Abstract
The aim of the present study was to characterize a novel electrophysiological assessment, the magnetically-evoked inter-enlargement response (MIER), by defining the anatomical location of the ascending axons that mediate the response and the relationship between the response and locomotor function following experimental spinal cord injury. Electromyographic (EMG) responses were recorded from the triceps muscles following magnetic stimulation of one hip. Short latency (∼ 6 ms) EMGs were recorded from triceps muscles in normal controls and following different laceration injuries (dorsal, lateral or dorsal and lateral hemisections) or a 150 kD contusion injury at the T9 level. The amplitude of the triceps MIER was significantly correlated to the area of spared white matter in the lateral funiculus and to hindlimb function during open field locomotion (r2=0.55). Following a complete lateral hemisection, MIERs were present in the triceps bilaterally following stimulation of either hip. Responses could also be recorded from the masseter muscles indicating that the influence of this pathway extends beyond the spinal cord. Anatomical evidence of a bilaterally distributed propriospinal pathway was found when biotinylated dextran amine (BDA) was injected into the lateral white matter on one side of the spinal cord at T9. BDA-labeled axons with varicosities were found bilaterally in the intermediate and ventral gray matter of the caudal region of the cervical enlargement. These observations suggest that MIERs may be useful to quantitatively assess neurotransmission and functional recovery over time after experimental spinal cord injury.
Keywords: electrophysiology, propriospinal neurons, pathway, spinal cord injury
Introduction
Propriospinal neurons that project intersegmentally in the mammalian spinal cord (rostrally, caudally or both) are well suited to influence a variety of physiological functions. While the anatomical organization of the propriospinal networks is fairly well described (Skinner et al., 1979, 1989; Matsushita, 1998; Miller et al., 1998; Wall et al., 2002), their functional organization is poorly understood (Miller et al., 1973; Schomburg et al., 1978; Sandkuhler et al., 1993; Jordan and Schmidt, 2002).
Anatomically, propriospinal pathways have been divided into two broad categories, those that project only a segment or two and those that project over longer distances, usually 3-5 segments or more (Chung and Coggeshall, 1983; Matsushita, 1998). A proportion of these might be divided into a third category namely inter-enlargement propriospinals which would include any ascending or descending neurons originating in one enlargement and terminating in the other (Giovanelli-Barilari and Kuypers, 1969; Matsushita et al., 1979; Yezierski et al., 1980). The locations of the long-propriospinal axons are thought to include the dorsolateral, lateral, ventrolateral and ventral white matter, however no discreet tracts have been characterized to date. It has been proposed that the relatively loose organization of ascending and descending long-propriospinal axons throughout the lateral and ventral white matter leads to the likelihood that significant numbers of these axons could be targeted with therapeutic interventions, seeking to enhance recovery of function following incomplete lesions to the spinal cord (Jordan and Schmidt, 2002; Conta and Stelzner, 2004).
Functionally, the roles of long propriospinal neurons are essentially unknown, although it is well established that disruption of the lateral, ventrolateral or ventral white matter tracks at the thoracic level have different effects on the motor behavior of adult rats as assessed by behavior tests like the BBB Open Field Locomotor Scale and grid walking tasks (Noble and Wrathall, 1989; Basso et al., 1995; Loy et al., 2002; Schucht et al., 2002). The majority of research in this area has focused on populations of corticospinal, rubrospinal and reticulospinal axons that are known to participate in various aspects of forelimb and hindlimb function, (Schmidt and Jordan, 2000; Jordan and Schmidt, 2002) however, the potential importance of long propriospinals in functional recovery following spinal cord injury has certainly been recognized (Conta and Stelzner, 2004). Importantly, a recent study showed that long propriospinal neurons are part of a new intraspinal circuit that arises after spinal cord injury that appears to relay output from the cortex to its original spinal targets (Bareyre et al., 2004). These observations give credence to the suggestions that long distance regeneration may not be necessary to achieve substantial improvements in function after spinal cord injury (Jordan and Schmidt, 2002; Bareyre et al., 2004).
The present study explores a novel electrophysiological response that involves connectivity between the lumbar and cervical enlargements in mammals which are known to contain the circuitry responsible for hindlimb and forelimb coordination during locomotor activities (Gernandt and Megirian, 1961; Miller et al., 1971; Jankowska et al., 1974; Alstermark and Sasaki, 1986; Loy et al., 2002; Leblond et al., 2003). In humans, reflexes in arm muscles have been recorded in response to both ankle displacement and cutaneous stimulation of the foot or the leg (Kearney and Chan, 1979, 1981; Pieseiur-Strehlow and Meinck, 1980). In addition, the extensive phase- and task- modulation of cutaneous reflexes was seen in multiple arm muscles during walking (Haridas and Zehr, 2003; Zehr et al., 2004), illustrating a strong ascending inter-enlargement pathway capable of modulating motor output. The aim of this study was to characterize the EMG responses in the triceps muscles following magnetic stimulation of one hip, an electrophysiological measure we are calling the magnetically-evoked inter-enlargement response (MIER). We have assessed animals before and after a thoracic contusion injury and following dorsal, lateral or dorsal and lateral hemisections, which allowed us to identify the white matter region that carries the ascending signal. The triceps are of interest because they are principle forelimb extensors involved in locomotion, posture and balance, activities which demand inter-enlargement coordination (forelimb-hindlimb coordination; Meinck and Piesiur-Strehlow, 1981; Jordan and Schmidt, 2002). In the same groups of animals we also recorded responses from the masseter muscles that are innervated by the fifth cranial nerve (trigeminal nerve; Mong et al., 1988), because they receive input from hindlimb sciatic type II propriospinal afferents (Deriu et al., 2001) and because they will allow the assessment of action potential conduction across a cervical injury where the majority of human injuries occur (SCI Information Network, 2005).
Methods
All animal care and surgical procedures were performed in accordance with the Public Health Service Policy on the Humane Care and Use of Laboratory Animals and with the approval of the University of Louisville Institutional Animal Care and Use Committee.
Electrophysiology
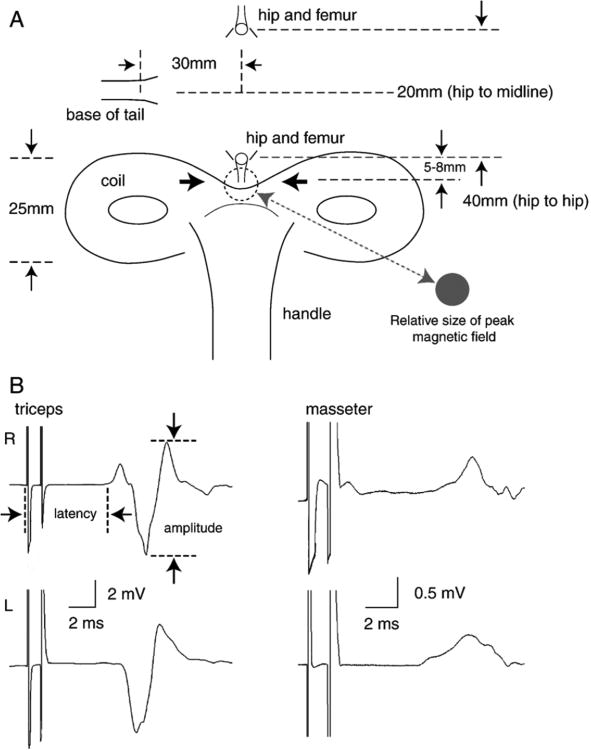
For electrophysiological assessment each rat was positioned on a piece of wood and was gently but securely held in place with a cloth stockinette pinned to the wood. (Magnuson et al., 1999). The position of the pins allowed the head and limbs to extend outside of the cloth. Magnetic stimuli were applied using a pair of Magstim 200 stimulators and a Bi-stimulation module (MagStim Inc., CA, USA). Each hindlimb was stimulated just distal to the hip via a 25mm, figure-8 magnetic transducer at 60, 70 and 80% of its maximum output, which was sufficient to induce maximal EMG responses. The transducer was hand-held and the same research assistant, who was blinded to the experimental groups, did all the magnetic stimulation. Figure 1A shows a diagram of the figure-8 transducer and the desired position relative to the hip for each stimulation. Pairs of stimuli were presented with a 1ms separation (1000Hz) which resulted in large, single-peaked EMG responses from the triceps or masseter muscles with latencies of about 6ms (Figure 1B). EMGs were recorded simultaneously, from both triceps muscles and alternately from both masseter muscles with 26G needle electrodes connected to AI 405 head stages and a CyberAmp 380 (Axon Instruments). The data was acquired in Axoscope running on a PC computer using a minimum 5 KHz sampling rate. The positive EMG electrodes were positioned in the belly of the triceps and masseter muscles and the negative electrodes were positioned near the distal tendon for the triceps and within the snout near the vibrissae for the masseter muscles. A reference electrode was positioned under the skin on the posterior portion of the neck. Acquired data were stored on a hard drive for off line analysis using Axograph 4.0 running on an iMac. EMG responses were measured for peak-to-peak amplitude and latency, as the start of the stimulus artifact to the first deflection from baseline in either direction. To measure the EMG responses, we used the peak-to-peak amplitude that occurs between 6 and 7.5 ms latency as shown in Figure 1B.
Figure 1.
A. Shown is a diagram of the 25mm figure-8 magnetic transducer made by MagStim, and the desired position of the coil relative to the hip and femur that were palpated and identified prior to each stimulation. B. Examples of MIER-evoked EMG responses recorded in a normal rat after bi-stimulation at 80% of the maximum unit stimulation from the left triceps (upper left trace) and right triceps (lower left trace) and right masseter (upper right trace) and left masseter (lower right trace). Calibration bars represent the time (ms) and the EMG amplitude (mV).
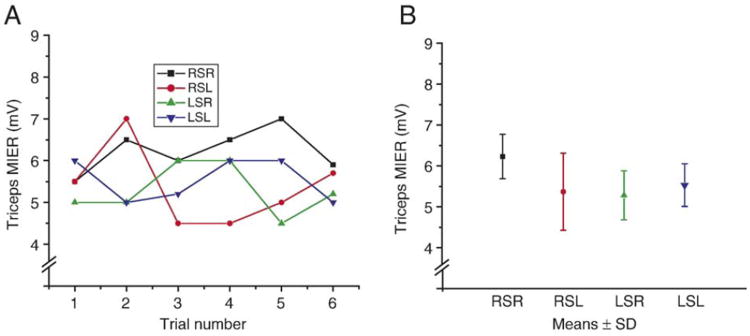
In an effort to assess the variability of the MIER, one normal animal was assessed 6 times over a 2 day period. Each assessment was performed independently; the animal was pinned-out, the electrodes placed and the stimulator re-positioned. Figure 2 shows the results from this animal with both the individual amplitudes for each stimulator/recording arrangement (A) and the means ± SD (B). An additional pair of otherwise uninjured animals were used to assess the effects of sciatic nerve transection on the MIER response. These animals were anesthetized with sodium pentobarbital (50mg/kg, i.p.) and the left sciatic nerve was cut just proximal to the hip socket. They were assessed at four days post-sciatic nerve transection by stimulating the right and left hindlimbs at the hip, and at various positions out to the knee.
Figure 2.
Six MIERs were recorded independently from a single animal over a 2 day period. The amplitudes of the individual responses for each recording/stimulating arrangement are plotted in A. The means ± SD for each are shown in B. RSR: right hip stimulation, right triceps recording; RSL: right hip stimulation, left triceps recording; LSR: left hip stimulation, right triceps recording; LSL: left hip stimulation, left triceps recording.
Spinal Cord Injuries
The rats were placed on a feedback controlled heating blanket to prevent a drop in temperature during the surgery. Ophthalmic ointment was placed on the eyes to prevent drying. Each animal received a 5 ml injection of sterile saline (sc) before and after the surgery to prevent dehydration. They also received prophylactic antibiotics (gentamicin, 50 mg/kg, i.m.) immediately following the surgery and again 2 and 4 days later. The bladder was emptied after spinal cord injury by gently massaging the lower abdomen twice daily until reflexive or voluntary emptying occurred. The animals were kept on a 12:12 h light/dark cycle and received water and food ad libitum. They were also given softened food in their cages and long sipper-tubes were used for their water bottles to ensure access to food and water.
Female Sprague-Dawley rats (n=32, 200-230 g. Charles River) were anesthetized with sodium pentobarbital (50 mg/kg, i.p.), and laminectomies were performed at the T8 and T9 vertebrae, exposing the T9 and T10 spinal cord segments. For contusion injuries (n=6), the vertebral columns were stabilized with clamps placed just rostral and caudal to the laminectomy. A 150 kilodyne contusion injury was produced using the Infinite Horizon spinal cord injury device (Precision Systems & Instrumentation, Lexington, KY, USA; Scheff et al., 2003). For the laceration injuries, the dura was cut and reflected. Mu-scissors were used to create dorsal hemisections that ranged in depth from 0.65mm (to the level of the dorsal corticospinal tract) to 1.2mm (to the level of the ventral sulcus) at the T9-T10 junction. Complete lateral hemisections (n=6) and a combination of a dorsal hemisection to the level of the central canal with a complete lateral hemisection, preserving only the right anterior quadrant of the cord (n=5) were also performed. In two animals, medial lesions were performed that preserved the lateral part of the spinal cord white matter, bilaterally.
Behavioral testing
Hindlimb movement during walking was assessed using the BBB Open Field Locomotor Scale. Briefly, animals were placed in a plastic wading pool with a nonskid surface and evaluated for 4 minutes by two investigators (blind to the lesion type) using the BBB scale that ranges from 0 (no hindlimb movement) to 21 (uninjured; Basso et al., 1995; Magnuson et al., 1999). All of the injured animals in the study had BBB scores of 12 or more indicating hindlimb plantar paw placement and weight-supported stepping at a minimum. Within the BBB Scale, forelimb-hindlimb coordination is evaluated as occasional (50% or less: BBB=12), frequent (51-94%: BBB=13) or consistent (95-100%) with rotated paw position or dorsal stepping (BBB=14). Animals that scored 15 or above had consistent forelimb-hindlimb coordination with plantar paw placement but only occasional toe clearance during the swing phase (BBB=15), frequent toe clearance and rotated paw position at lift off (BBB=16), frequent toe clearance and parallel paw position (BBB=17) or consistent toe clearance and a tail-down position (BBB = 18). Animals that scored 19 or 20 had completely normal hindlimb movement and forelimb-hindlimb coordination during locomotion but had minor deficits in tail position and trunk stability. Uninjured animals received a BBB score of 21.
Histology of the injured rats
Six weeks after injury or neuroanatomical tracing, the animals were euthanized with pentobarbital (250 mg/kg, ip) and perfused transcardially with oxygenated calcium free tyrodes, followed by 4% paraformaldehyde solution in 0.1 M phosphate buffer, pH 7.4. The spinal cords were removed, post-fixed overnight in 4% paraformaldehyde and transferred to a 30% sucrose solution for 2-4 days. Thereafter, the spinal cords were blocked by hand, placed in OCT tissue freezing medium and were stored at -20°C. Transverse sections were cut at 50μm on a Zeiss Mum cryostat, mounted onto charged glass slides and stored at -20°C.
Sections from the injury epicenter of each spinal cord were stained using the Cresyl Echt violet method (Powers and Clark, 1955; Magnuson et al., 2005). Slides were coverslipped with Permount (Fisher Scientific, PA) and the sections were photographed using a Spot Cooled Digital camera (Diagnostic Instruments, MI) attached to a Nikon Labophot muscope and a G4 Macintosh computer running the proprietary Spot image acquisition software. Digitized images were opened in Appleworks, traced using a Wacom Drawing Tablet (Vancouver, WA) and the traces were saved as bitmap (PICT) files. Bitmap images were opened in NIH Image and regions of interest were identified as measured as cross-sectional area (mm2). The spared white matter was identified as being evenly and lightly stained and lacking the stippling associated with damaged tissue and scarring. The area of spared white matter was determined in mm2, and converted to percent by comparison to an uninjured control (Magnuson et al., 2005).
BDA-neuroanatomical tracing
Three uninjured adult female rats were anesthesized with sodium pentobarbital (50mg/kg) and the T9 spinal cord was exposed by laminectomy at T10. BDA (10%, 0.18-0.33μl of lysine-fixable, mol. Wt. 10,000; Molecular Probes, Oregon) was injected into the right lateral funiculus (LF) at T9 from a glass mupipette (pulled and beveled to 25μm O.D.) using pressure pulses of 20-40 mmHg from a pneumatic Picopump (PV800, WPI). The injection coordinates used were just dorsal and lateral to those we used previously for injections of ethidium bromide into the ventrolateral funiculus. (Loy, 2002). After a survival period of 21 days, the animals were euthanized, perfused and their spinal cords were removed and processed as described above. Transverse sections of the cervical spinal cord were cut at 30μm on a cryostat and mounted onto glass slides. They were hydrated in 0.1M PBS, rinsed in sodium acetate, and processed using a standard avidin-biotinylated peroxidase complex (ABC, 1:100 Vector Laboratories, CA) with nickel ammonium sulfate (1.5g/50ml) enhanced 3,3′ diaminobenzidine tetrahydrochloride (80mg/50ml DAB, 30% H2O2) staining procedure. The sections were processed through a series of alcohols and xylene prior to being coverslipped with Permount.
Drawings of non-consecutive (150μm) tissue sections were reconstructed from the caudal cervical (C6-C8) spinal cord using 4-6 photomugraphs (10×) as described above. Digital photomugraphs were opened in Appleworks 6.0 and axons with variocosities were magnified (40×) and traced using a Wacom drawing tablet. Drawings were superimposed to determine the distribution pattern of axons with varicosities in the cervical gray matter from a thoracic (T9) lateral funiculus BDA injection.
Statistics
The mean amplitudes of the MIER responses were assessed using repeated measures analysis of variance (ANOVA). Data were subjected to a Tukey HSD post hoc t-tests comparison to determine significant differences between individual means when appropriate.
Results
MIER recorded from triceps and masseter muscles of normal rats
Magnetic stimulation of the hip was found to activate a short-latency pathway that resulted in large amplitude, bilateral EMG responses in both the triceps and masseter muscles (Figure 1B). The EMG response latencies for the control groups were 6.3 ± 0.6 ms (mean ± SD, n=32) for the triceps and 6.9 ± 0.9 ms (n=26) for the masseter muscles. The amplitudes of the triceps and masseter EMG responses following magnetic stimulation of one hip are shown in Table 1. The EMG responses from the masseter and triceps muscles were bilaterally symmetrical with respect to amplitude and latency following stimulation of either the right or left hip.
Table 1. Amplitudes in mV of magnetically evoked interenlargement responses (MIERs).
| (A) | Right hip stimulus | Left hip stimulus | ||
|---|---|---|---|---|
|
|
|
|||
| R triceps | L triceps | R triceps | L triceps | |
| Control (n = 5) | 6.9 ± 3.9 | 7.6 ± 3.3 | 6.6 ± 2.9 | 6.6 ± 3.2 |
| Lateral (n = 6) | 4.7 ± 2.0 | 4.7 ± 2.8 | 2.3 ± 1.0* | 2.8 ± 1.9* |
| Dors + lat (n = 5) | 0.5 ± 0.5* | 0.5 ± 0.6* | 0.4 ± 0.2* | 0.4 ± 0.4* |
| (B) | Right hip stimulus | Left hip stimulus | ||
|
|
|
|||
| R masseter | L masseter | R masseter | L masseter | |
|
| ||||
| Control (n = 5) | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.2 |
| Lateral (n = 6) | 0.4 ± 0.2 | 0.5 ± 0.3 | 0.2 ± 0.2* | 0.3 ± 0.2 |
| Dors + lat (n = 5) | 0.06 ± 0.08* | 0.09 ± 0.09* | 0.00 ± 0.00* | 0.05 ± 0.07* |
Data are given as means ± SD. Asterisks show responses that are significantly different from controls (p < 0.05).
Figure 2 shows the data generated by repeated, independent assessments made of 1 animal. The graph in a shows the amplitudes of individual responses following stimulation of one hip. The responses are designated at RS or LS (right or left stimulation) followed by R or L for right or left triceps recording. The graph in B shows the mean ± SD for all 6 responses for each stimulation/recording arrangement.
Transection of the left sciatic nerve proximal to the hip in 2 animals resulted in MIERs with decreased amplitude (∼2mV) and normal latency (∼6ms) when the stimulating magnet was placed normally as shown in Figure 1A (5-8mm distal to the hip).When the stimulating magnet was moved distally, towards the knee, the response rapidly decreased to 0. Stimulation of the knee on the right side resulted in normal bilateral responses in the triceps.
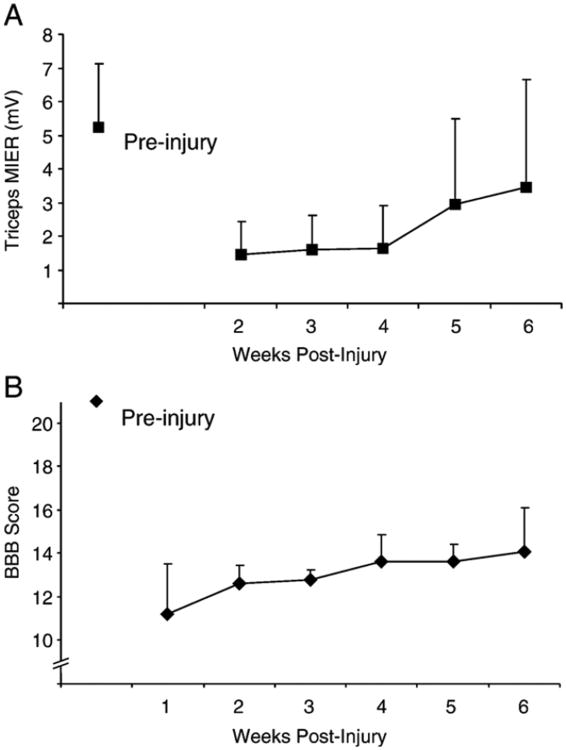
MIER after a 150 kD contusion injury
MIERs in the triceps were significantly smaller in amplitude compared to uninjured controls over a 6 week period following a 150 kD contusion injury at T9 (Figure 3; p<0.05). Some long-latency responses (>12ms) were recorded at two weeks after injury, but were not included in this study because their waveforms were inconsistent in profile, amplitude and latency. Only responses with latencies of 9ms or less were included in the analysis.
Figure 3.
Mean amplitude ± SD of MIERs recorded from the right and left triceps (averaged) of normal rats and then weekly from weeks 2 to 6 following a 150 kD injury at T9. The MIER amplitudes were significantly decreased for 6 weeks after the injury (p<0.05). MIER testing by repeating the electrode insertion and the magnet positioning on the same rat 6 times on two consecutive day.
MIER following graded dorsal hemisections
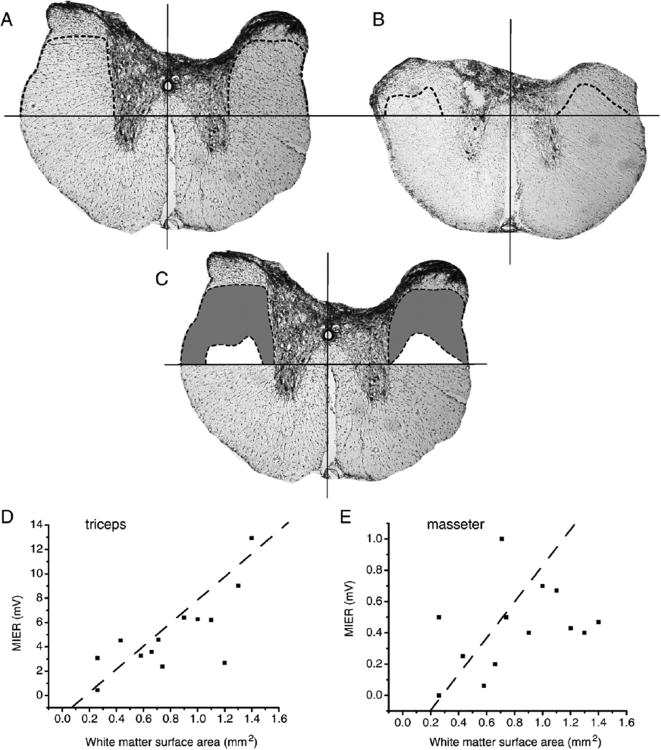
In order to determine the anatomical location of the propriospinal pathway transmitting the MIER at the thoracic level, dorsal hemisections of different depths were performed at T9. The spared lateral white matter was calculated for each spinal cord by determining the area of white matter (mm2) retained above a horizontal line positioned at the dorsal tip of the ventral sulcus, drawn perpendicularly to a line bisecting the ventral artery, as shown in Figure 4A. Figure 4A shows the maximum dorsal hemisection that had no apparent effect on the MIER amplitude and Figure 4B shows the anatomical location of the minimum dorsal hemisection depth required to completely abolish both the triceps and masseter MIER. In Figure 4C the spared white matter these two lesions (maximum – minimum) are superimposed to illustrate the region of white matter that carries the MIER (gray). A significant correlation was found for the triceps MIER response amplitudes and the spared lateral white matter surface area located dorsal to the ventral sulcus (r2= 0.56, n=13) as shown in Figure 4D. The masseter responses were not strongly correlated to spared lateral white matter (r2= 0.14, n=13) as shown in Figure 4E.
Figure 4.
Cross-section area at the T10 spinal cord level following a shallow dorsal hemisection (A, 0.65mm, to the CST) that did not alter the MIER-evoked triceps and masseters EMG responses compared to normal rats. A horizontal line was positioned at the top of the ventral sulcus, a vertical line was positioned in the middle of the cord connecting the central canal and the anterior central artery, and a dashed line was used to indicate spared white matter (region lacking gliotic stippling. The area of spared white matter above the ventral sulcus and below the dashed line for a deep dorsal hemisection (B, 1.2mm, to the ventral sulcus) that abolished the MIER-evoked triceps and masseter EMG responses. The difference between the shallow and deep hemisections is shown in C for comparison purposes. A significant linear correlation was found between the MIER-evoked average of summed left and right EMG amplitude (mV) in the triceps and the white matter surface area above the ventral sulcus (D, r2=0.56, n=13). The correlation for the masseter responses and white matter surface area above the ventral sulcus was not significant (E, r2=0.14, n=13).
MIER following lateral or lateral and dorsal hemisections
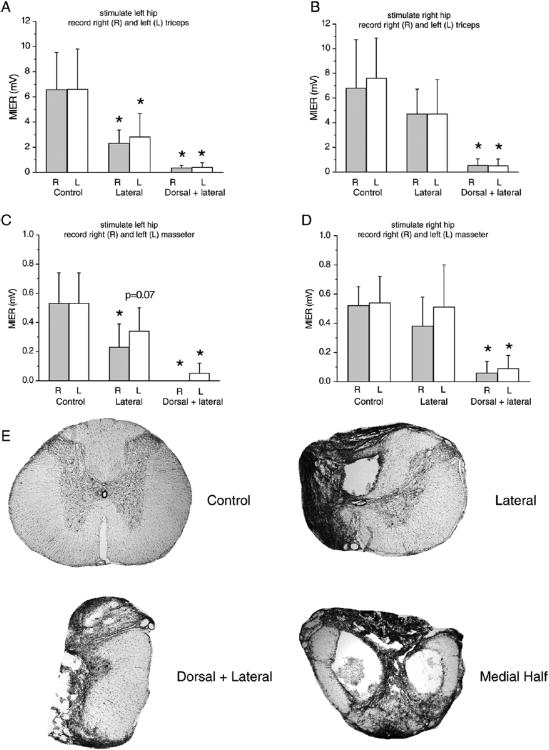
In this experiment we compared three groups of animals; uninjured controls (n=5), animals with left lateral hemisections (n=6) and animals with a combination of a dorsal hemisection (to the level of the central canal) and a left lateral hemisection (n=5). Triceps and masseter EMG responses to magnetic stimulation of the right hip were significantly smaller in amplitude following the combined lesion than following a lateral hemisection only (p<0.05). Animals with a lateral hemisection only had responses that were not significantly lower than uninjured controls as shown in Figure 5 and Table 1. No right-left differences were noted for either the triceps or masseter pairs under any of these conditions. The triceps EMG responses following magnetic stimulation of the left hip were significantly smaller in amplitude for the lesion groups compared to the uninjured control group (p<0.05), while no differences in response amplitude between left and right triceps were noted (Figure 5A). The responses from the masseter muscles were significantly larger in amplitude (p<0.05) for the uninjured group compared to the lesion groups for the right (uninjured) side and approached significance (p=0.07) for the left (injured) side (Figure 5C). Examples of the cross section area located at the injury epicenter in uninjured cord and following lateral, dorsal+lateral and mid-sagittal sections are shown in Figure 5E. In addition, the latency was 0.4 ± 0.1 ms longer for triceps and masseters following left hip stimulation compared to right hip stimulation after left lateral hemisection.
Figure 5.
MIERs following a bi-stimulation protocol when stimulating the left (A) or right (B) hip and recording from both triceps or the left (C) or right (D) hip and recording from both masseters. Normal rats had significantly higher EMG amplitudes than the injured groups (A, p<0.05). The group that received a combination of dorsal and lateral hemisection had significantly lower EMG amplitudes than the other groups (p<0.05). Normal rats had significantly higher EMG amplitudes than the injured groups for the right masseter (p<0.05) and approached significance for the left masseter (p=0.07). The group that received a combination of dorsal and lateral hemisection had a significantly lower EMG amplitudes than the other groups (p<0.05). E shows transverse sections at the T10 spinal cord level of a normal rat (top left) and the injury epicenter of rats that underwent a lateral hemisection (top right), a combination of lateral and dorsal hemisection (bottom left) or a mid-sagittal section (bottom right), the latter preserving only the lateral part of the white matter on both sides.
VLF Axonal tracing
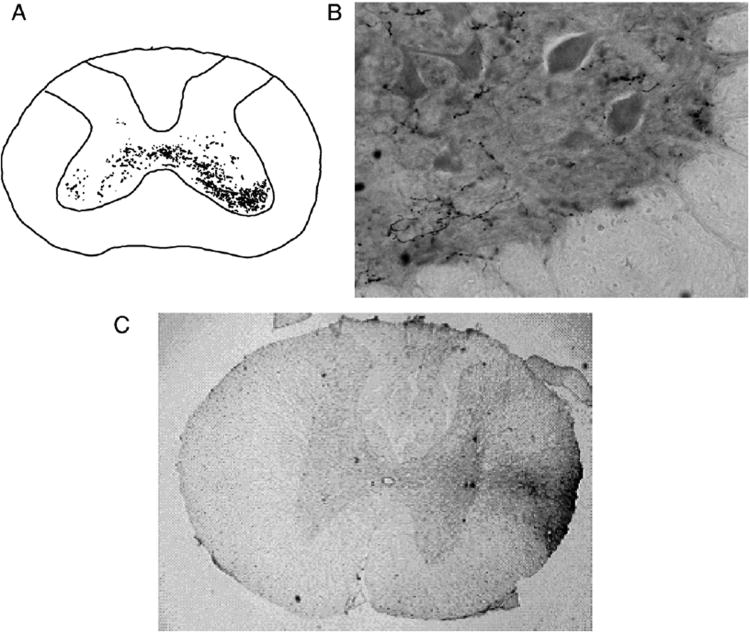
Terminals arising from axons located in the lateral funiculus at the level of the central canal were traced by injecting BDA into this location at T9 in 3 uninjured animals. All 3 animals showed bilaterally distributed axons with terminals in the intermediate and ventral gray matter at the (C6-8) spinal cord segments (Figure 6A) where the interneurons and motoneurons that serve the triceps muscles are located (McKenna et al., 2000). Representative photomugraphs of BDA labeled axons with varicosities and of the injection site located in the lateral white matter at the T9 level are shown in Figures 6B and 6C, respectively.
Figure 6.
A shows three superimposed camera lucida drawings of bilateral BDA-labeled axons with varicosities in the uninjured rat C6-C8 spinal cord following a unilateral T9 LF injection of BDA. B shows an example of BDA-labeled axons with varicosities in ipsilateral lamina IX of a transverse C6-C8 caudal cervical spinal cord section using a magnification of 40×. A representative section of the BDA injection site in the right lateral white matter at the T9 level is shown in C.
Relationship between the BBB score and the MIER response
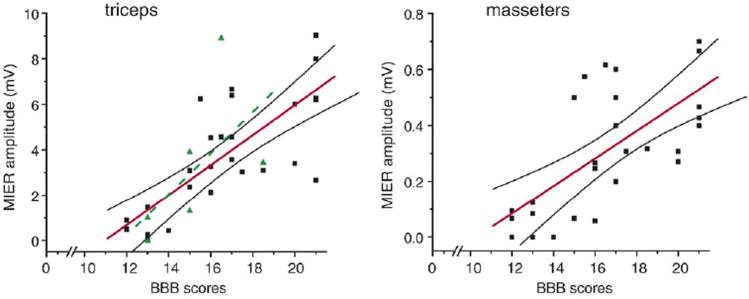
To assess a potential relationship between MIER responses and behavior, we plotted EMG response amplitudes against the BBB Open Field Locomotor scores for the uninjured controls and the laceration injured animals in this study. After plotting a line of best fit and 95% confidence intervals, we found significant (p<0.05) correlations with r2=0.55, n=35 for the triceps (Figure 7A) and r2=0.45, n=26 for the masseter (Figure 7B) EMG responses. We also plotted the triceps MIER response amplitudes vs BBB scores from the 6 animals with moderate contusion injuries at T9 and after drawing a line of best fit, we found a significant correlation of (r2=0.36, n=6; Figure 7A).
Figure 7.
Linear relationships exist between the MIER-evoked EMG amplitudes and the BBB Scores of normal rats and rats following laceration injuries (solid squares) for A) the triceps (r2=0.55, n=35) and B) the masseter (r2=0.45, n=26) muscles. A best linear fit (dashed line) in A was also calculated for the 150 kD contusion animals (r2=0.36, n=6, triangles) and a similar linear relationship was found for the triceps.
Discussion
This study provides an electrophysiological and anatomical characterization of a novel assessment tool, the magnetically-evoked inter-enlargement response or MIER. The pathway that mediates the MIER is ascending, originating near the hip and ending in the motoneuron pool in the cervical enlargement that serves the triceps muscles (Miller et al., 1973). We chose to use magnetic stimulation at the hip and acute EMG electrodes as opposed to electrical stimulation of the sciatic nerve and chronic implantable EMG electrodes in an effort to establish a protocol that did not require additional surgeries or anesthetics thus making it feasible to assess the same animals over time and/or following chronic regenerative and/or rehabilitative treatments. As a secondary consideration, we wanted to utilize techniques that would be easy to adapt for use in the clinical setting. We propose that the MIER will be a good adjunct to transcranial magnetic motor-evoked potentials (tcMMEPs; Linden et al., 1999; Magnuson et al., 1999) and somatosensory evoked potentials (SSEPs; Onifer et al., 2005) that are also recorded from unanesthetized animals following spinal cord injury. It is important to note that while tcMMEPs cannot be assessed in animals that have been chronically instrumented for SSEP recording (Onifer et al., 2005), MIERs are possible because they do not require magnetic stimulation of the head.
The magnetic stimulator we employed is a 25mm figure-8 transducer (Figure 1) made by the MagStim Corporation. It has a peak magnetic field of 4.0 Tesla with a deep and narrow focal region approximately 1cm wide and 1cm deep that is ideal for stimulating peripheral nerves (www.magstim-us.com). To establish that the MIER is initiated by magnetic stimulation of the sciatic nerve and not by activation of the spinal cord or cauda equina directly, we sectioned the sciatic nerves just proximal to the right hip in 2 different animals. Two days after nerve section we found the MIERs to be greatly reduced in amplitude, but with a normal latency of approximately 6ms when the stimulator was positioned normally, as shown in Figure 1. This suggests that the magnetic field was able to stimulate some of the axons in the stump of the sciatic nerve, or some of the small peripheral nerves that join the sciatic proximal to the cut. When the stimulator was moved towards the knee on the right side the amplitude quickly dropped to 0. Stimulation of the hip and knee on the left side induced responses of 5-7mV and 1-2mv in amplitude, respectively. We conclude that the sciatic nerve is the primary structure mediating the MIER in the otherwise intact adult rat.
Some of the drawbacks of our chosen procedure are that the EMG electrodes are re-positioned for each recording session and that the magnetic stimulator is hand-held and is re-positioned for each stimulation. In order to estimate the variability in the MIER responses due to these factors we independently assessed the same normal animal 6 times over a 2 day period. We found that the response amplitudes varied from 4.5 to 7mv while the latencies were consistently at 5.8-6.0ms. While the variability is greater than ideal, it is our opinion that a mean response of approximately 5.3 ± 1.0 mV (mean ± SD; Figure 2, RSL) representing the greatest variability recorded for this animal, is sufficient for the detection of changes in amplitude following spinal cord injury and subsequent treatment.
MIER and dorsal hemisections
Investigations into the relationship between spinal cord white matter tracts and function have been accomplished using laceration lesions (Schuct et al., 2002), contusion and compression injuries (Basso et al., 1996; 2002; Fehlings and Tator, 1995) and ethidium bromide demyelination (Loy et al., 2002). These and many other published works have lead to the conclusion that as little as 10-15% sparing of lateral and ventral white matter is sufficient for significant hindlimb locomotor function following SCI in rats. For the present study we used dorsal hemisections of varying depth to show that there is a correlation between the amplitude of the MIERs recorded from the triceps and the area of spared white matter located dorsal to the level of the ventral sulcus (r2= 0.56; Figure 3). The white matter in this region includes the lateral funiculus (LF) and the VLF, regions that were found by Miller and colleagues to be critical to the responses in cat forelimb musculature following electrical stimulation of the sciatic nerve (Miller et al., 1973). Since the relationship between the area of spared white matter and the amplitude of the MIER was linear, we can assume that the axons involved are not restricted to a specific tract, but are distributed throughout the LF and the most dorsal portion of the VLF. The finding that the responses in three animals were not attenuated following dorsal hemisections that completely severed the dorsal columns demonstrates convincingly that the dorsal column primary afferent system is not involved in the MIER.
Responses recorded from the masseter muscles were also correlated to spared white matter in the lateral funiculus at the level of the central canal, but less strongly (r2= 0.14). In three animals with deep dorsal horn lesions (to the top of the central canal), responses in the triceps were reduced by approximately 50% compared to controls while the masseter responses were reduced by 90%. In one additional animal with a slightly deeper lesion the triceps responses were reduced by 85% and the masseter responses were absent. These differences between the triceps and masseter responses, and the 0.6ms difference in latency suggests that the pathway to the masseter muscles may be a relay from the cervical spinal cord involving spinoreticular neurons or collaterals from cervical interneurons that participate in the triceps response locally and also project rostrally to the trigeminal motoneurons. Either way, it appears that the responses recorded from the masseter muscles are not as robust as those from the triceps.
MIER after lateral or combined lateral/dorsal hemisection
The MIER response was attenuated, but not abolished, following a complete left lateral hemisection of the spinal cord at the T9 level when stimulating the left hip. This suggests that while the pathways involved are bilaterally represented, there is some ipsilateral bias. It further suggests that a commissural component of the pathway exists at both the lumbar and cervical levels sufficient to result in a response of ∼ 40% of the normal amplitude bilaterally following unilateral stimulation of the left hip after a left lateral thoracic hemisection. The results also show that there is an increase in the response latency of about 0.4 ± 0.1ms in these animals compared to stimulation of the right hip. This is relevant for two reasons. First of all, the right-left latency difference shows that the magnet does not stimulate the lumbar spinal cord or cauda equina directly, but that the response is mediated by action potentials induced in the sciatic nerve on one side of the animal. Secondly, and in agreement with our previous work in the rat pup spinal cord in vitro preparation, it suggests that long propriospinal (inter-enlargement) neurons with ascending axons in the contralateral VLF receive input from lumbosacral dorsal roots (Magnuson et al., 1997; Antonino-Green et al., 2002).
Stimulation of the right hip following a left lateral hemisection resulted in responses that were attenuated for both muscle groups, but not significantly, compared to intact controls. In contrast to the situation described above where there was a right-left latency difference following stimulation of the hip ipsilateral to the lateral hemisection, the response latencies for the right and left triceps were identical following stimulation of the hip contralateral to the hemisection. It appears that contralateral activation at the lumbar level is sufficient to induce a bilateral response in the cervical spinal cord that is near-normal in amplitude and with a normal latency. The ipsilateral component is therefore redistributed bilaterally rostral to the level of the hemisection at T9, presumably in the cervical enlargement. This redistribution may be mediated, at least in part, by the long-ascending commissural propriospinal axons illustrated by the bilateral distribution of BDA-labeled axons with varicosities we observed following injection into the LF at T9 (Figure 6). This pattern of tracing indicates that neurons with cell bodies caudal to T9 and axons in the LF extend those axons into the intermediate gray matter of the lower cervical segments (C6-8) both ipsilateral and contralateral to the injection site. Whether or not these long-ascending propriospinal commissural neurons are responsible for all or just a proportion of the bilateral nature of the MIER is open to debate. Neurons analogous to these could be responsible for the bilateral responses seen in human arm muscles, including the triceps brachii, as described by Zehr and colleagues (2001). They found that trans-cutaneous stimulation of the peroneal nerve could elicit bilateral, short-latency responses in multiple arm and leg muscles in the normal human subject and proposed that these responses are mediated by a bilaterally distributed propriospinal network (Zehr et al., 2001). In the present study, the fact that the response latencies from the right and left triceps are identical when stimulating the uninjured side suggests that the contralateral component probably does not involve an additional synapse.
The animals that received a combination of dorsal and lateral lesions showed a pattern of responses similar to those that received a lateral hemisection only, however, with a significant decrease in the MIER amplitude. This drop in amplitude is explained by the removal of a portion of lateral white matter on the intact side as shown in Figure 4E.
MIER after a 150 kD contusion injury
Following a 150 kD contusion injury at T9 the MIER responses were still present, but with significantly attenuated amplitudes at two weeks post-injury. Over the subsequent 4 weeks these responses recovered to approximately 25% of the pre-injury levels for the triceps. We showed previously that tcMMEPs are completely abolished by a 12.5g-cm NYU Impactor injury at T9, which is thought to be a milder injury than the 150 kD IH injury (Magnuson et al., 1999; Cao et al., 2005). The descending axons transmitting the short-latency tcMMEP are located in the medial aspect of the VLF, (Adamson et al., 1989; Loy et al., 2002; Cao et al., 2005) and the absence of the tcMMEP signal following a 150 kD contusion injury indicates that this portion of the ventrolateral white matter was destroyed. Despite the loss of tcMMEPs, the MIER response is preserved but attenuated following a 150 kD contusion injury, suggesting that the ascending axons involved may be located somewhat more laterally in the white matter compared to the medial descending axons transmitting the tcMMEP. This suggestion is further supported by an observation we made in 2 animals following laceration of the central part of the spinal cord, which spared only the most lateral part of the VLF and DLF tracts on both sides. These animals had an average triceps MIER response of 1.1 mV with normal latencies. A transverse section of this injury epicenter is shown in Figure 5E.
Relation between the BBB score and the MIER response
The BBB Open Field Locomotor Scale has been used successfully to assess locomotor deficits and recovery of function following a wide variety of thoracic and lumbar spinal cord injuries (Benzel et al., 1990; Basso et al., 1995, 1996; Loy et al., 2002a,b; Schucht et al., 2002). We found that there is a linear relationship between the MIER amplitude and the BBB scores following thoracic laceration or contusion injuries (Figure 7). This suggests that the MIER may serve as a quantitative measure of the integrity of neuronal signaling from the hindlimb (lumbar) circuitry to the cervical forelimb motor pathways, possibly facilitating inter-limb (inter-enlargement) coordination. Indeed, in humans the electrophysiological measures MEP (motor-evoked potentials) and SSEP (somatosensory evoked potentials) are of similar significance in predicting functional outcomes with respect to ambulatory capacity following a traumatic spinal cord injury (Curt and Dietz, 1999; Iseli et al., 1999). Also, for uncooperative patients or in animal models the evaluation of action potential conduction across an injury site can contribute greatly to the selection of appropriate approaches for rehabilitation (Curt and Dietz, 1997).
It is worth noting that the amplitude of the triceps MIER appears to be particularly related to BBB scores in the 12 to 16 range, where detailed aspects of inter-limb coordination are critical to the scoring. For example, a BBB score of 12 shows that the animals have only occasional homologous and diagonal coupling of their limbs. We found that animals with this score had highly attenuated MIERs. However, animals that score 16 or better on the BBB Scale have consistent inter-limb coordination and we found that these animals had MIER responses with amplitudes of approximately half of the pre-injury level (BBB of 21 with MIER amplitude of 7mV). However, other ascending and descending pathways must be considered. The ascending dorsal columns and descending corticospinal tracts may be ruled out because of the observations by Schucht and colleagues (2002) who reported that lesions of the dorsal columns and corticospinal tracts in the thoracic spinal cord did not significantly change the BBB score from normal. Similarly, we found that the MIER is not altered by dorsal column lesions even when the dorsolateral funiculus (DLF) is involved as part of a shallow dorsal hemisection. In addition, we reported previously that discreet demyelinating lesions of the ventral columns, VLF or DLF do not reduce BBB scores below 16 which allows us to exclude the influence of ascending and descending axons in these regions (Loy et al., 2002a,b). However, we also found that combination lesions (VLF plus DLF, VLF plus ventral columns or complete ventral lesions) reduce average BBB scores to below 16 (Loy et al., 2002a,b; Schucht et al., 2002) suggesting that different combinations of axons in these regions may act in concert to efficiently regulate locomotion and coordination. Finally, BBB scores of 17 and 12 are reported for mild and moderate thoracic contusion injuries, respectively, but no differences were seen in the number of retrogradely labeled cervical spinal cord descending propriospinal neurons between these injury severities (Basso et al., 2002). This may rule them out as individually important players in coordination as well. Future studies using discrete lesions, neuroanatomical tracing, electrophysiology and behavioral assessments will need to be done to resolve this issue.
Conclusion
Early work by Miller and colleagues (1971) and by Jankowska and colleagues (1973) demonstrated that primary afferents originating from lumbar dorsal roots can influence the cervical spinal cord and are able to modulate a stepping pattern. More recently, Kohlheimer and colleagues (1998) and Deriu and colleagues (2001) showed in cats and rats respectively, that excitatory synaptic responses can be induced in masseter motoneurons by sciatic nerve stimulation. Taken together, these studies revealed that stimulation of hindlimb afferents could influence motor output from the cervical enlargement and medulla, however, the pathway(s) mediating these responses were not fully characterized. Our data demonstrates in normal adult rats that magnetic stimulation of the sciatic nerve at the level of the hip induces short latency EMG responses in the triceps muscles that result from activation of a long propriospinal pathway located in the lateral funiculus of the thoracic spinal cord and not via the dorsal column primary sensory pathway. These electrophysiological findings are supported by tract-tracing data, using BDA injections into the right lateral funiculus at T9, that resulted in the labeling of terminal axons with varicosities bilaterally in the intermediate and ventral gray matter in the cervical enlargement. In conclusion, we propose that these data illustrate an inter-enlargement pathway composed primarily of long ascending propriospinal neurons that are well suited to participate in inter-limb coordination in the intact rat. Long propriospinal neurons have been recognized recently as important targets for therapeutic intervention following spinal cord injury (Jordan and Schmidt, 2002; Conta and Stelzner, 2004; Bareyre et al., 2004) however methods to assess these pathways are lacking. We found that the MIER amplitudes are well correlated with recovery of hindlimb function as assessed by the BBB Open Field Locomotor Scale following both laceration and contusion injuries at T9. We propose that the relatively non-invasive nature of magnetic stimulation and EMG recording makes the MIER a potential tool that might be translated to the clinical situation to assess ascending conduction across a thoracic site of injury in the human. Furthermore, the observation that the responses are relayed to the masseter motoneurons in the brainstem suggests that conduction across a cervical spinal cord injury site might also be assessed.
Acknowledgments
This research was supported by the Kentucky Spinal Cord and Head Injury Research Trust and by NIH RR15576. The authors wish to acknowledge Ryan Baltzley, Jignesh Shah and Michael J. Wells for their efforts and Alice Shum-Siu, Darlene Burke, Kim Fentress, Christine Nunn and Aaron Puckett for their expert technical assistance. The authors wish to thank Dr. Larry M. Jordan for many helpful comments and suggestions.
References
- Adamson J, Zappulla RA, Fraser A, Ryder J, Malis LI. Effects of selective spinal cord lesions on the spinal motor evoked potential (MEP) in the rat. Electroencephalogr Clin Neurophysiol. 1989;74:469–480. doi: 10.1016/0168-5597(89)90038-5. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Sasaki S. Integration in descending motor pathways controlling the forelimb in the cat. 14. Differential projection to fast and slow motoneurones from excitatory C3-C4 propriospinal neurones. Exp Brain Res. 1986;63:530–542. doi: 10.1007/BF00237476. [DOI] [PubMed] [Google Scholar]
- Antonino-Green DM, Cheng J, Magnuson DS. Neurons labeled from locomotor-related ventrolateral funiculus stimulus sites in the neonatal rat spinal cord. J Comp Neurol. 2002;442:226–238. doi: 10.1002/cne.10081. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. Epub 2004 Feb 2015. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Descending systems contributing to locomotor recovery after mild or moderate spinal cord injury in rats: experimental evidence and a review of literature. Restor Neurol Neurosci. 2002;20:189–218. [PubMed] [Google Scholar]
- Benzel EC, Lancon JA, Thomas MM, Beal JA, Hoffpauir GM, Kesterson L. A new rat spinal cord injury model: a ventral compression technique. J Spinal Disord. 1990;3:334–338. [PubMed] [Google Scholar]
- Cao Q, Zhang YP, Iannotti C, Devries WH, Xu XM, Shields CB, Whittemore SR. Functional and electrophysiological changes after graded traumatic spinal cord injury in adult rat. Exp Neurol. 2005;191:S3–S16. doi: 10.1016/j.expneurol.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Chung K, Coggeshall RE. Propriospinal fibers in the rat. J Comp Neurol. 1983;217:47–53. doi: 10.1002/cne.902170105. [DOI] [PubMed] [Google Scholar]
- Conta AC, Stelzner DJ. Differential vulnerability of propriospinal tract neurons to spinal cord contusion injury. J Comp Neurol. 2004;479:347–359. doi: 10.1002/cne.20319. [DOI] [PubMed] [Google Scholar]
- Curt A, Dietz V. Ambulatory capacity in spinal cord injury: significance of somatosensory evoked potentials and ASIA protocol in predicting outcome. Arch Phys Med Rehabil. 1997;78:39–43. doi: 10.1016/s0003-9993(97)90007-1. [DOI] [PubMed] [Google Scholar]
- Curt A, Dietz V. Electrophysiological recordings in patients with spinal cord injury: significance for predicting outcome. Spinal Cord. 1999;37:157–165. doi: 10.1038/sj.sc.3100809. [DOI] [PubMed] [Google Scholar]
- Deriu F, Milia M, Podda MV, Chessa G, Tolu E. Jaw muscle response to stimulation of type II somatosensory afferents of limbs in the rat. Exp Brain Res. 2001;139:209–215. doi: 10.1007/s002210100755. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Tator CH. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp Neurol. 1995;132:220–228. doi: 10.1016/0014-4886(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Gernandt BE, Megirian D. Ascending propriospinal mechanisms. J Neurophysiol. 1961;24:364–376. doi: 10.1152/jn.1961.24.4.364. [DOI] [PubMed] [Google Scholar]
- Giovanelli Barilari M, Kuypers HG. Propriospinal fibers interconnecting the spinal enlargements in the cat. Brain Res. 1969;14:321–330. doi: 10.1016/0006-8993(69)90113-9. [DOI] [PubMed] [Google Scholar]
- Haridas C, Zehr EP. Coordinated interlimb compensatory responses to electrical stimulation of cutaneous nerves in the hand and foot during walking. J Neurophysiol. 2003;90:2850–2861. doi: 10.1152/jn.00531.2003. Epub 2003 Jul 2859. [DOI] [PubMed] [Google Scholar]
- Iseli E, Cavigelli A, Dietz V, Curt A. Prognosis and recovery in ischaemic and traumatic spinal cord injury: clinical and electrophysiological evaluation. J Neurol Neurosurg Psychiatry. 1999;67:567–571. doi: 10.1136/jnnp.67.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Lundberg A, Stuart D. Propriospinal control of last order interneurones of spinal reflex pathways in the cat. Brain Res. 1973;53:227–231. doi: 10.1016/0006-8993(73)90786-5. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Lundberg A, Roberts WJ, Stuart D. A long propriospinal system with direct effect on motoneurones and on interneurones in the cat lumbosacral cord. Exp Brain Res. 1974;21:169–194. doi: 10.1007/BF00234388. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Schmidt BJ. Propriospinal neurons involved in the control of locomotion: potential targets for repair strategies? Prog Brain Res. 2002;137:125–139. doi: 10.1016/s0079-6123(02)37012-2. [DOI] [PubMed] [Google Scholar]
- Kearney RE, Chan CW. Reflex response of human arm muscles to cutaneous stimulation of the foot. Brain Res. 1979;170:214–217. doi: 10.1016/0006-8993(79)90958-2. [DOI] [PubMed] [Google Scholar]
- Kearney RE, Chan CW. Interlimb reflexes evoked in human arm muscles by ankle displacement. Electroencephalogr Clin Neurophysiol. 1981;52:65–71. doi: 10.1016/0013-4694(81)90190-5. [DOI] [PubMed] [Google Scholar]
- Kohlmeier KA, Lopez-Rodriguez F, Morales FR, Chase MH. Effects of excitation of sensory pathways on the membrane potential of cat masseter motoneurons before and during cholinergically induced motor atonia. Neuroscience. 1998;86:557–569. doi: 10.1016/s0306-4522(98)00016-5. [DOI] [PubMed] [Google Scholar]
- Leblond H, L'Esperance M, Orsal D, Rossignol S. Treadmill locomotion in the intact and spinal mouse. J Neurosci. 2003;23:11411–11419. doi: 10.1523/JNEUROSCI.23-36-11411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden RD, Zhang YP, Burke DA, Hunt MA, Harpring JE, Shields CB. Magnetic motor evoked potential monitoring in the rat. J Neurosurg Spine. 1999;91:205–210. doi: 10.3171/spi.1999.91.2.0205. [DOI] [PubMed] [Google Scholar]
- Loy DN, Magnuson DS, Zhang YP, Onifer SM, Mills MD, Cao QL, Darnall JB, Fajardo LC, Burke DA, Whittemore SR. Functional redundancy of ventral spinal locomotor pathways. J Neurosci. 2002;22:315–323. doi: 10.1523/JNEUROSCI.22-01-00315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy DN, Talbott JF, Onifer SM, Mills MD, Burke DA, Dennison JB, Fajardo LC, Magnuson DS, Whittemore SR. Both dorsal and ventral spinal cord pathways contribute to overground locomotion in the adult rat. Exp Neurol. 2002;177:575–580. doi: 10.1006/exnr.2002.7959. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Trinder TC. Locomotor rhythm evoked by ventrolateral funiculus stimulation in the neonatal rat spinal cord in vitro. J Neurophysiol. 1997;77:200–206. doi: 10.1152/jn.1997.77.1.200. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Trinder TC, Zhang YP, Burke D, Morassutti DJ, Shields CB. Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp Neurol. 1999;156:191–204. doi: 10.1006/exnr.1999.7016. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Lovett R, Coffee C, Gray R, Han Y, Zhang YP, Burke DA. Functional consequences of lumbar spinal cord contusion injuries in the adult rat. J Neurotrauma. 2005;22:529–543. doi: 10.1089/neu.2005.22.529. [DOI] [PubMed] [Google Scholar]
- Matsushita M. Ascending propriospinal afferents to area X (substantia grisea centralis) of the spinal cord in the rat. Exp Brain Res. 1998;119:356–366. doi: 10.1007/s002210050351. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Ikeda M, Hosoya Y. The location of spinal neurons with long descending axons (long descending propriospinal tract neurons) in the cat: a study with the horseradish peroxidase technique. J Comp Neurol. 1979;184:63–80. doi: 10.1002/cne.901840105. [DOI] [PubMed] [Google Scholar]
- McKenna JE, Prusky GT, Whishaw IQ. Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: a retrograde carbocyanine dye analysis. J Comp Neurol. 2000;419:286–296. doi: 10.1002/(sici)1096-9861(20000410)419:3<286::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Meinck HM, Piesiur-Strehlow B. Reflexes evoked in leg muscles from arm afferents: a propriospinal pathway in man? Exp Brain Res. 1981;43:78–86. doi: 10.1007/BF00238812. [DOI] [PubMed] [Google Scholar]
- Miller KE, Douglas VD, Richards AB, Chandler MJ, Foreman RD. Propriospinal neurons in the C1-C2 spinal segments project to the L5-S1 segments of the rat spinal cord. Brain Res Bull. 1998;47:43–47. doi: 10.1016/s0361-9230(98)00065-3. [DOI] [PubMed] [Google Scholar]
- Miller S, Reitsma DJ, van der Meche FG. Excitatory ascending propriospinal actions between lumbosacral and cervical segments in the cat. J Physiol. 1971;218:76P–77P. [PubMed] [Google Scholar]
- Miller S, Reitsma DJ, van der Meche FG. Functional organization of long ascending propriospinal pathways linking lumbo-sacral and cervical segments in the cat. Brain Res. 1973;62:169–188. doi: 10.1016/0006-8993(73)90626-4. [DOI] [PubMed] [Google Scholar]
- Mong FS, Chen YC, Lu CH. Dendritic ramifications of trigeminal motor neurons innervating jaw-closing muscles of rats. J Neurol Sci. 1988;86:251–264. doi: 10.1016/0022-510x(88)90103-7. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Wrathall JR. Correlative analyses of lesion development and functional status after graded spinal cord contusive injuries in the rat. Exp Neurol. 1989;103:34–40. doi: 10.1016/0014-4886(89)90182-9. [DOI] [PubMed] [Google Scholar]
- Onifer SM, Zhang YP, Burke DA, Brooks DL, Decker JA, McClure NJ, Floyd AR, Hall J, Proffitt BL, Shields CB, Magnuson DS. Adult rat forelimb dysfunction after dorsal cervical spinal cord injury. Exp Neurol. 2005;192:25–38. doi: 10.1016/j.expneurol.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Piesiur-Strehlow B, Meinck HM. Response patterns of human lumbo-sacral motoneurone pools to distant somatosensory stimuli. Electroencephalog Clin Neurophysiol. 1980;48:673–682. doi: 10.1016/0013-4694(80)90424-1. [DOI] [PubMed] [Google Scholar]
- Powers MM, Clark G. An evaluation of cresyl echt violet acetate as a Nissl stain. Stain Technol. 1955;30:83–88. doi: 10.3109/10520295509113749. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, Stelzer B, Fu QG. Characteristics of propriospinal modulation of nociceptive lumbar spinal dorsal horn neurons in the cat. Neuroscience. 1993;54:957–967. doi: 10.1016/0306-4522(93)90587-6. SCI Information Network, 2005. http://www.spinalcord.uab.edu/ [DOI] [PubMed] [Google Scholar]
- Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Meinck HM, Haustein J, Roesler J. Functional organization of the spinal reflex pathways from forelimb afferents to hindlimb motoneurones in the cat. Brain Res. 1978;139:21–33. doi: 10.1016/0006-8993(78)90057-4. [DOI] [PubMed] [Google Scholar]
- Schucht P, Raineteau O, Schwab ME, Fouad K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp Neurol. 2002;176:143–153. doi: 10.1006/exnr.2002.7909. [DOI] [PubMed] [Google Scholar]
- Skinner RD, Coulter JD, Adams RJ, Remmel RS. Cells of origin of long descending propriospinal fibers connecting the spinal enlargements in cat and monkey determined by horseradish peroxidase and electrophysiological techniques. J Comp Neurol. 1979;188:443–454. doi: 10.1002/cne.901880307. [DOI] [PubMed] [Google Scholar]
- Skinner RD, Nelson R, Griebel M, Garcia-Rill E. Ascending projections of long descending propriospinal tract (LDPT) neurons. Brain Res Bull. 1989;22:253–258. doi: 10.1016/0361-9230(89)90050-6. [DOI] [PubMed] [Google Scholar]
- Wall PD, Kerr BJ, Ramer MS. Primary afferent input to and receptive field properties of cells in rat lumbar area X. J Comp Neurol. 2002;449:298–306. doi: 10.1002/cne.10294. [DOI] [PubMed] [Google Scholar]
- Yezierski RP, Culberson JL, Brown PB. Cells of origin of propriospinal connections to cat lumbosacral gray as determined with horseradish peroxidase. Exp Neurol. 1980;69:493–512. doi: 10.1016/0014-4886(80)90047-3. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Collins DF, Chua R. Human interlimb reflexes evoked by electrical stimulation of cutaneous nerves innervating the hand and foot. Exp Br Res. 2001;140:495–504. doi: 10.1007/s002210100857. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Carroll TJ, Chua R, Collins DF, Frigon A, Haridas C, Hundza SR, Thompson AK. Possible contributions of CPG activity to the control of rhythmic human arm movement. Can J Physiol Pharmacol. 2004;82:556–568. doi: 10.1139/y04-056. [DOI] [PubMed] [Google Scholar]