Abstract
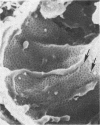
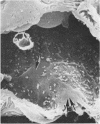
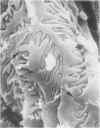
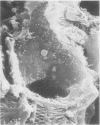
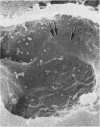
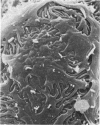
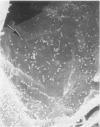
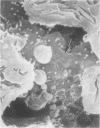
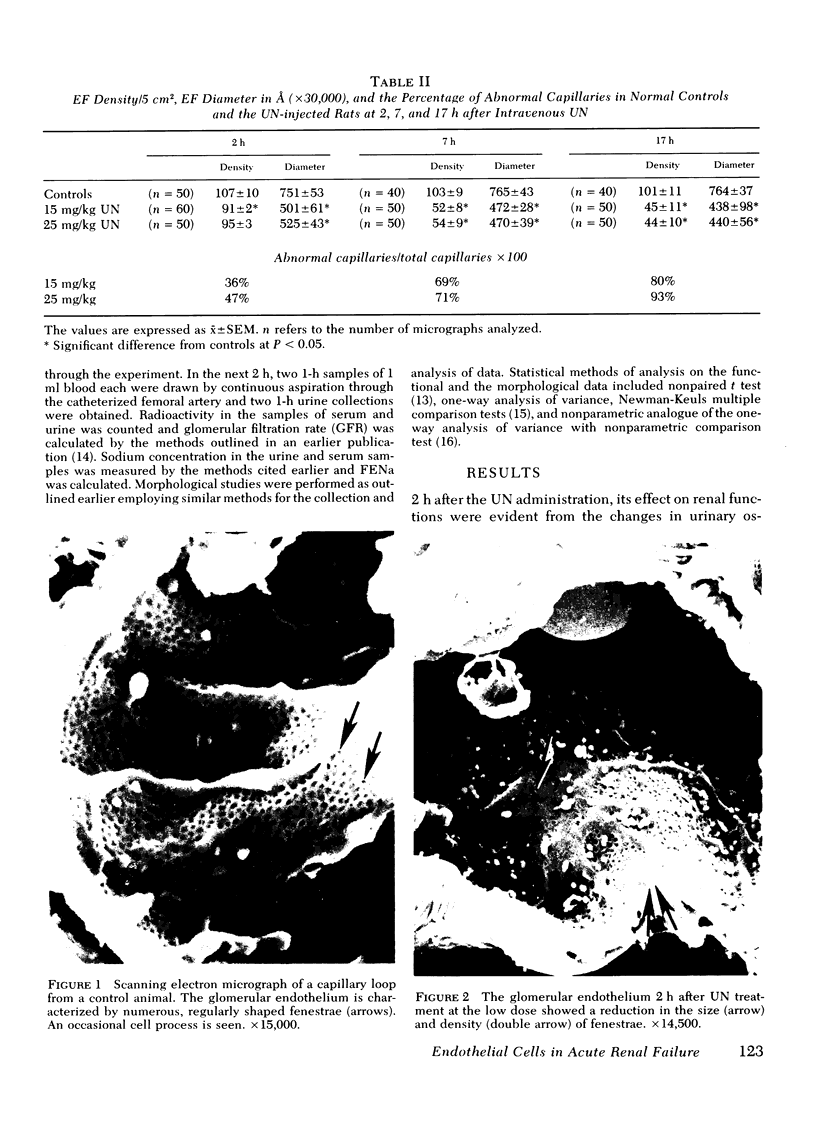
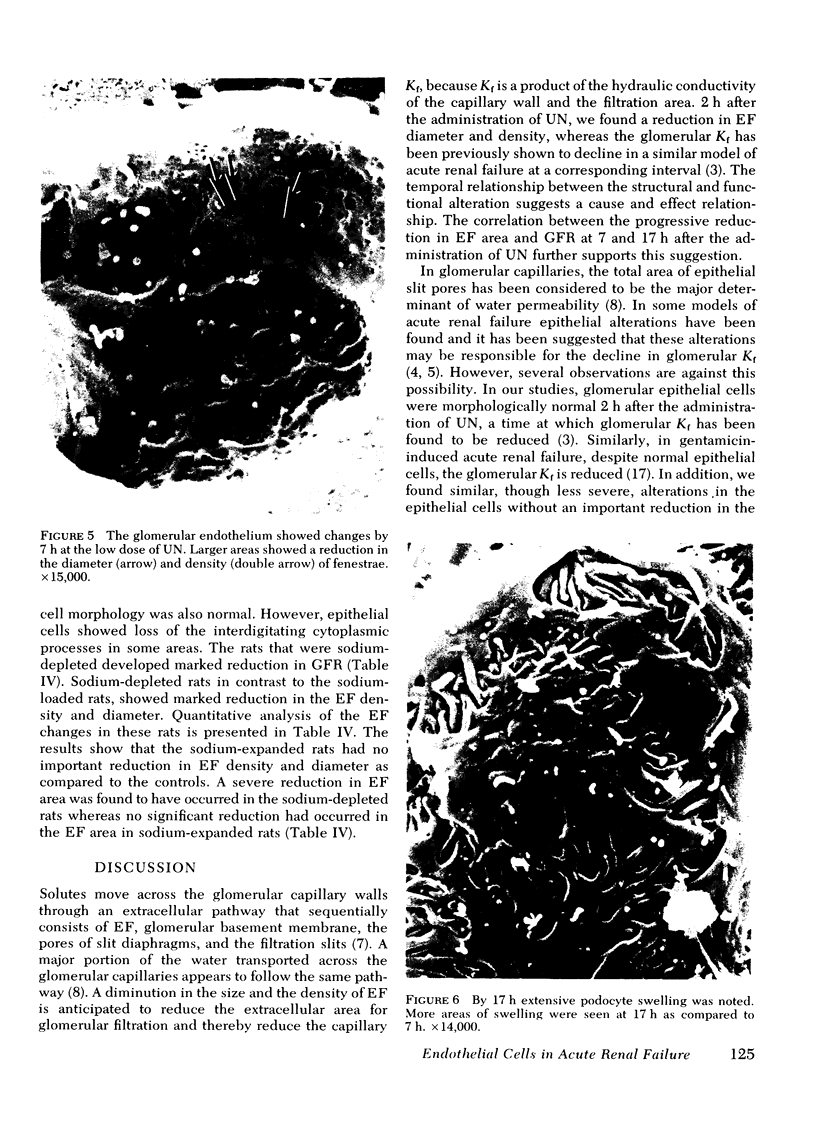
In uranyl nitrate (UN)-induced acute renal failure (ARF) glomerular ultrafiltration coefficient (Kf) decreases because of unknown reasons. Since transport of water across the glomerular capillary wall occurs predominantly extracellularly through the endothelial fenestrae (EF), a reduction in the diameter and/or the density of EF can reduce the extracellular filtration area and the glomerular Kf. To examine this possibility, ARF was induced in rats by intravenous administration of UN in low (15 mg/kg) and high doses (25 mg/kg). Fenestral density (¯x±SEM) per 5 cm2 from the scanning electron micrographs (×30,000) was 107±10, 103±9, and 101±11 at 2, 7, and 17 h after the intravenous administration of bicarbonate saline to the control rats. In the low-dose UN group the EF density was 91±2, 52±8, and 45±11 at 2, 7, and 17 h after the injection, whereas for the high-dose group at corresponding time intervals the EF density was 95±3, 54±9, and 44±10. Fenestral diameters, in Angstrom units (¯x±SEM), were 751±53, 765±43, and 764±37 at 2, 7, and 17 h after the injection of bicarbonate saline to control rats. At corresponding intervals after the administration of UN, the fenestral diameters were 501±61, 472±28, and 438±98 for the low-dose group and 525±43, 470±39, and 440±56 for the high-dose group. 2, 7, and 17 h after the injection of UN, fenestral area of the low-dose group decreased to 52.1, 30.1, and 24.6% of the controls, whereas in the high-dose group, the fenestral area declined to 54.3, 30.2, and 23.6% of the controls. Administration of UN (15 mg/kg) to sodium-loaded rats did not alter renal function or endothelial cell morphology. It is suggested that in UN-induced ARF the morphological alterations in endothelial cells reduce the Kf of glomerular capillaries by reducing the filtration area.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELE J. E. The physical background to freezing point osmometry and its medical-biological applications. Am J Med Electron. 1963 Jan-Mar;2:32–41. [PubMed] [Google Scholar]
- Avasthi P. S., Evan A. P. Glomerular permeability in aminonucleoside-induced nephrosis in rats. A proposed role of endothelial cells. J Lab Clin Med. 1979 Feb;93(2):266–276. [PubMed] [Google Scholar]
- Baehler R. W., Kotchen T. A., Burke J. A., Galla J. H., Bhathena D. Considerations on the pathophysiology of mercuric chloride-induced acute renal failure. J Lab Clin Med. 1977 Aug;90(2):330–340. [PubMed] [Google Scholar]
- Baylis C., Deen W. M., Myers B. D., Brenner B. M. Effects of some vasodilator drugs on transcapillary fluid exchange in renal cortex. Am J Physiol. 1976 Apr;230(4):1148–1158. doi: 10.1152/ajplegacy.1976.230.4.1148. [DOI] [PubMed] [Google Scholar]
- Baylis C., Rennke H. R., Brenner B. M. Mechanisms of the defect in glomerular ultrafiltration associated with gentamicin administration. Kidney Int. 1977 Nov;12(5):344–353. doi: 10.1038/ki.1977.121. [DOI] [PubMed] [Google Scholar]
- Blantz R. C. Dynamics of glomerular ultrafiltration in the rat. Fed Proc. 1977 Nov;36(12):2602–2608. [PubMed] [Google Scholar]
- Blantz R. C., Konnen K. S., Tucker B. J. Angiotensin II effects upon the glomerular microcirculation and ultrafiltration coefficient of the rat. J Clin Invest. 1976 Feb;57(2):419–434. doi: 10.1172/JCI108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blantz R. C. The mechanism of acute renal failure after uranyl nitrate. J Clin Invest. 1975 Mar;55(3):621–635. doi: 10.1172/JCI107970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. W., Baehler R. W., Sharma H., O'Dorisio T., Osgood R. W., Stein J. H., Ferris T. F. Studies of the mechanism of oliguria in a model of unilateral acute renal failure. J Clin Invest. 1974 Jun;53(6):1546–1558. doi: 10.1172/JCI107705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamenbaum W., Hamburger R. J., Huddleston M. L., Kaufman J., McNeil J. S., Schwartz J. H., Nagle R. The initiation phase of experimental acute renal failure: an evaluation of uranyl nitrate-induced acute renal failure in the rat. Kidney Int Suppl. 1976 Oct;6:S115–S122. [PubMed] [Google Scholar]
- Kleinman J. G., McNeil J. S., Schwartz J. H., Hamburger R. J., Flamenbaum W. Effect of dithiothreitol on mercuric chloride- and uranyl nitrate-induced acute renal failure in the rat. Kidney Int. 1977 Aug;12(2):115–121. doi: 10.1038/ki.1977.88. [DOI] [PubMed] [Google Scholar]
- Shea S. M., Morrison A. B. A stereological study of the glomerular filter in the rat. Morphometry of the slit diaphragm and basement membrane. J Cell Biol. 1975 Nov;67(2PT1):436–443. doi: 10.1083/jcb.67.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J. H., Gottschall J., Osgood R. W., Ferris T. F. Pathophysiology of a nephrotoxic model of acute renal failure. Kidney Int. 1975 Jul;8(1):27–41. doi: 10.1038/ki.1975.73. [DOI] [PubMed] [Google Scholar]
- Stein J. H. The glomerulus in acute renal failure. J Lab Clin Med. 1977 Aug;90(2):227–230. [PubMed] [Google Scholar]
- Venkatachalam M. A., Rennke H. G. The structural and molecular basis of glomerular filtration. Circ Res. 1978 Sep;43(3):337–347. doi: 10.1161/01.res.43.3.337. [DOI] [PubMed] [Google Scholar]