Abstract
Traumatic brain injury (TBI) is a major cause of acquired epilepsy, and significant resources are required to develop a better understanding of the pathologic mechanism as targets for potential therapies. Thus, we decided to investigate whether physical exercise after fluid percussion injury (FPI) protects from oxidative and neurochemical alterations as well as from behavioral electroencephalographic (EEG) seizures induced by subeffective convulsive doses of pentylenetetrazol (PTZ; 35 mg/kg). Behavioral and EEG recordings revealed that treadmill physical training increased latency to first clonic and tonic-clonic seizures, attenuated the duration of generalized seizures, and protected against the increase of PTZ-induced Racine scale 5 weeks after neuronal injury. EEG recordings also revealed that physical exercise prevented PTZ-induced amplitude increase in TBI animals. Neurochemical analysis showed that exercise training increased glutathione/oxidized glutathione ratio and glutathione levels per se. Exercise training was also effective against alterations in the redox status, herein characterized by lipid peroxidation (thiobarbituric acid reactive substances), protein carbonyl increase, as well as the inhibition of superoxide dismutase and Na+,K+-ATPase activities after FPI. On the other hand, histologic analysis with hematoxylin and eosin revealed that FPI induced moderate neuronal damage in cerebral cortex 4 weeks after injury and that physical exercise did not protect against neuronal injury. These data suggest that the ability of physical exercise to reduce FPI-induced seizures is not related to its protection against neuronal damage; however, the effective protection of selected targets, such as Na+/K+-ATPase elicited by physical exercise, may represent a new line of treatment for post-traumatic seizure susceptibility.
Key words: EEG, epilepsy, oxidative stress, post-traumatic seizure
Introduction
Traumatic brain injury (TBI) is a devastating disease that commonly causes disability and strongly affects quality of life in patients.1 This pathology is also frequently referred to as the “silent epidemic” because survivors are often left with significant behavioral disabilities and long-term medical complications, such as epilepsy.2,3 Epidemiologic studies have reported that TBI leads to post-traumatic epilepsy (PTE) in 9–42% of civilian head injuries.4,5 Most of the data concerning the progression of the damage collected during the first months suggest that a cascade of biological events, including reactive oxygen species (ROS) production, underlies the development and propagation of PTE.6–9
In the central nervous system (CNS), oxidative stress (OS) is involved in neuron degeneration in several rodent models of experimental epilepsy and seizure.10–14 Further, experimental findings from our group have demonstrated that a single fluid percussion injury (FPI) episode in rat parietal cortex decreases Na+/K+-ATPase activity, with a concomitant increase in levels of OS markers, 5 weeks after the injury.15,16 This alteration in lipid/protein oxidation, membrane fluidity, and Na+/K+-ATPase activity may be a significant component of PTE development and propagation.7 Of note, a large amount of information regarding the molecular alterations corresponding to the TBI acute phase are available, although relevant studies to assess the chronic epileptogenesis phase are still scarce.17
Concerns about increased seizure frequency and its potential damage after TBI have led health care professionals to adopt protective measures.18,19 In this context, studies have shown that animals and humans clearly undergo significant adaptive responses to regular endurance exercise, which involve increased endurance capacity resulting from mitochondrial biogenesis, oxidant production reduction, and antioxidant defense increase.20,21 Further, experimental findings in epilepsy models, such as temporal lobe epilepsy, reveal that exercise training increases the amount of stimulation necessary to reach the convulsive threshold,22 attenuates seizure frequency, and decreases susceptibility to seizures in the pilocarpine epilepsy model.23,24 These data indicate that exercise training may ameliorate the course of epileptic activity in the brain.
It is important to consider that exercise training may alter brain injury characteristics, depending on exercise type and intensity.25 Moreover, the appropriate window time and intensity of exercise training after TBI may have an influence on injury severity. For example, forced treadmill exercise after a unilateral sensorimotor cortex motor injury increases brain lesion size and results in behavioral impairment.26,27 On the other hand, aerobic exercise training presents neuroprotective properties counteracting OS increases that lead to ROS production.15,28 Although the determining factor for such a discrepancy is unknown, the apparent contradictions elicited by exercise training in this neurological disorder may be explained by different experimental conditions (e.g., species, tissue analyzed, types of exercise training, and TBI models).
Therefore, because there are caveats to consider when interpreting the effect of exercise training after TBI, we aimed to investigate the anticonvulsant effect elicited by exercise training in seizure induced by pentylenetetrazol (PTZ) after TBI. Further, we investigated whether the possible anticonvulsant effect of exercise training involves the decrease of ROS production and Na+/K+-ATPase activity homeostasis.
Methods
Animals and reagents
Adult male Wistar rats (250–250 g) were kept under controlled light-cycle and environmental conditions (12-h light/dark cycle, 24±1°C, and 55% relative humidity). Experimental protocols were designed to use as few animals as possible and also to refrain from suffering during the procedures. The animal experimentation reported on in this study has been carried out in accord with the policies of the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH publication no. 80-23; revised in 1996) as well as with the approval of the Ethics Committee for Animal Research of the Federal University of Santa Maria (23081.018516/2006-57). Reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Experimental design
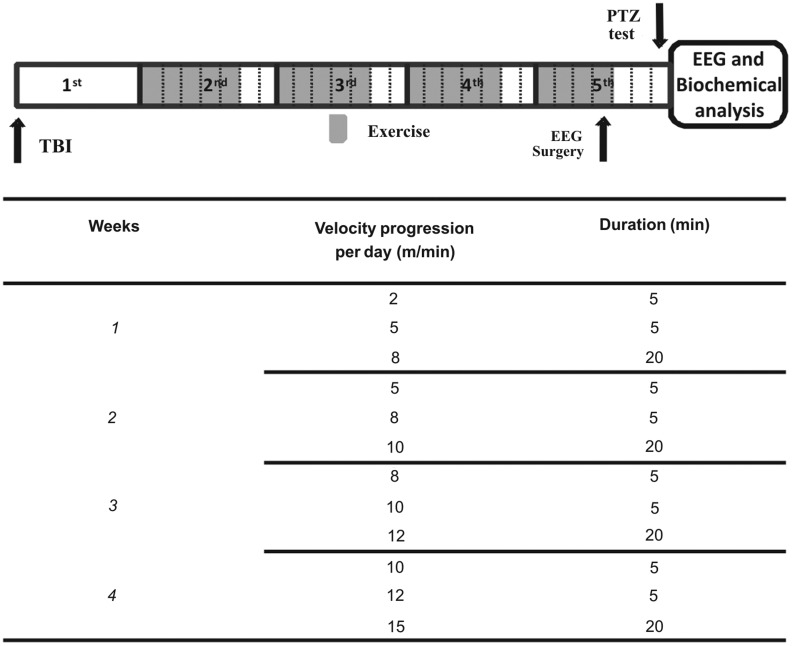
Initially, animals were submitted to TBI by FPI as described below. One week after the FPI procedure, animals were randomly assigned to either exercise or sedentary groups. After 4 weeks of exercise training, animals were submitted to electroencephalographic (EEG) surgery. Three days after the EEG procedure, animals were tested with a subeffective PTZ dose, as described in Figure 1. Immediately after EEG and behavioral evaluation, animals were killed by decapitation and cortical tissues were quickly removed for subsequent biochemical analysis, as described further below.
FIG. 1.
Representation of experimental design with exercise training protocol. Controls of all factors, such as sham and sedentary animals or saline administration, were not represented. Treadmill exercise started 7 days after TBI, for 4 weeks excluding the last 2 days of each week. In the afternoon of the last exercise day, animals were operated on for electrode implantation. Three days after this procedure, all animals were randomized to receive a subeffective dose of PTZ (see the text) or saline. Time and running velocity varied during the same day of training, as indicated. TBI, traumatic brain injury; EEG, electroencephalogram; PTZ, pentylenetetrazol.
TBI
FPI was carried out as previously described.15 Briefly, animals were anesthetized with a single injection of equithesin [6 mL/kg; intraperitoneally (i.p.)], a mixture containing sodium pentobarbital (58 mg/kg), chloralhydrate (60 mg/kg), magnesium sulfate (127.2 mg/kg), propyleneglycol (42.8%), and absolute ethanol (11.6%), and placed in a rodent stereotaxic apparatus. Heart rate, temperature, tale, and eyelid reflexes were used to measure anesthetic depth and duration. A burr hole of 3 mm in diameter was drilled on the right convexity, 2 mm posterior to the bregma and 3 mm lateral to the midline, avoiding dura mater injury. A plastic injury cannula was placed over the craniotomy with acrylic cement. When the cement hardened, the cannula was filled with chloramphenicol and closed with a proper plastic cap. The animal was then removed from the stereotaxic device and returned to the cage. After 24 h, animals were anesthetized with isofluorane, had the injury cannula attached to the fluid percussion device, and were placed in a heat pad maintained at 37±0.2°C. TBI was produced by a fluid percussion device developed in our laboratory. A brief (10–15-ms) transient pressure fluid pulse [4.05±0.17 atmosphere (atm)] impact was applied against the exposed dura mater. Pressure pulses were measured extracranially by a transducer (Fluid Control Automação Hidráulica, Belo Horizonte, MG, Brazil) and recorded on a storage oscilloscope (Gould Ltd., Essex, UK). The sham group underwent the same procedures and was coupled to the injury device; however, no fluid pulse was delivered.
Exercise training
Exercise training was carried out blindly and according to the protocol described by Arida and colleagues,29 with few modifications. Briefly, 1 week after FPI/sham procedures, animals from both groups were randomized and placed on the treadmill (Insight) for 5 days a week in the first 3 weeks and 4 days in the last week, as described in Figure 1. To provide a trainability measurement, treadmill performance was rated on a 1–5 scale according to Dishman and colleagues.30
Surgical procedure and behavioral evaluation
Animals were submitted to surgery 12 h after the last training session. In brief, rats were deeply anesthetized with equithesin (1% phenobarbital, 2% magnesium sulfate, 4% chloral hydrate, 42% propylene glycol, and 11% ethanol; 3 mL/kg, i.p.), and two screw electrodes were placed 4 mm bilaterally to the midline, 2 mm behind the posterior edge of the craniectomy along with a ground lead positioned over the nasal sinus. Electrodes were connected to a multi-pin socket fixed to the skull with acrylic cement. Experiments were performed 3 days after surgery.
Seizure evaluation
Seizures were monitored in all animals by EEG recording, and all behavioral and EEG analyses were carried out blindly by two researchers. Each animal was transferred to a Plexiglas cage (25×25×40 cm) and allowed to adapt for 20 min before EEG recording. Each animal was then connected to the lead socket in a swivel inside a Faraday's cage, and the EEG was recorded using a digital encephalographer (Neuromap EQSA260; Neuromap Ltda, Itajubá, Brazil). EEG signals were amplified, filtered (0.1–70.0 Hz, bandpass), digitalized (sampling rate, 256 Hz), and stored in a PC for off-line analysis. Routinely, a 10-min baseline recording was obtained to establish an adequate control period. After baseline recording, sedentary and trained animals received a saline injection (0.9% NaCl, 1 mL/kg, i.p) or PTZ (35 mg/kg, i.p.). Animals were observed for the appearance of generalized tonic-clonic convulsive episodes for 20 min according to Ferraro and colleagues, who describe clonic convulsions as episodes characterized by typical partial clonic activity affecting the face, head, vibrissae, and forelimbs. Generalized convulsive episodes were considered as broad whole-body clonus involving all four limbs and tail, rearing, and wild running and jumping, followed by sudden loss of upright posture and autonomic signs, such as hypersalivation and defecation, respectively. During the 20-min observation period, latencies for the first generalized tonic-clonic convulsions were measured. EEG recordings were visually analyzed for seizure activity, which were defined by the occurrence of the following alterations in the recording leads31: isolated sharp waves (≥1.5-fold baseline); multiple sharp waves (≥2-fold baseline) in brief spindle episodes (≥1 to ≥5 sec); multiple sharp waves (≥2-fold baseline) in long spindle episodes (≥5 sec); spikes (≥2-fold baseline) plus slow waves; multi-spikes (≥2-fold baseline, ≥3 spikes/complex) plus slow waves; and major seizure (repetitive spikes plus slow waves obliterating background rhythm, ≥5 sec). For quantitative analysis, EEG amplitude was averaged over the 20 min of observation.
Tissue processing for neurochemical analyses
After behavioral evaluation, animals were killed by decapitation and the brain was exposed by removing the parietal bone. Brains were quickly removed and a coronal section (7 mm) of the injured hemisphere corresponding to the impact site of injury was dissected. Cortical tissues surrounding the injured core were homogenized in cold 30-mM Tris-HCl buffer (pH, 7.4) and used for further biochemical analysis.
Fluorometric assay of reduced (GSH) and oxidized (GSSG) glutathione
GSH and GSSG levels were assayed as previously described.32 Briefly, homogenates were centrifuged at 4°C at 100,000g for 30 min and supernatants were separated in two different aliquots for GSH and GSSG measurements. For GSH, 500 μL of supernatant was added to 4.5 mL of phosphate buffer. The final assay mixture (2.0 mL) contained 100 μL of the diluted tissue supernatant, 1.8 μL of phosphate buffer, and 100 μL of o-phthalaldehyde (1 μg/μL). Mixtures were incubated at room temperature for 15 min, and their fluorescent signals were recorded in the luminescence spectrometer at 420-nm emission and 350-nm excitation wavelengths.
For GSSG, a 500-μL portion of the original supernatant was incubated at room temperature with 200 μL of N-ethylmaleimide (NEM; 0.04 M) for 30 min to react with free GSH to prevent its oxidation to GSSG. To this mixture, 4.3 μL of 0.1 N of NaOH was added. A 100-μL portion of this mixture was taken for GSSG measurement, using the procedure previously described for GSH assay, except that NaOH was employed as diluent rather than phosphate/ethylenediaminetetraacetic acid (EDTA) buffer. Results are expressed as GSH/GSSG ratio.
Measurement of protein carbonyl and thiobarbituric acid reactive substances (TBARS) content
Total protein carbonyl content was determined by the method described by Yan and colleagues33 and adapted for brain tissue.34 Briefly, homogenates were diluted to 750–800 μg/mL of protein in each sample, and 1-mL aliquots were mixed with 0.2 mL of 10 mM of 2,4-dinitrophenylhydrazine (DNPH) or 0.2 mL of 2 M of HCl. After incubation at room temperature for 1 h in a dark ambient, 0.6 mL of 150 mM of phosphate-buffered saline (PBS) denaturing buffer with 3% sodium dodecyl sulfate (SDS; pH, 6.8), 1.8 mL of heptane (99.5%), and 1.8 mL of ethanol (99.8%) were added sequentially and mixed with vortex agitation for 40 sec and centrifuged for 15 min. Next, protein isolated from the interface was washed twice with 1 mL of ethyl acetate/ethanol (1:1, v/v) and suspended in 1 mL of denaturing buffer. Each DNPH sample was read at 370 nm in a Hitachi U-2001 spectrophotometer (Hitachi, Tokyo, Japan) against the corresponding HCl sample (blank) and total carbonylation calculated using a molar extinction coefficient of 22,000 M−1 cm−1, as described by Levine and colleagues.35 For TBARS assay,36 a slice of cerebral cortex was homogenized in ultrapurified water, and the thiobarbituric acid (TBA) reagent [15% of trichloroacetic acid (TCA), 0.375% of TBA, and 2.5% (v/v) of HCl] was added. After 30 min of incubation, samples were centrifuged (3000g, 15 min) and TBARS levels were measured at 532 nm.37
Superoxide dismutase (SOD) activity
SOD activity was determined in the brain according to the method proposed by Misra and Fridovich.38 This method is based on the capacity of SOD to inhibit autoxidation of adrenaline to adrenochrome. In brief, the supernatant fraction (100 μL) was added to a medium containing 50 mM of sodium bicarbonate/carbonate buffer (pH, 10.2) and 0.4 mM of adrenaline. Kinetic analysis of SOD was started after adrenaline addition, and the color reaction was measured at 480 nm.
Na+/K+-ATPase activity
The reaction mixture for Na+/K+-ATPase activity assay contained 3 mM of MgCl, 125 mM of NaCl, 20 mM of KCl, and 50 mM of Tris-HCl (pH, 7.4) in a final volume of 500 μL. The reaction was started by the addition of adenosine triphosphate (ATP) to a final concentration of 3.0 mM. For obtaining ouabain-sensitive activity, samples were carried out under the same conditions with the addition of 0.1 mM of ouabain. Samples were incubated at 37°C for 30 min, and the incubation was stopped by adding TCA solution (10% TCA) with 10 mM of HgCl2. Na+/K+-ATPase activity was calculated by subtracting the ouabain-sensitive activity from the overall activity (in the absence of ouabain). Release dinorganic phosphate (Pi) was spectrofluorimetrically measured at 650 nm, as described by Wyse and colleagues,39 and Na+/K+-ATPase activity was expressed as nmol Pi/mg protein/min.
Tissue processing for histologic analysis
To determine the effect of TBI and physical exercise on neurological damage, animals were randomly assigned to either exercise or sedentary groups. After 4 weeks of exercise training, animals (sham/sedentary, n=7; exercise/sham, n=7; TBI/sedentary, n=7; and TBI/exercise group, n=7) were deeply anesthetized with 5% isoflurane and perfused transcardially with 20 mL of heparinized 0.9% saline in 0.1% phosphate buffer, then 20 mL of 4% paraformaldehyde (PFA) in 0.1% phosphate buffer. Brains were postfixed in 4% PFA in 0.1% phosphate buffer solution overnight, before being removed from the skull and returned to the same solution for 2 h before being transferred into 0.1% phosphate buffer solution overnight. Brains were then divided into 3-mm-thick coronal blocks for paraffin processing. Next, 4-μm-thick sections were cut and mounted in pairs on poly-L-lysine–coated slides.
Histologic analysis
Slides containing sections from the level corresponding to 2 mm posterior to the bregma and 3 mm lateral to the midline, according to Franklin and Paxinos,40 were selected for staining with hematoxylin and eosin (H&E) to determine the presence of neuronal damage. Cross-cuts were made identical in brains of rats (region trauma), forming a sample of the cortex, hippocampus, and thalamus. This sample was dehydrated in increasing concentrations of ethyl alcohol, cleared in xylene, and embedded in paraffin. After, 4-μm cuts were made and stained with H&E.
Statistical analyses
Neurochemical analyses (total carbonyl, TBARS levels, GSH, GSSG, SOD, and Na+/K+-ATPase activity) were analyzed by a three-way analysis of variance (ANOVA). All data are expressed as the mean±standard error of the mean.
Mann-Whitney's test was performed to evaluate exercise intensity in the sham and TBI groups. Latency to first clonic and generalized tonic-clonic seizures were analyzed by Scheirer-Ray-Hare's test and expressed as median±interquartile range. A one-sided Fisher's exact test was used to calculate risk for seizure susceptibility and adherence of sham and TBI groups to exercise protocol. A probability of p<0.05 was considered significant.
Results
Differences between sham and TBI groups in exercise intensity and adherence were evaluated. All animals allocated to perform the physical training protocol did it successfully and no difference in exercise intensity was observed between sham and TBI groups (data not shown).
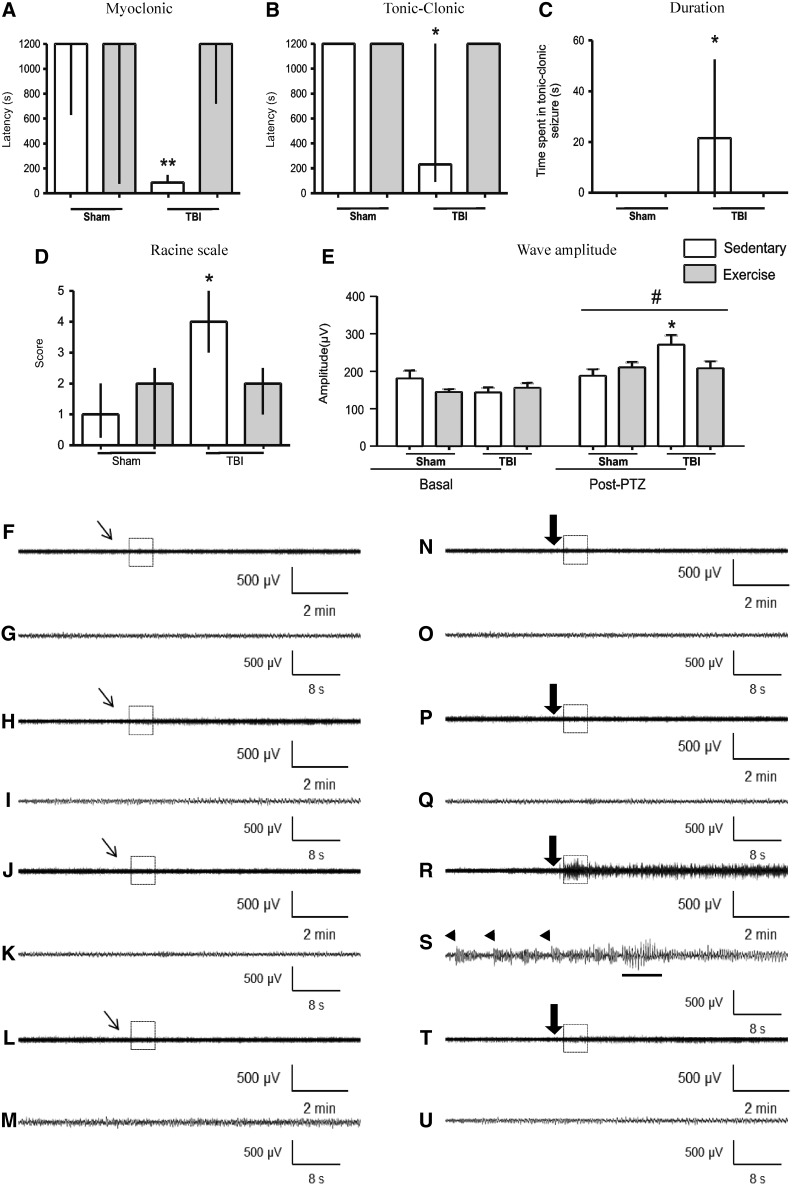
One of the main aims of this study was to evaluate the effect of the proposed exercise protocol on seizure susceptibility in TBI rats. In this context, statistical analysis revealed that 4 weeks of exercise training had significant statistical interaction with FPI, increasing latency to first myoclonic (H1=21.60; p<0.01; Fig. 2A) and tonic-clonic seizures (H1=7.00; p<0.05; Fig. 2B), decreasing time spent in generalized tonic-clonic seizures (H1=7.00; p<0.05; Fig. 2C) and score on Racine's scale (H1=4.36; p<0.05; Fig. 2D) induced by subeffective dose of PTZ (35 mg/kg). EEG recordings confirmed behavioral seizures elicited by PTZ and the effective prevention induced by exercise training (Fig. 2N–U) characterized by latency and duration of seizures as well as Racine scale. EEG recordings revealed that PTZ induced epileptogenic discharges in ipsi- and contralateral as well as increased wave amplitude in both sides, comparing with the basal period (F1,31=46.93; p<0.001; Fig. 2E]. Statistical analysis showed that the PTZ-induced amplitude increase was more pronounced in sedentary TBI animals (F3,31=7.23; p<0.001) and that treadmill physical exercise protected against this increase. The present protocol of physical training also decreased the number of myoclonic and tonic-clonic seizure induced by PTZ after neuronal injury (Table 1).
FIG. 2.
Effect of 4-week physical exercise started 1 week after TBI in seizure susceptibility with PTZ (35 mg/kg, intraperitoneally). Data from latency to the first myoclonic seizure (A), first tonic-clonic seizure (B), time spent in generalized tonic-clonic seizure (C), and Racine scale (D) are median±interquartile range. Data from wave amplitude analysis (E) are mean±standard error of the mean; all for n=8–11 per group. *p<0.05 and **p<0.01, compared with sham-sedentary, sham-exercise, and TBI-exercise (Scheirer-Ray-Hare and two-way ANOVA tests). #p<0.001, compared with basal period (two-way repeated measures ANOVA). Representative EEG recordings from ipsilateral parietal cortex after administration of saline (F-M) and PTZ (N-U), with their respective expanded waveforms outlined by boxes of sham-sedentary (F-G, N-O), sham-exercise (H-I, P-Q), TBI-sedentary (J-K, R-S), and TBI-exercise (L-M, T-U) groups. Inclined thin arrows indicate saline administration; large arrows indicate PTZ injection. Arrowheads indicate myoclonic seizure. Tonic-clonic seizure is indicated with an underlying trace. TBI, traumatic brain injury; ANOVA, analysis of variance; EEG, electroencephalogram; PTZ, pentylenetetrazol.
Table 1.
Occurrence of Myoclonic and Tonic-Clonic Seizure
| Variable | Sham/sedentary (%) | Sham/exercise (%) | TBI/sedentary (%) | TBI/exercise (%) |
|---|---|---|---|---|
| Myoclonic seizure | 2/8 (25) | 4/9 (44) | 10/11 (91)* | 2/9 (22) |
| Tonic-clonic seizure | 0/8 (0) | 0/9 (0) | 8/11 (72)* | 0/9 (0) |
Data from EEG and behavioral analysis after administered subconvulsant dose of PTZ (35 mg/kg, i.p.), revealing the number of animals in each group that developed myoclonic and tonic-clonic seizure.
p<0.01, compared to all other groups (Fisher's test).
TBI, traumatic brain injured rats; EEG, electroencephalographic; i.p., intraperitoneally.
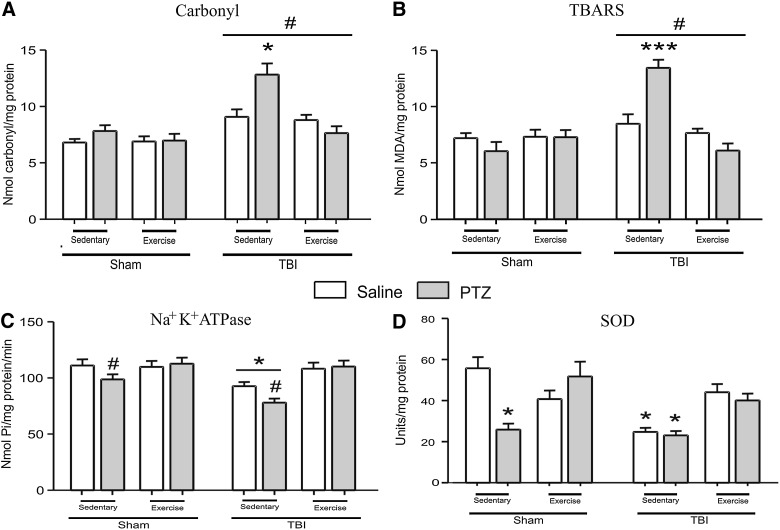
In the present study, the participation of GSH and GSSG levels and their ratio in the development and propagation of seizure susceptibility to PTZ was investigated (Table 2). Statistical analysis revealed a significant effect of exercise training, increasing GSH levels (F1,64=4.13; p<0.05) and GSH/GSSG ratio (F1,64=7.73; p<0.01). However, GSH/GSSG ratio and levels of GSH and GSSG were not altered in sham/TBI or saline/PTZ procedures. Statistical analysis also showed that FPI induced protein carbonyl (F1,64=30.44; p<0.001; Fig. 3A), TBARS increase (F1,64=18.57; p<0.001; Fig. 3B), and SOD activity decrease (F1,56=13.03; p<0.002; Fig. 3D), when compared to the sham group. It is important to note that the decrease in SOD activity was not worsened by PTZ in the TBI/sedentary group, demonstrating the lack of statistical interaction between PTZ and TBI factors in this assay. On the other hand, statistical analysis showed that this protocol of physical exercise had a significant interaction with TBI and PTZ procedures in TBARS (F1,64=17.65; p<0.001), protein carbonyl content (F1,64=4.98; p<0.05), and SOD activity (F1,56=13.81; p<0.002) in the ipsilateral cortex of FPI animals. Post-hoc analysis revealed that exercise training decreased protein carbonylation and TBARS production and maintained SOD activity in sham/TBI, saline/PTZ, and PTZ/TBI groups.
Table 2.
Effect of TBI on GSH, GSSG Content and GSH/GSSG Ratio
| |
GSH content (nmol g/tissue) |
GSSG content (nmol g/tissue) |
GSH/GSSG ratio (nmol g/tissue) |
|||
|---|---|---|---|---|---|---|
| Treatment | Sham | TBI | Sham | TBI | Sham | TBI |
| NaCI | 34.57±1.50 | 33.22±1.71 | 17.40±1.16 | 16.83±1.01 | 2.05±0.14 | 2.01±0.11 |
| PTZ | 31.14±1.64 | 34.59±2.04 | 15.75±0.64 | 18.30±0.96 | 1.99±0.10 | 1.91±0.10 |
| NaCl/Ex | 36.07±1.85* | 35.42±2.06* | 17.35±0.81 | 14.17±0.73 | 2.10±0.13* | 2.56±0.21* |
| PTZ/Ex | 35.80±1.52* | 37.36±2.07* | 15.68±0.71 | 17.49±0.94 | 2.34±0.18* | 2.18±0.15* |
Data from GSH and GSSG content. Data are mean±standard error of the mean for n=8–11 in each group.
p<0.05, comparing physical exercise groups to sedentary animals on increased GSH and GSH/GSSG content, with no significant effect of other factors (three-way analysis of variance and Student-Newman-Keuls SNK tests).
TBI, traumatic brain injury; GSH, reduced glutathione; GSSG, oxidized glutathione; PTZ, pentylenetetrazol; Ex, animals submitted to exercise protocol.
FIG. 3.
Data from total protein carbonylation (A) and TBARS (B). *p<0.05 and ***p<0.001, compared to all other groups, revealing the interaction between all factors and the protective effect of physical exercise in the TBI/PTZ group for this analysis; #p<0.001, compared to sham groups. Data from Na+/K+ATPase activity (C). *p<0.05, compared to the TBI/exercise group independently from saline/PTZ procedures; #p<0.05 compared, to exercise/PTZ groups, independently from sham/TBI procedures. Data from SOD activity (D). *p<0.05 compared to other groups, revealing the effect of physical exercise, increasing SOD activity even in the TBI and PTZ groups. All data are mean±standard error of the mean for n=8–11. TBI, traumatic brain injury; PTZ, pentylenetetrazol; saline, sterile NaCl 0.9% (vehicle of PTZ); TBARS, thiobarbituric acid reactive species; MDA, malondialdehyde; SOD, superoxide dismutase.
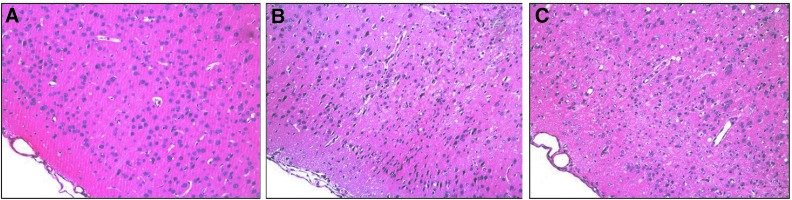
Considering that Na+/K+-ATPase enzyme plays a pivotal role in cellular ionic gradient maintenance and is particularly sensitive to free radicals,41 we decided to investigate the involvement of this enzyme in the progression and manifestation of seizure elicited by PTZ in this model of TBI. Statistical analysis revealed a decrease in Na+/K+-ATPase activity in the ipsilateral cerebral cortex of FPI (F1,64=9.56; p<0.01; Fig. 3C) and PTZ animals (F1,64=5.10; p<0.05; Fig. 3C), when compared to sham groups. Statistical analysis also revealed that PTZ administration had not additive effect on Na+/K+-ATPase activity inhibition in the TBI group (F1,64=0.05; p=0.818). On the other hand, post-hoc analysis revealed that treadmill exercise prevented the decrease in the Na+/K+-ATPase activity induced by PTZ (F1,64=18.10; p<0.001) or TBI (F1,64=6.37; p<0.05). Histological analysis (H&E staining) identified damaged neurons characterized by pyknosis, which were distinguishable from dark cell changes by their eosinophilic appearance and blebbing of nuclei (Fig. 4). In the cortex local to the craniotomy site, 7 of 7 brain-injured animals and 6 of 7 exercise-injured animals had neuronal loss with a marked gemistocytic amount of astrocytes and moderate gliosis. Four weeks after injury, no damage was noted in the hippocampus and thalamus (data not shown).
FIG. 4.
Cellular changes in the ipsilateral hemisphere 4 weeks after injury. No damage was observed in the cerebral cortex of the sham/sedentary group (A). Both TBI/exercise (B) and TBI/sedentary (C) animals showed damaged neurons in the cortex close to the injury site, as demonstrated by hematoxylin and eosin (H&E) staining.
Discussion
Results presented in this report confirmed and extended previous findings that a single FPI episode in rat parietal cortex increases OS markers, alters redox status, and decreases Na+/K+-ATPase activity after 5 weeks of injury.16 In addition, our data revealed that the injection of a subthreshold dose of PTZ (35 mg/kg, i.p.) after 4 weeks of injury induced by FPI decreased latency for first clonic seizures and increased the time of spent generalized tonic-clonic seizures. This behavioral seizure induced by PTZ was followed by Na+/K+-ATPase activity inhibition and concomitant increase in levels of OS markers. These experiential findings reinforce the assumption that OS after TBI contributes to the excitability of injured tissue and may be correlated with seizure susceptibility development.7 In fact, histologic analysis (H&E) revealed that FPI induced moderate neuronal damage in the cerebral cortex 4 weeks after injury. Taken together, these data demonstrated that there is increased histopathology vulnerability of the post-traumatic brain to periods of seizures elicited by PTZ. These results agree with epidemiologic studies that have demonstrated a significant association between brain damage with elevated risk of PTE.4 Further, experimental studies have demonstrated that lesions located in specific areas, such as the cortex (perirhinal, entorhinal, and postrhinal), are associated with lowered seizure threshold and seizures,42,43 indicating that severity of cortical injury correlates with epileptogenesis and epilepsy in the experimental model as it occurs in humans.
The present study shows, for the first time to our knowledge, that treadmill exercise training started 1 week after FPI protected against seizure susceptibility, EEG, OS markers, and neurochemical alterations induced by a subeffective dose of PTZ (35 mg/kg). Interestingly, our data revealed that this protocol of physical exercise had no effect on FPI-induced neuronal damage, suggesting that the antioxidant ability exerted by this protocol of physical exercise does not protect against TBI-induced neuronal damage. These data also suggest that the ability of physical exercise to reduce FPI-induced seizures is not related to its protection against neuronal damage. On the other hand, the effective protection elicited by physical exercise of selected targets for free radicals, such as Na+/K+-ATPase, may represent a new line of treatment for post-traumatic seizure.
Currently, TBI is a significant public health concern,1 and the understating of secondary brain injury after trauma is of critical need. In this context, the excessive free radical generation and consequent impairment of endogenous antioxidant mechanisms play a significant role in the secondary events elicited by TBI.44–47 In line with this view, the occurrence of lipid peroxidation (LPO), protein carbonylation, and SOD activity inhibition 5 weeks after TBI suggests that alterations in lipid/protein oxidation and SOD activity may be correlated with seizure susceptibility in this TBI model. On the other hand, it is important to consider that events after brain injury are not linear. The processes may start with an initial insult that may or may not involve acute seizure activity, but that lead to later epilepsy development.48 Bao and colleagues demonstrated that post-traumatic seizures induced by a subthreshold dose of PTZ exacerbate histopatologic damage 2 weeks after moderate FPI in rats, suggesting that the post-traumatic brain is extremely sensitive to patterns of abnormal activation. Results presented in this report revealed that animals that were more susceptible to seizures (TBI/sedentary group) presented higher levels of protein carbonyl and TBARS after PTZ injection, reinforcing the idea that seizures may be a delicate pathological state in which its prevention is of paramount importance in the secondary injury of TBI.49
It is also plausible to propose that free radical overproduction induces the development of epileptic focus by disruption of antioxidant activity50 and by oxidative inactivation of membrane Na+/K+-ATPase. This enzyme is the main one responsible for maintaining ion gradients across plasma membranes and is the main ATP consumer in neurons.41 Thus, the decrease of Na+/K+-ATPase activity in the ipsilateral cerebral cortex of TBI animals reinforces the idea that selected targets for oxidative damage, such as Na+/K+-ATPase, may be related to post-traumatic seizure susceptibility after TBI.7 However, it is worth pointing out that the simultaneous increase of free radical generation and convulsions in this model of TBI does not necessary imply a cause-effect relationship between these events. In this context, our data showed that PTZ caused an additional increase in lipid and protein oxidation markers in TBI animals; however, this additive effect was not observed in the decreased activity of Na+/K+-ATPase and SOD, with both PTZ and TBI causing this outcome independently. This lack of effect may be explained by a possible maximum reduction of the activities of these enzymes exerted by head trauma, and thus PTZ could not cause any additional decrement in their functions. However, reasons for these results are still unclear and additional studies are necessary to clarify the involvement of Na+/K+-ATPase in post-traumatic seizure after TBI.
Inhibition of SOD activity after neuronal injury induce an overproduction of superoxide anions into oxygen and hydrogen peroxide.51 The hydrogen peroxide generated could become further reduced by ferrous iron to hydroxyl radicals, which are even more reactive and capable of initiating LPO. Because SOD is a scavenger of ROS and adaptive responses to regular exercise involve oxygen uptake increment and mitochondrial biogenesis,20,52 we suggest that the increase of antioxidant defenses and free-radical-leak reduction during oxidative phosphorylation increase the amount of stimulation necessary to reach the convulsive threshold in this TBI model. In fact, although PTZ and TBI factors had no statistical interaction, exercise training protected against the SOD activity decrease induced by TBI and PTZ.
Nevertheless, our data disagree with previous studies (see earlier review53), because TBI had no effect on GSH/GSSG ratio after 5 weeks of neuronal injury. In the cortical contusion injury (CCI) model, experimental findings demonstrated a significant time-dependent change in GSH/GSSG ratio, with maximum depletion observed 24 h postinjury (see earlier review53). These contradictions may be supported by the differences between the two experimental models as well as because of the fact that the present study focused on the late follow-up (5 weeks) after TBI, whereas the other studies mentioned focused on the early TBI phase. On the other hand, the exercise protocol proposed in this report enhanced GSH level and GSH/GSSG ratio, revealing a possible positive factor related to the beneficial effects observed in animals submitted to physical training.
In this context, experimental and clinical studies have suggested that regular exercise after TBI promotes an alleviation of neuronal injury and cognitive deficits caused by a variety of phenomena, which include antiapoptosis, neurogenesis, as well as cardio- and cerebrovascular effects with focus on dynamic training.18 In addition, voluntary exercise performed 2 weeks after mild FPI increases molecular markers of plasticity such as brain-derived neurotrophic factor (BDNF), with animals exhibiting a significant enhancement in cognitive performance.54 On the other hand, animal studies have found that FPI and acute physical exercise may induce pronounced learning and memory deficits, compared to unexercised, injured rats.55 Taken together, it has been suggested that exercise has significant rehabilitative value after TBI, but an appropriate postinjury time window for reinstatement of exercise training is necessary.25 A recent study found that exercise training 6 weeks before injury increased the survival of cerebellar neurons after neuronal injury.56 Further, it has been demonstrated that aerobic exercise training exerts a prophylactic effect on TBI-induced inflammatory response, free radical generation, and Na+/K+-ATPase activity inhibition.15,57 These experimental findings suggest that adaptive responses to regular and moderate endurance exercise may protect against the failure of a few selected targets, such as Na+/K+-ATPase enzyme, in this TBI model. In line with this view, results presented in this report revealed a significant exercise training effect on GSH levels and effectiveness against the decrease of TBI-induced SOD activity. In fact, experimental findings revealed that the training protocol described herein protected against SOD inhibition after TBI and PTZ injection. This adaptive response may also result in improved ability of the neuron to withstand TBI-mediated energy depletion and, consequently, free radical production.45,58,59 It is important to consider that the fundamental aim of conducting experiments on animal models is to understand the main clinical complications arising from TBI.
In this context, our experimental findings agree with studies that have demonstrated that exercise performance increases the amount of stimulation necessary to reach the convulsive threshold,22 attenuates frequency of seizures, and decreases susceptibility to subsequently evoked seizures in the pilocarpine model of epilepsy.23,24 Further, considering that seizure activity raised oxidative damage in TBI rats and that physical exercise prevented seizures, it is plausible to conclude that physical exercise may also protect against secondary damage by preventing seizure onset, which is probably facilitated by previous TBI-generated OS increase and other enzymatic dysfunctions mentioned above.
In conclusion, the present study reports that a single FPI episode in rat parietal cortex induces free radical generation, alters redox status, and decreases Na+/K+-ATPase activity after 5 weeks of injury. These neuronal alterations contribute to the excitability of injured tissue and may be related to post-traumatic epilepsy development. However, seizure itself may also enhance OS in the already vulnerable injured brain, a fact that reveals the importance of preventing these events. Our experimental findings also revealed that treadmill exercise training increased the latency for the first convulsive episode and attenuated the duration of seizures induced by a subeffective dose of PTZ after TBI. Although significant resources are required for a better understanding of the pathophysiology of PTE, the protection exerted by this exercise protocol suggests that exercise training may be an important therapeutic approach to control seizure activity after TBI.
Acknowledgments
The authors thank Dr. Guilherme Bresciani, Dr. Leandro Rodrigo Ribeiro, Iuri Domingues Della-Pace, and Guilherme Busanello for sharing their expertise and knowledge to contribute to this study. This work was supported by FAPERGS/CNPq (grants: Pronem: 11/2082-4).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.De Silva M.J. Roberts I. Perel P. Edwards P. Kenward M.G. Fernandes J. Shakur H. Patel V. Patient outcome after traumatic brain injury in high-, middle- and low-income countries: analysis of data on 8927 patients in 46 countries. Int. J. Epidemiol. 2009;38:452–458. doi: 10.1093/ije/dyn189. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein L.H. Behavioural and cognitive-behavioural treatments for epilepsy: a progress review. Br. J. Clin. Psychol. 1990;29:257–269. doi: 10.1111/j.2044-8260.1990.tb00884.x. [DOI] [PubMed] [Google Scholar]
- 3.Vaishnavi S. Rao V. Fann J.R. Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic. Psychosomatics. 2009;50:198–205. doi: 10.1176/appi.psy.50.3.198. [DOI] [PubMed] [Google Scholar]
- 4.Annegers J.F. Hauser W.A. Coan S.P. Rocca W.A. A population-based study of seizures after traumatic brain injuries. N. Engl. J. Med. 1998;338:20–24. doi: 10.1056/NEJM199801013380104. [DOI] [PubMed] [Google Scholar]
- 5.Asikainen I. Kaste M. Sarna S. Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: brain injury factors causing late seizures and influence of seizures on long-term outcome. Epilepsia. 1999;40:584–589. doi: 10.1111/j.1528-1157.1999.tb05560.x. [DOI] [PubMed] [Google Scholar]
- 6.Clausen F. Marklund N. Lewen A. Enblad P. Basu S. Hillered L. Interstitial F(2)-isoprostane 8-iso-PGF(2alpha) as a biomarker of oxidative stress after severe human traumatic brain injury. J. Neurotrauma. 2012;29:766–775. doi: 10.1089/neu.2011.1754. [DOI] [PubMed] [Google Scholar]
- 7.Silva L.F. Hoffmann M.S. Rambo L.M. Ribeiro L.R. Lima F.D. Furian A.F. Oliveira M.S. Fighera M.R. Royes L.F. The involvement of Na+, K+-ATPase activity and free radical generation in the susceptibility to pentylenetetrazol-induced seizures after experimental traumatic brain injury. J. Neurol. Sci. 2011;308:35–40. doi: 10.1016/j.jns.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 8.Willmore L.J. Ballinger W.E., Jr. Boggs W. Sypert G.W. Rubin J.J. Dendritic alterations in rat isocortex within an iron-induced chronic epileptic focus. Neurosurgery. 1980;7:142–146. doi: 10.1227/00006123-198008000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Willmore L.J. Rubin J.J. Effects of antiperoxidants on FeCl2-induced lipid peroxidation and focal edema in rat brain. Exp. Neurol. 1984;83:62–70. doi: 10.1016/0014-4886(84)90046-3. [DOI] [PubMed] [Google Scholar]
- 10.Frantseva M.V. Perez Velazquez J.L. Tsoraklidis G. Mendonca A.J. Adamchik Y. Mills L.R. Carlen P.L. Burnham M.W. Oxidative stress is involved in seizure-induced neurodegeneration in the kindling model of epilepsy. Neuroscience. 2000;97:431–435. doi: 10.1016/s0306-4522(00)00041-5. [DOI] [PubMed] [Google Scholar]
- 11.Gluck M.R. Jayatilleke E. Shaw S. Rowan A.J. Haroutunian V. CNS oxidative stress associated with the kainic acid rodent model of experimental epilepsy. Epilepsy Res. 2000;39:63–71. doi: 10.1016/s0920-1211(99)00111-4. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A. Naorem T. Cognitive retraining in epilepsy. Brain Inj. 2003;17:161–174. doi: 10.1080/0269905021000010195. [DOI] [PubMed] [Google Scholar]
- 13.Gupta Y.K. Veerendra Kumar M.H. Srivastava A.K. Effect of Centella asiatica on pentylenetetrazole-induced kindling, cognition and oxidative stress in rats. Pharmacol. Biochem. Behav. 2003;74:579–585. doi: 10.1016/s0091-3057(02)01044-4. [DOI] [PubMed] [Google Scholar]
- 14.Patsoukis N. Zervoudakis G. Georgiou C.D. Angelatou F. Matsokis N.A. Panagopoulos N.T. Effect of pentylenetetrazol-induced epileptic seizure on thiol redox state in the mouse cerebral cortex. Epilepsy Res. 2004;62:65–74. doi: 10.1016/j.eplepsyres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Lima F.D. Oliveira M.S. Furian A.F. Souza M.A. Rambo L.M. Ribeiro L.R. Silva L.F. Retamoso L.T. Hoffmann M.S. Magni D.V. Pereira L. Fighera M.R. Mello C.F. Royes L.F. Adaptation to oxidative challenge induced by chronic physical exercise prevents Na+,K+-ATPase activity inhibition after traumatic brain injury. Brain Res. 2009;1279:147–155. doi: 10.1016/j.brainres.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 16.Lima F.D. Souza M.A. Furian A.F. Rambo L.M. Ribeiro L.R. Martignoni F.V. Hoffmann M.S. Fighera M.R. Royes L.F. Oliveira M.S. de Mello C.F. Na+,K+-ATPase activity impairment after experimental traumatic brain injury: relationship to spatial learning deficits and oxidative stress. Behav. Brain Res. 2008;193:306–310. doi: 10.1016/j.bbr.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Pitkänen A. Lukasiuk K. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav. 2009;14(Suppl. 1):16–25. doi: 10.1016/j.yebeh.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 18.Archer T. Influence of physical exercise on traumatic brain injury deficits: scaffolding effect. Neurotox. Res. 2012;21:418–434. doi: 10.1007/s12640-011-9297-0. [DOI] [PubMed] [Google Scholar]
- 19.Loane D.J. Faden A.I. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol. Sci. 2010;31:596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Packer L. Cadenas E. Oxidants and antioxidants revisited. New concepts of oxidative stress. Free Radic. Res. 2007;41:951–952. doi: 10.1080/10715760701490975. [DOI] [PubMed] [Google Scholar]
- 21.Sachdev S. Davies K.J. Production, detection, and adaptive responses to free radicals in exercise. Free Radic. Biol. Med. 2008;44:215–223. doi: 10.1016/j.freeradbiomed.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Arida R.M. de Jesus Vieira A. Cavalheiro E.A. Effect of physical exercise on kindling development. Epilepsy Res. 1998;30:127–132. doi: 10.1016/s0920-1211(97)00102-2. [DOI] [PubMed] [Google Scholar]
- 23.Arida R.M. Scorza F.A. Peres C.A. Cavalheiro E.A. The course of untreated seizures in the pilocarpine model of epilepsy. Epilepsy Res. 1999;34:99–107. doi: 10.1016/s0920-1211(98)00092-8. [DOI] [PubMed] [Google Scholar]
- 24.Setkowicz Z. Mazur A. Physical training decreases susceptibility to subsequent pilocarpine-induced seizures in the rat. Epilepsy Res. 2006;71:142–148. doi: 10.1016/j.eplepsyres.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Griesbach G.S. Exercise after traumatic brain injury: is it a double-edged sword? Am. Acad. Phys. Med. Rehab. 2011;3:564–572. doi: 10.1016/j.pmrj.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Humm J.L. Kozlowski D.A. James D.C. Gotts J.E. Schallert T. Use-dependent exacerbation of brain damage occurs during an early post-lesion vulnerable period. Brain Res. 1998;783:286–292. doi: 10.1016/s0006-8993(97)01356-5. [DOI] [PubMed] [Google Scholar]
- 27.Kozlowski D.A. James D.C. Schallert T. Use-dependent exaggeration of neuronal injury after unilateral sensorimotor cortex lesions. J. Neurosci. 1996;16:4776–4786. doi: 10.1523/JNEUROSCI.16-15-04776.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griesbach G.S. Hovda D.A. Gomez-Pinilla F. Sutton R.L. Voluntary exercise or amphetamine treatment, but not the combination, increases hippocampal brain-derived neurotrophic factor and synapsin I following cortical contusion injury in rats. Neuroscience. 2008;154:530–540. doi: 10.1016/j.neuroscience.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arida R.M. Scorza F.A. de Lacerda A.F. Gomes da Silva S. Cavalheiro E.A. Physical training in developing rats does not influence the kindling development in the adult life. Physiol. Behav. 2007;90:629–633. doi: 10.1016/j.physbeh.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Dishman R.K. Armstrong R.B. Delp M.D. Graham R.E. Dunn A.L. Open-field behavior is not related to treadmill performance in exercising rats. Physiol. Behav. 1988;43:541–546. doi: 10.1016/0031-9384(88)90206-5. [DOI] [PubMed] [Google Scholar]
- 31.McColl C.D. Horne M.K. Finkelstein D.I. Wong J.Y. Berkovic S.F. Drago J. Electroencephalographic characterisation of pentylenetetrazole-induced seizures in mice lacking the alpha 4 subunit of the neuronal nicotinic receptor. Neuropharmacology. 2003;44:234–243. doi: 10.1016/s0028-3908(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 32.Hissin P.J. Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976;74:214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- 33.Yan L.J. Traber M.G. Packer L. Spectrophotometric method for determination of carbonyls in oxidatively modified apolipoprotein B of human low-density lipoproteins. Anal. Biochem. 1995;228:349–351. doi: 10.1006/abio.1995.1362. [DOI] [PubMed] [Google Scholar]
- 34.Schneider Oliveira M. Flávia Furian A. Freire Royes L.F. Rechia Fighera M. J. de Carvalho Myskiw J. Gindri Fiorenza N. Mello C.F. Ascorbate modulates pentylenetetrazol-induced convulsions biphasically. Neuroscience. 2004;128:721–728. doi: 10.1016/j.neuroscience.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Levine R.L. Garland D. Oliver C.N. Amici A. Climent I. Lenz A.G. Ahn B.W. Shaltiel S. Stadtman E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 36.Ohkawa H. Ohishi N. Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 37.Rios C. Santamaria A. Quinolinic acid is a potent lipid peroxidant in rat brain homogenates. Neurochem. Res. 1991;16:1139–1143. doi: 10.1007/BF00966592. [DOI] [PubMed] [Google Scholar]
- 38.Misra H.P. Fridovich I. The generation of superoxide radical during the autoxidation of hemoglobin. J. Biol. Chem. 1972;247:6960–6962. [PubMed] [Google Scholar]
- 39.Wyse A.T. Streck E.L. Barros S.V. Brusque A.M. Zugno A.I. Wajner M. Methylmalonate administration decreases Na+,K+-ATPase activity in cerebral cortex of rats. Neuroreport. 2000;11:2331–2334. doi: 10.1097/00001756-200007140-00052. [DOI] [PubMed] [Google Scholar]
- 40.Franklin K.B.J. Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1997. [Google Scholar]
- 41.Ames A., 3rd CNS energy metabolism as related to function. Brain Res. Brain Res. Rev. 2000;34:42–68. doi: 10.1016/s0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- 42.Bao Y.H. Bramle H.M. Atkins C.M. Truettner J.S. Lotocki G. Alonso O.F. Dietrich W.D. Post-traumatic seiszures exacerbate histopathological damage after fluid-percussion brain injury. J. Neurotrauma. 2011;28:35–42. doi: 10.1089/neu.2010.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kharatishvili I. Pitakänen A. Association of the severity of cortical damage with the occurrence of spontaneous seizures and hyperexcitability in an animal model of posttraumatic epilepsy. Epilepsy Res. 2010;90:47–59. doi: 10.1016/j.eplepsyres.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Hall E.D. Detloff M.R. Johnson K. Kupina N.C. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J. Neurotrauma. 2004;21:9–20. doi: 10.1089/089771504772695904. [DOI] [PubMed] [Google Scholar]
- 45.Kontos H.A. Povlishock J.T. Oxygen radicals in brain injury. Cent. Nerv. Syst. Trauma. 1986;3:257–263. doi: 10.1089/cns.1986.3.257. [DOI] [PubMed] [Google Scholar]
- 46.Kontos H.A. Wei E.P. Superoxide production in experimental brain injury. J. Neurosurg. 1986;64:803–807. doi: 10.3171/jns.1986.64.5.0803. [DOI] [PubMed] [Google Scholar]
- 47.Opii W.O. Nukala V.N. Sultana R. Pandya J.D. Day K.M. Merchant M.L. Klein J.B. Sullivan P.G. Butterfield D.A. Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. J. Neurotrauma. 2007;24:772–789. doi: 10.1089/neu.2006.0229. [DOI] [PubMed] [Google Scholar]
- 48.Jensen F.E. Developmental factors in the pathogenesis of neonatal seizures. J. Pediatr. Neurol. 2009;7:5–12. doi: 10.3233/JPN-2009-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pitkänen A. Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011;10:173–186. doi: 10.1016/S1474-4422(10)70310-0. [DOI] [PubMed] [Google Scholar]
- 50.Rauca C. Zerbe R. Jantze H. Formation of free hydroxyl radicals after pentylenetetrazol-induced seizure and kindling. Brain Res. 1999;847:347–351. doi: 10.1016/s0006-8993(99)02084-3. [DOI] [PubMed] [Google Scholar]
- 51.DeKosky S.T. Taffe K.M. Abrahamson E.E. Dixon C.E. Kochanek P.M. Ikonomovic M.D. Time course analysis of hippocampal nerve growth factor and antioxidant enzyme activity following lateral controlled cortical impact brain injury in the rat. J. Neurotrauma. 2004;21:491–500. doi: 10.1089/089771504774129838. [DOI] [PubMed] [Google Scholar]
- 52.Boveris A. Navarro A. Systemic and mitochondrial adaptive responses to moderate exercise in rodents. Free Radic. Biol. Med. 2008;44:224–229. doi: 10.1016/j.freeradbiomed.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 53.Ansari M.A. Roberts K.N. Scheff S.W. A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. J. Neurotrauma. 2008;25:513–526. doi: 10.1089/neu.2007.0451. [DOI] [PubMed] [Google Scholar]
- 54.Griesbach G.S. Gomez-Pinilla F. Hovda D.A. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J. Neurotrauma. 2007;24:1161–1171. doi: 10.1089/neu.2006.0255. [DOI] [PubMed] [Google Scholar]
- 55.Griesbach G.S. Gomez-Pinilla F. Hovda D.A. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain Res. 2004;1016:154–162. doi: 10.1016/j.brainres.2004.04.079. [DOI] [PubMed] [Google Scholar]
- 56.Seo T.B. Kim B.K. Ko I.G. Kim D.H. Shin M.S. Kim C.J. Yoon J.H. Kim H. Effect of treadmill exercise on Purkinje cell loss and astrocytic reaction in the cerebellum after traumatic brain injury. Neurosci. Lett. 2010;481:178–182. doi: 10.1016/j.neulet.2010.06.087. [DOI] [PubMed] [Google Scholar]
- 57.Mota B.C. Pereira L. Souza M.A. Silva L.F. Magni D.V. Ferreira A.P. Oliveira M.S. Furian A.F. Mazzardo-Martins L. Silva M.D. Santos A.R. Ferreira J. Fighera M.R. Royes L.F. Exercise pre-conditioning reduces brain inflammation and protects against toxicity induced by traumatic brain injury: behavioral and neurochemical approach. Neurotox. Res. 2012;21:175–184. doi: 10.1007/s12640-011-9257-8. [DOI] [PubMed] [Google Scholar]
- 58.Soustiel J.F. Palzur E. Vlodavsky E. Veenman L. Gavish M. The effect of oxygenation level on cerebral post-traumatic apoptotsis is modulated by the 18-kDa translocator protein (also known as peripheral-type benzodiazepine receptor) in a rat model of cortical contusion. Neuropathol. Appl. Neurobiol. 2008;34:412–423. doi: 10.1111/j.1365-2990.2007.00906.x. [DOI] [PubMed] [Google Scholar]
- 59.Soustiel J.F. Sviri G.E. Monitoring of cerebral metabolism: non-ischemic impairment of oxidative metabolism following severe traumatic brain injury. Neurol. Res. 2007;29:654–660. doi: 10.1179/016164107X240017. [DOI] [PubMed] [Google Scholar]