Abstract
We tested whether the heritability of heart rate variability (HRV) under stress is different from rest and its dependency on ethnicity or gender. HRV indexed by root mean square of successive differences (RMSSD) and high-frequency (HF) power was measured at rest and during 3 stressors in 427 European and 308 African American twins. No ethnic or gender differences were found for any measures. There was a nonsignificant increase in heritability of RMSSD (from 0.48 to 0.58) and HF (from 0.50 to 0.58) under stress. Up to 81% and 60% of the heritabilities of RMSSD and HF under stress could be attributed to genes influencing rest levels. The heritabilities due to genes expressed under stress were 0.11 for RMSSD and 0.23 for HF. The findings suggest that, independent of ethnicity and gender, HRV regulation at rest and under stress is largely influenced by the same genes with a small but significant contribution of stress-specific genetic effects.
Descriptors: Heart rate variability, Stress, Ethnicity, Twin study
Heart rate variability (HRV), a simple noninvasive measurement of cardiac autonomic function, has been used as an indicator of cardiovascular health. Reduced HRV, reflecting a shift in cardiac sympathovagal balance from parasympathetic to sympathetic control of the heart rhythm (La Rovere et al., 2001; Schwartz, 1998), is a predictor of all-cause mortality, arrhythmic events, and sudden death after acute myocardial infarction (Reinhardt et al., 1996) as well as in the general population (Dekker et al., 1997).
There are large individual differences in HRV. Previous reports showed that several environmental factors, including lack of exercise (Gutin, Owens, Slavens, Riggs, & Treiber, 1997), unhealthy diet (Bouchard et al., 1990), chronic stress (Vrijkotte, van Doornen, & de Geus, 2000), coffee consumption, and smoking (Stolarz et al., 2003; Tsuji et al., 1996) can decrease HRV. However, it has now become clear that a large part of the differences can be attributed to genetic factors. Twin studies (Boomsma, van Baal, & Orlebeke, 1990; Kupper et al., 2004; Snieder, Boomsma, Van Doornen, & De Geus, 1997) and family studies (Singh et al., 1999; Sinnreich, Friedlander, Luria, Sapoznikov, & Kark, 1999) in Caucasian populations have found that up to 65% of the variance in HRV at rest can be explained by genetic influences. Recently, several lines of evidence suggest that genetic influence on HRV may be more pronounced when the subject is challenged by mentally and emotionally taxing tasks (Boomsma et al., 1990; Snieder et al., 1997). In one study (Boomsma et al., 1990), HRV indexed by respiratory sinus arrhythmia (RSA) was measured in 160 adolescent twin pairs during a rest period and two stressful laboratory tasks. During rest, only 25% of the variance in HRV was accounted for by genetic influences. But under task conditions, the genetic contribution increased up to 51%. A similar study was performed in 212 middle-aged twin pairs and also observed that the genetic variance of RSA increased from 31% at rest to up to 43% under stress (Snieder et al., 1997). However, in these two studies univariate models were used, which are unable to address the following two important questions. First, are the genes influencing HRV under stress the same or different from those at rest? Second, does the magnitude of the genetic influence differ significantly under rest and stress conditions? Furthermore, the above studies only involved Caucasian subjects. We recently conducted the first twin study to test and compare heritability of HRV at rest in African and European Americans twins (Wang, Thayer, Treiber, & Snieder, 2005). Although African Americans (AAs) had greater mean values of HRV than European Americans (EAs) at rest, the best fitting model did not show ethnic differences in the heritability of HRV. To the best of our knowledge, no study has explored the heritability of HRV under behavioral stress in AAs.
In this study, which includes a large number of EA and AA adolescent and young adult twin pairs who had beat-to-beat heart rate measured both at rest and during three acute behavioral stress tasks, we aim to determine whether the genetic influences on HRV under stress are different from those at rest and the extent to which they depend on ethnicity or gender using a multivariate model.
Methods
Participants
The present study comprised participants from the Georgia Cardiovascular Twin Study, which was established in 1996 (Ge, Dong, Wang, Treiber, & Snieder, 2006; Snieder & Treiber, 2002). It included roughly equal numbers of AAs and EAs (> 500 twin pairs) with the purpose of exploring the change in relative influence of genetic and environmental factors on the development of cardiovascular risk factors. All twin pairs were reared together and zygosity was determined using five standard microsatellite markers in DNA collected with buccal swabs (Jackson, Snieder, Davis, & Treiber, 2001). Participants were recruited from the southeastern United States and were overtly healthy and free of any acute or chronic illness based on parental report. Study design and selection criteria for this twin study have been described previously (Snieder & Treiber, 2002). Participants were classified as AAs if (1) both parents reported being of African heritage, (2) they and the child were born and raised in the United States, and (3) parents considered themselves and their child to be African American, Black, or Afro-American. Participants were classified as EAs if (1) both parents reported that they were of European ancestry, (2) they and the child were born and raised in the United States, and (3) they considered themselves and their child to be European American, White, or Caucasian (Snieder & Treiber, 2002).
For the current study, data were available from 427 EA (193 pairs and 41 singletons) and 308 AA (134 pairs and 40 singletons) twins (mean ± SD age: 17.8 ± 3.2 years; range: 11.9–32.9 years), who had HRV parameters measured at rest or during stress from 2004 to 2005 during a routine scheduled examination. The Institutional Review Board at the Medical College of Georgia had given approval for this study. Written informed consent was provided by all participants and by parents if participants were <18 years.
Experimental Procedures
On arrival at the laboratory, the participant was escorted to a quiet, temperature-controlled room where the participant’s anthropomorphic measurements (e.g., height, weight, and waist) were recorded using established protocols. The participant was then asked to lie on a hospital bed in a supine position to relax as completely as possible for 15 min. Following the resting period, the participant engaged in three 10-min laboratory stressors (viz., the virtual reality car driving, video game challenge, and the social competence interview) using standardized protocols. These three stressors have been successfully used in our laboratory studies for over 10 years (Jackson, Treiber, Turner, Davis, & Strong, 1999; Malpass et al., 1997; Treiber et al., 1996).
The virtual reality car driving stressor was administered using a protocol developed in our laboratory. Briefly, the participant wore a Kaiser-Optic Visual Immersion Monitor (VIM 500) fitted on his/her head. The VIM 500 was interfaced with a Panasonic Real 3DO Interactive Multiplayer System. The participant played ‘‘Need for Speed’’ under the condition of challenge (i.e., money incentive) without harassment for 10 minutes.
In the video game challenge, participants performed a modified version of the video game protocol of Murphy, Alpert, and Walker (1992). Briefly, after viewing a videotape with an adult female of the same ethnicity providing sex neutral instructions and a demonstration, the subject played ‘‘Breakout’’ under the condition of challenge (i.e., money incentive) without harassment for 10 min.
The social competence interview was administered using an established protocol (Ewart & Kolodner, 1991). Briefly, participants discussed a recent interpersonal interaction that resulted in significant anger and/or frustration. A 10-min structured interview was used to guide the participant in describing the event, including his/her affective and behavioral responses and summarization of outcome of the event.
Measurement of Beat-to-Beat Heart Rate at Rest and during Stress
The continuous RR intervals were recorded by BioZ (Cardio-Dynamics, San Diego, CA) impedance monitors. Four dual sensors connected to the BioZ were placed on the participant’s neck and thorax, which formed four ECG vectors. These ECG vectors can be detected by the BioZ. The BioZ then converted the RR interval into beat-to-beat heart rate with precision of two decimal places, including a record of the real time of each beat. The instrument and recording procedure were used both at rest and during stress. At the 10th, 12th, and 14th minute of the 15-min resting period, a 30-s continuous period of RR intervals was recorded. During each 10-min stressor, a 30-s continuous period of RR intervals was recorded once every 2min. It has been documented that HRV from short recordings can assess cardiac autonomic activity (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996) and predict all-cause mortality and cardiac death as accurately as the long recordings (Bigger, Fleiss, Rolnitzky, & Steinman, 1993).
Parameters of HRV
Prior to calculation of HRV parameters, the raw RR interval data were first processed for artifactual readings using the following two criteria: (1) RR intervals were between 300 and 2000 ms and (2) the successive RR interval ratios were between 0.8 and 1.2 (Timonen et al., 2006). The software (Kubios HRVanalysis), which was developed by Niskanen, Tarvainen, Ranta-Aho, and Karjalainen (2004), was used to generate the HRV parameters from the recorded RR intervals. One of the time-domain measures, root mean square of successive differences (RMSSD) of normal RR intervals, and one of the frequency-domain measures, high-frequency (HF) power (defined as the power between 0.15 and 0.40 Hz, using Fast Fourier Transformation), were used in the present study. Both measures specifically reflect vagal tone activity and have been recommended by the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). HRV parameters at rest and during each stressor were calculated separately using the combination of the three 30-s segments of continuous RR intervals at rest and five 30-s segments of RR intervals during the virtual reality car driving, video game challenge, and the social competence interview. Based on the study by Schroeder et al. (2004), which found that the RMSSD calculated from 10 s showed the same reproducibility as those obtained from 6 min, and the study by De Rivecourt, Kuperus, Post, and Mulder (2008), which observed a high correlation between the HF calculated from 240 s and 30, 60, or 120 s, the HRV parameters in our data set should be accurate enough to assess cardiac autonomic activity. Because aggregation over multiple tasks has been shown to enhance reliability due to its ability to reduce the relative influence of unique situational variance (Kamarck, Jennings, Pogue-Geile, & Manuck, 1994; Kamarck & Lovallo, 2003), the aggregated stress score, that is, the average HRV value across the three tasks, was used as the stress level. The responses of RMSSD and HF to stress were indexed by the difference between stress and resting levels.
Analytical Approach
The purpose of our analyses was to test whether the genetic influences on HRV under stress are different from those at rest and the extent to which they depend on ethnicity or gender. To this end we applied a bivariate model fitting technique in AA and EA twins separately to estimate ethnicity-specific genetic and environmental variance components and investigate gender differences. Eventually we combined both ethnic groups into one bivariate model to test for potential differences in AAs and EAs.
Genetic Modeling
Twin methodology makes use of the fact that monozygotic (MZ) twins share identical genotypes, whereas dizygotic (DZ) twins share on average 50% of their genes. It is assumed that both types of twins share their common family environment to the same extent, so any greater similarity between MZ compared with DZ twins reflects genetic influences. In this study, classical structural equation modeling (SEM) was used. SEM is based on the comparison of the variance–covariance matrices in MZ and DZ twin pairs and allows separation of the observed phenotypic variance into its genetic and environmental components: additive (A) genetic, common (C), and unique (E) environmental components. Dividing each of these components by the total variance yields the different standardized components of variance. For example, the heritability (h2) can be defined as the proportion of the total variance attributable to additive genetic variation (Neale & Cardon, 1992).
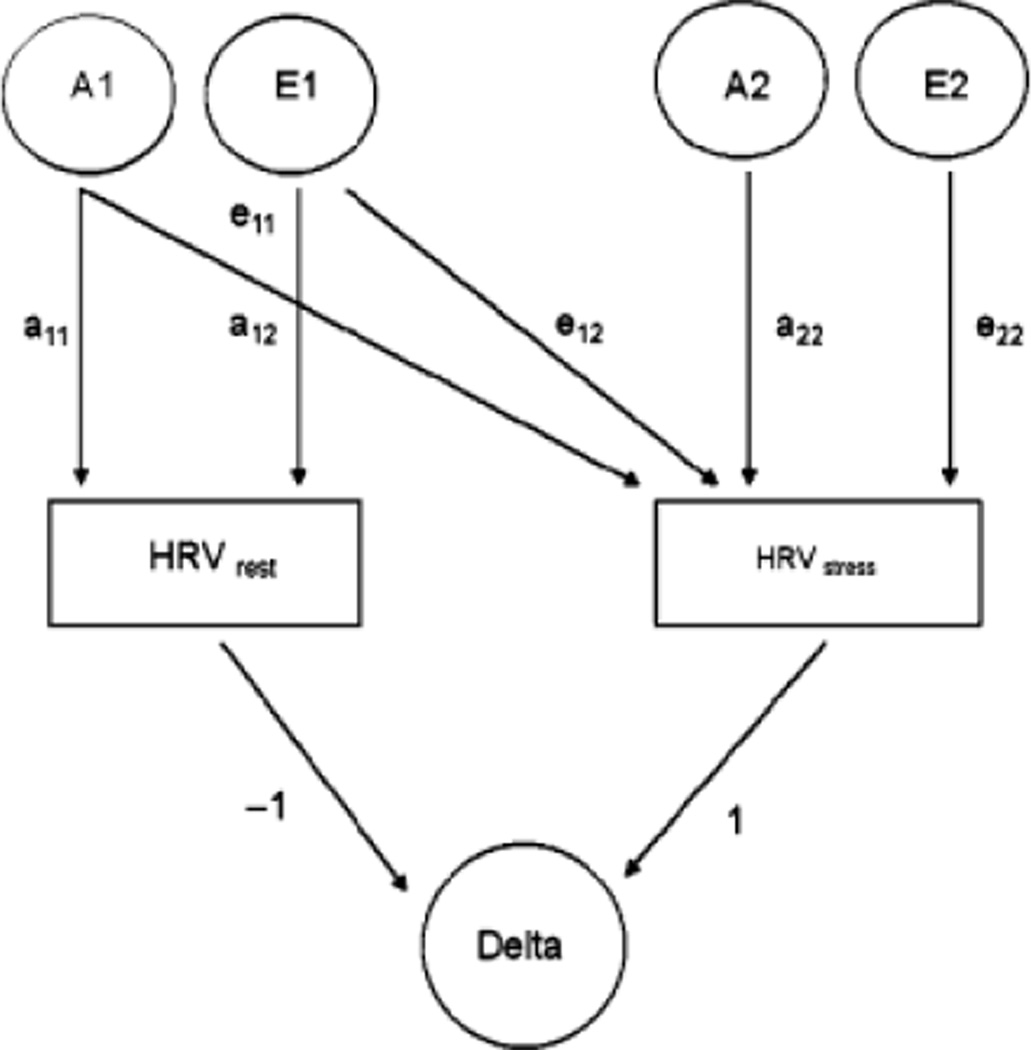
For the purpose of the current study, a bivariate extension of the basic path model, which is shown in Figure 1, was used. With this model, making use of the ‘‘Cholesky decomposition,’’ we can not only estimate the heritability of HRV at rest and during stress , but also can test whether the magnitude of the genetic influence differs in rest and stress conditions (i.e., ?). We can further test whether the genes influencing HRV under stress are the same (i.e., a22 = 0?), partly the same (i.e., a12 ≠ 0 and a22 ≠, 0?) or entirely different (i.e., a12 = 0?) from those at rest. If they are partly the same, this bivariate model allows further determination of the amount of overlap between genes influencing HRV at rest and during stress by calculating genetic correlation between the two HRV levels (rg = COVA(rest, stress)/ √(VArest *VAstress)). Shared and unique environmental correlations can be calculated in a similar fashion (Neale & Cardon, 1992). Within the best fitting bivariate model of the HRV at rest and during stress, we also calculated the heritability of reactivity as a change score, which can simply be derived from the parameter estimates of the phenotype levels in the bivariate model (Figure 1; De Geus, Kupper, Boomsma, & Snieder, 2007).
Figure 1.
Gender Differences
Gender differences were examined by comparing a full model in which parameter estimates are allowed to differ in magnitude between males and females with a reduced model in which parameter estimates are constrained to be equal across the genders. In addition to those models a scalar model was tested. In a scalar model heritabilities are constrained to be equal across genders, but total variances may be different (Neale & Cardon, 1992).
Ethnic Differences
Ethnic differences were, just like gender differences, examined by comparing a full model in which parameter estimates are allowed to differ in magnitude between AAs and EAs with a reduced model in which parameter estimates are constrained to be equal across ethnicity. In addition to those models a scalar model was tested in a similar fashion as done for gender (Neale & Cardon, 1992).
Model Fitting Procedure
Prior to analysis, effects of age (a), gender (g), ethnicity (e), and their interactions (a × g, a × e, g × e, a × g × e) were regressed out for all variables before using the residuals in model fitting. The significance of variance components A, C, and E was assessed by testing the deterioration in model fit after each component was dropped from the full model. Standard hierarchic χ2 tests were used to select the best fitting models in combination with Akaike’s information criterion (AIC = χ2 – 2df). The model with the lowest AIC reflects the best balance of goodness of fit and parsimony (Neale & Cardon, 1992).
Statistical Software
Prior to analysis, all HRV parameters were log transformed to obtain a better approximation of the normal distribution. Preliminary analyses were done using STATA 8 (StataCorp, College Station, TX). Genetic modeling was carried out with Mx, a computer program specifically designed for the analysis of twin and family data (Neale, Boker, Xie, & Maes, 1999).
Results
Table 1 presents the general characteristics of the twins by ethnicity and gender. Males were taller and had a lower body mass index (BMI) than females. AAs had a higher BMI and weight than EAs.
Table 1.
General Characteristics of Study Subjects by Ethnicity and Gender
| EAa |
AAb |
P value |
||||
|---|---|---|---|---|---|---|
| Characteristics | Male | Female | Male | Female | Ethnicity | Gender |
| n | 210 | 217 | 126 | 182 | ||
| Age (years) | 17.3 ± 4.0 | 18.4 ± 3.8 | 17.6 ± 3.6 | 18.0 ± 3.8 | n.s. | n.s. |
| Height (cm)c | 171.9 ± 10.5 | 161.8 ± 7.4 | 173.0 ± 8.5 | 162.2 ± 6.4 | n.s. | <.001 |
| Weight (kg)c | 68.9 ± 20.8 | 62.0 ± 17.5 | 72.8 ± 22.2 | 69.5 ± 21.0 | <.001 | <.001 |
| BMI (kg/m2)c | 23.1 ± 5.7 | 23.5 ± 5.7 | 24.1 ±6.1 | 26.4 ± 7.5 | <.001 | .027 |
Data are mean ± SD. EA: European Americans, AA: African Americans, and BMI: body mass index.
Including 91 monozygotic (MZ) and 102 dizygotic (DZ) pairs as well as 41 singletons.
Including 50 MZ and 84 DZ pairs as well as 40 singletons.
Age was adjusted for the evaluation of ethnic and gender effects.
Table 2 presents means and SD of heart period and HRV parameters at rest and during stress, stratified by ethnicity and gender. Condition (rest vs. stress) × Ethnicity × Gender analysis of variance (ANOVA) showed significant main effects for ethnicity on RMSSD and HF. Independent of experimental condition, AAs had higher RMSSD and HF than EAs. A significant main effect of gender was found for RR interval, with females having a shorter RR interval both at rest and during stress. Gender significantly interacted with Condition for RMSSD and HF, with females showing larger decreases of HRV during stress than males. Significant main effects of Condition were found for heart period, RMSSD and HF, showing significant reactivity of these parameters to the stressors.
Table 2.
HRV Parameters of Study Subjects by Ethnicity, Gender, and Conditions
| EA |
AA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rest |
Stress |
Rest |
Stress |
P value |
|||||||
| Variables | Male | Female | Male | Female | Male | Female | Male | Female | Condition | Ethnicity | Gender |
| n | 210 | 217 | 210 | 217 | 126 | 182 | 126 | 182 | |||
| RR (ms) | 995.8 ± 171.5 | 920.4 ± 152.9 | 892.8 ± 153.5 | 833.7 ± 126.5 | 1024.9 ± 155.0 | 919.4 ± 145.5 | 914.7 ± 135.0 | 827.6 ± 117.0 | <.001 | n.s. | <.001 |
| RMSSD (ms) | 76.9 ± 38.0 | 74.8 ± 38.8 | 61.5 ± 30.6 | 55.4 ± 29.9 | 84.5 ± 36.3 | 81.1 ± 39.0 | 73.6 ± 31.5 | 64.0 ± 31.2 | <.001 | .014 | n.s. |
| HF (ms2) | 2239.8 ± 1951.3 | 2347.3 ± 2214.0 | 1470.4 ± 1436.2 | 1285.8 ± 1308.6 | 2361.1 ±2077.7 | 2464.7 ± 2240.3 | 1875.2 ± 1581.8 | 1519.0 ± 1293.4 | <.001 | .002 | n.s. |
Age was adjusted for the evaluation of condition, ethnic, and gender effects.
Table 3 presents twin correlations for HRV parameters for each ethnic and zygosity group. In both ethnic groups, twin correlations in MZ twin pairs were larger than those in DZ twin pairs, indicating genetic influences. We present the correlations collapsed over gender, because models that best explained the variance and covariance of these variables did not show any gender differences (see below).
Table 3.
Twin Correlations of Heart Rate Variability Parameters by Ethnicity and Zygosity
| EA |
AA |
|||
|---|---|---|---|---|
| MZ | DZ | MZ | DZ | |
| Rest | ||||
| n | 89 | 100 | 49 | 83 |
| RMSSD | .48 | .26 | .47 | .26 |
| HF | .51 | .20 | .51 | .34 |
| Stress | ||||
| n | 91 | 102 | 50 | 83 |
| RMSSD | .54 | .37 | .71 | .29 |
| HF | .58 | .37 | .65 | .34 |
| Reactivity | ||||
| n | 89 | 100 | 49 | 82 |
| RMSSD | .19 | .02 | .37 | .07 |
| HF | .23 | .09 | .50 | .16 |
RMSSD: root mean square of successive difference; HF: high frequency; EA: European Americans; AA: African Americans; MZ: monozygotic twins; and DZ: dizygotic twins.
Strong positive correlations between HRV parameters at rest and during stress were observed for both RMSSD (r = .72) and HF (r = .60). Table 4 presents these correlations by ethnicity and zygosity. The higher cross twin correlations between HRV levels at rest and during stress in MZ twin pairs than those in DZ twin pairs indicate shared genetic influences between HRV at rest and during stress.
Table 4.
Correlations of Heart Rate Variability Parameters between Rest and Stress by Ethnicity and Zygosity
| Stress |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| EA |
AA |
||||||||
| MZ |
DZ |
MZ |
DZ |
||||||
| Rest | Twin 1 | Twin 2 | Twin 1 | Twin 2 | Twin 1 | Twin 2 | Twin 1 | Twin 2 | |
| RMSSD | Twin1 | .80 | .49 | .65 | .36 | .68 | .45 | .72 | .21 |
| Twin2 | .44 | .67 | .23 | .75 | .51 | .67 | .30 | .74 | |
| HF | Twin1 | .67 | .43 | .50 | .34 | .61 | .32 | .61 | .26 |
| Twin2 | .40 | .59 | .25 | .64 | .46 | .51 | .31 | .65 | |
RMSSD: root mean square of successive difference; HF: high frequency; EA: European Americans; AA: African Americans; MZ: monozygotic twins; and DZ: dizygotic twins.
Results from bivariate testing, using the model depicted in Figure 1, are shown in Table 5. Models including only an additive genetic and unique environmental component (AE model) gave the overall best fit to the data for both RMSSD (ACE vs. AE model, χ2(3) = 1.00, p = .80; ACE vs. CE model, χ2(3) = 9.83, p = .02) and HF (ACE vs. AE model, χ2 (3) = 2.46, p = .48; ACE vs. CE model, χ2(3) = 12.26, p = .01). Furthermore, for these two measures, the best fitting model showed no significant differences in genetic and environmental variance components estimates between males and females or between AAs and EAs, indicating males and AAs show similar heritabilities to females and EAs in HRV parameters.
Table 5.
Heritability Estimates of Best-Fitting Bivariate Models for Heart Rate Variability Parameters
| RMSSD | HF | ||
|---|---|---|---|
| Rest level | h2 (CI) | .48 (.36–.58) | .50 (.39–.60) |
| e2 (CI) | .52 (.42–.64) | .50 (.40–.61) | |
| Stress level | h2 (CI) | .58 (.48–.66) | .58 (.48–.66) |
| e2 (CI) | .42 (.34–.52) | .42 (.34–.52) | |
| Rest and stress | rg (CI) | .90 (.82–.98) | .78 (.67–.88) |
| re (CI) | .51 (.40–.61) | .39 (.26–.50) | |
| Stress-specific | h2 (CI) | .11 (.03–.19) | .23 (.13–.32) |
| e2 (CI) | .31 (.25–.38) | .36 (.29–.44) | |
| Reactivity | h2 (CI) | .18 (.04–.31) | .49 (.37–.59) |
| e2 (CI) | .82 (.69–.96) | .51 (.41–.63) |
RMSSD: root mean square of successive difference; HF: high frequency.
As shown in Table 5, significant heritability was found for RMSSD and HF levels at rest and during stress. Although there was an increase in heritability of RMSSD (from 0.48 to 0.58) and HF (from 0.50 to 0.58) under stress, this increase in heritability did not reach statistical significance (ps = .07 and .20 for RMSSD and HF, respectively). Furthermore, both a12 and a22 cannot be set to 0 (a12 ≠0 model vs. a12 = 0 model, χ2(1) = 69.81, p < .001 for RMSSD and χ2(1) = 65.54, p < .001 for HF; a22 ≠0 model vs. a22 5 0 model, χ2 (1) = 6.36, p = .01 for RMSSD and χ2(1) = 16.07, p <.01 for HF). This indicates that the genes influencing HRV under stress are partly shared with those at rest. Genetic correlations were .90 (95% CI: .82 to .98) between resting and stress RMSSD, and .78 (95% CI, .67 to .88) between resting and stress HF, indicating a significant overlap in genes influencing HRV at rest and during stress. As shown in Table 5, Table 81% of the stress RMSSD heritability ([.58 – .11]/.58) and 60% of the stress HF heritability ([.58 – .23]/.58) were attributed to genes that also influenced their resting levels. The specific heritabilities due to novel genetic effects emerging during stress were .11 (.03–.19) for RMSSD and .23 (.13–.32) for HF. The heritabilities of RMSSD and HF reactivity to stress were .18 (.04–.31) and .49 (.37–.59), respectively.
Discussion
The important findings in this study are that independent of ethnicity and gender, HRV regulation at rest and during stress is largely influenced by the same genes with a small but significant contribution of stress-specific genetic effects. There was an increase in heritability of HRV under stress, which did not reach statistical significance.
Two previous studies, one in adolescent (Boomsma et al., 1990) and one in middle-aged Dutch twins (Snieder et al., 1997), observed that the genetic influence on HRV indexed by RSA increased under laboratory stressors, but whether this increase was statistically significant was not tested. Recently, De Geus et al. (2007) revisited these two data sets using a bivariate model. The results showed that not only was HRV at rest and under stress influenced by the same genes (i.e., a22 = 0), but also that there was a significant increase in heritability of RSA under stress for adolescent twins (from .31 to .54) and a nonsignificant increase in middle-aged twins (from .33 to .43). This has important implications for attempts to find the genes influencing HRV through linkage or association approaches. The increased genetic influence under stress in combination with the lack of stress-specific genetic effect will increase the power to locate susceptibility genes and may be the optimal phenotype for gene finding studies. In the current study, which includes subjects of the same age range as the Dutch adolescent cohort, we did observe an increase in heritability for HRV under stress and a substantial overlap with genes that influence HRV at rest. However, the increase in heritability did not reach significance, and there were new genes emerging during stress. The identification of novel genes during stress might be due to the increased power of the current study, which involves 189 EA and 131 AA twin pairs, much larger than the number of Dutch adolescent twin pairs (n5160). However, the inconsistent findings might also be caused by two important differences between these two studies. First, different stressors were used. Two stressors, including a speeded reaction time task and a mental arithmetic task, were conducted in the Dutch twin study whereas three stressors, including a videogame challenge, a virtual reality car driving simulation, and a social stressor interview, were applied in our study. Although we observed a high stability of RMSSD (r2 ranged from .82 to .88) and HF (r2 ranged from .63 to .74) across the three tasks we used, whether this high stability can be further extended to other stressors, including the speeded reaction time task and the mental arithmetic task, remains an open question. Second, different HRV indices were used. Although RSA, RMSSD, and HF all specifically reflect cardiac vagal tone and are closely related, they are not entirely comparable. RSA reflects the variation of heart period with respiration rate. The correlation (ranges from .93 to .96) between RMSSD and HF is higher than the correlation of RSA with RMSSD or HF (ranges from .70 to .88) at different conditions (Goedhart, van der Sluis, Houtveen, Willemsen, & de Geus, 2007). Future studies with larger sample size, HRV measured during more stressors, and HRV indexed by both RSA and RMSSD or HF are needed to clarify these issues.
Ethnic difference in HRV at rest has been noted, with AAs having higher mean values than EAs (Guzzetti et al., 2000; Liao et al., 1995; Wang et al., 2005). We confirmed this observation in the current study and further extended it to HRV levels under stress. It is noteworthy that our study is the first twin study to test heritabilities of HRV both at rest and under stress in AAs and found they were similar to EAs. The classic twin study is established as the ideal study design to estimate the relative importance of genetic and environmental factors to the variance of traits and diseases in human populations, but without actual measurement of specific genes or environments, the ethnic difference in mean values cannot be attributed to either of these factors (Snieder & MacGregor, 2003). However, our study does show that the observed difference in mean values did not translate into many differences in genetic and environmental variability within each ethnic group. The fact that a similar amount of variation is explained by genetic factors within different ethnic groups does not exclude the possibility, however, that the actual genes or the number responsible for these effects may differ between ethnic groups.
Physiological reactivity to behavioral or psychological stressors has long been regarded as a potential contributor to individual differences in cardiovascular disease risk (Treiber et al., 2003). Studies investigating the genetic contribution to individual differences in response to stress have traditionally analyzed stress reactivity as a change score calculated as the difference between stress and resting levels of the physiological variables of interest (Busjahn, Faulhaber, Viken, Rose, & Luft, 1996; Snieder, van-Doornen, & Boomsma, 1995; Turner & Hewitt, 1992). In the current study, the heritability of HRV reactivity was calculated within the bivariate model, which has one advantage over the univariate analysis on the change scores. It avoids the increased error term induced by the change score, which contains potential measurement error of both the baseline measure and of the response measure. Our results showed that heritabilities of RMSSD and HF reactivity were 18% and 49%, respectively. Previously, there was only one study trying to address the heritability of HRV reactivity (Snieder et al., 1997). In this study, change scores were used to index HRV reactivity, and the observed twin correlations were incompatible with any biologically plausible model for three of the four tasks. Therefore, further model fitting was not carried out. The reasons for the uninterpretable pattern of twin correlations in that study might be that (1) change scores were used, which include more measurement error than the HRV levels; and (2) reactivity scores calculated from each stressor instead of the aggregated score across the four tasks were used, although the latter is more reliable due to its ability to reduce the relative influence of unique situational variance (Kamarck et al., 1994; Kamarck & Lovallo, 2003).
An overall summary of our findings is that, independent of ethnicity and gender, substantial overlap exists between genes that influence HRVat rest and during stress as well as a small but significant genetic variation that is specific to the stress condition. The genetic variation that emerges during stress can only be found in gene finding studies that have attempted to measure the stress levels of HRV. We have designed a currently ongoing gene-wide association study (Neale & Sham, 2004) with the objective of identifying genes contributing to HRV at rest and during stress. Different from previous candidate gene studies that only focus on one or two polymorphisms, a gene-wide approach will consider all variants within a gene jointly. We chose to focus on eight key genes involved in biosynthesis, transport, breakdown, and receptor binding of acetylcholine, which is the neurotrans mitter of the vagal pathway. Previous studies (Neumann et al., 2006; Neumann, Halder, Ferrell, & Manuck, 2008; Neumann, Lawrence, Jennings, Ferrell, & Manuck, 2005) have found that variants in the genes involved in the acetylcholine pathway were associated with resting HRV. Our ongoing study will add further information to this by investigating specific genetic contributions to HRV under stress. The identification of susceptibility genes for HRV may lead to a more accurate prediction of individuals at risk for cardiovascular disease. Furthermore, understanding the genetic basis of phenotypes representing regulatory mechanisms of the cardiovascular system could bring us closer to an understanding of the way in which disturbance of these mechanisms leads to pathology.
Acknowledgments
Research was supported by HL086530 from the National Heart, Lung and Blood Institute as well as 0730156N from the American Heart Association.
REFERENCES
- Bigger JT, Fleiss JL, Rolnitzky LM, Steinman RC. The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation. 1993;88:927–934. doi: 10.1161/01.cir.88.3.927. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, van Baal GC, Orlebeke JF. Genetic influences on respiratory sinus arrhythmia across different task conditions. Acta Geneticae Medicae et Gemellologiae (Rome) 1990;39:181–191. doi: 10.1017/s0001566000005419. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Tremblay A, Despres JP, Nadeau A, Lupien PJ, Theriault G, et al. The response to long-term overfeeding in identical twins. New England Journal of Medicine. 1990;322:1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- Busjahn A, Faulhaber HD, Viken RJ, Rose RJ, Luft FC. Genetic influences on blood pressure with the cold-pressor test: A twin study. Journal of Hypertension. 1996;14:1195–1199. doi: 10.1097/00004872-199610000-00007. [DOI] [PubMed] [Google Scholar]
- De Geus EJ, Kupper N, Boomsma DI, Snieder H. Bivariate genetic modeling of cardiovascular stress reactivity: Does stress uncover genetic variance? Psychosomatic Medicine. 2007;69:356–364. doi: 10.1097/PSY.0b013e318049cc2d. [DOI] [PubMed] [Google Scholar]
- De Rivecourt M, Kuperus MN, Post WJ, Mulder LJM. Cardiovascular and eye activity measures as indices for momentary changes in mental effort during simulated flight. Ergonomics. 2008;51:1295–1319. doi: 10.1080/00140130802120267. [DOI] [PubMed] [Google Scholar]
- Dekker JM, Schouten EG, Klootwijk P, Pool J, Swenne CA, Kromhout D. Heart rate variability from short electrocar-diographic recordings predicts mortality from all causes in middle-aged and elderly men. The Zutphen Study. American Journal of Epidemiology. 1997;145:899–908. doi: 10.1093/oxfordjournals.aje.a009049. [DOI] [PubMed] [Google Scholar]
- Ewart CK, Kolodner KB. Social competence interview for assessing physiological reactivity in adolescents. Psychosomatic Medicine. 1991;53:289–304. doi: 10.1097/00006842-199105000-00003. [DOI] [PubMed] [Google Scholar]
- Ge D, Dong Y, Wang X, Treiber FA, Snieder H. The Georgia Cardiovascular Twin Study: Influence of genetic predisposition and chronic stress on risk for cardiovascular disease and type 2 diabetes. Twin Research and Human Genetics. 2006;9:965–970. doi: 10.1375/183242706779462877. [DOI] [PubMed] [Google Scholar]
- Goedhart AD, van der Sluis S, Houtveen JH, Willemsen G, de Geus EJ. Comparison of time and frequency domain measures of RSA in ambulatory recordings. Psychophysiology. 2007;44:203–215. doi: 10.1111/j.1469-8986.2006.00490.x. [DOI] [PubMed] [Google Scholar]
- Gutin B, Owens S, Slavens G, Riggs S, Treiber F. Effect of physical training on heart-period variability in obese children. Journal of Pediatrics. 1997;130:938–943. doi: 10.1016/s0022-3476(97)70280-4. [DOI] [PubMed] [Google Scholar]
- Guzzetti S, Mayet J, Shahi M, Mezzetti S, Foale RA, Sever PS. Absence of sympathetic overactivity in Afro-Caribbean hypertensive subjects studied by heart rate variability. Journal of Human Hypertension. 2000;14:337–342. doi: 10.1038/sj.jhh.1001009. [DOI] [PubMed] [Google Scholar]
- Jackson RW, Snieder H, Davis H, Treiber FA. Determination of twin zygosity: A comparison of DNA with various questionnaire indices. Twin Research. 2001;4:12–18. doi: 10.1375/1369052012092. [DOI] [PubMed] [Google Scholar]
- Jackson RW, Treiber FA, Turner JR, Davis H, Strong WB. Effects of race, sex, and socioeconomic status upon cardiovascular stress responsivity and recovery in youth. International Journal of Psychophysiology. 1999;31:111–119. doi: 10.1016/s0167-8760(98)00044-0. [DOI] [PubMed] [Google Scholar]
- Kamarck TW, Jennings JR, Pogue-Geile M, Manuck SB. A multidimensional measurement model for cardiovascular reactivity: Stability and cross-validation in two adult samples. Health Psychology. 1994;13:471–478. doi: 10.1037//0278-6133.13.6.471. [DOI] [PubMed] [Google Scholar]
- Kamarck TW, Lovallo WR. Cardiovascular reactivity to psychological challenge: Conceptual and measurement considerations. Psychosomatic Medicine. 2003;65:9–21. doi: 10.1097/01.psy.0000030390.34416.3e. [DOI] [PubMed] [Google Scholar]
- Kupper NH, Willemsen G, van den Berg M, de Boer D, Post huma D, Boomsma DI, et al. Heritability of ambulatory heart rate variability. Circulation. 2004;110:2792–2796. doi: 10.1161/01.CIR.0000146334.96820.6E. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mor tara A, Nohara R, et al. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: Implications for clinical trials. Circulation. 2001;103:2072–2077. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- Liao D, Barnes RW, Chambless LE, Simpson RJ, Jr, Sorlie P, Heiss G. Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability—The ARIC study. Atherosclerosis Risk in Communities. American Journal of Cardiology. 1995;76:906–912. doi: 10.1016/s0002-9149(99)80260-4. [DOI] [PubMed] [Google Scholar]
- Malpass D, Treiber FA, Turner JR, Davis H, Thompson W, Levy M, et al. Relationships between children’s cardiovascular stress responses and resting cardiovascular functioning 1 year later. International Journal of Psychophysiology. 1997;25:139–144. doi: 10.1016/s0167-8760(96)00736-2. [DOI] [PubMed] [Google Scholar]
- Murphy JK, Alpert BS, Walker SS. Ethnicity, pressor reactivity, and children’s blood pressure. Five years of observations. Hypertension. 1992;20:327–332. doi: 10.1161/01.hyp.20.3.327. [DOI] [PubMed] [Google Scholar]
- Neale BM, Sham PC. The future of association studies: Gene-based analysis and replication. American Journal of Human Genetics. 2004;75:353–362. doi: 10.1086/423901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. Richmond, VA: Department of Psychiatry, Virginia Commonwealth University; 1999. [Google Scholar]
- Neale MC, Cardon LR. Methodologies for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- Neumann SA, Brown SM, Ferrell RE, Flory JD, Manuck SB, Hariri AR. Human choline transporter gene variation is associated with corticolimbic reactivity and autonomic-cholinergic function. Biological Psychiatry. 2006;60:1155–1162. doi: 10.1016/j.biopsych.2006.03.059. [DOI] [PubMed] [Google Scholar]
- Neumann SA, Halder I, Ferrell RE, Manuck SB. Cholinergic mechanisms potentially underlying shared genetic vulnerability for depression and coronary artery disease. Psychosomatic Medicine. 2008;70:A4. [Abstract] [Google Scholar]
- Neumann SA, Lawrence EC, Jennings JR, Ferrell RE, Manuck SB. Heart rate variability is associated with polymorphic variation in the choline transporter gene. Psychosomatic Medicine. 2005;67:168–171. doi: 10.1097/01.psy.0000155671.90861.c2. [DOI] [PubMed] [Google Scholar]
- Niskanen JP, Tarvainen MP, Ranta-Aho PO, Karjalainen PA. Software for advanced HRV analysis. Computer Methods and Programs in Biomedicine. 2004;76:73–81. doi: 10.1016/j.cmpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Reinhardt L, Makijarvi M, Fetsch T, Martinez-Rubio A, Bocker D, Block M, et al. Reduced beat-to-beat changes of heart rate: An important risk factor after acute myocardial infarction. Cardiology. 1996;87:104–111. doi: 10.1159/000177071. [DOI] [PubMed] [Google Scholar]
- Schroeder EB, Whitsel EA, Evans GW, Prineas RJ, Chambless LE, Heiss G. Repeatability of heart rate variability measures. Journal of Electrocardiology. 2004;37:163–172. doi: 10.1016/j.jelectrocard.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ. The autonomic nervous system and sudden death. European Heart Journal. 1998;19(Suppl F):F72–F80. [PubMed] [Google Scholar]
- Singh JP, Larson MG, O’Donnell CJ, Tsuji H, Evans JC, Levy D. Heritability of heart rate variability: The Framing ham Heart Study. Circulation. 1999;99:2251–2254. doi: 10.1161/01.cir.99.17.2251. [DOI] [PubMed] [Google Scholar]
- Sinnreich R, Friedlander Y, Luria MH, Sapoznikov D, Kark JD. Inheritance of heart rate variability: The kibbutzim family study. Human Genetics. 1999;105:654–661. doi: 10.1007/s004399900189. [DOI] [PubMed] [Google Scholar]
- Snieder H, Boomsma DI, Van Doornen LJ, De Geus EJ. Heritability of respiratory sinus arrhythmia: Dependency on task and respiration rate. Psychophysiology. 1997;34:317–328. doi: 10.1111/j.1469-8986.1997.tb02402.x. [DOI] [PubMed] [Google Scholar]
- Snieder H, MacGregor AJ. Twin methodology. In: Cooper DN, editor. Encyclopedia of the human genome. London: Nature Publishing Group; 2003. [Google Scholar]
- Snieder H, Treiber FA. The Georgia Cardiovascular Twin Study. Twin Research. 2002;5:497–498. doi: 10.1375/136905202320906354. [DOI] [PubMed] [Google Scholar]
- Snieder H, vanDoornen LJP, Boomsma DI. Development of genetic trends in blood pressure levels and blood pressure reactivity to stress. In: Turner JR, Cardon LR, Hewitt JK, editors. Behavior genetic approaches in behavioral medicine. New York: Plenum Press; 1995. pp. 105–130. [Google Scholar]
- Stolarz K, Staessen JA, Kuznetsova T, Tikhonoff V, State D, Babeanu S, et al. Host and environmental determinants of heart rate and heart rate variability in four European populations. Jounral of Hypertension. 2003;21:525–535. doi: 10.1097/00004872-200303000-00018. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Timonen KL, Vanninen E, de Hartog J, Ibald-Mulli A, Brunek-reef B, Gold DR, et al. Effects of ultrafine and fine particulate and gaseous air pollution on cardiac autonomic control in subjects with coronary artery disease: The ULTRA study. Journal of Exposure Science & Environmental Epidemiology. 2006;16:332–341. doi: 10.1038/sj.jea.7500460. [DOI] [PubMed] [Google Scholar]
- Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosomatic Medicine. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Treiber FA, Turner JR, Davis H, Thompson W, Levy M, Strong WB. Young children’s cardiovascular stress responses predict resting cardiovascular functioning 2 1/2 years later. Journal of Cardiovascular Risk. 1996;3:95–100. [PubMed] [Google Scholar]
- Tsuji H, Venditti FJ, Jr, Manders ES, Evans JC, Larson MG, Feldman CL, et al. Determinants of heart rate variability. Journal of the American College of Cardiology. 1996;28:1539–1546. doi: 10.1016/s0735-1097(96)00342-7. [DOI] [PubMed] [Google Scholar]
- Turner JR, Hewitt JK. Twin studies of cardiovascular response to psychological challenge: A review and suggested future directions. Annals of Behavioral Medicine. 1992;14:12–20. [Google Scholar]
- Vrijkotte TG, van Doornen LJ, de Geus EJ. Effects of work stress on ambulatory blood pressure, heart rate, and heart rate variability. Hypertension. 2000;35:880–886. doi: 10.1161/01.hyp.35.4.880. [DOI] [PubMed] [Google Scholar]
- Wang X, Thayer JF, Treiber F, Snieder H. Ethnic differences and heritability of heart rate variability in African- and European American youth. American Journal of Cardiology. 2005;96:1166–1172. doi: 10.1016/j.amjcard.2005.06.050. [DOI] [PubMed] [Google Scholar]