Abstract
Reproduction and locomotion are essential features of animals that help to facilitate their interaction with the surrounding environment. Previous studies have produced inconsistent results on behavioral response to spaceflight by the model animal Caenorhabditis elegans (C. elegans) in liquid culture. Using standard agar-based nematode growth medium (NGM), we show here that both reproductive and locomotory capacities of C. elegans were not significantly changed by centrifuge-produced hypergravity or clinostat-simulated microgravity. To investigate the effect of actual spaceflight on C. elegans, a nematode test unit was specifically designed to maintain its normal growth on solid NGM slides and to allow automatic RNA fixation on board the Shenzhou-8 spaceflight. We did not detect alteration in either brood size of immediate progenies from postflight nematodes or locomotory behavior, including speed of locomotion, frequency of reversals, and rate of body bends of space-flown nematodes collected directly from nematode test units. Our results provide clear evidence that the nematode test unit is an appropriate apparatus for nematode growth on standard NGM and can be used for on-orbit analysis of C. elegans, including onboard RNA fixation for molecular analysis and real-time video acquisition for behavioral analysis, which are critical for further studies in unmanned spaceflight and outer space exploration. Key Words: Spaceflight—Hypergravity—Microgravity—Caenorhabditis elegans—Behavior—Reproduction. Astrobiology 13, 617–625.
1. Introduction
An organism often initiates a complex response when exposed to a given stress but may become resistant to multiple stresses once tolerance to the specific stress is achieved. For example, certain organisms among nematodes, rotifers, and tardigrades can survive almost complete desiccation by entering anhydrobiosis (“life without water”) even though water is essential for life on Earth. Interestingly, this robust cryptobiotic state of suspended animation also enables anhydrobiotic organisms to survive a wide range of other chemical and physical extremes such as high/low temperature, oxygen, irradiation, pressure, acidity, alkalinity, or salinity (Rothschild and Mancinelli, 2001; Convey and Stevens, 2007; Gladyshev and Meselson, 2008; Hengherr et al., 2009). As a survival strategy in nature, this type of anhydrobiotic ability may help an organism endure severe adversities in a variety of extreme habitats, including exposure to outer space (Wang et al., 2010; Fontaneto et al., 2012).
Both anhydrobiotic and non-anhydrobiotic nematodes have been used in studies of desiccation and other stress response. Although Caenorhabditis elegans (C. elegans) is not an anhydrobiotic nematode in a strict sense, as a model animal it can be used to explore many aspects otherwise unachievable in anhydrobiotic but intractable organisms (Huang et al., 2010; Banton and Tunnacliffe, 2012). Therefore, C. elegans is useful for gaining insights into the general nature of interaction between animals and their environment. In fact, as a major model system in biology, this nematode has been used in a wide range of cutting-edge biomedical research areas and is powerful at both molecular and organismal levels. Caenorhabditis elegans contains more than two-thirds potential counterparts of human disease genes and yet is a tiny organism (∼1 mm in length) with a short life span and a large number of progenies. Thus, it is particularly sensible to use this highly tractable model animal in experiments where space is extremely limited, for example, characterization of behavioral and reproductive changes in response to spaceflight.
The rapid development of aerospace technology has made space exploration and the search for extraterrestrial life-forms a reality. However, a variety of spaceflight hazards for Earth life need to be addressed, for example, temperature and pressure extremes, microgravity, cosmic radiation, and high-speed micrometeorites. These detrimental aspects of spaceflight may induce behavioral, reproductive, physiological, and biochemical changes. For instance, weightlessness, one of the most significant impact factors on life in space, may produce harmful effects on the human body, including cardiovascular and sensory-motor deconditioning, decrease of bone density and muscle mass, and changes in the immune system (Pietsch et al., 2011). Nevertheless, although a number of spaceflight stresses have been previously assessed, some of the findings are inconclusive. For example, earlier studies have suggested that cosmic radiation can cause an increased rate of mutations in C. elegans (Nelson et al., 1994; Hartman et al., 2001), but no significant difference in mutation rate was found in another spaceflight study (Zhao et al., 2006). Also, a decreased expression of myogenic transcription factors and myosin heavy chains has been detected, but no significant abnormality in muscle development was observed in C. elegans after spaceflight (Higashibata et al., 2006; Selch et al., 2008). Further, little information is available on the alteration of reproductive capacity and locomotory ability of C. elegans in response to variable gravities and spaceflight.
Here, we first studied the reproductive and behavioral responses of C. elegans on solid nematode growth medium (NGM) to centrifuge-produced hypergravity and clinostat-simulated microgravity and then investigated the effect of actual spaceflight on the responses of nematodes also grown on standard NGM in the Shenzhou-8 mission, which was an unmanned spaceflight launched on 1 November 2011 by a Long March 2F rocket from Jiuquan Satellite Launch Center in China. The mission included two automatic rendezvous and docking with the Tiangong-1 space module on 3 November 2011 during orbital darkness and on 14 November 2011 in full sunlight.
2. Materials and Methods
2.1. Nematode strains and general procedures
All C. elegans strains, including Bristol N2 (wild-type), HA759 {pqe-1(rt13) III; rtIs11[osm-10p::GFP+osm-10p::HtnQ150+Dpy-20(+)]}, and AM141 {rmIs133 [P(unc-54)Q40::YFP]}, were obtained from the Caenorhabditis Genetics Center, University of Minnesota. The nematodes were grown on solid NGM plates with Escherichia coli OP50 lawn as food at 23±1°C following standard protocols. Synchronization of nematodes was performed by using the standard alkaline hypochlorite method.
2.2. Treatment of nematodes with centrifuge-produced hypergravity and clinostat-simulated microgravity
Synchronized nematodes in NGM plates (3.5 cm) seeded with OP50 bacteria were subjected to hypergravity treatment in a tailor-made centrifuge by Huazhong University of Science and Technology (Wuhan, China). The hypergravity centrifuge was set at 37 Hz to produce a gravitational force of 10g (a=104.19 m/s2), as shown in Fig. 1A. Microgravity was simulated in a desktop 2-D clinostat SM-X1 (Fig. 1B) with a radius of 5 cm made by the Center for Space Science and Applied Research, the Chinese Academy of Sciences (Beijing, China). NGM plates containing OP50 bacteria and nematodes were rotated at 15 rpm around a horizontal axis in the clinostat. Control nematodes were cultured at the same conditions but under normal gravity (1g).
FIG. 1.

Hypergravity and microgravity simulations. (A) Hypergravity produced by a tailor-made centrifuge. The curve displays the resultant acceleration (a) with the centrifuge set at various frequencies (r=21 cm). (B) Image of 2-D clinostat SM-X1 used for producing simulated microgravity. Color images available online at www.liebertonline.com/ast
2.3. Brood size assay
Sextuplicate NGM plates (3.5 cm) containing OP50 bacteria and five nematode larvae (L1) were treated with centrifuge-produced hypergravity or clinostat-simulated microgravity at 23±1°C. After 48 h (time zero) treatment, the nematodes were transferred to fresh plates every 12 h, and the same treatment was continued until without progeny production. Progenies were counted under a dissecting microscope after 2 days when the nematodes reached the young adult stage (Hughes et al., 2007).
2.4. Locomotory behavior assay
Synchronized nematodes in NGM plates (3.5 cm) seeded with OP50 bacteria were subjected to treatment with centrifuge-produced hypergravity or clinostat-simulated microgravity at 23±1°C for the indicated times. After collection from the plates with M9 buffer, the nematodes were transferred onto unseeded NGM plates (3.5 cm), and their movement was recorded with a CCD microscope camera (Samsung, Seongnam City, South Korea). The captured images were converted to an uncompressed, high-contrast, grayscale (8-bit) movie at a resolution of 640×480 pixels in AVI format. The movie was then analyzed for speed of locomotion with Worm Tracker & Track Analyzer software as described (Romot et al., 2008) and for rate of reversals and frequency of body bends by visual counting (Tsalik and Hobert, 2003).
2.5. Spaceflight of nematodes in the Shenzhou-8 mission
The N2 nematodes for the Shenzhou-8 spaceflight mission were prepared at the Jiuquan Satellite Launch Center (Jiuquan, China) 15 days before the launch date (1 November 2011), and the L1 larvae in the nematode test unit were loaded into the pre-arranged SIMBOX experimental platform (Astrium Services, Friedrichshafen, Germany) on the Shenzhou-8 spacecraft 1 day prior to the launch. The SIMBOX facility is an intelligent incubator that contains a centrifuge and provides two types of gravitational states on board—microgravity versus 1g. The centrifuge was started after the rocket launch, and the temperature of SIMBOX was maintained at 23±0.5°C. At 9.5 days into the flight, one set of nematode cultures was fixed by injection of RNAlater solution (Ambion, Austin, TX). After 16.5 days of spaceflight, the Shenzhou-8 spacecraft landed, and the nematode samples were brought to the laboratory of the General Establishment of Space Science and Application (GESSA), the Chinese Academy of Sciences (Beijing, China). The RNAlater-fixed samples were collected and stored at−20°C, while the nonfixed nematode samples were collected for survival assay and locomotory analysis as well as for RNAlater fixation. The nematodes for ground controls were prepared at the GESSA laboratory following the same procedure.
2.6. Statistical analysis
Data were presented as mean±standard error of the mean and evaluated by one-way ANOVA followed by Bonferroni's post hoc test, unless otherwise stated, for which GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA) was used. Probability values of p<0.05 were considered to be significant.
3. Results
3.1. Effect of centrifuge-produced hypergravity and clinostat-simulated microgravity on the brood size of wild-type and proteotoxic C. elegans models
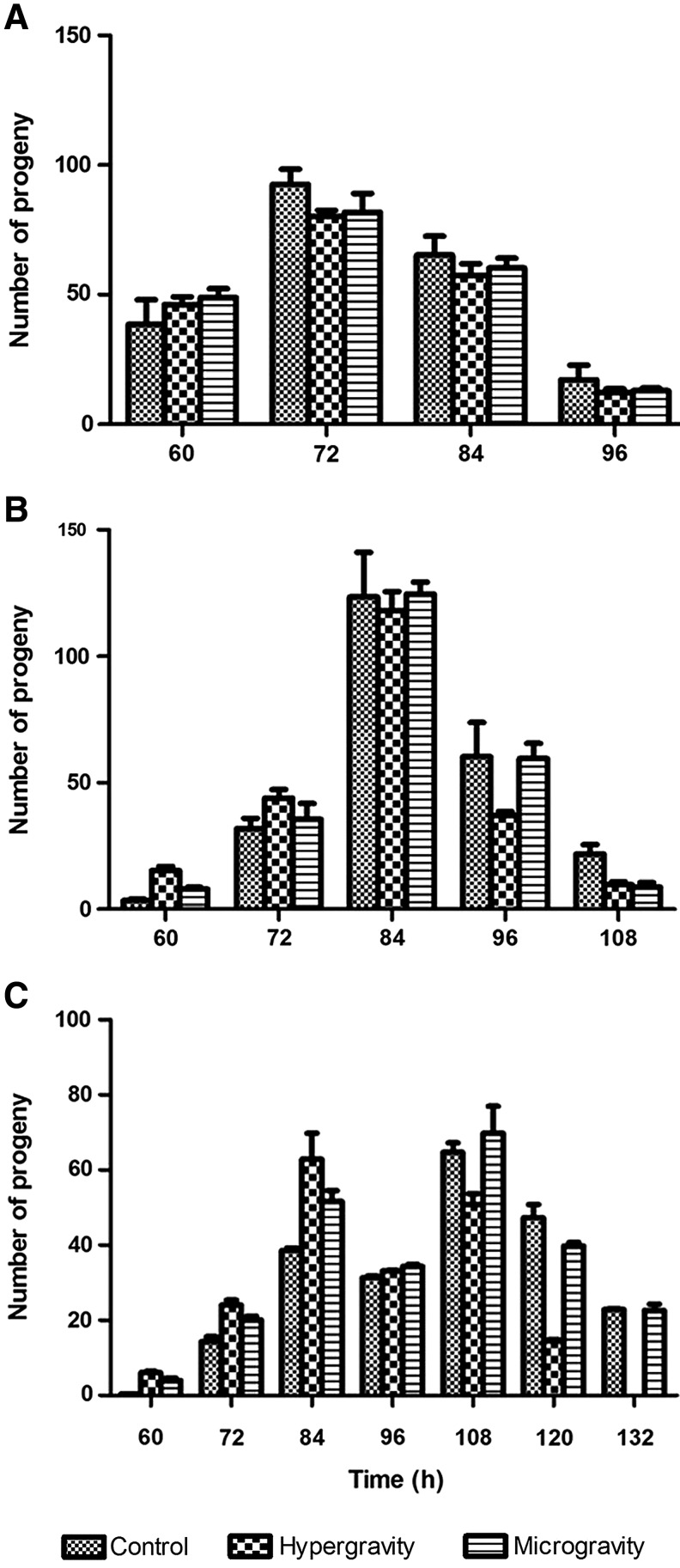
Since reproduction is an important event for organisms facing environmental stress, we tested the effect of different gravities on the brood size of the model animal C. elegans. The hypergravity and simulated microgravity were achieved via centrifugation and clinorotation, respectively, and the nematodes were treated under hypergravity or microgravity from L1 stage to the end of egg production on standard NGM plates with sufficient OP50 bacteria as food. As shown in Fig. 2A, the brood size of wild-type N2 nematodes after either hypergravity or microgravity treatment was almost the same as that in the control, with a total of 213.4±9.5, 211.9±13.5, and 203.5±8.4 progenies for control, hypergravity, and microgravity, respectively, suggesting that the reproductive efficiency of nematodes was not affected by the gravities tested.
FIG. 2.
Effect of centrifuge-produced hypergravity and clinostat-simulated microgravity on the brood size of C. elegans. Synchronized N2 (wild-type; A), HA759 (polyQ150 in sensory ASH neurons; B), and AM141 (polyQ40 in body wall muscle cells; C) nematodes in NGM plates containing OP50 bacteria were treated with hypergravity or microgravity from L1 to the end of egg production. After egg-laying started, the nematodes were transferred to fresh plates every 12 h, and the number of progenies were counted 2 days later in the original plates. Results are means±standard error of the mean from three independent experiments.
It is known that proteotoxic stress caused by protein misfolding and aggregation is a major threat to living organisms during aging and environmental stress, and C. elegans is a powerful model system that has been successfully used for in vivo studies of proteotoxic response and regulation (Zhang et al., 2012). Thus, we further tested the effect of centrifuge-produced hypergravity and clinostat-simulated microgravity on the brood size of C. elegans proteotoxic models imposed by transgenic polyglutamine (polyQ), including AM141 (polyQ40 aggregates in body wall muscle cells) and HA759 (polyQ150 in sensory ASH neurons) nematodes. As shown in Fig. 2B and 2C, however, the total progeny numbers of both nematode models were not affected by hypergravity or microgravity, although the egg production in AM141 nematodes appears to have started at a slightly earlier stage as compared to the control; for example, the progenies of hypergravity treatment (62.9±6.9) were more than those of the control (38.6±0.7) at 84 h, while the progenies of the control (64.7±2.5) were more than those of the hypergravity treatment (50.9±2.8) at 108 h.
3.2. Effect of centrifuge-produced hypergravity and clinostat-simulated microgravity on the locomotory behavior of C. elegans
The capacity to control its own behavior is obviously critical for an animal to successfully adapt to its surrounding environment. Since locomotion is an essential feature of animal behavior and is influenced by multiple factors, including stress from the external environment (Jordan et al., 2007; Schwabe and Wolf, 2009), we examined whether different gravities affected the locomotory behavior patterns of wild-type C. elegans. After treatments with centrifuge-produced hypergravity or clinostat-simulated microgravity from L4 stage for up to 4 days on solid NGM plates, the locomotory patterns of the adult nematodes, including speed of locomotion, frequency of reversals, and rate of body bends, were not significantly changed (well within 2-fold difference) as compared to the control (Fig. 3), with a speed of ∼0.02 mm/s, a frequency of ∼10 reversals in 2 min, and a rate of ∼30 body bends in 30 s on Day 2. Since earlier-stage nematodes may have a more sensitive response to stress (Castro et al., 2012), we then investigated the response of larval nematodes to hypergravity and microgravity. After 6 h of treatment from L1 and L4 stages (Fig. 4A and 4B) with either hypergravity or microgravity, the locomotory behavior patterns of the nematodes were also not changed as compared to those of the control nematodes. Similar results were also observed after treatment of L1 and L4 nematodes with hypergravity or microgravity for 3 and 9 h (data not shown). Together, these data suggest that the ground-based simulation of hypergravity and microgravity did not have much impact on the locomotory behavior of C. elegans.
FIG. 3.
Effect of centrifuge-produced hypergravity and clinostat-simulated microgravity on the locomotory behavior of C. elegans. Synchronized wild-type N2 nematodes were treated with hypergravity or microgravity from L4 at 23±1°C for 4 days with 5-FUdR added in the NGM plates to prevent production of progenies. After collection from the plates with M9 buffer, the nematodes were transferred on a daily basis onto unseeded NGM plates (3.5 cm) and recorded with a CCD camera. Locomotory pattern of nematodes was analyzed from the recorded movies with Worm Tracker & Track Analyzer software for speed of locomotion and by visual counting for rate of reversals and frequency of body bends. The locomotion (A), reversal (B), and body bend (C) indices were, respectively, the ratios of locomotion speed, reversal rate, and body bend frequency of nematodes treated with simulated variable gravities divided by those in the controls. The results are shown as means±standard error of the mean of three independent experiments. An index value <0.5 or >2.0 is considered to be effective on the locomotory behavior.
FIG. 4.
Effect of centrifuge-produced hypergravity and clinostat-simulated microgravity on the locomotory behavior of C. elegans larvae. Synchronized wild-type N2 nematodes were treated with hypergravity or microgravity from L1 (A) or L4 (B), and the locomotory behavior indices were determined after 6 h. See Fig. 3 for details.
3.3. Successful spaceflight of C. elegans in the unmanned Shenzhou-8 spacecraft
Microgravity is only one of the stresses that may affect the performance and health of spaceflight crew (Morphew, 2001). To examine the overall effect of actual spaceflight, an experimental platform SIMBOX was designed and built for the Shenzhou-8 spaceflight mission to test the effect of spaceflight stress on a number of organisms, including C. elegans. The platform provided 1g centrifugation and RNAlater fixation facilities on board. The C. elegans spaceflight experiment was performed in nematode-specific test units (Fig. 5), which were loaded into the SIMBOX experimental platform 1 day before the Shenzhou-8 spaceflight. The nematode test unit contained two nematode culture chambers, each of which had two windows of biofoil membrane to allow gas exchange and ensure a suitable growth condition. Each culture chamber held two slides of solid NGM seeded with adequate OP50 bacteria and synchronized L1 wild-type nematodes. One set of the nematode samples was fixed with RNAlater solution at 9.5 days by the SIMBOX facility. The ground control nematodes were grown in the nematode test units on the ground and treated in the same way as the spaceflight nematodes.
FIG. 5.
Nematode test unit used in the Shenzhou-8 spaceflight mission. Mimetic diagram (A), cross-sectional profile (B), and real picture (C) of nematode test unit are presented. 1, cover of nonfixated chamber; 2, cover of fixated chamber; 3, mounted-on container lid; 4, fixative/waste bag; 5, pump; 6, cover with snap ring; 7, pump; 8, sealing; 9, tray system. The nematodes were grown on both the top and bottom slides (3.2×2.3 cm), which were held in the tray system (9). The nematode samples were fixed on orbit automatically with RNAlater solution through an electrical pump (7) and a tubing system, which connected the culture chamber to a tank (4) containing a fixative bag and a waste bag. Color images available online at www.liebertonline.com/ast
After 16.5 days of spaceflight, the flown animals were apparently alive and appeared healthy, similar to those of the ground control nematodes (Fig. 6), demonstrating a successful spaceflight of the animals in nematode test units and the SIMBOX experimental platform. However, for the automatic onboard RNA fixation experiment in which RNAlater solution was used, only the spaceflight microgravity sample was fixed on board successfully at 9.5 days (Fig. S1; Supplementary Data are available online at www.liebertonline.com/ast), while the spaceflight control (onboard centrifuge, 1g) nematodes failed to be fixed. Also, although total RNA was successfully isolated from both onboard and ground nematode samples (Fig. S1), an attempt to perform digital gene expression profiling was not successful due to a limited amount of RNA. Nevertheless, these efforts clearly demonstrate the potential of the nematode test unit to automatically fix onboard nematodes with RNAlater solution for real-time studies in unmanned spaceflight.
FIG. 6.

Survival of C. elegans from the Shenzhou-8 spaceflight mission. Synchronized L1 nematodes (N2) in the nematode test unit were prepared 1 day prior to the launch. After 16.5 days of spaceflight, the nematodes were collected from the test unit and filmed with a CCD camera. Images show photographs of nematodes from nematode test units under 1g conditions on the ground (ground control; A), under 1g gravity in space (spaceflight control; B), and under microgravity in space (spaceflight microgravity; C), respectively. Color images available online at www.liebertonline.com/ast
3.4. Effect of spaceflight on the brood size of postflight C. elegans progenies
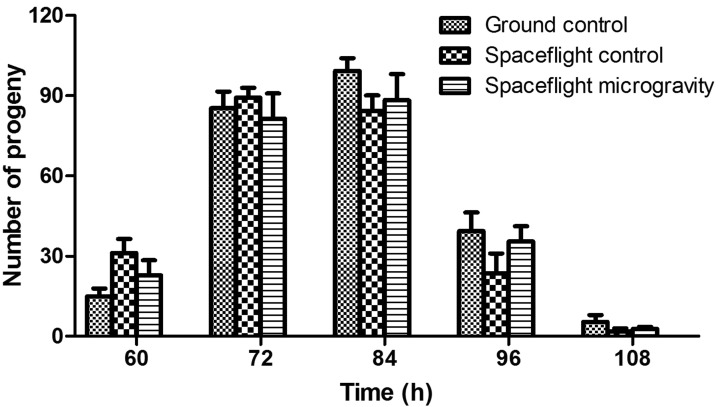
To examine the effect of spaceflight on the reproductivity of C. elegans, L1 progenies from the space-flown nematodes were further grown on NGM plates seeded with OP50 bacteria, and their brood size was determined. As shown in Fig. 7, the brood size of the immediate progenies from the postflight animals, including those after onboard centrifuge (spaceflight control, 1g) and spaceflight microgravity treatments, was roughly the same as that of the 1g ground control nematodes (e.g., about 80–90 progenies at 72 h), suggesting the spaceflight did not affect the reproductive ability of the nematodes.
FIG. 7.
Effect of spaceflight on the brood size of postflight C. elegans progenies. Brood size was determined as in Fig. 2 by using L1 progenies of nematodes under 1g conditions on the ground (ground control), under 1g gravity in space (spaceflight control), and under microgravity in space (spaceflight microgravity), respectively. Data are presented as means±standard deviation and were evaluated by the Student t test with GraphPad Prism.
3.5. Effect of spaceflight on the locomotory behavior of postflight C. elegans
To examine the effect of spaceflight on C. elegans locomotory behavior, the nematodes collected directly from the nematode test units were immediately filmed with a CCD camera. The frequency of body bends and rate of reversals of the nematodes were then counted from the video, and the average speed of locomotion was also analyzed by using the video data with a MATLAB-based parallel worm tracker system (Romot et al., 2008). As shown in Fig. 8, the space-flown nematodes did not display significantly different patterns of postflight locomotion as compared to the ground control nematodes. However, this experiment itself strongly suggests the feasibility of onboard tracking and real-time analysis of C. elegans behavior when using an auto-imaging system.
FIG. 8.
Effect of spaceflight on the locomotory behavior of C. elegans. The behavioral analysis was performed as in Fig. 3 by using the video data of nematodes as stated in Fig. 6. Results are presented as averages of locomotory behavior indices of mixed-stage nematodes.
4. Discussion
As a well-characterized and well-recognized model animal, C. elegans has been previously used in space biology studies. The nematodes in most of the previous spaceflights came back alive except those from the 1998 NASA STS-95 mission study, in which both space and ground nematodes were dead, presumably because of bioincompatibility of hardware materials or anoxia (Szewczyk et al., 2008). In the present study, biocompatibility of the materials used in the nematode test unit had been fully tested, which showed no detrimental effects on the nematodes (data not shown). To avoid potential anoxic conditions to the nematodes, the culture chambers of the nematode test units were equipped with windows of biofoil membrane to allow sufficient gas exchange (Fig. 5). Prior to the Shenzhou-8 mission, the growth of nematodes was assessed on solid NGM in the test units six times on the ground, and the nematodes were grown normally as compared to the controls grown in normal Petri dishes (data not shown). After the actual spaceflight in the Shenzhou-8 mission, the nematodes in the culture chambers of both test units that were not fixed by RNAlater were apparently alive and appeared as healthy as the ground controls (Fig. 6). These results demonstrate that the nematode test unit used in this study is a reliable apparatus for spaceflight of C. elegans.
To study the real-time response of animals to spaceflight stress, it is critical to obtain samples during the actual flight. However, most of the biological samples in previous spaceflight studies were treated after the flight rather than in real time in orbit. Only in rare situations the nematode samples were frozen during manned spaceflight with the help of the flight crews (Higashitani et al., 2009). In the Shenzhou-8 mission, we attempted to fix the nematode samples at a selected time point during the spaceflight with RNAlater solution through an automatic fixation system in the SIMBOX experimental platform. Since RNAlater is a RNA stabilization solution that can rapidly permeate nematodes to stabilize and protect RNA at room temperature, it is ideal for injecting fixative into the culture chamber to fix nematodes on solid NGM. Although the fixation of the spaceflight control (onboard centrifuge, 1g) sample failed, the onboard microgravity nematode sample was fixed successfully. The fixation failure of the onboard centrifuge sample may have been due to blockage of the tubing system by RNAlater crystallization, which could have been avoided by pre-loading a tiny amount of sterilized water into the tubing or by giving adequate pumping time of RNAlater solution. Other occurrences such as an error of spacecraft command may also result in the failure of sample fixation. Nonetheless, our attempt has clearly demonstrated the usefulness of the nematode test unit in combination with sample-fixing reagents (e.g., RNAlater solution) for real-time automatic fixation of onboard samples, and thus has great potential in unmanned spaceflight and outer space exploration.
Successful reproduction is a prerequisite for animal fitness in an evolutionary context. For the model animal C. elegans, reproduction is a simple yet effective behavior that is continuously modulated in response to environmental stimuli. A neuromuscular circuit containing 8 neurons and 16 vulval and uterine muscles, together with multiple neurotransmitters, is required for C. elegans reproduction (Ringstad and Horvitz, 2008). Therefore, in this report we examined the reproductivity of L1 progenies from the space-flown nematodes and found that the brood size of the nematodes after spaceflight, including both spaceflight control (onboard centrifuge, 1g) and spaceflight microgravity treatments, was not changed as compared to the ground control (Fig. 7). This is in agreement with the experiments of centrifuge-produced hypergravity and clinostat-simulated microgravity (Fig. 2) and with a previous observation on brood size of recultured nematodes after spaceflight (Szewczyk et al., 2005). Together, these data suggest that spaceflight does not affect the reproductive efficiency of nematodes. However, the AM141 nematodes appear to have laid eggs slightly earlier under hypergravity produced by centrifuge on the ground than the control (Fig. 2C). This is interesting because similar early-onset phenomena have also been observed in other organisms; for example, brine shrimp instar develops more rapidly during spaceflight (Spooner et al., 1994), while bacteria exhibit a shorter lag phase in both space and clinorotation (Brown et al., 2002) as compared to ground controls.
In addition to reproduction, movement is also an essential feature of an animal. Locomotion of C. elegans reflects its overall behavior and is influenced by multiple factors, including external stimuli. In this study, the locomotory behavior of the nematode populations was experimentally assessed immediately after the spaceflight in the Shenzhou-8 mission. As shown in Fig. 8, the locomotion behavior of the nematodes, including speed of locomotion, rate of reversals, and frequency of body bends, was not significantly changed in the space-flown nematodes as compared to the ground controls. Although this is inconsistent with a previous observation showing reduced rates of movement after a 10-day spaceflight (Higashibata et al., 2006), it is in agreement with a recent study of over 12 generations in a spaceflight mission (Oczypok et al., 2012) and with our experimental results of hypergravity and microgravity simulations (Figs. 3 and 4). It is also worth noting that the nematodes in the previous studies were cultured in liquid medium but in the current study were grown on solid NGM, which is the routinely used C. elegans culture medium that mimics its natural surroundings in soil. Moreover, since all these spaceflight data were obtained from the nematodes after the landing of spacecrafts, how they would respond in real time to the spaceflight environment in orbit remains largely unknown. Nevertheless, the locomotion behavioral analysis in the current study, in which the MATLAB-based parallel worm tracker system was used, demonstrates the potential of using such devices to monitor the real-time behavioral response of nematodes on solid medium in unmanned spaceflight experiments through video recording if equipped with an appropriate auto-imaging system.
In summary, we report here that both actual spaceflight in the Shenzhou-8 mission and variable gravity simulation on the ground did not significantly affect the reproductive and locomotory capacities of the nematode C. elegans (Table S1). However, our results clearly demonstrate that the nematode test unit used in this study is a reliable apparatus for nematode growth on normal NGM solid medium and can be used for in-orbit analysis of the nematodes, including onboard RNA fixation for molecular analysis and potential real-time acquisition of video data for behavioral analysis, which are critical for further studies in unmanned spaceflight.
Supplementary Material
Acknowledgments
This work was supported by the Project of Chinese Manned Spaceflight, the National Natural Science Foundation of China (Grants 30970688 and 81274048), and the Fundamental Research Funds for the Central Universities (Grant 20083060101000071). We thank DLR/EADS-Astrium and GESSA/CAS for help in SIMBOX/Shenzhou-8 mission experiments. The strains used in this study were obtained from the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources.
Abbreviations
GESSA, General Establishment of Space Science and Application; NGM, nematode growth medium.
References
- Banton M.C. Tunnacliffe A. MAPK phosphorylation is implicated in the adaptation to desiccation stress in nematodes. J Exp Biol. 2012;215:4288–4298. doi: 10.1242/jeb.074799. [DOI] [PubMed] [Google Scholar]
- Brown R.B. Klaus D. Todd P. Effects of space flight, clinorotation, and centrifugation on the substrate utilization efficiency of E. coli. Microgravity Sci Technol. 2002;13:24–29. doi: 10.1007/BF02881678. [DOI] [PubMed] [Google Scholar]
- Castro P.V. Khare S. Young B.D. Clarke S.G. Caenorhabditis elegans battling starvation stress: low levels of ethanol prolong lifespan in L1 larvae. PLoS One. 2012;7:e29984. doi: 10.1371/journal.pone.0029984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convey P. Stevens M.I. Antarctic biodiversity. Science. 2007;317:1877–1878. doi: 10.1126/science.1147261. [DOI] [PubMed] [Google Scholar]
- Fontaneto D. Bunnefeld N. Westberg M. Long-term survival of microscopic animals under desiccation is not so long. Astrobiology. 2012;12:863–869. doi: 10.1089/ast.2012.0828. [DOI] [PubMed] [Google Scholar]
- Gladyshev E. Meselson M. Extreme resistance of bdelloid rotifers to ionizing radiation. Proc Natl Acad Sci USA. 2008;105:5139–5144. doi: 10.1073/pnas.0800966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman P.S. Hlavacek A. Wilde H. Lewicki D. Schubert W. Kern R.G. Kazarians G.A. Benton E.V. Benton E.R. Nelson G.A. A comparison of mutations induced by accelerated iron particles versus those induced by low Earth orbit space radiation in the FEM-3 gene of Caenorhabditis elegans. Mutat Res. 2001;474:47–55. doi: 10.1016/s0027-5107(00)00154-8. [DOI] [PubMed] [Google Scholar]
- Hengherr S. Worland M.R. Reuner A. Brümmer F. Schill R.O. High-temperature tolerance in anhydrobiotic tardigrades is limited by glass transition. Physiol Biochem Zool. 2009;82:749–755. doi: 10.1086/605954. [DOI] [PubMed] [Google Scholar]
- Higashibata A. Szewczyk N.J. Conley C.A. Imamizo-Sato M. Higashitani A. Ishioka N. Decreased expression of myogenic transcription factors and myosin heavy chains in Caenorhabditis elegans muscles developed during spaceflight. J Exp Biol. 2006;209:3209–3218. doi: 10.1242/jeb.02365. [DOI] [PubMed] [Google Scholar]
- Higashitani A. Hashizume T. Sugimoto T. Mori C. Nemoto K. Etheridge T. Higashitani N. Takanami T. Suzuki H. Fukui K. Yamazaki T. Ishioka N. Szewczyk N. Higashibata A. C. elegans RNAi space experiment (CERISE) in Japanese Experiment Module Kibo. Biol Sci Space. 2009;23:183–187. doi: 10.2187/bss.23.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z. Banton M.C. Tunnacliffe A. Modeling anhydrobiosis: activation of the mitogen-activated protein kinase ERK by dehydration in both human cells and nematodes. J Exp Zool. 2010;313A:660–670. doi: 10.1002/jez.637. [DOI] [PubMed] [Google Scholar]
- Hughes S.E. Evason K. Xiong C. Kornfeld K. Genetic and pharmacological factors that influence reproductive aging in nematodes. PLoS Genet. 2007;3:e25. doi: 10.1371/journal.pgen.0030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan K.W. Carbone M.A. Yamamoto A. Morgan T.J. Mackay T.F. Quantitative genomics of locomotor behavior in Drosophila melanogaster. Genome Biol. 2007;8:R172. doi: 10.1186/gb-2007-8-8-r172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morphew M.E. Psychological and human factors in long duration spaceflight. McGill Journal of Medicine. 2001;6:74–80. [Google Scholar]
- Nelson G.A. Schubert W.W. Kazarians G.A. Richards G.F. Benton E.V. Benton E.R. Henke R. Radiation effects in nematodes: results from IML-1 experiments. Adv Space Res. 1994;14:87–91. doi: 10.1016/0273-1177(94)90455-3. [DOI] [PubMed] [Google Scholar]
- Oczypok E.A. Etheridge T. Freeman J. Stodieck L. Johnsen R. Baillie D. Szewczyk N.J. Remote automated multi-generational growth and observation of an animal in low Earth orbit. J R Soc Interface. 2012;9:596–599. doi: 10.1098/rsif.2011.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietsch J. Bauer J. Egli M. Infanger M. Wise P. Ulbrich C. Grimm D. The effects of weightlessness on the human organism and mammalian cells. Curr Mol Med. 2011;11:350–364. doi: 10.2174/156652411795976600. [DOI] [PubMed] [Google Scholar]
- Ringstad N. Horvitz H.R. FMRFamide neuropeptides and acetylcholine synergistically inhibit egg-laying by C. elegans. Nat Neurosci. 2008;11:1168–1176. doi: 10.1038/nn.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romot D. Johnson B.E. Berry T.L., Jr. Carnell L. Goodman M.B. The Parallel Worm Tracker: a platform for measuring average speed and drug-induced paralysis in nematodes. PLoS One. 2008;3:e2208. doi: 10.1371/journal.pone.0002208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild L.J. Mancinelli R.L. Life in extreme environments. Nature. 2001;409:1092–1101. doi: 10.1038/35059215. [DOI] [PubMed] [Google Scholar]
- Schwabe L. Wolf O.T. Stress prompts habit behavior in humans. J Neurosci. 2009;29:7191–7198. doi: 10.1523/JNEUROSCI.0979-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selch F. Higashibata A. Imamizo-Sato M. Higashitani A. Ishioka N. Szewczyk N.J. Conley C.A. Genomic response of the nematode Caenorhabditis elegans to spaceflight. Adv Space Res. 2008;41:807–815. doi: 10.1016/j.asr.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner B.S. Metcalf J. DeBell L. Paulsen A. Noren W. Guikema J.A. Development of the brine shrimp Artemia is accelerated during spaceflight. J Exp Zool. 1994;269:253–262. doi: 10.1002/jez.1402690310. [DOI] [PubMed] [Google Scholar]
- Szewczyk N.J. Mancinelli R.L. McLamb W. Reed D. Blumberg B.S. Conley C.A. Caenorhabditis elegans survives atmospheric breakup of STS-107, Space Shuttle Columbia. Astrobiology. 2005;5:690–705. doi: 10.1089/ast.2005.5.690. [DOI] [PubMed] [Google Scholar]
- Szewczyk N.J. Tillman J. Conley C.A. Granger L. Segalat L. Higashitani A. Honda S. Honda Y. Kagawa H. Adachi R. Higashibata A. Fujimoto N. Kuriyama K. Ishioka N. Fukui K. Baillie D. Rose A. Gasset G. Eche B. Chaput D. Viso M. Description of International Caenorhabditis elegans Experiment first flight (ICE-FIRST) Adv Space Res. 2008;42:1072–1079. doi: 10.1016/j.asr.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsalik E.L. Hobert O. Functional mapping of neurons that control locomotory behavior in Caenorhabditis elegans. J Neurobiol. 2003;56:178–197. doi: 10.1002/neu.10245. [DOI] [PubMed] [Google Scholar]
- Wang G. Hao Z. Huang Z. Chen L. Li X. Hu C. Liu Y. Raman spectroscopic analysis of a desert cyanobacterium Nostoc sp. in response to UVB radiation. Astrobiology. 2010;10:783–788. doi: 10.1089/ast.2009.0407. [DOI] [PubMed] [Google Scholar]
- Zhang H. Pan N. Xiong S. Zou S. Li H. Xiao L. Cao Z. Tunnacliffe A. Huang Z. Inhibition of polyglutamine-mediated proteotoxicity by Astragalus membranaceus polysaccharide through the DAF-16/FOXO transcription factor in Caenorhabditis elegans. Biochem J. 2012;441:417–424. doi: 10.1042/BJ20110621. [DOI] [PubMed] [Google Scholar]
- Zhao Y. Lai K. Cheung I. Youds J. Tarailo M. Tarailo S. Rose A. A mutational analysis of Caenorhabditis elegans in space. Mutat Res. 2006;601:19–29. doi: 10.1016/j.mrfmmm.2006.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.