Abstract
Previously, we have shown that vascular smooth muscle cells (VSMCs) exhibit varied physiological responses when exposed to altered gravitational conditions. In the present study, we focused on elucidating whether the cell surface glycocalyx could be a potential gravity sensor. For this purpose, a roller culture apparatus was used with the intent to provide altered gravitational conditions to cultured rat aortic smooth muscle cells (RASMCs). Heparinase III (Hep.III) was applied to degrade cell surface heparan sulfate proteoglycans (HSPG) selectively. Sodium chlorate was used to suppress new synthesis of HSPG. Glycocalyx remodeling, nitric oxide synthase (NOS) activation, and F-actin expression induced by gravity alteration were assessed by flow cytometry, reverse transcription polymerase chain reaction (RT-PCR), and Western blot. Results indicate that the exposure of cultured RASMCs to altered gravitational conditions led to a reduction in cell surface HSPG content and the activation of NOS. It also down-regulated the expression of glypican-1, constitutive NOS (NOSI and NOSIII), and F-actin. On the other hand, Hep.III followed by sodium chlorate treatment of HSPG attenuated the aforementioned NOS and F-actin modulation under altered gravitational conditions. All these findings suggest that the glycocalyx, and HSPG in particular, may be an important sensor of gravitational changes. This may play an important role in the regulation of NOS activation, F-actin modulation, and HSPG remodeling in VSMCs. Key Words: Glycocalyx—Gravity sensor—Gravity alteration—Roller culture apparatus. Astrobiology 13, 626–636.
1. Introduction
Orthostatic intolerance, the symptoms of which include increased heart rate, orthostatic hypotension, and frank syncope, may be one of the highest risks to astronaut safety, well-being, and overall agility on return to Earth (Nicogossian et al., 1996; Sides et al., 2005). Unfortunately, after several decades of investigation, the underlying mechanisms still remain disputed. Multiple factors that include hypovolemia, cardiovascular structural changes, alterations in central integration, baroreceptor function, and neurohumoral regulation have been proposed to be involved (Jin et al., 2003). Microgravity-induced vascular structural and functional remodeling may be one of the key contributors to post-spaceflight orthostatic intolerance (Zhang et al., 1997; Delp et al., 2000).
Vascular smooth muscle cells (VSMCs) normally reside in the tunica media of the vessel wall and are essential for optimal cardiovascular system performance. Under physiological conditions, via contraction and relaxation, smooth muscle cells (SMCs) alter the lumen diameter to maintain normal blood pressure of the vasculature. SMCs also play an important role in vessel remodeling during pregnancy, exercise, and vascular injury (Owens et al., 2004; Rensen et al., 2007). When pathological conditions are present, such as intimal hyperplasia, restenosis, and atherosclerosis, SMCs lose their “contractile” phenotype; increase their proliferation, migration, and extracellular matrix production (Beamish et al., 2010); and shift toward a “synthetic” phenotype. It has been well established that SMCs exhibit a remarkable capability to transform between “contractile” and “synthetic” phenotypes and a continuum of states in between (Halayko and Solway, 2001). Moreover, the transformation can be modulated by mechanical factors like stretch and shear stress (Davies et al., 1988). Recently, a study in our laboratory demonstrated that VSMCs can react to gravity alteration at the single cellular level. Long time-exposure (≥72 h) of SMCs to altered gravity conditions can induce a contractile phenotype tendency (Kang et al., 2013). Nevertheless, it remains unclear how SMCs could sense the mechanical stimuli (gravitational vector alteration) and transform to biochemical responses.
The glycocalyx of vascular cells is a surface layer composed primarily of proteoglycan core proteins (syndecans, glypicans, and perlecans), with their associated glycosaminoglycans (GAGs), which include heparan sulfate (HS), chondroitin sulfate, and hyaluronic acid, and glycoproteins bearing acidic oligosaccharides with terminal sialic acids (Pahakis et al., 2007). On the SMC surface, about 50–60% of the GAGs are chondroitin sulfate, and the rest are predominantly HS (Nilsson et al., 1983). An in vitro study by Ainslie et al. (2005) shows that HS and chondroitin sulfate components of the SMC glycocalyx play an important role in the mechanotransduction of shear stress to control SMC contraction. Kang et al. (2011) also demonstrated that the SMC glycocalyx might participate in mechanotransduction of shear stress to modulate cell proliferation, migration, and NO production. Recently, the SMC glycocalyx has been reported to play a dominant role in 3-D interstitial flow mechanosensing to modulate SMC phenotype gene expression (Shi et al., 2010) and motility (Shi et al., 2011). Although the role of the glycocalyx in flow-induced mechanotransduction has been extensively studied, its potential gravity-sensing role has never been investigated.
It has been shown that the cytoplasmic domains of syndecans, the most common core protein of the glycocalyx, can associate with molecules, such as ezrin, dynamin-2, syntenin, syndesmos, and a-actinin, which link it to cytoskeletal elements (Tarbell and Pahakis, 2006). Glypicans, the second most abundant proteoglycan core proteins, along with their HS chains, localize in caveolae (Tarbell and Pahakis, 2006). Interestingly, both the cytoskeleton and caveolae constituents have been shown to be involved in mechanosensing after microgravity adaptation at the single cellular level (Ingber, 1999; Spisni et al., 2006). Therefore, we postulated that the glycocalyx might be involved in gravity sensing due to its close association with the cytoskeleton and caveolae constituents.
In an attempt to elucidate whether the glycocalyx could be a potential gravity sensor, we used a roller culture apparatus with the intent to apply altered gravitational conditions to cultured rat aortic smooth muscle cells (RASMCs). Heparinase III (Hep.III) was applied to degrade cell surface HS selectively. Sodium chlorate was added to DMEM culture medium to suppress new synthesis of the glycocalyx. We evaluated possible responses of glycocalyx to the altered gravitational conditions including changes in its GAG content and core protein backbone expression. Particularly, we assessed the potential gravity-sensing role of the glycocalyx, its ability to adapt in response to gravitational changes, and its possible role in the regulation of RASMC nitric oxide synthase (NOS) activity and F-actin expression.
2. Materials and Methods
2.1. Antibodies and reagents
The primary antibody for heparin sulfate used in immunofluorescence and flow cytometry studies was HepSS-1 (US Biological, Swampscott, MA). The primary antibodies used for Western blotting were a polyclonal anti-NOSI (BD Transduction Laboratories, Lexington, KY), polyclonal anti-F-actin (Bioss, Beijing), monoclonal anti-NOSII (BD Transduction Laboratories, Lexington, KY), and monoclonal anti-GAPDH (Bioss, Beijing). Alexa Fluor 488–labeled secondary antibody (goat anti-mouse IgM) for immunofluorescence and flow cytometry was obtained from Molecular Probes (Eugene, OR). Peroxidase-conjugated goat anti-mouse or rabbit IgG was obtained from ZSGB-BIO (Beijing, China). The following chemicals were obtained from Invitrogen (Camarillo, CA): Collagenase II, Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), penicillin-streptomycin. Trypsin, paraformaldehyde, glutaraldehyde, BSA, PI, and RNAase were purchased from Sigma (St. Louis, MO). Type I rat-tail collagen was obtained from Millipore (Bedford, MA). TRNzol reagent was purchased from Tiangen Biotech (Beijing, China). RevertAid First Strand cDNA Synthesis Kit and DreamTaq Green PCR Master Mix were obtained from Fermentas (Ontario, Canada). 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI), Antifade mounting medium, and Nitric Oxide Synthase Assay Kit were obtained from Beyotime Biotech of China (Jiangsu, China).
2.2. Cell culture and experimental setup
Rat aortic smooth muscle cells were isolated from the thoracic aortas of 200–250 g male Sprague-Dawley rats by collagenase digestion with the use of established techniques (Kang et al., 2011). These cells were maintained in DMEM supplemented with 10% (v/v) FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin, at 37°C in 5% CO2. The studies were performed with RASMCs between passages 6 and 10. To achieve altered gravitational conditions, a roller culture apparatus (Wheaton, NJ) in a temperature-controlled incubator (37°C, 5% CO2) was employed. Flasks (12.5 cm2, Becton Dickinson, USA) seeded with RASMCs were filled completely with DMEM plus 10% FBS, capped, and fastened to the edge of the roller around the horizontal axis with a rotational radius of about 21 mm. The rotation speed was adjusted to 20 rpm so that time-averaged gravitational vector acting on these cellular assemblies at the outer radius is reduced to about 10−2g, which is regarded as microgravity conditions (Schwarz et al., 1992). A 1g group was used as the control. These flasks were filled with the same DMEM complete medium and were cultured under stationary conditions.
2.3. Heparan sulfate depletion
Heparinase III was used to cleave HS on RASMC surface. The enzyme was used at a concentration of 0.2 U/mL in DMEM at 37°C for 30 min as previously described (Kang et al., 2011). To suppress new synthesis of HS, 30 mM sodium chlorate was then added to DMEM complete culture medium as employed elsewhere (Humphries and Silbert, 1988). Before the experiment, the negative effect of sodium chlorate on RASMC growth was tested. The efficiency of Hep.III and sodium chlorate treatment after 2-day culture was assessed by immunofluorescence staining as previously described (Kang et al., 2011). Negative controls without HepSS-1 were stained under the same protocol. The fluorescence intensity was quantified by Image J (National Institutes of Health, Bethesda, MD).
2.4. Flow cytometry analysis of surface HSPG
Rat aortic smooth muscle cells cultured under 1g or altered gravitational conditions for 4 or 6 days were incubated in 0.04% EDTA for 30 min at room temperature. Then, cells were scratched from the bottom of the flask and fixed in 4% paraformaldehyde and 0.5% glutaraldehyde for 20 min. After filtering through 300-nylon mesh, cells were incubated with HepSS-1 (1:50) at 37°C for 15 min and washed in phosphate-buffered saline once. Afterward, 100 μL Alexa Fluor 488–labeled secondary antibody (1:50) was added to cells for another 15 min in the dark. After washing with phosphate-buffered saline three times at 5 min intervals, cells were analyzed by flow cytometry. The expression of HSPG was normalized to the 1g group by geometric mean fluorescence intensity. As negative controls, RASMCs were incubated without HepSS-1 but with all other conditions kept the same.
2.5. Reverse transcription polymerase chain reaction (RT-PCR)
For RT-PCR experiments, the total RNA of RASMCs from each sample was isolated with TRNzol reagent according to the manufacturer's instructions. The amount of total RNA was quantified by a NanoDrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE). Thereafter, 2000 ng total RNA of each sample was reverse transcripted into cDNA with the RevertAid First Strand cDNA Synthesis Kit. The cDNA was amplified by using DreamTaq Green PCR Master Mix and gene-specific primer pairs (Table 1). PCR conditions were as follows: 95°C for 2 min, followed by 30 cycles of denaturation (95°C, 30 s), annealing at indicated temperature for 30 s, and extension at 72°C for 45 s. PCR products were exposed to agarose gel electrophoresis and stained with ethidium bromide before being imaged with a Gel Image system (Tanon, Shanghai, China). The total gray value of each sample was evaluated by BandScan (Glyko Biomedical Limited, USA). Each experiment was repeated three to six times.
Table 1.
Primers for PCR Amplification
| Gene | Sequence of primers (5′-3′) | Annealing temperature (°C) | Product size (bp) |
|---|---|---|---|
| Heparanase | Forward: TTTGATCCCAACAAGGAACCCAC Reverse: GTAGTGATGCCAGGTGAGAGAGTC |
55 | 585 |
| Syndecan-1 | Forward: TGCAACCGGCAACTCCGACC Reverse: TCCCTAGGCCTGGTGGTGGC |
58 | 445 |
| Glypican-1 | Forward: GAATGACTCGGAGCGTACACT Reverse: CCTTTGAGCACATTTCGGCAA |
55 | 489 |
| NOSI | Forward: CTGGCTCAACAGAATACAGGCT Reverse: GCAGTGTACAGCTCTCTGAAGA |
56 | 294 |
| NOSII | Forward: TCACCTTCGAGGGCAGCCGA Reverse: CAGACGCCATGGTGCAGGGG |
61 | 562 |
| NOSIII | Forward: GCCCAGCAGCGTGGAGTGTT Reverse: GCGGGTCAAAGGACCAGGGC |
61 | 397 |
| GAPDH | Forward: GCTCTCTGCTCCTCCCTGTTCT Reverse: CAGGCGTCCGATACGGCCAAA |
58 | 117 |
2.6. Nitric oxide synthase assay
Nitric oxide synthase activity was measured by the Nitric Oxide Synthase Assay Kit according to the manufacturer's instructions. Briefly, after exposure to 1g or altered gravitational conditions for 6, 12, or 24 h, cells from each flask were trypsinized and centrifuged at 800 rpm for 5 min. Then the cells were resuspended in 1 mL NOS assay buffer (1×) and transferred to a 96-well plate (100 μL per well). Cell density was evaluated by a hemocytometer. Thereafter, 100 μL NOS assay reaction solution (50% NOS assay buffer, 39.8% Milli-Q water, 5% L-arginine solution, 5% 0.1 mM NADPH, 0.2% DAF-FMDA) was added to each well and incubated for 1 h at 37°C. Three parallel wells were set for each experiment, and experiments were repeated at least three times. Fluorescence was measured with a Thermo Scientific Varioskan Flash (Thermo Scientific, Beverly, MA) at excitation of 495 nm and emission of 515 nm. NOS activity was expressed as the fluorescence intensity divided by the cell number.
2.7. Western blotting
Rat aortic smooth muscle cells from each flask were lysed in 300 μL RIPA Lysis Buffer containing 1 mM phenylmethanesulfonyl fluoride at 4°C for 30 min and centrifuged at 14,000g for 10 min. Protein content in the supernatant was determined by an Enhanced BCA Protein Assay Kit according to the manufacturer's instructions. The protein samples (91 μg per lane) were then boiled for 5 min after mixing with 5×SDS-PAGE loading buffer and separated in denaturing SDS/8.0% polyacrylamide gels. Thereafter, proteins were transferred to PVDF membranes (Millipore, Bedford, MA) and blocked for 90 min at room temperature with 5% nonfat dry milk in TBS-T. The membranes were incubated overnight at 4°C with primary antibodies against NOSI (diluted at 1:250), NOSII (diluted at 1:1000), F-actin (diluted at 1:200), and GAPDH (diluted at 1:500). After rinsing in TBS-T three times (10 min each), the membranes were incubated in ECL horseradish peroxidase–conjugated secondary antibodies (diluted at 1:5000) for 1.5 h at room temperature and washed three times (10 min each) in TBS-T. The proteins on PVDF membranes were then detected by using SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL) and the universal imaging hood 2 with the Quantity One software (Bio-Rad, Berkeley, CA). Each experiment was repeated at least three times.
2.8. Statistical analysis
Data are presented as means±standard error of the mean. Statistical analysis was performed by repeated measures analysis of variance and the Student t test (two groups). Differences between means were considered significant if P<0.05.
3. Results
3.1. Altered gravitational stimulation reduces the geometric mean of HSPG and downregulates glypican-1 mRNA level significantly
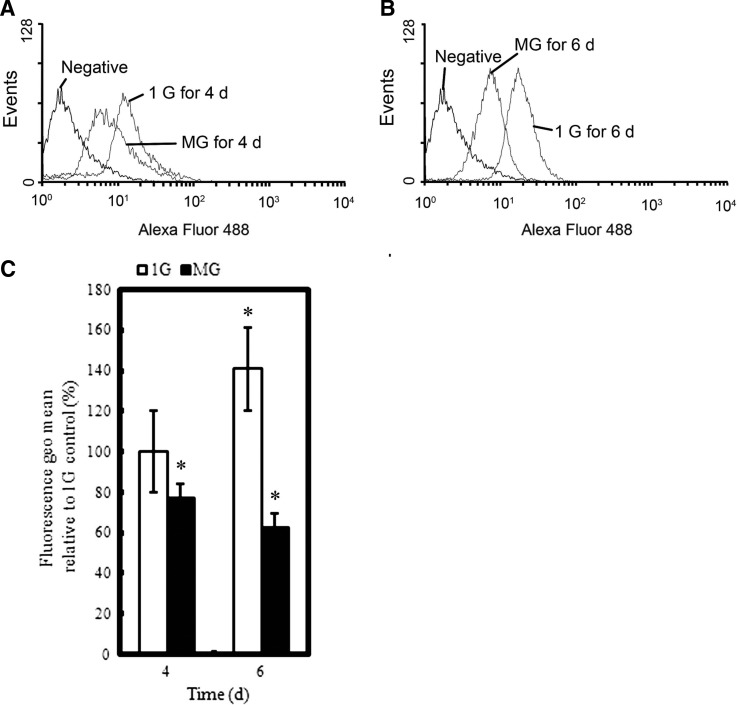
As evident from Fig. 1, the relative fluorescence geo mean of HSPG was dramatically reduced from 75.4±5.17% (4 days) to 44.4±2.05% (6 days) after exposure to altered gravitational conditions. To study the effect of altered gravitational stimulation on HSPG core protein expression, we semiquantified the mRNA levels of syndecan-1 and glypican-1 and found that the mRNA level of glypican-1 was significantly reduced when exposed to altered gravitational conditions (P<0.05), while syndecan-1 mRNA level reduced without significant differences. We also tested the mRNA level of endogenous heparanase, an enzyme that specifically degrades HS. As demonstrated in Fig. 2, 6-day altered gravitational stimulation significantly enhanced heparanase expression.
FIG. 1.
Flow cytometry analysis of cell surface HSPG under 1g or altered gravitational conditions. (A and B) Representative histograms of Alexa Fluor 488 fluorescence of RASMCs exposed to 1g or MG for 4–6 days. (C) The fluorescence geo mean relative to the 1g condition on Day 4, showing that MG exposure reduced HSPG content from 75.4±5.17% (4 days) to 44.4±2.05% (6 days). *P<0.05 vs. 1g group on Day 4 (two-tailed t test, n=3). MG, altered gravitational stimulation.
FIG. 2.
Effect of altered gravitational stimulation on the mRNA expression of heparanase, syndecan-1, and glypican-1 of RASMCs. (A) Gel panel data from RT-PCR. (B, C, and D) Bar graph data from BandScan analysis of each panel. Each mRNA level relative to GAPDH was normalized to the value of 1g conditions on Day 3. *P<0.05 vs. 1g group (two-tailed t test, n=3). MG, altered gravitational stimulation.
3.2. Heparinase III and sodium chlorate treatment remove HS from RASMC surface and inhibit new synthesis of HS
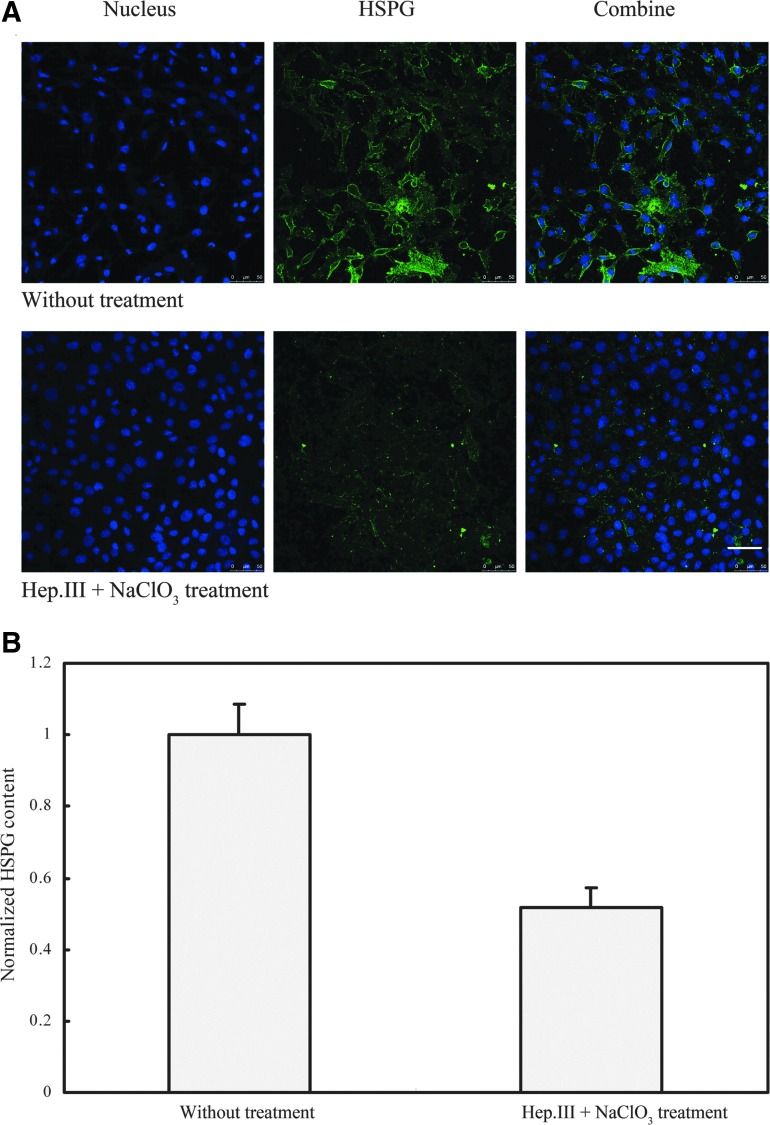
To verify the effectiveness of Hep.III and sodium chlorate treatment, both the control and the treated cells were cultured for 2 days, exposed to an antibody specific to HS, and then visualized by a fluorescently labeled secondary antibody. Figure 3 shows a representative immunostaining. As shown in Fig. 3, the Hep.III and sodium chlorate treatment caused a nearly 50% (48.34±5.70%) reduction in fluorescence intensity relative to the untreated control (Fig. 3B).
FIG. 3.
(A) Fluorescence immunostaining of RASMCs with nucleus in blue and HSPG in green. (B) Hep.III+NaClO3 treatment (2 days) caused a 48.34±5.70% reduction in HSPG content relative to the untreated control. NaClO3, sodium chlorate. Scale bar: 50 μm. Color images available online at www.liebertonline.com/ast
3.3. Heparinase III and sodium chlorate treatment attenuate NOS activation induced by altered gravitational stimulation
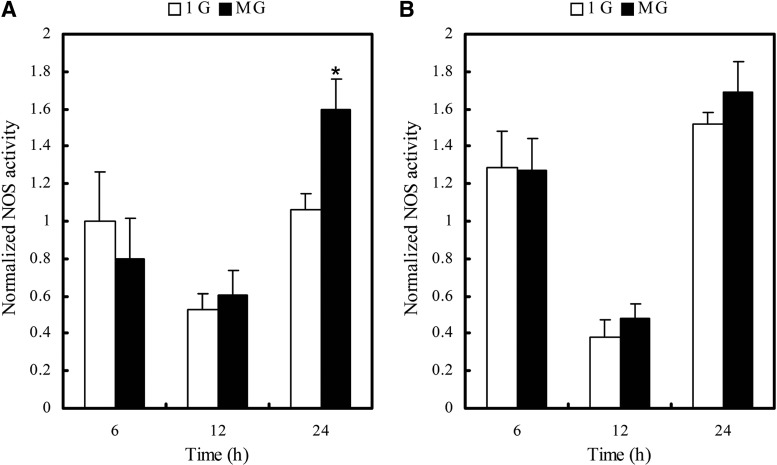
We exposed RASMCs to 1g or altered gravitational conditions for 6, 12, and 24 h, then collected cells and measured the fluorescence of each reaction system. As indicated in Fig. 4, without Hep.III and sodium chlorate treatment, NOS was significantly activated (P<0.05) after 24 h altered gravitational stimulation, while this activation was attenuated when the glycocalyx was disrupted by Hep.III and sodium chlorate treatment.
FIG. 4.
Nitric oxide synthase (NOS) activity of RASMCs exposed to 1g or MG for 6, 12, and 24 h. (A) 24 h MG exposure activates NOS significantly. (B) Hep.III+NaClO3 treatment attenuates MG-induced NOS activation. MG, altered gravitational stimulation. *P<0.05 vs. 1g group (two-tailed t test, n=3). Data were normalized to the 1g group without treatment at 6 h.
3.4. Downregulation of constitutive NOS (NOSI and NOSIII) mRNA and protein levels by altered gravitational stimulation is HSPG-dependent
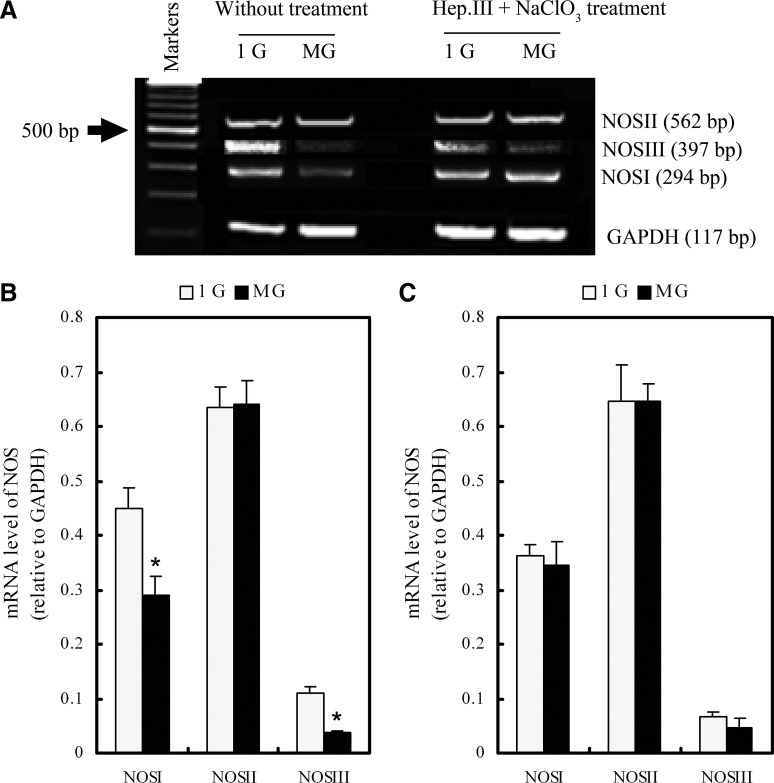
Up to now, we have shown that NOS activity could be affected by gravity alteration and glycocalyx disruption. To further detect the underlying mechanism of NOS activity regulation, we measured the mRNA levels of the three NOS isoforms, namely, NOSI, NOSII, and NOSIII. Evidence from Fig. 5 indicates that 24 h altered gravitational stimulation significantly downregulated the mRNA level of NOSI and NOSIII while insignificantly affecting the NOSII mRNA expression. However, after Hep.III and sodium chlorate treatment, the mRNA expressions of all three NOS isoforms under both 1g and altered gravitational conditions showed no significant differences. We also quantified NOSI and NOSII protein levels by Western blotting. Figure 6 shows that 24 h altered gravitational stimulation can downregulate the NOSI protein expression significantly while upregulating the NOSII expression slightly. After Hep.III and sodium chlorate treatment, the expressions of both NOSI and NOSII under altered gravitational conditions showed the same level as the 1g group, suggesting that HSPG may play a pivotal role in the sensing of gravity changes to regulate constitutive NOS mRNA and protein expression.
FIG. 5.
The mRNA expression of NOSI, NOSII, and NOSIII after 24 h 1g or MG exposure. (A) Gel panel data from RT-PCR. (B) 24 h MG exposure downregulated the mRNA level of constitutive NOS (NOSI and NOSIII) while insignificantly affecting NOSII. (C) NOS mRNA expression, showing no significant differences under 1g or MG conditions after Hep.III+NaClO3 treatment. MG, altered gravitational stimulation; NaClO3, sodium chlorate. *P<0.05 vs. 1g group (two-tailed t test, n=3–6).
FIG. 6.
(A) Western blotting analysis of NOSI and NOSII after 24 h 1g or MG exposure. (B) Normalized NOSI expression level relative to GAPDH, showing that MG-induced NOSI downregulation was attenuated after Hep.III+NaClO3 treatment. (C) Normalized NOSII expression level relative to GAPDH, showing that MG did not affect NOSII expression significantly. MG, altered gravitational stimulation; NaClO3, sodium chlorate. *P<0.05 vs. 1g group without treatment (two-tailed t test, n=3). Data were normalized to the 1g group without treatment.
3.5. The downregulation of F-actin expression by altered gravitational stimulation depends on HSPG
As shown in Fig. 7, 96 h altered gravitational stimulation reduced the total amount of F-actin of the cells to 61.38% of the 1g group. More interestingly, after Hep.III and sodium chlorate treatment, the F-actin levels of both the 1g and the altered gravity groups showed no significant differences. This suggests that HSPG may be an important gravity sensor that mediates F-actin downregulation under conditions of altered gravity.
FIG. 7.
(A) Western blotting analysis of F-actin after 4 days 1g or MG exposure. (B) 4 days MG exposure reduced the amount of F-actin to the level of approximately 70% of the 1g group, while after Hep.III+NaClO3 treatment, the expression of F-actin showed no significant differences between the MG and the 1g groups. F-actin, filamentous actin; MG, altered gravitational stimulation; NaClO3, sodium chlorate. *P<0.05 vs. 1g group without treatment (two-tailed t test, n=3). Data were normalized to the 1g group without treatment.
4. Discussion
In this study, we used a roller culture apparatus to achieve altered gravitational conditions for SMCs. This 2-D clinostat method can null the gravity vector by continuous averaging and mimic the near-zero gravity force encountered in spaceflight (Cogoli and Cogoli-Greuter, 1997). This method has been widely used as an effective ground-based tool to test the effects of microgravity on mammalian cell physiology (Sarkar et al., 2000; Kacena et al., 2002). During rotation, however, other forces, especially shear stress, turbulence, or hydrostatic pressure, may be important factors that affect our results (Herranz et al., 2013). We cannot eliminate the above-mentioned factors completely. However, we filled each flask with culture medium to eliminate air bubbles, thus diminishing the turbulence and shear forces during rotation. As computed by other researchers, the shear level produced in this manner is less than 1 dyn/cm2 (Goodwin et al., 1993), which may be insufficient to trigger responses of SMCs. Furthermore, the effect of the hydrostatic pressure may be neglected because the cells are placed very close to the rotation axis (Albrecht-Buehler, 1992). Therefore, the altered gravitational stimulation may be the most likely factor to induce the observed variation in physiological responses of SMCs.
The net steady-stage glycocalyx dimension is determined by two main factors: the local synthesis and the disruption of its constituents. Previous studies have demonstrated that the highly negative-charged glycocalyx can be disrupted by some atherogenic stimuli, like oxygen-derived free radicals (Vink and Duling, 1996) and oxidized low-density lipoproteins (Vink et al., 2000), which results in elevated capillary tube hematocrit (Constantinescu et al., 2001) and immobilization of leukocytes and platelets at the endothelial surface (Vink et al., 2000; Constantinescu et al., 2003). More recently, van den Berg et al. (2009) uncovered that a high-fat diet could impair the composition and dimension of the endothelial glycocalyx, leading to a 2- to 3-fold increase of low-density lipoprotein transendothelial leakage (van den Berg et al., 2009). Additionally, biomechanical factors like fluid shear stress were reported to stimulate hyaluronan synthesis and protect the blood vessel against pro-inflammatory and pro-atherosclerotic stimuli (Gouverneur et al., 2006). In the present study, our results indicate that gravity alteration may be another biomechanical factor modulating the content of the SMC glycocalyx. The decreased content of HSPG in the altered gravity group may be from the result of a reduction in the synthesis of the glypican-1 core protein and a simultaneous increase in the synthesis of the endogenous heparanase, which disrupted the synthesis-disruption balance and cut down the net steady-stage glycocalyx of the RASMCs.
In the present study, we hypothesized that the glycocalyx might also be involved in gravity sensing due to its close association with the cytoskeleton and caveolae constituents. To elucidate the gravity-sensing role of the SMC glycocalyx, sodium chlorate was employed to inhibit glycocalyx biosynthesis. It has been established that sodium chlorate can competitively bind to the active site of the 3′-phosphoadenosine-5′-phosphosulfate synthase and inhibit the formation of 3′-phosphoadenosine-5′-phosphosulfate, the most important sulfate donor, thereby affecting the sulfation events of the glycocalyx in the Golgi (Safaiyan et al., 1999). Results from our previous studies (Kang et al., 2011) demonstrate that Hep.III pretreatment causes a 39.67±1.3% reduction in fluorescence intensity (or HSPG content) relative to the untreated controls. Moreover, 6 h after Hep.III treatment, cell surface HSPG regrew by 23.78±0.9% relative to the fresh Hep.III exposure cells (Kang et al., 2011). Data obtained in the present study indicate that Hep.III treatment followed by 2-day sodium chlorate exposure can keep the HSPG fluorescence intensity at a 48.34±5.70% reduction relative to the untreated controls, which clearly demonstrates the effectiveness of sodium chlorate treatment on HS-biosynthesis inhibition. Nevertheless, it should be mentioned that, although sodium chlorate treatment has been widely used to define the biological functions of sulfated GAG chains in biological systems, long-time (>24 h) exposure of cells to 30 mM sodium chlorate may inevitably interfere with the growth of RASMCs (Humphries and Silbert, 1988). However, because the same sodium chlorate treatment had been applied to both the control and the experimental groups, the results obtained in the present study should remain valid.
To date, three major isoforms of NOS have been identified in VSMCs, which modulate the vascular tone in an endothelium-independent manner (Buchwalow et al., 2002). One subtype is the inducible NOS (NOSII), which is isolated originally from macrophages and activated in a Ca2+-independent way. The other two isoforms (NOSI and NOSIII) are constitutively expressed and Ca2+-dependent. Our observation that 24 h altered gravitational stimulation significantly enhanced NOS activity might be the result of multiple regulative mechanisms. Among these, the modulation of NOS mRNA and protein expressions can be excluded because, in our experiments, the mRNA and protein levels of NOS (NOSI and NOSIII) were not increased but declined significantly after 24 h altered gravity exposure. Therefore, a posttranslational regulation mechanism (Fleming and Busse, 2003) or the enzyme association with some regulative proteins at the catalytic sites (Spisni et al., 2006) may be more likely to happen. More experiments are required to elucidate the precise mechanism of NOS activation induced by gravity alteration.
Previous studies have established the mechanosensing role of the glycocalyx on cardiovascular cells (EC and SMC) in shear stress-induced NO production (Florian et al., 2003), cell cytoskeleton reorganization (Thi et al., 2004), and cell contraction responses (Ainslie et al., 2005). Nevertheless, its potential mechanosensing role in other mechanical stimuli has never been reported. Data from the present study suggests that the glycocalyx may also be a pivotal gravity sensor in the sensing of gravity changes at the single cellular level.
With regard to the transduction mechanism, one possible mechanism could be that gravity alteration induces syndecan displacement, which results in F-actin reorganization that, in turn, regulates L-arginine transport and affects NO production, as reported by Zharikov et al. (2001). In addition, the mechanical stimuli can be decentralized to different cell regions via syndecans (the primary common proteoglycan core proteins), particularly to the cell junctions (Davies, 1995). Moreover, glypicans (the second most common proteoglycan core proteins) reside in caveolae, coexisted with inactive NOS, G-proteins, receptor and nonreceptor tyrosine kinases, Ca2+ and Na+-K+ ATPase pumps, PKC isoforms, the L-arginine recycling enzymes and its transporter, and a number of other NOS-interacting proteins (Tarbell and Pahakis, 2006). Therefore, simply by their location, any modulation of glypicans induced by gravity alteration could be involved in the downstream NOS activity regulation.
5. Conclusion
In conclusion, the exposure of the cultured RASMCs to altered gravitational conditions led to a reduction in cell surface HSPG content and the activation of NOS. It also downregulated the expression of glypican-1, constitutive NOS (NOSI and NOSIII), and F-actin. On the other hand, Hep.III followed by sodium chlorate treatment of HSPG attenuated the modulation of NOS and F-actin under altered gravitational conditions. All these findings suggest that HSPG or the whole glycocalyx may be an important sensor of gravitational changes that plays important roles in the regulation of NOS activation, F-actin modulation, and HSPG remodeling in VSMCs.
Acknowledgments
This work is supported by Grants-in-Aid from the National Natural Science Research Foundation of China (No. 11072023, 31170904, 61190123, 11228205), the Innovation Foundation of BUAA for PhD Graduates, and Specialized Research Fund for the Doctoral Program of Higher Education (20121102110031).
Author Disclosure Statement
No competing financial interests exist.
Abbreviations
DMEM, Dulbecco's modified Eagle's medium; EC, endothelial cells; FBS, fetal bovine serum; GAGs, glycosaminoglycans; Hep.III, heparinase III; HS, heparan sulfate; HSPG, heparan sulfate proteoglycans; NOS, nitric oxide synthase; RASMCs, rat aortic smooth muscle cells; RT-PCR, reverse transcription polymerase chain reaction; SMCs, smooth muscle cells; VSMCs, vascular smooth muscle cells.
References
- Ainslie K.M. Garanich J.S. Dull R.O. Tarbell J.M. Vascular smooth muscle cell glycocalyx influences shear stress-mediated contractile response. J Appl Physiol. 2005;98:242–249. doi: 10.1152/japplphysiol.01006.2003. [DOI] [PubMed] [Google Scholar]
- Albrecht-Buehler G. The simulation of microgravity conditions on the ground. ASGSB Bull. 1992;5:3–10. [PubMed] [Google Scholar]
- Beamish J.A. He P. Marchant K.K. Marchant R.E. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng Part B Rev. 2010;16:467–491. doi: 10.1089/ten.teb.2009.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwalow I.B. Podzuweit T. Boecker W. Samoilova V.E. Thomas S. Wellner M. Baba H.A. Robenek H. Schnekenburger J. Lerch M.M. Vascular smooth muscle and nitric oxide synthase. FASEB J. 2002;16:500–508. doi: 10.1096/fj.01-0842com. [DOI] [PubMed] [Google Scholar]
- Cogoli A. Cogoli-Greuter M. Activation and proliferation of lymphocytes and other mammalian cells in microgravity. Adv Space Biol Med. 1997;6:33–79. doi: 10.1016/s1569-2574(08)60077-5. [DOI] [PubMed] [Google Scholar]
- Constantinescu A.A. Vink H. Spaan J.A. Elevated capillary tube hematocrit reflects degradation of endothelial cell glycocalyx by oxidized LDL. Am J Physiol Heart Circ Physiol. 2001;280:H1051–H1057. doi: 10.1152/ajpheart.2001.280.3.H1051. [DOI] [PubMed] [Google Scholar]
- Constantinescu A.A. Vink H. Spaan J.A. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol. 2003;23:1541–1547. doi: 10.1161/01.ATV.0000085630.24353.3D. [DOI] [PubMed] [Google Scholar]
- Davies P.F. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P.F. Olesen S.P. Clapham D.E. Morrel E.M. Schoen F.J. Endothelial communication. State of the art lecture. Hypertension. 1988;11:563–572. doi: 10.1161/01.hyp.11.6.563.a. [DOI] [PubMed] [Google Scholar]
- Delp M.D. Colleran P.N. Wilkerson M.K. McCurdy M.R. Muller-Delp J. Structural and functional remodeling of skeletal muscle microvasculature is induced by simulated microgravity. Am J Physiol Heart Circ Physiol. 2000;278:1866–1873. doi: 10.1152/ajpheart.2000.278.6.H1866. [DOI] [PubMed] [Google Scholar]
- Fleming I. Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- Florian J.A. Kosky J.R. Ainslie K. Pang Z. Dull R.O. Tarbell J.M. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93:e136–e142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- Goodwin T.J. Prewett T.L. Wolf D.A. Spaulding G.F. Reduced shear stress: a major component in the ability of mammalian tissues to form three-dimensional assemblies in simulated microgravity. J Cell Biochem. 1993;51:301–311. doi: 10.1002/jcb.240510309. [DOI] [PubMed] [Google Scholar]
- Gouverneur M. Spaan J.A. Pannekoek H. Fontijn R.D. Vink H. Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2006;290:H452–H458. doi: 10.1152/ajpheart.00592.2005. [DOI] [PubMed] [Google Scholar]
- Halayko A.J. Solway J. Molecular mechanisms of phenotypic plasticity in smooth muscle cells. J Appl Physiol. 2001;90:358–368. doi: 10.1152/jappl.2001.90.1.358. [DOI] [PubMed] [Google Scholar]
- Herranz R. Anken R. Boonstra J. Braun M. Christianen P.C. de Geest M. Hauslage J. Hilbig R. Hill R.J. Lebert M. Medina F.J. Vagt N. Ullrich O. van Loon J.J. Hemmersbach R. Ground-based facilities for simulation of microgravity: organism-specific recommendations for their use, and recommended terminology. Astrobiology. 2013;13:1–17. doi: 10.1089/ast.2012.0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries D.E. Silbert J.E. Chlorate: a reversible inhibitor of proteoglycan sulfation. Biochem Biophys Res Commun. 1988;154:365–371. doi: 10.1016/0006-291x(88)90694-8. [DOI] [PubMed] [Google Scholar]
- Ingber D. How cells (might) sense microgravity. FASEB J. 1999;13:S3–S15. doi: 10.1096/fasebj.13.9001.s3. [DOI] [PubMed] [Google Scholar]
- Jin M.A. Kahwaji I.C. Zhenmin N.I. Vaziri D.N. Purdy R. Effects of simulated microgravity on arterial nitric oxide synthase and nitrate and nitrite content. J Appl Physiol. 2003;94:83–92. doi: 10.1152/japplphysiol.00294.2002. [DOI] [PubMed] [Google Scholar]
- Kacena M.A. Todd P. Gerstenfeld L.C. Landis W.J. Experiments with osteoblasts cultured under varying orientations with respect to the gravity vector. Cytotechnology. 2002;39:147–154. doi: 10.1023/A:1023936503105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H. Fan Y. Deng X. Vascular smooth muscle cell glycocalyx modulates shear-induced proliferation, migration and NO production responses. Am J Physiol Heart Circ Physiol. 2011;300:76–83. doi: 10.1152/ajpheart.00905.2010. [DOI] [PubMed] [Google Scholar]
- Kang H. Fan Y. Sun A. Jia X. Deng X. Simulated microgravity exposure modulates the phenotype of cultured vascular smooth muscle cells. Cell Biochem Biophys. 2013;66:121–130. doi: 10.1007/s12013-012-9460-0. [DOI] [PubMed] [Google Scholar]
- Nicogossian A.E. Sawin C.F. Huntoon C.L. Overall physiologic responses to space flight. In: Nicogossian A.E., editor. Space Physiology and Medicine. Lea & Fibiger; Philadelphia: 1996. pp. 213–227. [Google Scholar]
- Nilsson J. Ksiazek T. Thyberg J. Wasteson A. Cell surface components and growth regulation in cultivated arterial smooth muscle cells. J Cell Sci. 1983;64:107–121. doi: 10.1242/jcs.64.1.107. [DOI] [PubMed] [Google Scholar]
- Owens G.K. Kumar M.S. Wamhoff B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Pahakis M.Y. Kosky J.R. Dull R.O. Tarbell J.M. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun. 2007;355:228–233. doi: 10.1016/j.bbrc.2007.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensen S.S.M. Doevendans P.A.F.M. van Eys G.J.J.M. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 2007;15:100–108. doi: 10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaiyan F. Kolset S.O. Prydz K. Gottfridsson E. Lindahl U. Salmivirta M. Selective effects of sodium chlorate treatment on the sulfation of heparan sulfate. J Biol Chem. 1999;274:36267–36273. doi: 10.1074/jbc.274.51.36267. [DOI] [PubMed] [Google Scholar]
- Sarkar D. Nagaya T. Koga K. Nomura Y. Gruener R. Seo H. Culture in vector-averaged gravity under clinostat rotation results in apoptosis of osteoblastic ROS 17/2.8 cells. J Bone Miner Res. 2000;15:489–498. doi: 10.1359/jbmr.2000.15.3.489. [DOI] [PubMed] [Google Scholar]
- Schwarz R.P. Goodwin T.J. Wolf D.A. Cell culture for three-dimensional modeling in rotating-wall vessels: an application of simulated microgravity. J Tissue Cult Methods. 1992;14:51–57. doi: 10.1007/BF01404744. [DOI] [PubMed] [Google Scholar]
- Shi Z.D. Abraham G. Tarbell J.M. Shear stress modulation of smooth muscle cell marker genes in 2-d and 3-d depends on mechanotransduction by heparan sulfate proteoglycans and erk1/2. PLoS One. 2010;5:e12196. doi: 10.1371/journal.pone.0012196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z.D. Wang H. Tarbell J.M. Heparan sulfate proteoglycans mediate interstitial flow mechanotransduction regulating mmp-13 expression and cell motility via fak-erk in 3d collagen. PLoS One. 2011;6:e15956. doi: 10.1371/journal.pone.0015956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sides M.B. Vernikos J. Convertino V.A. Stepanek J. Tripp L.D. Draeger J. Hargens A.R. Kourtidou-Papadeli C. Pavy-LeTraon A. Russomano T. Wong J.Y. Buccello R.R. Lee P.H. Nangalia V. Saary M.J. The Bellagio report: cardiovascular risks of spaceflight: implications for the future of space travel. Aviat Space Environ Med. 2005;76:877–895. [PubMed] [Google Scholar]
- Spisni E. Toni M. Strillacci A. Galleri G. Santi S. Griffoni C. Tomasi V. Caveolae and caveolae constituents in mechanosensing: effect of modeled microgravity on cultured human endothelial cells. Cell Biochem Biophys. 2006;46:155–164. doi: 10.1385/CBB:46:2:155. [DOI] [PubMed] [Google Scholar]
- Tarbell J.M. Pahakis M.Y. Mechanotransduction and the glycocalyx. J Intern Med. 2006;259:339–350. doi: 10.1111/j.1365-2796.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- Thi M.M. Tarbell J.M. Weinbaum S. Spray D.C. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a “bumper-car” model. Proc Natl Acad Sci USA. 2004;101:16483–16488. doi: 10.1073/pnas.0407474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg B.M. Spaan J.A. Vink H. Impaired glycocalyx barrier properties contribute to enhanced intimal low-density lipoprotein accumulation at the carotid artery bifurcation in mice. Pflugers Arch. 2009;457:1199–1206. doi: 10.1007/s00424-008-0590-6. [DOI] [PubMed] [Google Scholar]
- Vink H. Duling B.R. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res. 1996;79:581–589. doi: 10.1161/01.res.79.3.581. [DOI] [PubMed] [Google Scholar]
- Vink H. Constantinescu A.A. Spaan J.A. Oxidized lipoproteins degrade the endothelial surface layer: implications for platelet-endothelial cell adhesion. Circulation. 2000;101:1500–1502. doi: 10.1161/01.cir.101.13.1500. [DOI] [PubMed] [Google Scholar]
- Zhang L.F. Ma J. Mao Q.W. Yu Z.B. Plasticity of arterial vasculature during simulated weightlessness and its possible role in the genesis of postflight orthostatic intolerance. J Gravit Physiol. 1997;4:P97–P100. [PubMed] [Google Scholar]
- Zharikov S.I. Sigova A.A. Chen S. Bubb M.R. Block E.R. Cytoskeletal regulation of the L-arginine/NO pathway in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L465–L473. doi: 10.1152/ajplung.2001.280.3.L465. [DOI] [PubMed] [Google Scholar]