and standard deviations (n=3). Asterisks indicate LD90 and SIC values that were significantly different (P values of ≤0.05) than the respective values obtained from spores of B. subtilis 168.
LD90 value, i.e., time (in seconds) needed to reduce one order of magnitude of the initial spore population.
FIG. 3.
Inactivation kinetics of spores of B. subtilis strain 168 (circles) and B. pumilus strain SAFR-032 (squares) on stainless steel screws obtained by direct plasma exposure [20 sccm hydrogen plasma, 400 W, 5 Pa (A)], vaporized 30% hydrogen peroxide treatment (B), and a two-step decontamination process of direct plasma exposure [20 sccm hydrogen plasma, 400 W, 5 Pa, and vaporized 30% hydrogen peroxide treatment (C)]. Data are expressed as averages and standard deviations (n=3). The open symbols indicate survival below the threshold of detection (i.e., complete spore reduction).
3.2. Effects of hydrogen peroxide as a process gas as single or combined low-pressure treatment
To determine the influence of hydrogen peroxide as an additional effecter for spore inactivation, two different concentrations (30%, 300 g/L and 60%, 600 g/L) of hydrogen peroxide were tested in a single treatment, and 30% H2O2 was used as a process gas in a combined low-pressure hydrogen plasma treatment. Again, as seen before in the plasma treatment without H2O2, B. pumilus strain SAFR-032 spores were up to 2.5-fold more resistant to hydrogen peroxide treatments than spores of B. subtilis strain 168 (Fig. 3B, Table 2), whereas a 60% H2O2 exposure yielded a more rapid spore reduction. When spore-contaminated screws were treated in a two-step decontamination process of direct plasma exposure (20 sccm of hydrogen plasma, 400 W, 5 Pa) and vaporized 30% hydrogen peroxide, there was a rapid spore reduction obtained with the only differences due to genotype (Fig. 3C).
4. Discussion
Since the beginning of the development and utilization of plasma application for sterilization purposes, a number of microbiological tests with selected indicator microorganisms have been carried out (Moisan et al., 2001, 2002; Rossi et al., 2006; Benedikt et al., 2008b; Kong et al., 2009; von Keudell et al., 2010; Ehlbeck et al., 2011). Low-pressure plasma sterilization processes have been under intensive study for a number of chemical and biological applications for many years due to the effective and efficient generation of cellular-damaging radicals and reactive species (Moisan et al., 2001; Rossi et al., 2006, 2009; Ehlbeck et al., 2011). Although a great deal of effort has been taken to investigate plasma sterilization, there is only one low-pressure plasma sterilization system commercially available. It is integrated in a pharmaceutical filling line as described by Denis et al. (2012) and recently achieved approval by the European Medicines Agency. Other so-called plasma sterilization systems, that is, Sterrad, Johnson & Johnson, use vaporized hydrogen peroxide as the sterilizing agent (Jacobs and Kowatsch, 1993; Rutala et al., 1999; Okpara-Hofmann et al., 2005). The plasma is ignited between a perforated, powered electrode and the grounded chamber wall; active plasma species can diffuse through the perforated electrode (Jacobs and Kowatsch, 1993), but the mean free path of all radicals at the pressure applied is much too short to reach the samples. According to Lerouge et al. (2000), Sterrad and comparable systems are not plasma sterilizers but instead involve purely chemical cycles that sterilize. However, in the case of this study, we used hydrogen peroxide as a process gas, delivering hydrogen and oxygen species as useful sterilizing agents (e.g., OH and O radicals). Moreover, the plasma described within this contribution is in direct contact with the surface. Additionally, evaporated hydrogen peroxide was combined with hydrogen or oxygen as a process gas, to enhance either UV radiation or oxidation. In our study, hydrogen and oxygen gases were used for plasma production. Several different components were produced by the plasma discharge and delivered to the treated sample, that is, charged particles (electrons and ions), reactive species (e.g., hydroxyl radicals), photons (UV and visible), and heat. A pure hydrogen discharge offers a high amount of UV radiation, whereas the combination of hydrogen and oxygen leads to more chemically active species. In our investigations, an exclusive effect of UV radiation on the spore inactivation can be ruled out, as the UV dose rates were on average approximately 6 J/(m2×s), reaching maximal values of 2000 J/m2 (total UV dose for 200–400 nm) after 5 min exposure time (Table 1). Thermal effects on the spore inactivation should be considered as a potential synergistic effect, as a temperature increase of 20–60°C, depending on the plasma treatment, was determined after 60 s, and high temperatures above 100°C were obtained after 5 min exposure (Table 1). It should be kept in mind that spores have increased heat resistance, compared to their vegetative counterparts; though the decimal reduction values may vary due to spore treatment with wet or dry heat, they have approximately a 1000-fold higher dry heat resistance (reviewed in Nicholson et al., 2000; Setlow, 2006). In our studies, we cannot exclude the potential damaging effects of the increased temperature alone or in combination with UV radiation, especially if the generated plasma components induce synergistic effects when reducing the spore bioburden.
Due to the high resistance of bacterial spores to sterilization processes and concerns in the biomedical and food-processing industries over their persistent contamination, there is an ongoing interest in studying methods of bacterial spore inactivation and the mechanisms by which spores resist the lethal effects of various disinfection treatments. Spores are generally significantly more resistant than growing cells to a wide variety of toxic chemicals, such as hydrogen peroxide (Bagyan et al., 1998; Setlow, 2006, 2007). In growing cells, specific enzymes (e.g., catalases, superoxide dismutase, or hydroperoxides) are sometimes capable of detoxifying chemical agents (Farr and Kogoma, 1991; Reder et al., 2012); however, this appears not to be a factor in dormant spore resistance, presumably because of the inactivity of enzymes in the spore core (Casillas-Martinez and Setlow, 1997; Bagyan et al., 1998; Setlow, 2006). Bioinformatic comparative analysis of the B. pumilus strain SAFR-032 genome (Gioia et al., 2007) with that of B. subtilis strain 168 shows both high similarities [e.g., genes involved in sporulation, especially in spore coat assembly and formation of small, acid-soluble spore proteins (SASP)] as well as significant differences, for example, in catalase formation. Bacillus subtilis produces two vegetative catalases, KatA and KatE, and one germination catalase, KatX, which is present in spores and protects germinating cells from H2O2 (Bagyan et al., 1998). Gioia et al. (2007) showed that B. pumilus has no homologue to either vegetative catalase of B. subtilis; however, it has two KatX homologues (each with a manganese catalase domain). Checinska et al. (2012) identified two manganese catalases of B. pumilus strain SAFR-032, both of which are localized in the spore coat layer along with a laccase (a copper-containing oxidase enzyme) and a superoxide dismutase. They proposed that the increased resistance of B. pumilus spores to hydrogen peroxide is due to the synergistic activity of both manganese catalases with other coat oxidoreductases; however, further work to prove this synergism is needed. Other (morphological) factors important in spore resistance to hydrogen peroxide have been identified, while others have been proposed, including the presence of spore coats, the impermeability of the spore core to hydrophilic chemicals, low spore core water content, and protection of spore DNA by α/β-type SASP (Setlow and Setlow, 1993; Popham et al., 1995; Paidhungat et al., 2000; Granger et al., 2011). The various layers of proteinaceous spore coats (and possibly the outer spore membrane), which surround the spore cortex, certainly protect the spore from attack by very large molecules, such as lytic enzymes that can hydrolyze the spore cortex (Riesenman and Nicholson, 2000; Driks, 2002). Both the spore coats and the membrane act as permeability barriers to chemicals by direct interaction and thus reduce the amount of toxic agents that are able to attack more-central spore molecules, such as enzymes or DNA, in the spore core (Nicholson et al., 2000; Setlow, 2006, 2007; Griffiths and Setlow, 2009). Popham et al. (1995) found a decrease in spore resistance to H2O2 with increasing core water content, whereas the details of the interaction of the core water content with hydrogen peroxide are still unknown. For several types of toxic chemicals, such as H2O2, there is strong evidence that one factor in spore resistance is the protection of spore DNA from attack by the binding of the major SASP (reviewed in Setlow, 2006, 2007). However, several of these features are poorly characterized, in particular their involvement in spore resistance to single and combined parameters of plasma treatments.
Relatively little work has been done on the role of specific repair systems or on the nature of the damage caused by an exposure to different sporicidal plasma treatments. In 2009, Roth et al. (2010) showed in their experiments with microwave-induced low-pressure, low-temperature nitrogen-oxygen plasma that B. subtilis spore reduction is mainly due to DNA and protein damage, as well as deficiencies in the major SASP formation and spore coat assembly that lead to high sensitivity. To systematically analyze the roles of all known features of the spore morphology, protective mechanisms, and DNA repair pathways and gain a better understanding of the processes that lead to spore inactivation or destruction by low pressure with regard to atmospheric plasma treatments, there is a need to conduct further experiments with the astrobiological model system B. subtilis strain 168, due to the availability of different mutant strains (Moeller et al., 2012b, 2012c). Also, further efforts need to be taken to investigate plasma sterilization on thermolabile materials (e.g., sensitive spacecraft hardware such as electronic component) with a more complex surface geometry (e.g., control circuit boards) in order to improve the utilization of plasma sterilization for planetary protection purposes (as indicated by Schuerger et al., 2008; Pottage et al., 2012).
Acknowledgments
The authors are very grateful to Andrea Schröder for her skillful technical assistance during parts of this work and Samantha M. Waters for her critical proofreading of the manuscript. We express thanks to the two anonymous reviewers for insightful comments. This study was supported in part by grants from the German Research Foundation (DFG) Paketantrag (PlasmaDecon PAK 728) to P.A. (AW 7/3-1), R.M. (MO 2023/2-1), and the German Aerospace Center (DLR) grant DLR-FuE-Projekt ISS-Nutzung in der Biodiagnostik, Programm RF-FuW, Teilprogramm 475 (to R.M. and G.R.).
Author Disclosure Statement
No competing financial interests exist.
Abbreviations
CFU, colony-forming units; PVA, polyvinyl alcohol solution; SAF, spacecraft assembly facility; SASP, small, acid-soluble spore proteins; SIC, spore inactivation constant; VHF-CCP, very high frequency capacitively coupled plasma reactor; VUV, vacuum UV.
References
- Bagyan I. Casillas-Martinez L. Setlow P. The katX gene, which codes for the catalase in spores of Bacillus subtilis, is a forespore-specific gene controlled by sigmaF, and KatX is essential for hydrogen peroxide resistance of the germinating spore. J Bacteriol. 1998;180:2057–2062. doi: 10.1128/jb.180.8.2057-2062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt U. Schumann P. Stieglmeier M. Pukall R. Augustin J. Spröer C. Schwendner P. Moissl-Eichinger C. Ulrich A. Characterization of heterotrophic nitrifying bacteria with respiratory ammonification and denitrification activity—description of Paenibacillus uliginis sp. nov., an inhabitant of fen peat soil and Paenibacillus purispatii sp. nov., isolated from a spacecraft assembly clean room. Syst Appl Microbiol. 2010;33:328–336. doi: 10.1016/j.syapm.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Benardini J.N. Vaishampayan P.A. Schwendner P. Swanner E. Fukui Y. Osman S. Satomi M. Venkateswaran K. Paenibacillus phoenicis sp. nov., isolated from the Phoenix lander assembly facility and a subsurface molybdenum mine. Int J Syst Evol Microbiol. 2011;61:1338–1343. doi: 10.1099/ijs.0.021428-0. [DOI] [PubMed] [Google Scholar]
- Benedikt J. Flötgen C. Kussel G. Raball V. von Keudell A. Etching of Bacillus atrophaeus by oxygen atoms, molecules and argon ion. J Phys Conf Ser. 2008a;133 doi: 10.1088/1742-6596/133/1/012012. [DOI] [Google Scholar]
- Benedikt J. Raballand V. Halfmann H. Awakowicz P. von Keudell A. Kylian O. Hasiwa M. Rossi F. Muranyi P. Wunderlich J. Comoy E. Schell J. Deslys J.P. BIODECON—European project on plasma inactivation of bacteria and biomolecules. GMS Krankenhhyg Interdiszip. 2008b. www.egms.de/en/journals/dgkh/2008-3/dgkh000102.shtml. www.egms.de/en/journals/dgkh/2008-3/dgkh000102.shtml doc04. Available online at. [PMC free article] [PubMed]
- Bruckner J.C. Osman S. Conley C. Venkateswaran K. Space microbiology: planetary protection, burden, diversity and significance of spacecraft associated microbes. In: Schaechter M., editor. Encyclopedia of Microbiology. Elsevier; Oxford: 2009. pp. 52–65. [Google Scholar]
- Casillas-Martinez L. Setlow P. Alkyl hydroperoxide reductase, catalase, MrgA, and superoxide dismutase are not involved in resistance of Bacillus subtilis spores to heat or oxidizing agents. J Bacteriol. 1997;179:7420–7425. doi: 10.1128/jb.179.23.7420-7425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checinska A. Burbank M. Paszczynski A.J. Protection of Bacillus pumilus spores by catalases. Appl Environ Microbiol. 2012;78:6413–6422. doi: 10.1128/AEM.01211-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Preventing the Forward Contamination of Mars. Preventing the Forward Contamination of Mars. National Research Council, The National Academies Press; Washington, DC: 2006. [Google Scholar]
- COSPAR. COSPAR; Paris: 2011. COSPAR Planetary Protection Policy (20 October 2002, as amended to 24 March 2011) [Google Scholar]
- De Geyter N. Morent R. Nonthermal plasma sterilization of living and nonliving surfaces. Annu Rev Biomed Eng. 2012;14:255–274. doi: 10.1146/annurev-bioeng-071811-150110. [DOI] [PubMed] [Google Scholar]
- Demidov V.V. Goncharov A.A. Osipov V.B. Trofimov V.I. Modern aspects of planetary protection and requirements to sterilization of space hardware. Adv Space Res. 1995;15:251–255. doi: 10.1016/s0273-1177(99)80093-3. [DOI] [PubMed] [Google Scholar]
- Denis B. Steves S. Semmler E. Bibinov N. Novak W. Awakowicz P. Plasma sterilization of pharmaceutical products: from basics to production. Plasma Process Polym. 2012;9:619–629. [Google Scholar]
- Driks A. Maximum shields: the assembly and function of the bacterial spore coat. Trends Microbiol. 2002;10:251–254. doi: 10.1016/s0966-842x(02)02373-9. [DOI] [PubMed] [Google Scholar]
- Ehlbeck J. Schnabel U. Polak M. Winter J. von Woedtke T. Brandenburg R. von dem Hagen T. Weltmann K.D. Low temperature atmospheric pressure plasma sources for microbial decontamination. J Phys D Appl Phys. 2011;44 doi: 10.1088/0022-3727/44/1/013002. [DOI] [Google Scholar]
- Farr S.B. Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991;55:561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia J. Yerrapragada S. Qin X. Jiang H. Igboeli O.C. Muzny D. Dugan-Rocha S. Ding Y. Hawes A. Liu W. Perez L. Kovar C. Dinh H. Lee S. Nazareth L. Blyth P. Holder M. Buhay C. Tirumalai M.R. Liu Y. Dasgupta I. Bokhetache L. Fujita M. Karouia F. Eswara Moorthy P. Siefert J. Uzman A. Buzumbo P. Verma A. Zwiya H. McWilliams B.D. Olowu A. Clinkenbeard K.D. Newcombe D. Golebiewski L. Petrosino J.F. Nicholson W.L. Fox G.E. Venkateswaran K. Highlander S.K. Weinstock G.M. Paradoxical DNA repair and peroxide resistance gene conservation in Bacillus pumilus SAFR-032. PLoS One. 2007;2 doi: 10.1371/journal.pone.0000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger A.C. Gaidamakova E.K. Matrosova V.Y. Daly M.J. Setlow P. Effects of Mn and Fe levels on Bacillus subtilis spore resistance and effects of Mn2+, other divalent cations, orthophosphate, and dipicolinic acid on protein resistance to ionizing radiation. Appl Environ Microbiol. 2011;77:32–40. doi: 10.1128/AEM.01965-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths K.K. Setlow P. Effects of modification of membrane lipid composition on Bacillus subtilis sporulation and spore properties. J Appl Microbiol. 2009;106:2064–2078. doi: 10.1111/j.1365-2672.2009.04176.x. [DOI] [PubMed] [Google Scholar]
- Heinlin J. Isbary G. Stolz W. Morfill G. Landthaler M. Shimizu T. Steffes B. Nosenko T. Zimmermann J. Karrer S. Plasma applications in medicine with a special focus on dermatology. J Eur Acad Dermatol Venereol. 2011;25:1–11. doi: 10.1111/j.1468-3083.2010.03702.x. [DOI] [PubMed] [Google Scholar]
- Höller C. Martiny H. Christiansen B. Rüden H. Gundermann K.O. The efficacy of low temperature plasma (LTP) sterilization, a new sterilization technique. Zentralbl Hyg Umweltmed. 1993;194:380–391. [PubMed] [Google Scholar]
- Hong Y.F. Kang J.G. Lee H.Y. Uhm H.S. Moon E. Park Y.H. Sterilization effect of atmospheric plasma on Escherichia coli and Bacillus subtilis endospores. Lett Appl Microbiol. 2009;48:33–37. doi: 10.1111/j.1472-765X.2008.02480.x. [DOI] [PubMed] [Google Scholar]
- Horneck G. Rettberg P. Reitz G. Wehner J. Eschweiler U. Strauch K. Panitz C. Starke V. Baumstark-Khan C. Protection of bacterial spores in space, a contribution to the discussion on Panspermia. Orig Life Evol Biosph. 2001;31:527–547. doi: 10.1023/a:1012746130771. [DOI] [PubMed] [Google Scholar]
- Horneck G. Klaus D.M. Mancinelli R.L. Space microbiology. Microbiol Mol Biol Rev. 2010;74:121–156. doi: 10.1128/MMBR.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horneck G. Moeller R. Cadet J. Douki T. Mancinelli R.L. Nicholson W.L. Panitz C. Rabbow E. Rettberg P. Spry A. Stackebrandt E. Vaishampayan P. Venkateswaran K.J. Resistance of bacterial endospores to outer space for planetary protection purposes—experiment PROTECT of the EXPOSE-E mission. Astrobiology. 2012;12:445–456. doi: 10.1089/ast.2011.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys P.N. Testing standards for sporicides. J Hosp Infect. 2011;77:193–198. doi: 10.1016/j.jhin.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Hury S. Vidal D.R. Desor F. Pelletier J. Lagarde T. A parametric study of the destruction efficiency of Bacillus spores in low pressure oxygen-based plasmas. Lett Appl Microbiol. 1998;26:417–421. doi: 10.1046/j.1472-765x.1998.00365.x. [DOI] [PubMed] [Google Scholar]
- Jacobs P. Kowatsch R. Sterrad Sterilization System: a new technology for instrument sterilization. Endosc Surg Allied Technol. 1993;1:57–58. [PubMed] [Google Scholar]
- Kempf M.J. Chen F. Kern R. Venkateswaran K. Recurrent isolation of hydrogen peroxide-resistant spores of Bacillus pumilus from a spacecraft assembly facility. Astrobiology. 2005;5:391–405. doi: 10.1089/ast.2005.5.391. [DOI] [PubMed] [Google Scholar]
- Kerney K.R. Schuerger A.C. Survival of Bacillus subtilis endospores on ultraviolet-irradiated rover wheels and Mars regolith under simulated martian conditions. Astrobiology. 2011;11:477–485. doi: 10.1089/ast.2011.0615. [DOI] [PubMed] [Google Scholar]
- Klämpfl T.G. Isbary G. Shimizu T. Li Y.F. Zimmermann J.L. Stolz W. Schlegel J. Morfill G.E. Schmidt H.U. Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Appl Environ Microbiol. 2012;78:5077–5082. doi: 10.1128/AEM.00583-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong M.G. Kroesen G. Morfill G. Nosenko T. Shimizu T. van Dijk J. Zimmermann J.L. Plasma medicine: an introductory review. New J Phys. 2009;11 doi: 10.1088/1367-2630/11/11/115012. [DOI] [Google Scholar]
- La Duc M.T. Satomi M. Venkateswaran K. Bacillus odysseyi sp. nov., a round-spore-forming Bacillus isolated from the Mars Odyssey spacecraft. Int J Syst Evol Microbiol. 2004a;54:195–201. doi: 10.1099/ijs.0.02747-0. [DOI] [PubMed] [Google Scholar]
- La Duc M.T. Kern R. Venkateswaran K. Microbial monitoring of spacecraft and associated environments. Microb Ecol. 2004b;47:150–158. doi: 10.1007/s00248-003-1012-0. [DOI] [PubMed] [Google Scholar]
- Lerouge S. Wertheimer M.R. Marchand R. Tabrizian M. Yahia L. Effect of gas composition on spore mortality and etching during low-pressure plasma sterilization. J Biomed Mater Res. 2000;51:128–135. doi: 10.1002/(sici)1097-4636(200007)51:1<128::aid-jbm17>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Link L. Sawyer J. Venkateswaran K. Nicholson W.L. Extreme spore UV resistance of Bacillus pumilus isolates obtained from an ultraclean spacecraft assembly facility. Microb Ecol. 2004;47:159–163. doi: 10.1007/s00248-003-1029-4. [DOI] [PubMed] [Google Scholar]
- Moeller R. Horneck G. Rettberg P. Mollenkopf H.J. Stackebrandt E. Nicholson W.L. A method for extracting RNA from dormant and germinating Bacillus subtilis strain 168 endospores. Curr Microbiol. 2006;53:227–231. doi: 10.1007/s00284-006-0099-1. [DOI] [PubMed] [Google Scholar]
- Moeller R. Stackebrandt E. Reitz G. Berger T. Rettberg P. Doherty A.J. Horneck G. Nicholson W.L. Role of DNA repair by nonhomologous-end joining in Bacillus subtilis spore resistance to extreme dryness, mono- and polychromatic UV, and ionizing radiation. J Bacteriol. 2007;189:3306–3311. doi: 10.1128/JB.00018-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller R. Setlow P. Reitz G. Nicholson W.L. Roles of small, acid-soluble spore proteins and core water content in survival of Bacillus subtilis spores exposed to environmental solar UV radiation. Appl Environ Microbiol. 2009;75:5202–5208. doi: 10.1128/AEM.00789-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller R. Reitz G. Berger T. Okayasu R. Nicholson W.L. Horneck G. Astrobiological aspects of the mutagenesis of cosmic radiation on bacterial spores. Astrobiology. 2010;10:509–521. doi: 10.1089/ast.2009.0429. [DOI] [PubMed] [Google Scholar]
- Moeller R. Reitz G. Nicholson W.L. PROTECT Team. Horneck G. Mutagenesis in bacterial spores exposed to space and simulated martian conditions: data from the EXPOSE-E spaceflight experiment PROTECT. Astrobiology. 2012a;12:457–468. doi: 10.1089/ast.2011.0739. [DOI] [PubMed] [Google Scholar]
- Moeller R. Reitz G. Li Z. Klein S. Nicholson W.L. Multifactorial resistance of Bacillus subtilis spores to high-energy proton radiation: role of spore structural components and the homologous recombination and non-homologous end joining DNA repair pathways. Astrobiology. 2012b;12:1069–1077. doi: 10.1089/ast.2012.0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller R. Schuerger A.C. Reitz G. Nicholson W.L. Protective role of spore structural components in determining Bacillus subtilis spore resistance to simulated Mars surface conditions. Appl Environ Microbiol. 2012c;78:8849–8853. doi: 10.1128/AEM.02527-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisan M. Barbeau J. Moreau S. Pelletier J. Tabrizian M. Yahia L.H. Low-temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. Int J Pharm. 2001;226:1–21. doi: 10.1016/s0378-5173(01)00752-9. [DOI] [PubMed] [Google Scholar]
- Moisan M. Barbeau J. Crevier M.C. Pelletier J. Philip N. Saoudi B. Plasma sterilization: methods and mechanisms. Pure Appl Chem. 2002;74:349–358. [Google Scholar]
- Moreau M. Orange N. Feuilloley M.G. Non-thermal plasma technologies: new tools for bio-decontamination. Biotechnol Adv. 2008;26:610–617. doi: 10.1016/j.biotechadv.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Morris A.D. McCombs G.B. Akan T. Hynes W. Laroussi M. Tolle S.L. Cold plasma technology: bactericidal effects on Geobacillus stearothermophilus and Bacillus cereus microorganisms. J Dent Hyg. 2009;83:55–61. [PubMed] [Google Scholar]
- NASA. National Aeronautics and Space Administration; Washington, DC: 2005. Apr, 2005. Planetary protection provisions for robotic extraterrestrial missions. NPR 8020.12C. [Google Scholar]
- Nicholson W.L. Galeano B. UV resistance of Bacillus anthracis spores revisited: validation of Bacillus subtilis spores as UV surrogates for spores of B. anthracis Sterne. Appl Environ Microbiol. 2003;69:1327–1330. doi: 10.1128/AEM.69.2.1327-1330.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson W.L. Setlow P. Sporulation, germination, and outgrowth. In: Harwood C.R., editor; Cutting S.M., editor. Molecular Biological Methods for Bacillus. John Wiley and Sons; Sussex, England: 1990. pp. 391–450. [Google Scholar]
- Nicholson W.L. Munakata N. Horneck G. Melosh H.J. Setlow P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev. 2000;64:548–572. doi: 10.1128/mmbr.64.3.548-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson W.L. Schuerger A.C. Race M.S. Migrating microbes and planetary protection. Trends Microbiol. 2009;17:389–392. doi: 10.1016/j.tim.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Okpara-Hofmann J. Knoll M. Dürr M. Schmitt B. Borneff-Lipp M. Comparison of low-temperature hydrogen peroxide gas plasma sterilization for endoscopes using various Sterrad models. J Hosp Infect. 2005;59:280–285. doi: 10.1016/j.jhin.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Osman S. Satomi M. Venkateswaran K. Paenibacillus pasadenensis sp. nov. and Paenibacillus barengoltzii sp. nov., isolated from a spacecraft assembly facility. Int J Syst Evol Microbiol. 2006;56:1509–1514. doi: 10.1099/ijs.0.64085-0. [DOI] [PubMed] [Google Scholar]
- Paidhungat M. Setlow B. Driks A. Setlow P. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J Bacteriol. 2000;182:5505–5512. doi: 10.1128/jb.182.19.5505-5512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popham D.L. Sengupta S. Setlow P. Heat, hydrogen peroxide, and UV resistance of Bacillus subtilis spores with increased core water content and with or without major DNA-binding proteins. Appl Environ Microbiol. 1995;61:3633–3638. doi: 10.1128/aem.61.10.3633-3638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottage T. Macken S. Giri K. Walker J.T. Bennett A.M. Low-temperature decontamination with hydrogen peroxide or chlorine dioxide for space applications. Appl Environ Microbiol. 2012;78:4169–4174. doi: 10.1128/AEM.07948-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raballand V. Benedikt J. Wunderlich J. von Keudell A. Inactivation of Bacillus atrophaeus and Aspergillus niger using beams of argon ions, of oxygen molecules and of oxygen atoms. J Phys D Appl Phys. 2008;41 doi: 10.1088/0022-3727/41/11/115207. [DOI] [Google Scholar]
- Rauscher H. Stapelmann K. Kylián O. Denis B. Rossi F. Monitoring plasma etching of biomolecules by imaging ellipsometry. Vacuum. 2009;84:75–78. [Google Scholar]
- Rauscher H. Kylián O. Benedikt J. von Keudell A. Rossi F. Elimination of biological contaminations from surfaces by plasma discharges: chemical sputtering. Chemphyschem. 2010;11:1382–1389. doi: 10.1002/cphc.200900757. [DOI] [PubMed] [Google Scholar]
- Reder A. Höper D. Gerth U. Hecker M. Contributions of individual σB-dependent general stress genes to oxidative stress resistance of Bacillus subtilis. J Bacteriol. 2012;194:3601–3610. doi: 10.1128/JB.00528-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesenman P.J. Nicholson W.L. Role of the spore coat layers in Bacillus subtilis spore resistance to hydrogen peroxide, artificial UV-C, UV-B, and solar UV radiation. Appl Environ Microbiol. 2000;66:620–626. doi: 10.1128/aem.66.2.620-626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F. Kylián O. Hasiwa M. Decontamination of surfaces by low pressure plasma discharges. Process Polym. 2006;3:431–442. [Google Scholar]
- Rossi F. Kylián O. Rauscher H. Hasiwa M. Gilliland D. Low pressure plasma discharges for the sterilization and decontamination of surfaces. New J Phys. 2009;11 doi: 10.1088/1367-2630/11/11/115017. [DOI] [Google Scholar]
- Roth S. Feichtinger J. Hertel C. Characterization of Bacillus subtilis spore inactivation in low-pressure, low-temperature gas plasma sterilization processes. J Appl Microbiol. 2010;108:521–531. doi: 10.1111/j.1365-2672.2009.04453.x. [DOI] [PubMed] [Google Scholar]
- Rummel J.D. Planetary protection in the time of astrobiology: protecting against biological contamination. Proc Natl Acad Sci USA. 2001;98:2128–2131. doi: 10.1073/pnas.061021398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutala W.A. Weber D.J. New disinfection and sterilization methods. Emerg Infect Dis. 2001;7:348–353. doi: 10.3201/eid0702.010241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutala W.A. Gergen M.F. Weber D.J. Sporicidal activity of a new low-temperature sterilization technology: the Sterrad 50 sterilizer. Infect Control Hosp Epidemiol. 1999;20:514–516. doi: 10.1086/501662. [DOI] [PubMed] [Google Scholar]
- Satomi M. La Duc M.T. Venkateswaran K. Bacillus safensis sp. nov., isolated from spacecraft and assembly-facility surfaces. Int J Syst Evol Microbiol. 2006;56:1735–1740. doi: 10.1099/ijs.0.64189-0. [DOI] [PubMed] [Google Scholar]
- Schaeffer P. Millet J. Aubert J.P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA. 1965;54:704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuerger A.C. Richards J.T. Hintze P.E. Kern R.G. Surface characteristics of spacecraft components affect the aggregation of microorganisms and may lead to different survival rates of bacteria on Mars landers. Astrobiology. 2005;5:545–559. doi: 10.1089/ast.2005.5.545. [DOI] [PubMed] [Google Scholar]
- Schuerger A.C. Trigwell S. Calle C.I. Use of non-thermal atmospheric plasmas to reduce the viability of Bacillus subtilis on spacecraft surfaces. International Journal of Astrobiology. 2008;7:47–57. [Google Scholar]
- Setlow P. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol. 2006;101:514–525. doi: 10.1111/j.1365-2672.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- Setlow P. I will survive: DNA protection in bacterial spores. Trends Microbiol. 2007;15:172–180. doi: 10.1016/j.tim.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Setlow B. Setlow P. Binding of small, acid-soluble spore proteins to DNA plays a significant role in the resistance of Bacillus subtilis spores to hydrogen peroxide. Appl Environ Microbiol. 1993;59:3418–3423. doi: 10.1128/aem.59.10.3418-3423.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapelmann K. Kylián O. Denis B. Rossi F. On the application of inductively coupled plasma discharges sustained in Ar/O2/N2 ternary mixture for sterilization and decontamination of medical instruments. J Phys D Appl Phys. 2008;41 doi: 10.1088/0022-3727/41/19/192005. [DOI] [Google Scholar]
- Venkateswaran K. Kempf M. Chen F. Satomi M. Nicholson W.L. Kern R. Bacillus nealsonii sp. nov., isolated from a spacecraft-assembly facility, whose spores are gamma-radiation resistant. Int J Syst Evol Microbiol. 2003;53:165–172. doi: 10.1099/ijs.0.02311-0. [DOI] [PubMed] [Google Scholar]
- Venkateswaran K. Chung S. Allton J. Kern R. Evaluation of various cleaning methods to remove Bacillus spores from spacecraft hardware materials. Astrobiology. 2004;4:377–390. doi: 10.1089/ast.2004.4.377. [DOI] [PubMed] [Google Scholar]
- Vaishampayan P. Probst A. Krishnamurthi S. Ghosh S. Osman S. McDowall A. Ruckmani A. Mayilraj S. Venkateswaran K. Bacillus horneckiae sp. nov., isolated from a spacecraft-assembly clean room. Int J Syst Evol Microbiol. 2010;60:1031–1037. doi: 10.1099/ijs.0.008979-0. [DOI] [PubMed] [Google Scholar]
- Vaishampayan P.A. Rabbow E. Horneck G. Venkateswaran K.J. Survival of Bacillus pumilus spores for a prolonged period of time in real space conditions. Astrobiology. 2012;12:487–497. doi: 10.1089/ast.2011.0738. [DOI] [PubMed] [Google Scholar]
- von Keudell A. Awakowicz P. Benedikt J. Raballand V. Yanguas-Gil A. Opretzka J. Flötgen C. Reuter R. Byelykh L. Halfmann H. Stapelmann K. Denis B. Wunderlich J. Muranyi P. Rossi F. Kylián O. Hasiwa N. Ruiz A. Rauscher H. Sirghi L. Comoy E. Dehen C. Challier L. Deslys J.P. Inactivation of bacteria and biomolecules by low-pressure plasma discharges. Plasma Process Polym. 2010;7:327–352. [Google Scholar]
- Wolfson R.P. Craven C.W. Contamination of planets by nonsterile flight hardware. Environ Biol Med. 1971;1:99–120. [PubMed] [Google Scholar]
- Yasuda H. Miura T. Kurita H. Takashima K. Mizuno A. Biological evaluation of DNA damage in bacteriophages inactivated by atmospheric pressure cold plasma. Plasma Process Polym. 2010;7:301–308. [Google Scholar]