Abstract
This review attempts to provide an overview of the current knowledge of TRP proteins and their possible role in bladder function and disease. At present, there are 28 transient receptor potential (TRP) channels (subdivided into 7 categories or families) which are involved in a number of functions [G.A. Hicks, TRP channels as therapeutic targets: hot property, or time to cool down? Neurogastroenterology and Motility 18, (2006) 590–594., J.D. Levine, N. Alessandri-Haber, TRP channels: targets for the relief of pain, Biochimica et Biophysica Acta 1772, (2007) 989–1003.]. Of those belonging to the group 1 subfamily, a number of TRPV, TRPM and TRPA proteins associated with osmoregulation, thermal, chemical and mechanical signaling mechanisms have been shown to be expressed within the lower urinary tract. Though the biological role of many of these channels in urinary bladder function still remains elusive, TRPV1 is by far the best characterized and is thought to be involved in a number of bladder disorders
Keywords: Capsaicin, Urothelium, Non-neuronal, Urinary bladder, neurogenic bladder
1. Introduction
TRPV1 previously known as the vanilloid receptor type 1 or the capsaicin receptor, is a ligand gated ion channel activated by capsaicin, heat, acidosis and endogenous agonists (such as anandamide, 12-hydroxyeicosatetranoic acid) [4–6]. Interest in TRP channel structure and function was stimulated by early studies of the actions agents such as capsaicin and resiniferatoxin (RTX) [1–3]. Capsaicin (first isolated from chili peppers in 1919) became a ‘hot’ topic when it was found to have specific binding sites in a number of tissues including sensory nerves [7–9]. This culminated in the cloning of the vanilloid receptor [5], identified by using capsaicin as a ligand.
2. TRPV1 expression and function in the urinary bladder
Both the upper and lower urinary tract are densely innervated by capsaicin-sensitive primary afferent neurons in a number of species including man [7]. Early functional studies revealed that capsaicin-sensitive C type bladder fibers play a role in micturition [7,10]. It was shown that capsaicin sensitive nerves exhibit both a sensory and also an “efferent” function which is determined by release of peptides including tachykinins such as substance P as well as other peptides such as calcitonin gene-related peptide [3,11]. The sensory function includes the regulation of the micturition threshold and the perception of pain from the urinary bladder, while the efferent function controls nerve excitability, smooth muscle contractility and plasma protein extravasation.
The expression of capsaicin-sensitive nerve fibers in the lower urinary tract was first demonstrated following binding of radiolabeled resiniferatoxin in the bladder and urethra of the rat [7,9]. Subsequently the use of antibodies to TRPV1 revealed TRPV1-immunoreactive nerve fibers in subpopulations of bladder nerves including unmyelinated (C-fiber) nerves that detect bladder distension or the presence of irritant chemicals [12–14]. These fibers could be localized near and within the bladder mucosa, in addition to close proximity to blood vessels and smooth muscle cells [12,15]. Within the muscular layer, these nerve fibers appear close to the smooth muscle cells and are separated by a narrow cleft. These anatomical relations suggest that TRPV1 bladder nerves may potentially modulate urothelial function and/or smooth muscle contractility via the release of sensory peptides contained in TRPV1 bladder fibers.
In the urinary bladder, one of the more remarkable findings is that TRPV1 is not only expressed by afferent nerves that form close contact with the bladder epithelium but also in non-neuronal cells including urothelial cells and myofibroblasts (Fig. 1) (Table 1) [12,13]. The urothelium is a multilayered structure which has been shown to express a number of receptors/ion channels similar to that of mechanoreceptor and nociceptor neurons [16–18].Urothelial cells may be actively engaged in communicating with bladder nerves, adjacent urothelial cells, smooth muscle or even cells of the immune systems. For example, urothelial cells can release a variety of products, such as ATP, prostaglandins and nitric oxide, which can alter excitability of bladder afferent nerves [16]. In turn, nociceptive afferent activation releases a number of factors, such as substance P that can activate urothelial cells. These data raise the possibility of bidirectional chemical communication between urothelium and bladder nerves. TRPV1 could play a role in the communication because activation of urothelial TRPV1 receptors with capsaicin or resiniferatoxin increases intracellular calcium and evokes transmitter (nitric oxide, NO or ATP) release in cultured cells [12,13]. As noted in sensory neurons, these responses are enhanced by low pH, blocked by TRPV1 antagonists and eliminated in TRPV1 null mice [13]. In neurons, TRPV1 is thought to integrate/amplify the response to various stimuli and to play an essential role in the development of inflammation-induced hyperalgesia. Thus, it seems likely that urothelial-TRPV1 might participate in a similar manner, in the detection of irritant stimuli following bladder inflammation or infection.
Fig.1.
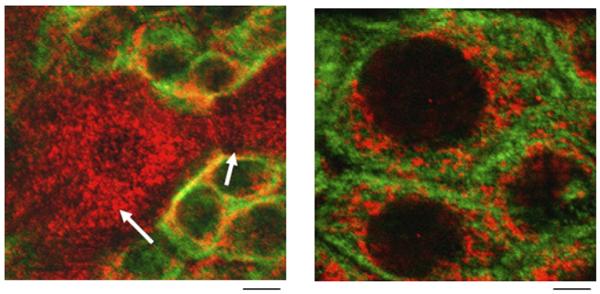
TRPV1 is detected in urothelial cells of the rat urinary bladder. Left panel, confocal image of urinary bladder urothelium reveals TRPV1 (cy3, red) and cytokeratin (FITC, green) expression. Arrow indicates apical cells within the field from a single plane of focus. Right panel, enlarged image of basal cells depicting TRPV1 (cy3, red) and cytokeratin (FITC, green) in urothelium. Scale bar: left, 15 μm; right, 5 μm.
Table 1.
Comparison of TRP channel properties in urinary bladder urothelial cells, interstitial cells, afferent nerves and skin keratinocytes
| TRPV1 | TRPV2 | TRPV3 | TRPV4 | TRPM8 | TRPA1 | |
|---|---|---|---|---|---|---|
| Urinary bladder urothelium |
Expression; Function [12,13,15,16,64] |
Expression [12,16] | Not shown | Expression; Function [16,47,48] |
Expression; Function [16,58] |
Expression; Function [16] |
| Urinary bladder afferent nerves |
Expression; Function [3,5,8–10,12–15] |
Not shown | Not shown | Function [16,47,48] | Function [50,52,53,54,56,57] | Function [50,52–54,56,57,61] |
| Urinary bladder interstitial cells |
Expression [19] | Not shown | Not shown | Not shown | Not shown | Not shown |
| Skin keratinocytes | Expression; Function [73] | Not shown | Expression; Function [73,74] |
Expression; Function [73] |
Not shown | Not shown |
Cultured urothelial cells from TRPV1 null mice also exhibit a reduction in stretch-evoked and hypotonic-evoked ATP release and stretch-evoked increase in membrane capacitance [13]. In addition, TRPV1 knockout mice have a higher frequency of low-amplitude, non-voiding bladder contractions suggesting the possibility of a small but ongoing role for TRPV1 in normal micturition [13]. This is also accompanied by a reduction in bladder distension-evoked c-fos expression in the spinal cord and reflex voiding. These relatively benign changes may result from TRPV1 expression not only in afferent nerves that form close contacts with bladder epithelial (urothelial) cells but also in urothelial cells themselves. These findings demonstrate that the functional significance of TRPV1 in the bladder extends beyond pain sensation to include participation in normal voiding function, and is essential for mechanically evoked purinergic signaling by the urothelium.
TRPV1 immunoreactivity has also been identified in a population of cells located in the sub-urothelial space [19]. These cells have morphological characteristics of myofibroblasts and stain intensively for vimentin and the gap junction protein connexin 43. It has been proposed that these “myofibroblast” or “interstitial” type cells may function as “pacemaker” cells in the bladder and have the capacity to modulate bladder sensations [20–22]. This hypothesis is given added support by the fact that many nerve fibers terminate on these cells, thus providing a means to modulate the sensitivity of bladder filling sensations. Understanding the mechanisms contributing to and maintaining these types of cell–cell interactions may provide important insight into development of novel targets for clinical management of a number of bladder disorders.
3. Role of TRP channels in bladder disorders
TRPV1 has attracted a lot of attention as an important player in bladder diseases. TRPV1 knockout mice display differences in the response to inflammation or injury as compared to their wild-type counterparts. For example, TRPV1 knockout mice do not develop bladder overactivity during acute bladder inflammation, suggesting that TRPV1 is involved in bladder hyperreflexia in inflammatory states [23,24]. In addition, patients suffering from neurogenic detrusor overactivity exhibit significant increases in the number of TRPV1-expressing nerves as well as TRPV1 expression within the urothelium [25,26]. These and other data which show a link between TRP channel expression or sensitivity and disease symptoms provide further support for a role of TRPV1 in the development of visceral hyperalgesia in the bladder.
In pathological conditions such as inflammation or following a spinal cord injury, increased reflex bladder contractions and emptying are triggered by activation of capsaicin sensitive C fibers. This emergence of a capsaicin-sensitive, C-fiber mediated spinal micturition reflex is thought to be due to reorganization of synaptic connections in the spinal cord and changes in the properties of the afferents [27,28]. Systemic administration of capsaicin is effective in animal models to block the hyperreflexia associated with neurogenic bladder dysfunctions following spinal cord injury (Fig. 2) [29–31]. This effect of capsaicin is attributable to induction of a long lasting refractory state in primary afferent neurons termed “capsaicin desensitization”. It is in this context that intravesical treatments with vanilloid compounds have been demonstrated to be beneficial in bladder disorders such as neurogenic bladder in patients with multiple sclerosis or following a spinal cord injury or hypersensitivity disorders such as interstitial cystitis (IC) [29,32–42]. Intravesical vanilloids have also been shown to dramatically reduce the number of bladder sensory fibers immunoreactive for TRPV1, substance P or CGRP in patients with painful bladder symptoms [25,43]. This is due in part to the either a depletion of afferent transmitters or degeneration of the peripheral nerve endings in the wall of the urinary bladder.
Fig. 2.
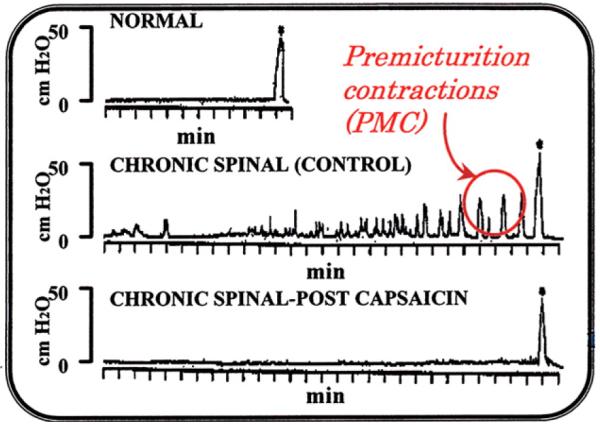
Cystometrograms (CMGs) in anesthetized CNS-intact and unanesthetized chronic spinal rats recorded under isotonic conditions with the bladder outlet open and the animals able to void. Top panel: CMG pattern with an infusion rate of 0.052 ml/min in anesthetized CNS-intact rat. Middle panel: CMG pattern (infusion rate 0.104 ml/min) in a chronic spinal rat before capsaicin treatment. Note relatively small nonvoiding contractions occurring during bladder filling and their amplitude progressively increasing with filling (Premicturition contractions, PMC). Bottom panel: the same chronic spinal rat after capsaicin (125 mg/kg s.c.) pretreatment 4 days before the CMG study. Note the nonvoiding contractions were eliminated but the voiding contraction was not altered. Vertical calibrations: intravesical pressure in cm H2O and horizontal calibrations: time in min. Asterisks (*) indicate voiding.
Though there is evidence that intravesical vanilloid therapy is successful, with a net effect to increase bladder capacity and to diminish bladder urge sensations associated with these bladder disorders [40], its use has been largely abandoned due to a high incidence of patient discomfort, poor bioavailability in addition to concerns about irreversible neurotoxicity. The use of resiniferatoxin (RTX) was met with initial enthusiasm as this compound is thought to exhibit an effect similar to capsaicin on bladder afferents but with lower toxicity. In patients with neurogenic detrusor overactivity exhibiting increased TRPV1 expression in both bladder nerves and the urothelium, intravesical treatment with RTX reduced TRPV1 immunoreactivity in both suburothelial afferent nerve and urothelial cells [25,44]. In addition, RTX has also been used in patients with IC [33,45]. While initial results with RTX were encouraging with improvements in bladder capacity and patients tolerating RTX better than capsaicin, analysis of clinical trial data revealed that RTX was ineffective and additional studies would not be pursued.
4. Additional TRP channels
Much less is known about the involvement of other TRPs in bladder function or disease. TRPV4 which is a nonselective cation channel activated by a number of stimuli including heat, shear stress, changes in osmolarity and lipid ligands is expressed in the urinary bladder [46–48]. A definitive role for TRPV4 in bladder function has not been established. However, in other systems, TRPV4 seems to play a role in hypo-osmotic hyperalgesia and in the development of neuropathic pain [49].
While little is known about the involvement of the cold-sensing TRP channels (TRPM8 and TRPA1) in bladder function, the effects of cold temperatures on lower urinary tract function has long been of interest [50,51]. The instillation of cold solutions (known as the ice water test) elicits involuntary detrusor contractions in patients with either chronic spinal cord lesions or following bladder outlet obstruction [51–54]. This reflex is believed to be mediated by activation of C-type afferent fibers within the pelvic nerve sensitive to cold temperatures. The finding that intravesical instillation of menthol facilitates the bladder cooling reflex in both the cat and human suggests that TRPM8, a cold- and menthol-sensing channel [55–57], may be involved in triggering the reflex. In the urinary bladder, TRPM8-positive immunoreactivity has been demonstrated within bladder nerves as well as the urothelium [58]. Because of the increased expression of TRPM8 in prostate cancer cells, this channel may also function as a potential tumor marker with utility in both the diagnosis and possible therapy for the treatment of cancer [59,60].
TRPA1 (formerly named ANKTM1) has been characterized as a thermoreceptor activated by noxious cold. Recent studies have shown that this channel is localized in sensory nerves that innervate the urinary bladder and mediates a contractile effect on bladder smooth muscle, likely due to release of tachykinins and cyclooxygenase metabolites [61]. The effect on smooth muscle contractility of agents capable of stimulating TRPA1 was comparable in potency to capsaicin, supporting the speculation that this channel may play a role in bladder function.
5. New directions for therapeutic treatments
The use of botulinum neurotoxin type A (BoNT/A) has been successful in the treatment of lower urinary tract symptoms such as frequency and urgency incontinence due to neurogenic or idiopathic overactivity of detrusor smooth muscle [62]. The mechanism of action was initially thought to involve blocking the vesicular release of neurotransmitters from efferent nerves resulting in reduced smooth muscle contractility. Because of patient reports of dramatic reductions in the sensation of urgency, it is now believed that the mechanism of action of this toxin in the overactive bladder may be more complicated and could involve modulation of purinergic or TRPV1-signaling mechanisms in afferent pathways. It has also been shown that the expression levels of TRPV1 may be regulated by nerve growth factor (NGF). Thus, a decrease in NGF production by botulinum toxin may in turn result in reduced TRPV1-mediated peptide release and prolonged desensitization of bladder afferents [63]. In addition, the reduction in nerve staining of the purinergic receptor P2X3 and TRPV1 after botulinum treatment may be due to a reduction in NGF levels which in turn, could reduce purinergic and/or TRP channel trafficking [64]. It is proposed that the decreased levels of sensory receptors may contribute to the clinical efficacy of BoNT/A in detrusor overactivity.
6. Complex regulation of TRP channels
TRP channels are complex not only because they are polymodal, i.e., respond to multiple external stimuli, but also because they are modulated by intracellular signaling mechanisms such as protein kinase induced phosphorylation and changes in intracellular Ca2+ concentration. In addition homomeric and heteromeric interactions between different TRP isoforms/splice variants and other accessory proteins could influence TRP channel function [65,66]. Whether co-assembly occurs between subunits belonging to TRPV1 and TRPV4 or TRPA1 is not presently known but would be one explanation for the sensitivity of TRPV1 to mechanical stimuli. Recent studies have also highlighted the complex effects of TRP channel agonists such as menthol, camphor and cinnamaldehyde on multiple temperature sensitive TRPs [67]. The activation and inhibition of multiple TRP channels by these and other agents may occur via a direct effect on the channel or indirect, via modification of membrane structure, binding to other receptors or activation of second messengers. Though it is still unclear as to how one TRP channel may have multiple functions or how various TRP channel proteins interact within a cell, these channels will undoubtedly continue to be a major focus of research into the mechanisms underlying diseases of the lower urinary tract.
7. Conclusions
The vast numbers of TRP channels, their broad expression patterns and redundancy of receptor expression in both neuronal and non-neuronal cells, the ability to respond to a number of stimulus modalities and the lack of specific antagonists have made their study in bladder function difficult. Intravesical instillation of vanilloids (capsaicin or resiniferatoxin, RTX) has demonstrated improvement in a number of urodynamic parameters in patients with detrusor overactivity and patients with hypersensitivity disorders such as IC, presumably by desensitizing bladder nerves. However, the high incidence of side effects in patients following intravesical administration with capsaicin has made this treatment a “double-edged sword” thus providing concerns regarding the selectivity of TRPV1 agonists for clinical use. In addition, the diverse mechanisms underlying many bladder disorders have promoted interest in the identification of small molecule TRP antagonists (in particular TRPV1) for the treatment of many of these conditions.
There is particular interest in TRPV1 due to the number of endogenous ligands known to accumulate in diseased tissue that could activate this channel. Besides moderate heat and low pH, TRPV1 can also be activated by the cannabinoid receptor ligand anandamide, the eicosanoids 12-(S)-HPETE, 15-(S)-HPETE, 5-(S)-HETE, and leukotriene B2, endogenous lipids and the so-called endovanilloids such as the endogenous compounds arachidonylethanolamide and 2-arachidonoyl-glycerol [6,68,69]. In addition to these endogenous activators which are generated during inflammation, TRPV1 channel activation can also be sensitized by phosphorylation such as by protein kinase C (PKC), which itself can be activated through a number of G protein coupled receptor pathways including bradykinin, ATP, acetylcholine or substance P, whose levels may be augmented during inflammation [70,71]. In support of this idea are demonstrations of abnormal TRPV1 responses in a cat model for IC [72]. In this model it was reported that afferent neurons exhibit abnormal capsaicin responses due in part to enhanced endogenous activity of PKC. Thus, it can be imagined that in the near future new antagonist based therapies that are selective for abnormal TRPV1 receptors sparing those with physiological functions may be used in the treatment in a number of bladder disorders.
Acknowledgements
The author is grateful to Drs. William C. de Groat and Ann Hanna-Mitchell for helpful suggestions and critical reading of this manuscript. This work was supported by grants to L. Birder from the NIH (RO1 DK54824 and RO1 DK57284).
References
- [1].Hicks GA. TRP channels as therapeutic targets: hot property, or time to cool down? Neurogastroenterology and Motility. 2006;18:590–594. doi: 10.1111/j.1365-2982.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- [2].Levine JD, Alessandri-Harber N. TRP channels: targets for the relief of pain. Biochimica et Biophysica Acta. 2007;1772:989–1003. doi: 10.1016/j.bbadis.2007.01.008. [DOI] [PubMed] [Google Scholar]
- [3].Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacological Reviews. 1999;51:150–221. [PubMed] [Google Scholar]
- [4].Nagy I, Santha P, Jansco G, Urban L. The role of the vanilloid (capsaicin) receptor (TRPV1) in physiology and pathology. European Journal of Pharmacology. 2004;500:351–369. doi: 10.1016/j.ejphar.2004.07.037. [DOI] [PubMed] [Google Scholar]
- [5].Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in pain pathway. Nature. 1997;89:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- [6].Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh Y. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proceedings of the National Academy of Sciences. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lecci A, Maggi CA. Tachykinins as modulators of the micturition reflex in the central and peripheral nervous system. Regulatory Peptides. 2001;101:1–18. doi: 10.1016/s0167-0115(01)00285-3. [DOI] [PubMed] [Google Scholar]
- [8].Dasgupta P, Fowler CJ. Chillies from antiquity to urology. British Journal of Urology. 1997;80:845–852. doi: 10.1046/j.1464-410x.1997.00424.x. [DOI] [PubMed] [Google Scholar]
- [9].Szallasi A, Conte B, Goso C, Blumberg PM, Manzini S. Characterization of a peripheral vanilloid (capsaicin) receptor in the rat urinary bladder. Life Sciences. 1993;52:PL221–PL226. doi: 10.1016/0024-3205(93)90051-4. [DOI] [PubMed] [Google Scholar]
- [10].Maggi CA, Barbanti G, Santicioli P, Beneforti P, Misuri D, Meli A. Cystometric evidence that capsaicin-sensitive nerves modulate the afferent branch of micturition reflex in humans. Journal of Urology. 1989;142:150–154. doi: 10.1016/s0022-5347(17)38701-3. [DOI] [PubMed] [Google Scholar]
- [11].Maggi CA. The dual function of capsaicin-sensitive sensory nerves in the bladder and urethra. Ciba Foundation Symposium. 1990;151:77–83. doi: 10.1002/9780470513941.ch5. [DOI] [PubMed] [Google Scholar]
- [12].Birder LA, Kanai AJ, Groat W.C. de, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proceedings of the National Academy of Sciences. 2001;98:13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, Groat W.C. de, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nature Neuroscience. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- [14].Avelino A, Cruz C, Nagy L, Cruz F. Vanilloid receptor 1 expression in the rat urinary tract. Neuroscience. 2002;109:787–797. doi: 10.1016/s0306-4522(01)00496-1. [DOI] [PubMed] [Google Scholar]
- [15].Avelino A, Cruz F. TRPV1 (vanilloid receptor) in the urinary tract: expression, function and clinical applications Naunyn–Schmiedeberg’s. Archives of Pharmacology. 2006;373:287–299. doi: 10.1007/s00210-006-0073-2. [DOI] [PubMed] [Google Scholar]
- [16].Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nature Clinical Practice Urology. 2007;4:46–54. doi: 10.1038/ncpuro0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Apodaca G. The urothelium: not just a passive barrier. Traffic. 2000;5:1–12. doi: 10.1046/j.1600-0854.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- [18].Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. American Journal of Physiology. 2000;278:F867–F874. doi: 10.1152/ajprenal.2000.278.6.F867. [DOI] [PubMed] [Google Scholar]
- [19].Ost D, Roskams T, Van derAa F, Ridder D. De. Topography of the vanilloid receptor in the human bladder: more than just the nerve fibers. Journal of Urology. 2002;168:293–297. [PubMed] [Google Scholar]
- [20].Sui GP, Wu C, Fry CH. Electrical characteristics of suburothelial cells isolated from the human bladder. Journal of Urology. 2004;171:938–943. doi: 10.1097/01.ju.0000108120.28291.eb. [DOI] [PubMed] [Google Scholar]
- [21].Aa F. Van der, Roskams T, Blyweert W, Ost D, Bogaert G, De Ridder D. Identification of kit positive cells in the human urinary tract. Journal of Urology. 2004;171:2492–2496. doi: 10.1097/01.ju.0000125097.25475.17. [DOI] [PubMed] [Google Scholar]
- [22].Brading AF, McCloskey KD. Mechanisms of disease: specialized interstitial cells of the urothelium: an assessment of current knowledge. Nature Clinical Practice Urology. 2005;2:546–555. doi: 10.1038/ncpuro0340. [DOI] [PubMed] [Google Scholar]
- [23].Silva C, Charrua A, Dinis P, Cruz F. TRPV1 knockout mice do not develop bladder overactivity during acute chemical bladder inflammation. Society for Neuroscience Abstracts. 2004:288–16. [Google Scholar]
- [24].Szallasi A, Cruz F, Geppetti P. TRPV1: a therapeutic target for novel analgesic drugs? Trends in Molecular Medicine. 2006;12:545–554. doi: 10.1016/j.molmed.2006.09.001. [DOI] [PubMed] [Google Scholar]
- [25].Apostolidis A, Brady CM, Yiangou Y, Davis J, Fowler CJ, Anand P. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology. 2005;65:400–405. doi: 10.1016/j.urology.2004.10.007. [DOI] [PubMed] [Google Scholar]
- [26].Brady CM, Apostolidis A, Harper M, Yiangou Y, Beckett A, Jacques TS, Freeman A, Scaravilli F, Fowler CJ, Anand P. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU International. 2004;93:770–776. doi: 10.1111/j.1464-410X.2003.04722.x. [DOI] [PubMed] [Google Scholar]
- [27].de Groat WC. A neurologic basis for the overactive bladder. Urology. 1997;50:36–52. doi: 10.1016/s0090-4295(97)00587-6. [DOI] [PubMed] [Google Scholar]
- [28].de Groat WC, Kawatani M, Hisamitsu T, Cheng C-L, Ma CP, Thor K, Steers W, Roppolo JR. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. Journal of the Autonomic Nervous System. 1990;30:S71–S78. doi: 10.1016/0165-1838(90)90105-r. [DOI] [PubMed] [Google Scholar]
- [29].Chancellor MB, Groat W.C. de. Intravesical capsaicin and resiniferatoxin therapy: spicing up the ways to treat the overactive bladder. Journal of Urology. 1999;162:3–11. doi: 10.1097/00005392-199907000-00002. [DOI] [PubMed] [Google Scholar]
- [30].Cheng C-L, Liu JC, Chang SY, Ma CP, de Groat WC. Effect of capsaicin on the micturition reflex in normal and chronic spinal cord-injured cats. American Journal of Physiology. 1999;277:R786–R794. doi: 10.1152/ajpregu.1999.277.3.R786. [DOI] [PubMed] [Google Scholar]
- [31].Cheng C-L, Ma CP, de Groat WC. Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Research. 1995;678:40–48. doi: 10.1016/0006-8993(95)00212-9. [DOI] [PubMed] [Google Scholar]
- [32].Lazzeri M, Spinelli M, Zanollo A, Turini D. Intravesical vanilloids and neurogenic incontinence: 10 years experience. Urology International. 2004;72:145–149. doi: 10.1159/000075969. [DOI] [PubMed] [Google Scholar]
- [33].Payne CK, Mosbaugh PG, Forrest JB. Intravesical resiniferatoxin for the treatment of interstitial cystitis: a randomized, double-blind, placebo controlled trial. Journal of Urology. 2005;173:1590–1594. doi: 10.1097/01.ju.0000154631.92150.ef. [DOI] [PubMed] [Google Scholar]
- [34].Cruz F, Guimaraes M, Silva C, Rio ME, Coimbra A, Reis M. Desensitization of bladder sensory fibers by intravesical capsaicin has long lasting clinical and urodynamic effects in patients with hyperactive or hypersensitive bladder dysfunction. Journal of Urology. 1997;157:585–589. [PubMed] [Google Scholar]
- [35].De seze M, Wiart L, Joseph PA, Dosque JP, Mazaux JM, Barat M. Capsaicin and neurogenic detrusor hyperreflexia. A double blind placebo controlled study in 20 patients with spinal cord lesions. Neurourology and Urodynamics. 1998;17:513–523. doi: 10.1002/(sici)1520-6777(1998)17:5<513::aid-nau7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- [36].Dinis P, Silva J, Ribeiro MJ, Avelino A, Reis M, Cruz F. Bladder C-fiber desensitization induces a long-lasting improvement of BPH-associated storage. LUTS: a pilot study, European Urology. 2004;46:88–93. doi: 10.1016/j.eururo.2004.01.016. [DOI] [PubMed] [Google Scholar]
- [37].Fowler CJ, Jewkes D, McDonald WI, Lynn B, de Groat WC. Intravesical capsaicin for neurogenic bladder dysfunction. Lancet. 1992;339:1239. doi: 10.1016/0140-6736(92)91186-c. [DOI] [PubMed] [Google Scholar]
- [38].Geirsson G, Fall M, Sullivan L. Clinical and urodynamic effects of intravesical capsaicin treatment in patients with chronic traumatic spinal detrusor hyperreflexia. Journal of Urology. 1995;154:1825–1829. [PubMed] [Google Scholar]
- [39].Lazzeri M, Beneforti P, Spinelli M, Zanollo A, Bargagli G, Turini D. Intravesical resiniferatoxin for the treatment of hypersensitive disorder: a randomized placebo controlled study. Journal of Urology. 2000;164:646–679. doi: 10.1097/00005392-200009010-00014. [DOI] [PubMed] [Google Scholar]
- [40].Apostolidis A, Gonzales GE, Fowler CJ. Effect of intravesical resiniferatoxin (RTX) on lower urinary tract symptoms, urodynamic parameters, and quality of life of patients with urodynamic increased bladder sensation. European Urology. 2006;50:1299–1305. doi: 10.1016/j.eururo.2006.04.006. [DOI] [PubMed] [Google Scholar]
- [41].Mukerji G, Yiangou Y, Agarwal SK, Anand P. Transient receptor potential vanilloid receptor subtype 1 in painful bladder syndrome and its correlation with pain. Journal of Urology. 2006;176:797–801. doi: 10.1016/j.juro.2006.03.074. [DOI] [PubMed] [Google Scholar]
- [42].Kalsi V, Fowler CJ. Therapy insight: bladder dysfunction associated with multiple sclerosis. Nature Clinical Practice Urology. 2005;2:492–501. doi: 10.1038/ncpuro0323. [DOI] [PubMed] [Google Scholar]
- [43].Brady CM, Apostolidis A, Harper M, Yiangou Y, Beckett A, Jacques TS, Freeman A, Scaravilli F, Fowler CJ, Anand P. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU International. 2004;93:770–776. doi: 10.1111/j.1464-410X.2003.04722.x. [DOI] [PubMed] [Google Scholar]
- [44].Apostolidis A, Brady CM, Yiangou Y, Davis J, Fowler CJ, Anand P. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology. 2005;65:400–405. doi: 10.1016/j.urology.2004.10.007. [DOI] [PubMed] [Google Scholar]
- [45].Chai TC, Keay S. New theories in interstitial cystitis. Nature Clinical Practice Urology. 2004;1:85–89. doi: 10.1038/ncpuro0057. [DOI] [PubMed] [Google Scholar]
- [46].Liedtke W. TRPV4 plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli in live animals. Pflugers Archiv. 2005;451:176–180. doi: 10.1113/jphysiol.2005.088963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gevaert T, Vriens J, Everaerts W, Nilius B, De Ridder D. TRPV4 is localized on urothelium: does it play a role in afferent bladder signaling? European Urology. 2007;(Suppl 6):38. [Google Scholar]
- [48].Barrick S, Lee H, Caterina M, Kanai AJ, de Groat WC, Birder LA. Expression and function of TRPV4 in urinary bladder urothelium. Society for Neuroscience Abstracts. 2003:608–6. [Google Scholar]
- [49].Alessandri-Haber N, Dina OA, Joseph EK, Reichling D, Levine JD. A transient receptor potential vanilloid 4-dependent mechanism of hyperalgesia is engaged by concerted action of inflammatory mediators. Journal of Neuroscience. 2006;26:3864–3874. doi: 10.1523/JNEUROSCI.5385-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cheng C-L, Chai CY, de Groat WC. Detrusor-sphincter dyssnergia induced by cold stimulation of the urinary bladder of rats. American Journal of Physiology. 1997;41:R1271–R1282. doi: 10.1152/ajpregu.1997.272.4.R1271. [DOI] [PubMed] [Google Scholar]
- [51].Wein AJ. In: Neuromuscular dysfunction of the lower urinary tract. Walsh PC, Retik AB, Stamey TA, Vaughn ED, editors. Campbell’s Urology, Saunders; Philadelphia: 1992. pp. 573–642. [Google Scholar]
- [52].Chai TC, Gray J, Steers W. The incidence of a positive ice water test in bladder outlet obstructed patients: evidence for bladder neural plasticity. Journal of Urology. 1998;160:34–38. [PubMed] [Google Scholar]
- [53].Gotoh M, Yoshikawa Y, Kondo AS, Kondo A, Ono Y, Ohshima S. Positive bladder cooling reflex in patients with bladder outlet obstruction due to benign prostatic hyperplasia. World Journal of Urology. 1999;17:126–130. doi: 10.1007/s003450050118. [DOI] [PubMed] [Google Scholar]
- [54].Hirayama A, Fujimoto K, Matumoto Y, Ozono S, Hirao Y. Positive response to ice water test associated with high-grade bladder outlet obstruction in patients with benign prostatic hyperplasia. Urology. 2003;62:909–913. doi: 10.1016/s0090-4295(03)00588-0. [DOI] [PubMed] [Google Scholar]
- [55].Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- [56].Jiang CH, Mazieres L, Lindstrom S. Cold- and menthol-sensitive C afferents of cat urinary bladder. Journal of Physiology. 2002;543:211–220. doi: 10.1113/jphysiol.2002.019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Geirsson G, Lindstrom S, Fall M. The bladder cooling reflex and the use of cooling as stimulus to the lower urinary tract. Journal of Urology. 1999;162:1890–1896. doi: 10.1016/S0022-5347(05)68062-7. [DOI] [PubMed] [Google Scholar]
- [58].Stein RJ, Santos S, Nagatomi J, Hayashi Y, Minnery BS, Xavier M, Patel AS, Nelson JB, Futrell WJ, Yoshimura N, Chancellor MB, De Miguel F. Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. Journal of Urology. 2004;172:1175–1178. doi: 10.1097/01.ju.0000134880.55119.cf. [DOI] [PubMed] [Google Scholar]
- [59].Beck B, Bidaux G, Bavencoffe A, Lemonnier L, Thebault S, Shuba Y, Barrit G, Skryma R, Prevarskaya N. Prospects for prostate cancer imaging and therapy using high-affinity TRPM8 activators. Cell Calcium. 2007;41:285–294. doi: 10.1016/j.ceca.2006.07.002. [DOI] [PubMed] [Google Scholar]
- [60].Zhang L, Barritt GJ. TRPM8 in prostate cancer cells: a potential diagnostic and prognostic marker with a secretory function? Endocrine-Related Cancer. 2006;13:27–38. doi: 10.1677/erc.1.01093. [DOI] [PubMed] [Google Scholar]
- [61].Andrade EL, Ferreira J, Andre E, Calixto JB. Contractile mechanisms coupled to TRPA1 receptor activation in rat urinary bladder. Biochemical Pharmacology. 2006;72:104–114. doi: 10.1016/j.bcp.2006.04.003. [DOI] [PubMed] [Google Scholar]
- [62].Chancellor MB. Urgency, botulinum toxin and how botulinum toxin can help urgency. Journal of Urology. 2005;174:818. doi: 10.1097/01.ju.0000175099.01082.d0. [DOI] [PubMed] [Google Scholar]
- [63].Agiannantoni SM, Distisi SM, Nardicchi V, Zucchi A, Marchioni L, Bini V, Goracci G, Forena M. Botulinum toxin A injections into the detrusor muscle decreases nerve growth factor bladder tissue levels in patients with neurogenic detrusor overactivity. Journal of Urology. 2006;175:2341–2344. doi: 10.1016/S0022-5347(06)00258-8. [DOI] [PubMed] [Google Scholar]
- [64].Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, Dasgupta P, Fowler CJ, Anand P. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. Journal of Urology. 2005;174:977–982. doi: 10.1097/01.ju.0000169481.42259.54. [DOI] [PubMed] [Google Scholar]
- [65].Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer M. Homo- and heteromeric assembly of TRPV channel subunits. Journal of Cell Science. 2005;118:917–928. doi: 10.1242/jcs.01675. [DOI] [PubMed] [Google Scholar]
- [66].Liapi A, Wood JN. Extensive co-localization and heteromultimer formation of the vanilloid receptor-like protein TRPV2 and the capsaicin receptor TRPV1 in the adult rat cerebral cortex. European Journal of Neuroscience. 2005;22:825–834. doi: 10.1111/j.1460-9568.2005.04270.x. [DOI] [PubMed] [Google Scholar]
- [67].Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Molecular and Cellular Neurosciences. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- [68].Calixto JB, Kassuya CAL, Andre E, Ferreira J. Contribution of natural products to the discovery of the transient receptor potential (TRP) channels family and their functions. Pharmacology and Therapeutics. 2005;106:179–208. doi: 10.1016/j.pharmthera.2004.11.008. [DOI] [PubMed] [Google Scholar]
- [69].Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proceedings of the National Academy of Sciences. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- [71].Jung J, Shin JS, Lee SY, Hwang SW, Koo J, Cho H, Oh U. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. Journal of Biological Chemistry. 2004;279:7048–7054. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- [72].Sculptoreanu A, Groat W.C. de, Buffington CA, Birder LA. Protein kinase C contributes to abnormal capsaicin responses in DRG neurons from cats with feline interstitial cystitis. Neuroscience Letters. 2005;381:42–46. doi: 10.1016/j.neulet.2005.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lee H, Caterina MJ. TRPV channels as thermosensory receptors in epithelial cells. Pflugers Archiv. 2005;451:160–167. doi: 10.1007/s00424-005-1438-y. [DOI] [PubMed] [Google Scholar]
- [74].Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, bevan S, Patapoutian A. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]


