Abstract
The ability of a small molecule to bind and modify the activity of a protein target at a specific site greatly impacts the success of drugs in the pharmaceutical industry. One of the most important tools for evaluating these interactions has been high-field solution NMR because of its unique ability to examine even weak protein-drug interactions at high resolution. NMR can be used to evaluate the structural, thermodynamic and kinetic aspects of a binding reaction. The basis of NMR screening experiments is that binding causes a perturbation in the physical properties of both molecules. Unique properties of small and macromolecules allow selective detection of either the protein target or ligand, even in a mixture of compounds. This review outlines current methodologies for assessing protein-ligand interactions from the perspectives of the protein target and ligand and delineates the fundamental principles for understanding NMR approaches in drug research. Advances in instrumentation, pulse sequences, isotopic labeling strategies, and the development of competition experiments support the study of higher molecular weight protein targets, facilitate higher-throughput and expand the range of binding affinities that can be evaluated, enhancing the utility of NMR for identifying and characterizing potential therapeutics to druggable protein targets.
Introduction
One of the most important uses of high-field solution nuclear magnetic resonance (NMR) in the pharmaceutical industry relies on its unique ability to examine protein-drug interactions at very high resolution. NMR experiments have provided crucial data to assess the ability of a small molecule drug to bind and modify the activity of a disease-modifying protein at a structurally defined binding site.1 Upon the interaction of a small molecule ligand with a protein target, a perturbation of the NMR spectrum occurs, and detection of the perturbation is the basis of ligand binding analysis by NMR. The perturbation assay is divided into two categories based on whether the signals from the protein target or the ligand are observed. An expanding set of NMR experiments is available to map either the small or macromolecule in a site-specific manner to chemically and physically characterize small molecule ligand interactions with protein targets. A significant advantage of these NMR assays is that they are useful for identifying binding partners from a mixture of compounds (NMR screening). Each method will be discussed in further detail in the appropriate sections, and a summary of the advantages and disadvantages is outlined in Table 1.
Table 1. Summary of current NMR screening methods.
The table outlines the capabilities limitations and requirements of each method as it pertains to the examination of ligand binding to protein targets.
| Protein-Target Observed | Limits and Requirements | Permits Identification of: | ||||
|---|---|---|---|---|---|---|
| Target MW |
KD | Isotopic Labeling |
Target Binding Site |
Ligand Binding Epitope |
Ligand Selectivity in a Mixture |
|
| aChemical Shift Perturbations | b30 kDa | 10−9-10−3 | 13C and/or 15N | c✓ | ||
| 19F Relaxation and Chemical Shift Perturbations | unlimited | 10−6-10−3 | 19F | d✓ | ||
| Ligand Observed | ||||||
| Relaxation Rates | unlimited | e10−6-10−3 | none | ✓ | ✓ | |
| Diffusion Coefficients | unlimited | 10−6-10−3 | none | ✓ | ||
| Nuclear Overhauser Effects | unlimited | 10−6-10−3 | none | ✓ | ✓ | |
| Magnetization Transfer | unlimited | e10−6-10−3 | none | ✓ | ✓ | |
| 19F Relaxation and Chemical Shift Perturbations | unlimited | e10−6-10−3 | fnone | ✓ | ||
This method requires the use of two-dimensional 1H-15N HSQC or 1H-13C HSQC experiments.
Higher molecular weight protein-targets may be studied with use of advanced pulse sequences and/or isotopic labeling strategies.
Partial or complete resonance assignments are required.
19F resonances must be assigned if more than one aromatic residue occurs in the protein.
A wider range of binding affinities may be studied if the method is used as a competition based screening experiment.
Isotopic labeling is not required if the ligand naturally contains an 19F moiety.
Solution NMR is often segregated into two fields. Low-field NMR is primarily utilized for small molecule analysis, while macromolecular studies require high-field instruments (greater than 500 MHz). Higher field spectrometers offer advantages for the study of both small and large compounds. First, the probes accompanying higher fields allow selective detection of bonds or atoms to obtain site-specific information about the protein target or small molecule. Additionally, increased field strength improves sensitivity and provides better resolution and narrower line widths, which are necessary to analyze large molecules that generate many signals. Improved sensitivity allows lower concentrations of analytes to be detected. Although small molecule analysis traditionally does not require high-field spectrometers, the higher field instruments are advantageous because the increased resolution allows for improved ability to examine small molecules, specifically in the context of their interactions with macromolecules where they are undergoing exchange processes, which decrease resolution by increasing line width.2
High-field solution NMR can provide supplementary or additional information about a binding reaction in addition to facilitating determination of the association constant between a drug and binding partner, as can be obtained with traditional functional assays. Most notably, NMR provides detailed structural information about the protein-ligand binding site, as well as data from which the KD value can be determined, providing a structure-activity relationship (SAR).3 Moreover, high-field NMR can be used to structurally characterize binding of two or more ligands simultaneously.4,5 The experimental setup is straightforward and provides a universal process, applicable to virtually any protein, for the detection of intermolecular interactions.4 Two unique advantages of analyzing molecular interactions by NMR are that: 1) it requires no target-specific assay or knowledge of the target protein’s function6 and 2) NMR experiments can detect species that interact weakly with each other (millimolar dissociation constants). The ability to detect direct interactions between ligand and target over a broad KD range illustrates the robustness of this technique.5
In brief, high-field solution NMR offers the ability to easily evaluate the structural, thermodynamic and kinetic aspects of a binding reaction.7 This review outlines the current methodologies used to assess protein-ligand interactions from both the perspectives of the protein target and ligand molecule. Appropriate background information is provided to describe the NMR experiments available for assessing ligand binding and the corresponding data interpretation. Finally, advances in NMR technology and experimentation are presented to illustrate the utility of solution NMR in the identification and characterization of potential therapeutics to protein targets.
“Protein target” Detected Methods
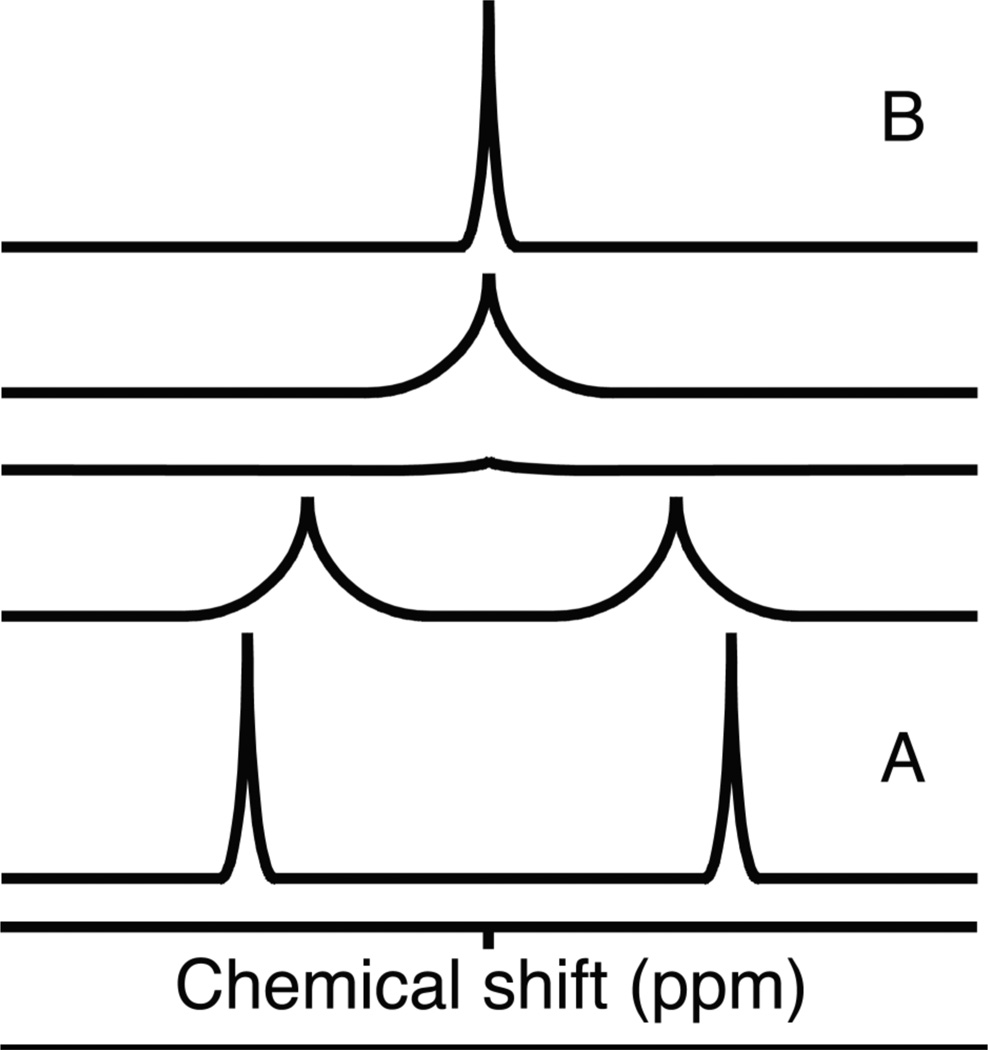
Most target-observed screening applications rely on chemical shift changes as indicators for intermolecular binding.6 This type of screening generally is referred to as a perturbation assay, and it is used to evaluate the resonances of the protein target as a function of ligand concentration in a titration experiment.8 Changes are observed upon binding because chemical shift reports on the local electronic environment of a nucleus. NMR commonly detects spin ½ nuclei (1H, 13C, 15N, 19F), and the type of nucleus is a major determinant of the chemical shift value.9 Additionally, the dielectric properties of the solvent, covalent or hydrogen bonds, van der Waals interactions and bond angles influence the electronic environment of an atom, which impact its chemical shift.10 Changes in NMR chemical shifts can result from any physical or chemical process that changes the magnetic environment of a nucleus, including dynamic processes such as conformational changes and solvent effects. The chemical shifts of surface exposed nuclei are affected by bulk solution. Proteins are virtually always analyzed in aqueous solution, where the main contribution to solvent effects comes from the pH and ionic species presen. Generally, low ionic strength and slightly acidic conditions are more conducive to generating high-quality spectra, but the requirements for each protein are unique.11 On the interior of the protein, chemical shift values are dictated largely by packing interactions and bond geometries. Chemical shifts corresponding to regions experiencing dynamic fluctuations may be substantially influenced by both structure and solvent contact. When interactions occur between analytes in solution different values of NMR chemical shifts reflect the different nuclear environments undergoing exchange and depend on the nature of the exchange or binding process.12 A nucleus involved in a binding reaction will experience a different environment in its free and bound form, generating signal that reflects the combination of both. The appearance of the NMR spectrum also depends on the rate at which the exchange phenomenon occurs (Figure 1). A slowly exchanging system exists when the exchange occurs more slowly than the difference in chemical shift in frequency units. When the exchange is faster than the difference in chemical shift, the system is classified as a fast-exchange system. NMR experiments detect processes that occur on the microsecond to millisecond time scale. If the nuclear environments change slowly on the NMR timescale, the spectrum will capture two distinct sets of resonances for the free and bound states (Figure 1A), whereas rapidly-exchanging nuclei will produce a single set of resonances representing a weighted average of the chemical shifts of the free and bound signals (Figure 1B).9,12
Figure 1. Exchange regimens observed with NMR.
A) Two resonances appear because the observed nucleus exchanges slower than the NMR scale. B) One resonance is observed because the observed nucleus exchanges faster than the NMR time scale. The peak represents a weighted averaged of the two exchanging environments.
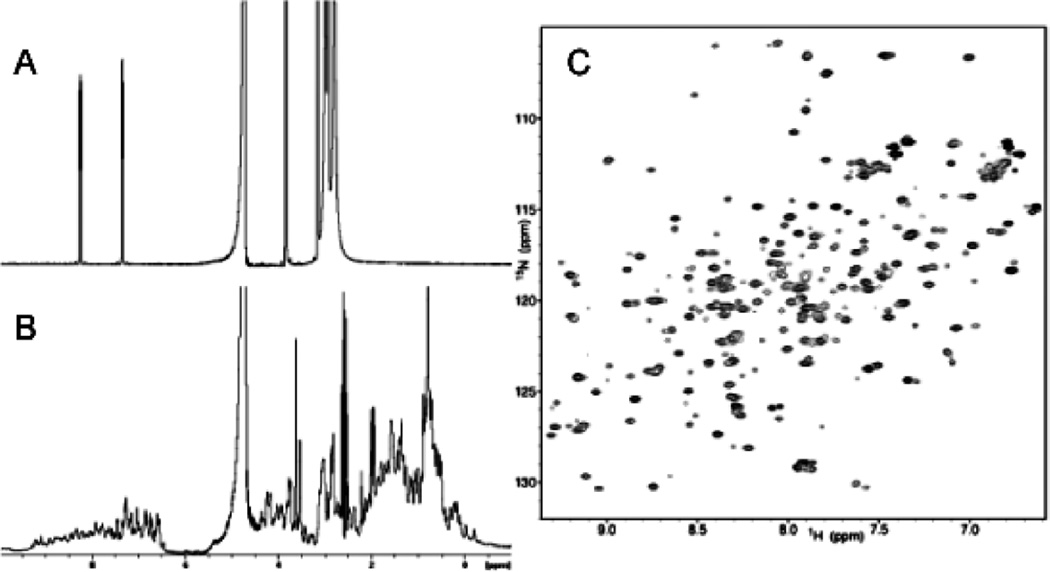
The ability to interpret a NMR spectrum becomes increasingly difficult for higher molecular weight species. A small molecule with relatively few atoms will produce well-resolved, sharp NMR signals with large chemical shift dispersion (Figure 2A). In contrast, a simple one-dimensional spectrum of a protein contains thousands of signals generated by numerous similar chemical moieties that appear in the same regions of the spectrum. The presence of secondary and tertiary structure elements provides improved dispersion, distinguishing the chemical shifts from those of the random state as the microenvironment of a particular nucleus dictates its chemical shift in an extremely sensitive manner. Nonetheless, due to the large number of signals, one-dimensional observation typically lacks sufficient resolution to evaluate structure and dynamics and quantify binding (Figure 2B).13 Consequently, this has led to the development of multidimensional correlation experiments. These experiments resolve overlapping signals by expanding the spectrum into two or three dimensions based on different nuclei or differences in connectivity.12
Figure 2. Multidimensional NMR.
A) 1H NMR spectrum of para-Nitrophenyl phosphate in 50mM HEPES, pH=7.5. The two resonances at approximately 7 and 8 ppm correspond to the two aromatic protons. The water peak resonates at 4.703 ppm and the remaining peaks below 4 ppm correspond to HEPES. B) 1H NMR spectrum of phosphatase of regenerating liver-1 (PRL-1), a protein target implicated in cancer metastasis. The peaks above 6.0 ppm are the protein’s backbone amides and side chains. The water peak resonates at 4.703 ppm and the remaining signals represent the protein’s aliphatic protons. The signals are too numerous and overlapped to make any specific assignments. C) 1H-15N HSQC of PRL-1. Each peak represents a single NH group in the protein, including backbone and side-chain amides. Using three-dimensional correlation experiments, each peak can be assigned to a given amino acid in the protein sequence to yield structural information that can guide structure-based inhibitor design to the protein target.
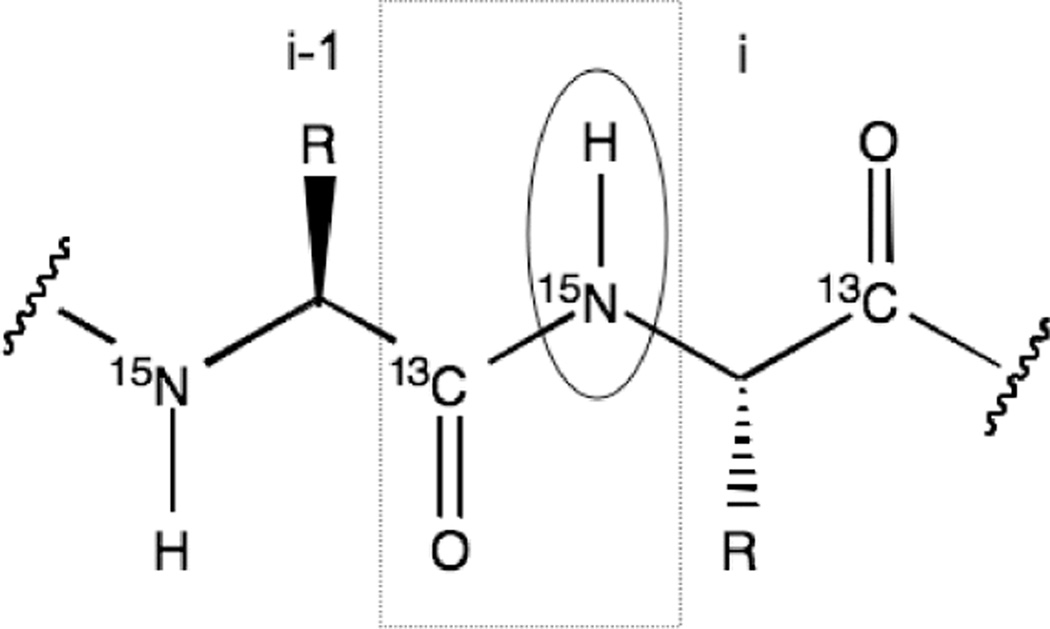
Two-dimensional correlation spectroscopy refers to a class of experiments that probe the connectivity between nuclei. Each axis shows the chemical shift of a specific nucleus, and cross peaks result only for those that are correlated to each other.14 In general, the experiment can be applied to investigate homo or heteronuclear associations. The Heteronuclear Single Quantum Correlation (HSQC) experiment is particularly useful for the analysis of proteins. The 1H-15N HSQC experiment exploits the repeating nature of the protein’s primary sequence and three-dimensional structure. The amide bond connects the amino acids that compose the protein sequence, creating a repeating series of NH groups that become chemically unique in the context of the protein’s tertiary structure (Figure 3). The experiment only detects those protons directly coupled to nitrogen, so the resulting spectrum lacks any overlap from proton signals associated with carbon (typically aromatics in the relevant ppm range).12 The spectrum displays one cross peak for every amide in the protein at a position characterized by its 1H and 15N chemical shifts.15 In total, the spectrum contains one signal for each residue except proline, which lacks an HN, two paired peaks generated by the asparagine and glutamine NH2 groups and additional signals from NH containing side chains (Figure 2C).7 The HSQC spectrum provides an overall map or fingerprint of the protein target and serves as the basis for assessing ligand binding and the overall fold and behavior of a protein. This experiment is fast and easily interpreted but requires isotopic labeling of the protein with 15N because of its low natural abundance (0.37%).10 When labeling is not practical, a 1H-13C version of the HSQC can be performed using unlabeled protein to evaluate the aliphatic protons; however, this experiment requires longer data acquisition. Both experiments reveal information about the protein’s structure. The 1H-15N-HSQC has the advantage of probing each amino acid and revealing whether the protein is folded, as the amide proton chemical shift distribution for each amino acid is significantly influenced by its secondary structure and collapses to a narrow ppm range upon unfolding.16 Also, the 13Cα alpha and 13Cβ and, to a lesser extent, 1Hα chemical shifts correlate with α-helical and β-sheet secondary structure elements, but the spectrum is more difficult to interpret with respect to structural changes than the 15N version.
Figure 3. Protein backbone.
Each amino acid is connected via a peptide bond between the carbonyl carbon of the first amino acid and the amide of the next amino acid, which is highlighted by the dashed square box. The 1H-15N HSQC experiment detects protons directly coupled to nitrogen (designated by the circle), and the resulting spectrum contains one peak for every amino acid in the protein. The HNCO experiment (see Isotopic Labeling Techniques under Advances in NMR) correlates the carbonyl carbon of amino acid (i) to the amide proton of the proceeding amino acid (i-1).
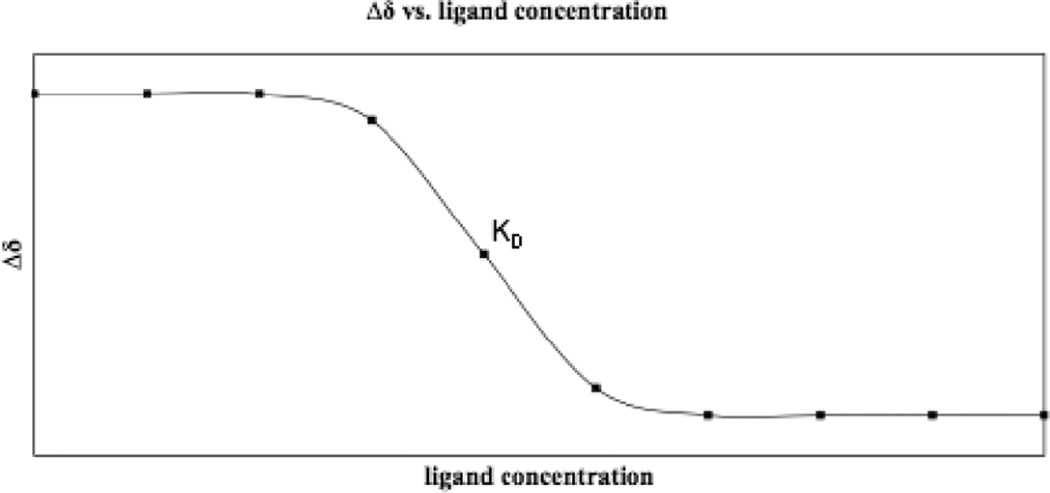
The HSQC experiment is a powerful tool for binding studies because of the sensitivity of coupled nuclei to changes in the environment. The 1H-15N HSQC detects changes in the backbone amide bonds upon addition of ligand, whereas the 1H-13C HSQC detects changes in aliphatic and aromatic chemical shifts of the side chains. Monitoring the chemical shift changes as a function of ligand concentration can be performed to accurately measure the affinity constant between the ligand and the target (Figure 4).7,17–19 The magnitude of the protein’s chemical shift change will differ depending on how the small molecule interacts with the protein. For example, hydrogen bonding will have a different effect on the chemical shift than covalent attachment.20 Knowledge of the protein’s resonance assignments reveals the residues involved in binding. In this case, most studies employ the 1H-15N HSQC because backbone resonances are more easily obtained.7 Moreover, binding site identification allows differentiation of specific from nonspecific binding, which is characterized by small environmental changes in the NMR spectrum similar to the effects of a change in temperature.21 NMR is, thus, a unique tool with the ability to immediately identify false positives.4 It is important to note that even if the protein target resonances have not been assigned, target-observed assays may still be used to identify ligands that bind strongly to the target and reveal whether multiple ligands bind using the same or different binding sites.22 In addition to detecting tight binding, this method can detect interactions that are as least as weak as millimolar binding KDs because observation of the protein target does not rely on tight binding to acquire bound-state information.21,23 A consequence of weaker binding is that the time scale on which the ligand exchanges leads to line broadening. For systems exchanging on this intermediate timescale, the line width of the protein resonances will sharpen as saturation binding is approached. To confirm that a ligand is binding in a single conformation the concentration of the ligand should be titrated until saturation is reached. If no change in the line width is observed, structural investigations may be complicated, as the ligand likely occupies the binding site in multiple conformations of comparable energy.24
Figure 4. Δδ vs [ligand].
Changes in chemical shift are monitored over a series of ligand concentrations and plotted to determine the dissociation constant.
Upon the addition of ligand, the signals of those amides whose environments are perturbed by ligand binding change position.15 Specific binding of a small molecule and protein target can take place using two modes.6 A lock and key interaction of the ligand to the protein has little or no effect on residues that are not directly involved. Consequently, only those involved experience a different environment and resonate at a different frequency. Some have reported that as few as eight out of 107 resonances change upon compound binding.25 Induced fit binding leads to a conformational change, often resulting in a global change of the protein as a whole, where the majority of the resonances are altered.7,26 In this case, a series of experiments that compare chemical shift changes induced by a series of closely related ligands may be used to identify the binding site.20 After mapping the changes in chemical shifts from the titration of a first ligand, a second, structurally-related ligand differing by a functional group is added to the protein target. Most of the perturbations will parallel the changes of first ligand except for the resonances at the binding site. This occurs because of the proximity of the protein’s binding site to the substituted functional group on the ligand and because the magnitude of the chemical shift change depends on the ligand moiety involved with binding.7,20
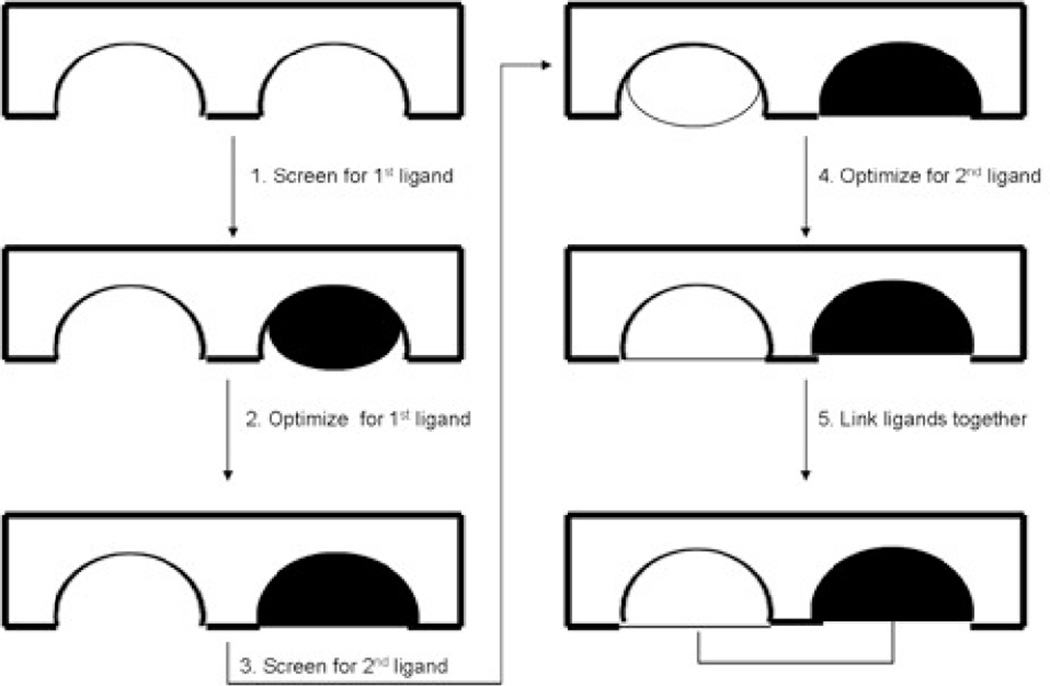
The chemical shift perturbation assay has been used extensively to identify the ligand binding site of many important protein targets and receptors. For example, cyclosporine A bound to human cyclophillin,27 interleukin-8 complexed with the N-terminal region of the human interleukin-8 receptor,28 the Fab fragment of IgG interacting with streptococcal protein G29 and NADP+ bound to the MurB enzyme30 have all been characterized using the chemical shift perturbation method. Importantly, these studies helped lead to the advent of the SAR (structure- activity relationship) by NMR method developed by S. Fesik at Abbott Laboratories.25 SAR by NMR detects changes in the chemical shifts of the protein target upon addition of ligands. Weakly interacting compounds that bind at adjacent sites on the protein target are identified and combined with structural information about the orientation of the bound ligands to guide a linked-fragment approach to generate lead drug compounds with greatly increased binding. Figure 5 outlines the experimental procedure. The first step requires screening a library of ligands using the perturbation method with the assumption that a change in chemical shift greater than 0.1 ppm for at least two peaks in the spectrum constitutes a significant change and indicates binding.31 After hit identification, monitoring chemical shift changes as a function of ligand concentration in a titration experiment is performed to accurately deduce the binding constant.32 Following identification of a binding partner to the first site, screening of related compounds is conducted to increase and optimize the binding affinity for that site. The same perturbation method is applied to identify a second low-affinity hit in the presence of the optimized first ligand. Following identification and optimization of the second binding site, structural data obtained by additional experiments is used to determine the location and orientation of the protein target in complex with its two low affinity hits. Maintaining the spatial orientation of the compounds with respect to each other and the protein target, the ligands are synthetically linked together to produce the high-affinity ligand.25
Figure 5. SAR by NMR.
Binding of a first ligand is determined by detecting changes in the 1H-15N HSQC of the protein target upon titration of a mixture of ligands. After a binding ligand is identified and optimized, a mixture is screened for a second low affinity binder to an adjacent site on the protein target. Once optimized, the ligands are synthetically linked together to create one high-affinity lead drug compound.
The SAR by NMR method has facilitated the development of inhibitors of proteins, for example, stromelysin, to combat its role in arthritis and tumor metastasis.32 Additionally, the methodology has supported the characterization and development of inhibitors that block DNA binding by the human papillomavirus E2 protein, to treat cervical cancer,33 and antagonists of Erm Methyltransferases to fight resistant bacteria.34 More currently, the SAR by NMR method served as the primary tool for characterizing protein-ligand complexes of the Bcl-2 family, a class of proteins involved in the apoptotic pathways.35 Additionally, Variations of this method have been developed and utilized recently to examine higher molecular weight proteins and analyze selective binding among related proteins, including SAR by NMR with 13C-labeled methyl groups (see Isotopic Labeling Techniques in Advances in NMR)36 and RAMPED-UP NMR.26 RAMPED-UP NMR (Rapid Analysis and Multiplexing of Experimentally Discriminated Uniquely labeled Proteins using NMR) is an important advance, because it provides a way of simultaneously evaluating the specificity of a ligand for a protein among a mixture or class of proteins. Each protein target is labeled with one unique amino acid type and studied simultaneously in a mixture using a ligand titration experiment. The ability to multiplex the protein target not only increases the screening throughput but also reveals whether the ligand of interest will target a single family member in a specific manner or bind to several members from a class of proteins.
Although the perturbation assay and SAR by NMR are powerful techniques for characterizing ligand-protein interactions, the size, solubility and stability of the protein, as well as the ability to isotopically label the protein, may limit the ability to screen for binding using the protein target resonances.4 The line width of NMR signals increases with increasing molecular weight, resulting in spectral overlap and loss of signal and resolution.37 To acquire protein spectra with reasonable signal-to-noise ratios in a timely manner, the protein must stay soluble at concentrations around 0.1–1 millimolar without degradation for the duration of the experiments. The length of the NMR experiments that detect the protein target depend on the type of experiment, which can last anywhere from a few minutes to several days.38 Furthermore, the 1H-15N HSQC requires isotopic labeling of the protein target.39 Although, the 13C nucleus occurs at 1% natural abundance, studies without 13C enrichment can provide valuable information, but this requires longer data acquisition. Although isotopically labeled protein production can increase cost, labeling facilitates the observation of specific nuclei or bonds of one species. With protein target detected NMR, for example, because the ligand is left unlabeled, ligand resonances do not complicate the protein target spectrum, eliminating the need for spectral subtraction (difference spectroscopy) (see Diffusion Experiments under Ligand-Detected Methods).40 15N and 13C isotopic labeling permits the assignment of backbone amides using a series of three-dimensional NMR experiments.13,16,38 A description of how assignments are completed is beyond the scope of this paper, and a detailed discussion of the topic can be found elsewhere.38 Completion of sequential assignments is necessary to obtain structural information about the protein target’s binding site and can be used to develop structure activity relationships. As the size of the protein increases, resonance assignment becomes more difficult to obtain, and as such, different labeling strategies are used to facilitate the evaluation of larger protein targets with molecular weights greater than 30,000 kDa (see Isotopic Labeling section under Advances in NMR).
Target observation also may result in lower through-put as a result of more time-consuming, multidimensional spectral analysis and the need to deconvolute hits from a small molecule compound library.4 Selecting an optimal mixture size from the library to be screened will have a great impact on the efficiency of the perturbation assay. A comprehensive review of the determinants has been presented by Mercier and Powers.41 The time needed to deconvolute hits from a mixture depends largely on the number of compounds in a mixture but also on the physicochemical properties of the mixture’s components. For example, the main goals to achieve when selecting a compound mixture include minimizing reactivity and interactions of compounds, achieving structural diversity, maximizing solubility, and preserving constant physical properties (pH and ionic strength). Deconvoluting mixtures of ligands also has been addressed by an automated system in which 306 individual compounds were screened for binding to a 15N-labeled target. This robotic sample preparation, in conjunction with the automated data acquisition and analysis tool is applicable to many systems. It can be used to evaluate a large number of ligand-protein interactions without the need to deconvolute hits from the larger library.42,43
An alternative approach to minimizing the spectral complexity of proteins is to introduce a spin probe. 19F is a unique spin ½ nucleus that can be incorporated into a protein at Tyr, Phe, His, or Trp residues using selective labeling techniques.44 Aromatic residues are useful probes of structural integrity and binding because, respectively, they are often buried in the hydrophobic core of the proteins and/or located at the interface of intermolecular interactions.45 Several useful properties of the 19F nucleus make it an ideal label for NMR studies, which have been described previously.46,47 First, 19F is present at 100% natural abundance and has 83% of the sensitivity of 1H providing a reasonable alternative to 1H NMR. Next, 19F incorporation generally produces no dramatic structural effects when substituted for hydrogen in an amino acid side chain. Although, 19F is substantial larger than 1H, the two have similar covalent radii (1.3Å for 19F and 1.2Å for 1H).46 Third, the considerably different dipole moment means that fluorine lone pair electrons primarily control the 19F chemical shift, resulting in a 100-fold larger chemical shift range than 1H. This also makes the nucleus especially responsive to local changes in van der Waals environments, electrostatic fields and hydrogen bonding.47 As such, small chemical shift perturbations are easily detected in one-dimensional 19F spectra. Finally, 19F does not occur naturally in proteins so no background signals complicate the spectrum. A protein labeled with 19F-labeled amino acid will exhibit NMR peaks due only to the label, not to the backbone of the protein permitting the use of simple one-dimensional NMR experiments to detect changes in the protein structure upon addition of ligand. Because one-dimensional experiments are faster, 19F NMR studies can be performed with lower protein concentrations, and thus, spectral acquisition is shorter than traditional protein detection techniques. Furthermore, 19F-NMR can be used to examine structural aspects of much higher molecular weight proteins, which traditional multidimensional NMR experiments fail to resolve. For example, selective 19F labeling and subsequent 19F NMR analysis of the 497 residue XIAP (X-linked inhibitor of apoptosis protein) with and without the Smac peptide revealed the interaction of the peptide to protein in a 2:1 ratio and the ability of the Smac peptide to inhibit the interaction of XIAP with its downstream partner.48,49 Additionally, in an investigation of inhibitors to the anti-apoptotic protein Bcl-xL (181 amino acids), selective 19F-Phe labeling was used to obtain supplementary structural constraints for defining the orientation of the inhibitor in the Bcl-xL-inhibitor complex.45
Ligand Detected Methods
Ligand observation experiments offer a useful alternative to protein target detection for larger proteins or those that oligomerize in solution at higher concentrations23 or have no suitable expression host that permits isotopic labeling.21 The use of simple one-dimensional spectra and ability to screen mixtures without deconvolution, results in higher throughput and its use as a well-suited primary screening technique.4 Ligand examination has the technical advantage of requiring a smaller amount of unlabeled protein9 and may be used to identify the binding epitope of the ligand.4 Furthermore, the size of the protein target does not limit this technique, and in many examples, larger proteins provide better sensitivity because binding of a large protein causes a more distinguishable change to the observable NMR signals.3 This broader approach to NMR screening relies on either detection of an altered hydrodynamic property of the ligand upon addition of protein target or exchange-mediated transfer of bound state information to the free state.50
Hydrodynamic Property Experiments
The NMR spectral properties of proteins and small molecules differ because of differences in hydrodynamic properties, which affect the translational and rotational mobility of a molecule in solution. Experiments in this category exploit the fact that a bound ligand transiently adopts the NMR properties characteristic of a large protein target, which are manifest in the spectrum of the ligand.21,40
Relaxation Experiments
Comparison of the ligand’s relaxation rate in the presence and absence of the protein target represents the most well-established class of NMR binding assays. Relaxation reflects the hydrodynamic radius and rotational tumbling rate of species in solution.9,21,23 Following delivery of a radio frequency pulse of energy, the system is perturbed and must re-establish its equilibrium by dissipating the absorbed energy supplied by the pulse. This loss occurs by transferring energy to either its surroundings or to other spins. Relaxation simply implies the movement of bulk nuclear magnetization towards equilibrium and occurs through two distinct mechanisms. Thermal equilibrium governs the recovery of magnetization in line with the magnetic field, meaning that the nuclear spin exchanges energy, in the form of heat, with its surroundings (lattice) to return to its ground state.39 The rate at which relaxation proceeds via the spin-lattice mechanism is called R1 and effectively occurs at a similar rate for small and large molecules.10 The second mechanism by which energy is transferred is more complex but fundamentally depends on the size of the molecule, such that relaxation proceeds more rapidly with increasing size.51 After an rf-pulse, the bulk magnetization becomes perpendicular to the magnetic field (transverse plane). Although the pulse initially aligns the nuclei in the same plane, spin diffusion ensues and the transfer of energy from one spin to another spin (dipolar coupling) proceeds until the net magnetization dissipates to zero (Figure 6).10 The rate at which relaxation occurs via the spin-spin mechanism is called R2 (transverse relaxation) and occurs much faster for large, slowly tumbling molecules.51 The rate of transfer strongly depends on the chemical environments of the different nuclei and how efficiently the nuclei exchange energy; the larger the molecule, the more efficient the exchange and the faster the rate of relaxation.37 Additionally, the rate of transverse relaxation determines the line width of the observed resonances. Line width is directly proportional to the rate of spin-spin relaxation (line width=R2/π), which is a consequence of detecting the NMR signal in the transverse plane, perpendicular to the magnetic field. Transverse relaxation governs the rate at which the signal or Free Induction Decay (FID) falls off, such that a large R2 results in a FID that decays rapidly. Therefore, upon Fourier transformation to the frequency domain, faster relaxing species produce broader, less resolved peaks in the spectral window.10
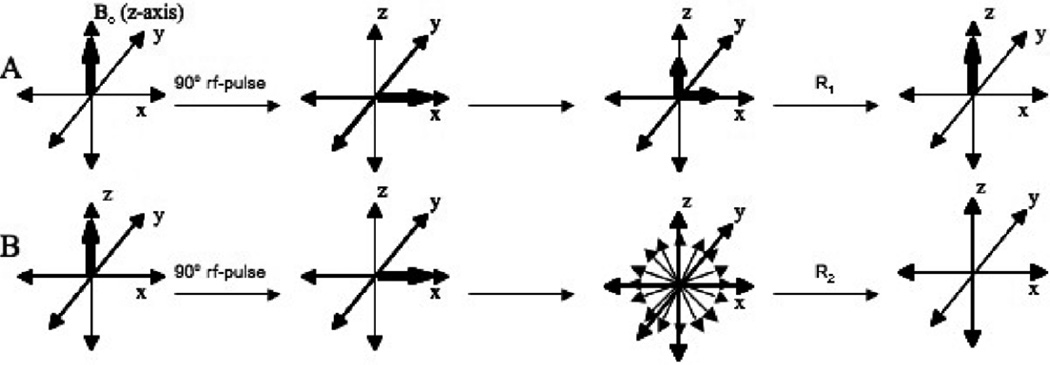
Figure 6. Nuclear relaxation.
A) Longitudinal Relaxation. After an rf-pulse of energy is applied to the nuclei in solution, the atoms must relax back to their equilibrium position in line with the z-axis by releasing energy in the form of heat to its surrounding. B) Transverse Relaxation. After an rf-pulse of energy is applied to the nuclei in solution, the signals corresponding to the different nuclei fan out away from the x-axis at different rates due to chemical shift dispersion and diffusion and the transfer of energy from one spin to another spin, which proceeds until the net magnetization becomes zero. For small molecules the rate of transverse relaxation often equals the rate of longitudinal relaxation because there lacks sufficient interaction between the atoms to permit the transfer of excitation energy. For larger molecules, especially proteins, the rate of transverse relaxation is generally faster than longitudinal relaxation because the transfer of energy between spins occurs much more quickly and efficiently than the transfer of energy to the surroundings.
When a small molecule binds to a large molecule, the small molecule transiently possesses similar NMR properties as the large molecule and assumes fast transverse relaxation rates leading to spectral line broadening of the small molecule signal.9 The extent of ligand binding and size of the protein target directly correlates with line width changes.52 As such, larger proteins usually produce stronger effects, increasing the sensitivity of the experiment.3 For ligands with weaker dissociation constants (1–10 millimolar) to smaller protein targets, it is difficult to obtain reproducible and reliable line-broadening data, while binding to a larger protein (greater than 60 kDa) with the same affinity for the ligand is easily detected.53 The most basic method to evaluate binding in this manner involves the acquisition of a ligand spectrum (or mixture of compounds) in the absence and presence of a protein target, from which the differences in line shape and relaxation rates are determined. Enhanced transverse relaxation of ligand upon the addition of the protein target indicates the transient formation of a bound complex.21,23,54 For example, relaxation experiments were used to obtain information regarding the interaction of the FK506/FKBP complex (FK506-binding protein) with its downstream target calcineurin to improve the structure-based design of immunosuppressive agents. By detecting relaxation differences in different regions of the FK506/FKBP complex upon binding to calcineurin, the region of the complex that interacts with the protein target was identified, which was important because the immunophilin/drug complex (FKBP/FK506 respectively), not the drug or protein alone, inhibits an immune response.55
Differential line broadening assays form the basis of the SHAPES screening method. A small but diverse library known as the SHAPES library is screened against any protein target of interest. The library consists of molecular shapes found commonly among commercially available therapeutics.53 This method is similar in concept to the SAR by NMR technique. Once two shapes have been identified as weak binders by differential line broadening assays, a non-binding shape is used to link the two binding components to yield a higher affinity lead compound. To demonstrate the value of the SHAPES screening method, molecules from the SHAPES library were screened against p38 MAP kinase, an important protein target involved in cellular responses to external stress signals. The p38 MAP kinase served as a model protein target with which the utility of the SHAPES method could be established, because several inhibitors of p38 had been developed previously. SHAPES screening was utilized to discover a unique tri-substituted imidazole lead compound. The hit came from the same family of drugs as other p38 inhibitors that have important anti-inflammatory effects. The effectiveness of SHAPES screening against p38 MAP kinase indicates its potential to be applied to other novel protein targets to develop inhibitors to their function.53
Although most ligand screening methods rely on 1H-NMR, the favorable NMR properties of the fluorine nucleus make 19F-NMR an extremely useful method for detecting the binding of fluorinated compounds to a target.3,56 The properties of fluorine that make it useful for protein target detected screening apply to ligand-detected methods as well. Most notably, the extraordinary sensitivity of 19F to its environment and local shielding effects means that its resonances are dispersed over a large chemical shift range and it is extremely sensitive to relaxation changes caused by binding, which can provide more resolution than 1H-NMR relaxation experiments. Furthermore, because 19F does not occur naturally in proteins, no background signals complicate the one-dimensional spectrum, which eliminates the need for difference spectroscopy (see below).4 Most NMR solvent and buffer components lack fluorine moieties, which means buffers and solvents also do not interfere with data acquisition.57 Additionally, the broad chemical shift dispersion allows the use of large compound libraries minimizing overlap of individual ligand resonances.21 Although protons are found more ubiquitously,4 approximately 12% of compounds (equating to 200,000 compounds) in the Available Chemical Directory of Screening Compounds58 and 17% of the MDDR database45 contain fluorine moieties, making it quite possible to obtain a 19F-containing lead compound without resorting to special synthetic efforts.57 For example, 19F-NMR has been used to characterize the multifunctional calcium-dependent Calmodulin protein bound to the fluorinated antipsychotic inhibitor, trifluoperazine (TFP) to determine that TFP binding can occur without calcium present.59,60 Additionally, fluorine incorporation often improves the pharmacokinetic properties and potency of the drug.21,45 Binding interactions between the plasma protein human serum albumin (HSA) and drugs can have detrimental effects on the drug’s pharmacokinetic properties. 19F-NMR has been used to report on specific interactions between HSA and fluorinated dugs prior to lengthy pharmacokinetic studies.61 This has recently been applied to the interactions between niflumic acid (a potent analgesic anti-inflammatory drug prescribed for rheumatoid arthritis) and HAS, providing insight into its pharmacokinetic and toxicological properties.62 19F NMR also has been applied to detect metabolism of cytochrome P450 substrates.63 Cytochrome P450 enzymes are largely responsible for drug metabolism, and analysis of fluorinated drug interactions with cytochrome P450 enzymes by NMR can be used to avoid adverse pharmacodynamic effects resulting from the accumulation of metabolites. Additionally, 19F-NMR facilitated the determination of the metal to substrate distances and geometries within the active site of P450s, providing valuable structural information for drug optimization.64 Quantitative information about substrate turnover by enzymes, including P450s, can be obtained using the recently developed 3-FABS (three Fluorine Atoms for Biochemical Screening) technique. The substrate is labeled with a CF3 moiety and detecting by using 19F NMR. The substrate and product concentrations are monitored as a function of time to determine inhibition constants.65–67 For a more extensive analysis of liganddetected 19F-NMR methods, a comprehensive review recently published is recommended.68
Another useful but less common spin probe used for analyzing protein-ligand interactions is 31P. 31P can be used to evaluate enzyme activity and inhibition. Screening for inhibitors can be conducted using this unique nucleus when the enzyme binds a phosphorylated cofactor, which would be perturbed by interactions with ligand molecules. A family of small molecule inhibitors of Ras was examined for their ability to stabilize the inactive Ras conformation by monitoring the 31P signals from the bound GTP.69
Diffusion Experiments
Comparison of the ligand’s diffusion coefficient, which reflects the translational mobility of the molecule, in the presence and absence of the protein target, may be used to accurately examine intermolecular interactions between protein targets and ligands. Lin, Shapiro, and Wareing illustrated the use of diffusion-edited NMR (affinity NMR) spectroscopy for screening compound libraries in 1997.70–72 The spectral differences upon the addition of protein target result from the inverse dependence of diffusion coefficients on the hydrodynamic radius of small and large molecules. Diffusion experiments follow the same basic principles as relaxation experiments but rely on differences in translational instead of rotational motion. The small molecule transiently adopts the properties of the large molecule and, as such, exhibits a decrease in translational diffusion, which can be readily assessed by pulsed-field gradient NMR.73 Because the signal intensity of the ligand resonances depends strongly on the strength of the gradient pulse, diffusion-edited NMR experiments distinguish between molecules that interact with the protein target and those that do not by increasing the strength of the gradient pulse, which selects for resonances from bound species (Diffusion Encoded Spectroscopy-DECODES).22,74 The Diffusion Ordered Spectroscopy (DOSY) experiment extrapolates the one-dimensional chemical shift information into a pseudo-second dimension of diffusion coefficients and eliminates the need for deconvolution of the signals from a mixture of ligands (Figure 7).75,76 A change in the second dimension indicates binding in a straightforward manner, as the data acquisition does not differ from simple one-dimensional experiments because the second dimension encodes a property of the molecule, not a nucleus.52 As with relaxation experiments, diffusion experiments can be used to examine compound mixtures or libraries for protein target interactions in a powerful, high-throughput manner. This method has been applied to the identification of peptide compounds that bind to vancomycin to study antibiotic resistance. Vancomycin is a potent antibiotic for streptococcal and staphylococcal bacterial infections because it specifically binds to the carboxy-terminal D-Ala-D-Ala sequence of the bacterial peptidoglycan, inhibiting cell wall synthesis. Antibiotic resistance results because of a substitution to one of the amino acids in the target bacterial cell wall, which inhibits binding of vancomycin. The ability to study binding interactions between vancomycin (or its derivatives) and peptides that mimic the bacterial cell wall will facilitate the development of additional agents that may overcome bacterial resistance. In this example, the limits of diffusion-edited NMR were challenged because the vancomycin receptor was only three times the size of the peptides tested. The results obtained, however, produced a spectrum with adequate resolution to identify interacting ligands.77
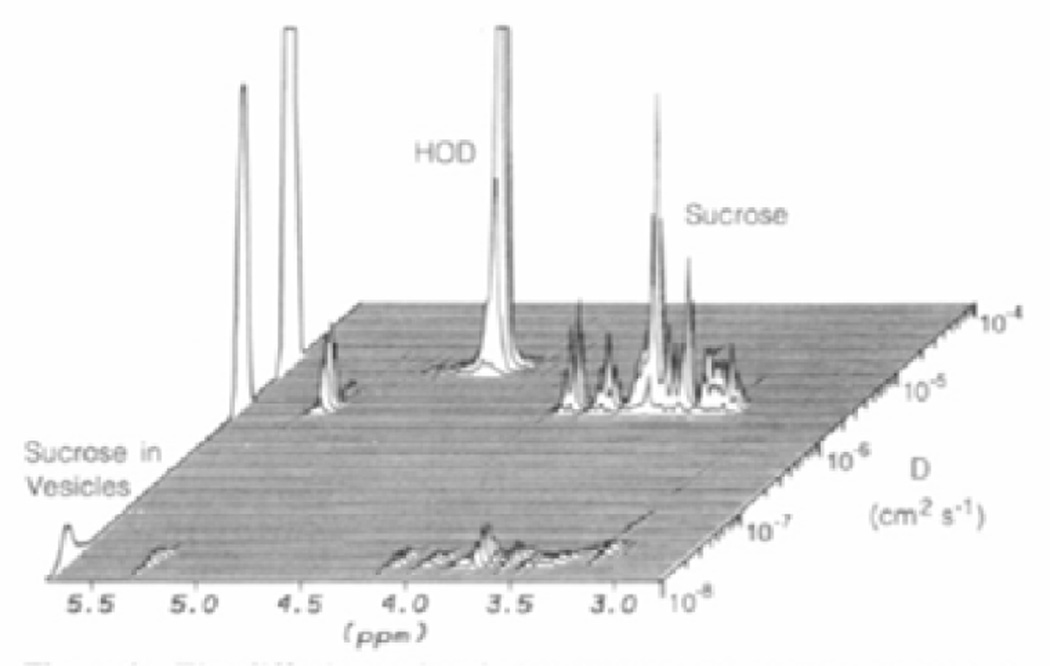
Figure 7. Diffusion Ordered SpectroscopY (DOSY).
A one-dimensional NMR experiment is extrapolated into a pseudo second dimension that encodes the diffusion coefficient of the molecule. In the above figure, the diffusion coefficient is used to distinguish free sucrose from sucrose bound to phospholipid vesicles. Reproduced with permission from J. Am. Chem. Soc. 1992, 114, 3139–3134. © 1992 American Chemical Society.
Relaxation and diffusion experiments have great utility, but both experiments require the use of spectral subtraction (difference spectroscopy), which can have disadvantages. When examining smaller proteins, the signal intensity from the protein approaches that of the ligand, and the protein resonances may overlap in the region of the ligand signals, such that they obscure the signal from the ligand. Consequently, a large excess of ligand is required to ensure ligand observation. Most studies of this nature require a minimum of ten-fold ligand molar excess.21 The experimental setup requires a one-dimensional spectrum of the protein alone and subsequent subtraction of the protein resonances from a spectrum of ligand in the presence of protein target. Subtraction of the resonances may lead to artifacts in the resulting spectrum when the protein’s chemical shifts change upon binding. Relaxation and diffusion filtering eliminates the signals of the protein target and bound ligands and simplifies the subtraction process, minimizing the number of artifacts observed.74 Relaxation filtered experiments employ the use of spin-locks, while diffusion filtered experiments utilize pulsed field gradients to attenuate the protein target and bound ligand signals. These edited experiments work because the rotational or translational motion properties of the bound ligand transfer to the detected free ligand. To detect binding, a spectrum of the ligand in the presence of the protein target is subtracted from a spectrum of the ligand in the absence of the protein target. If the ligand interacts with the protein, the resulting difference spectrum should resemble that of the ligand alone with decreased signal intensities. For ligands that do not bind to the protein target, the resonances will be completely eliminated upon subtraction. Figure 8 displays a hallmark example of ligand binding using relaxation filtering. Diffusion filtering experiments yield similar results as observed, for example, when applied to the same system shown in Figure 8.74
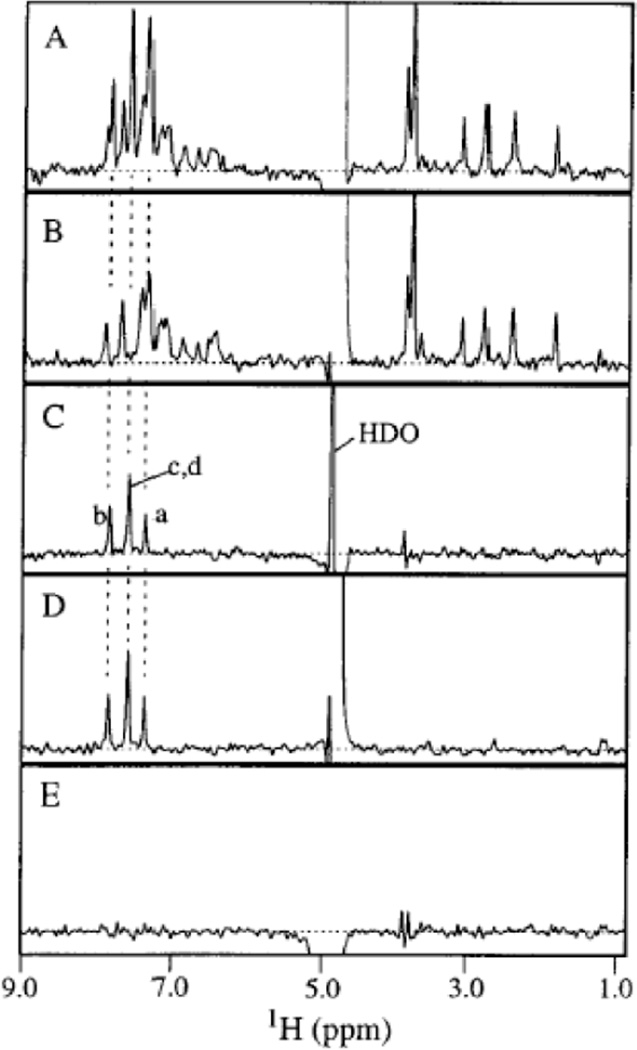
Figure 8. Difference spectroscopy with relaxation filtering.
A) Relaxation-edited 1H NMR spectrum of a mixture of nine compounds in the absence of protein. B) Relaxation-edited 1H NMR spectrum of a mixture of nine compounds in the presence of protein after correcting for residual protein signals by subtracting a spectrum of protein alone. C) Difference spectrum obtained by subtracting B from A, which reveals only signal for the bound ligand. D) Reference spectrum of the binding ligand alone. E) Difference spectrum obtained by screening a mixture of non-binding ligands. This served as a negative control. Reproduced with permission from J. Am. Chem. Soc. 1997, 194, 12257–12261. © 1992 American Chemical Society.
Both relaxation and diffusion experiments require a fast-exchange regimen between the ligand and the protein target. If a ligand briefly exchanges into another environment by binding to a protein and obtains a very different chemical shift during NMR detection, the signals from the ligand will dramatically decrease and broaden. The accumulation of these exchange events with time leads to destructive interference in the FID and loss of signal. Slowly exchanging nuclei cause these errors to accumulate and result in broad NMR signals indistinguishable from the noise. Fast-exchanging nuclei will average out these errors and produce one resonance line.10,39 This limits the technique to weakly binding ligands in rapid exchange, and consequently, these experiments cannot differentiate the nature of the binding reaction (specific from non-specific binding),78 leading to more frequent detection of false positives.3,21 On the other hand, because relaxation and diffusion experiments rely on detection of the ligand resonances, the size, composition and oligomeric status of the protein target are essentially unlimited, which is advantageous over protein target detected methods.23 Furthermore, the extent of ligand binding and size of the protein target bound directly correlates to line width changes.52 As such, larger proteins usually produce stronger effects, increasing the sensitivity of relaxation experiments.3,53 Additionally, there is no need for multidimensional or correlation experiments, simplifying the experimental setup.
Exchange Transferred NOEs
To determine the specific conformation of weak to medium affinity ligands when bound to a large protein target, the one- and two-dimensional exchange transferred NOE experiments (etNOE) have been used extensively.52,79 Like relaxation and diffusion-based experiments, the use of the transferred nuclear Overhauser effects (NOEs) also depends on the ability of the ligand to acquire the relaxation properties of the protein target.50 Upon binding of a ligand to a protein target, the NOEs observed for the free ligand change because the molecule retains a “memory” of its bound state properties. The ligand acquires distinct magnetic properties while bound to the protein, which are retained even when the complex dissociates.80
Direct magnetic interactions between nuclear dipoles located close to each other in space result in dipolar coupling, leading to time-dependent mutual cross-relaxation. These time-dependent dipolar couplings provide an important source of nuclear relaxation in solution. They also provide incoherent magnetization transfer pathways that result in direct through-space interactions between nuclei called nuclear Overhauser effects (NOEs). Experimental observation of the NOE requires a change in signal intensity from one spin after the perturbation of a nearby spin with radio-frequency excitation. The rate constants governing cross-relaxation depend on the distance between two nuclei and the rotational mobility of the vector connecting the two atoms.22,54 NOEs are sensitive probes of short-range, through-space, intramolecular and intermolecular interactions,9 because the intensity of the NOE falls off rapidly with increasing distance (1/r6).37 As such, the NOE provides relative spatial information for structure determination, and allows the use of solution NMR to examine the interactions between nuclei that are not necessarily covalently bound but are within five angstroms of each other.22 Differences in Brownian motions of the molecular structure also intimately reflect alterations in NOE patterns. For rapidly tumbling small molecules, two-dimensional 1H-1H nuclear Overhauser spectroscopy (NOESY) experiments result in negative NOE cross peaks with weak intensity. For slowly tumbling large molecules, stronger positive cross peaks result.6,9,22 The differences in sign and intensity of the NOE serve as the basis for extracting information about binding from etNOE experiments.
When a ligand binds to the protein target, the ligand protons experience additional dipole-dipole interactions from the protein’s protons. In addition, the ligand transiently adopts the tumbling properties of the large protein target. Detection of binding relies on intraligand NOEs that develop in the bound state, where the dipole-dipole interaction caused by the decreased molecular tumbling rate occurs much more efficiently than in the free state. As such, positive NOE cross peaks for small molecules in the presence of the protein target clearly indicate binding. Unbound ligand molecules produce weak, negative NOE cross peaks typical of small molecules. These so-called exchange transferred NOEs have great utility in the examination of the interactions between ligands and protein targets.3,9,22,54,79 etNOEs were used to examine the interactions between the antibody SM3 and the aberrantly glycosylated MUC-1 protein (which is associated with breast cancer) because specificity of the SM3 antibody to cancerous MUC-1 glycoproteins may result from decreased carbohydrate chain length or a conformational change. etNOES were used to determine the conformation of differentially glycosylated MUC-1 peptides bound to the SM3 antibody and found that the less glycosylated form is bound preferentially because the peptide acquires a more knob-like structure. The ability to determine the conformation of the MUC-1 peptides bound to SM3 has facilitated the development of SM3 for diagnostic and therapeutic purposes.81
Once a ligand has been confirmed to bind to a protein by methods such as etNOEs, two recently developed techniques may be used to facilitate identification of the bound ligand’s orientation without the need for complete structure determination of the protein-ligand complex under investigation. The INPHARMA (Protein Mediated Interligand NOEs for Pharmacophore Mapping) method was developed to investigate the binding orientation of a competing ligand when a structure of a similar protein-ligand complex is known.82 Interligand NOEs are used to gain information about the binding mode of one ligand with respect to the other. Interligand NOEs arise between the known binder and new compound, which are used to determine the orientation of the new complex without the need for complete structure identification. The SAR by ILOEs (interligand nuclear Overhauser effect) approach uses etNOEs to identify small molecule ligands bound to the protein target simultaneously in close proximity to each other. Interligand NOEs then are used to provide information about the orientation of ligands, which subsequently may be linked to yield a high-affinity lead compound in the correct conformation.83 The SAR by ILOEs approach is advantageous over the SAR by NMR method previously described because it provides information about the relative orientation of the two compounds in the binding pocket without needing to solve the structure of the ternary complex.80
Limitations to the etNOE method’s utility include the sensitivity of the 1H-1H NOESY experiment itself. The low-sensitivity of the two-dimensional homonuclear NOE experiment requires that relatively long acquisition times are used to provide spectra of sufficient quality and precludes the high-throughput analysis of large libraries of compounds.3,7 Furthermore, the spectrum contains strong diagonal peaks that prohibit the observation of cross peaks between ligand resonances having similar chemical shifts. The strong diagonal peaks may also introduce noise or baseline problems that interfere with the observation of weak cross peaks.52 An additional constraint is that the transferred-NOE experiment most efficiently characterizes ligands with a KD value in the micromolar to millimolar range. To observe changes in NOEs, the dissociation of the ligand must occur quickly enough such that a sufficient percentage of the free ligand will remember the bound state and generate intense, positive NOE cross peaks. If the on rate is too slow (much slower than diffusion allows), the transfer NOE will be too weak for detection.6,23 The dissociation constants of slowly exchanging systems can be determined using the etNOE experiment but requires a complex mathematical treatment, which has been reviewed previously and is beyond the scope of this paper.80,84 This limits the transfer NOE technique to weakly binding ligands in fast-exchange and, as such, requires large ligand concentrations in excess of the protein target (10:1–20:1 ligand:target ratio). This large ratio introduces the possibility of secondary binding, which may hamper unambiguous interpretation of the results. Also, compounds of limited solubility can aggregate in solution and exhibit large positive NOE cross peaks in the absence of the protein target, producing false positives.9,52,85 False positives are easily identified by examining control samples containing only the small molecules. Nonetheless, the transferred NOE method has great advantages because of its ability to generate information on both the binding affinity and the geometry of the ligand in the bound state from the same experiment. etNOE experiments are often employed as one of the final steps in the SAR by NMR method, when sequential assignments of the labeled protein target are known, to determine the orientation of two adjacently bound ligands to a protein target.25 Although it is possible to attain even higher resolution information when coupled with sequential assignments, the transferred NOE method always retains the important advantage of being able to provide structural information about ligands interacting with unlabeled protein targets of unlimited molecular weight.
Exchange-Mediated Saturation Transfer Experiments
The second category of ligand-detected methods relies on exchange-mediated transfer of another type of bound-state information to the free state.50 The basis of this type of NMR experiment is similar to transferred NOE experiments in that magnetization transfer can occur between ligand and target through NOEs.23,86 The difference from etNOE experiments is the fact that detection of binding does not rely on the bound ligand exhibiting transient relaxation properties of the protein target. Instead, the protein target is saturated with radio frequency energy, which is transferred to an exchanging ligand to affect the one-dimensional proton signal intensities of the ligand. The Saturation Transfer Difference (STD) experiment utilizes this form of magnetization transfer in conjunction with difference spectroscopy and manifests the binding interaction as an apparent decrease in signal intensity of the ligand. The STD experiment is advantageous because it provides more sensitivity than previously described ligand-detected methods and may be used to quantify binding affinities between ligand and target and identify the binding epitope of the ligand.87
STD experiments require the application of a train of selective radio-frequency pulses until the protons in the protein target can no longer absorb any more energy (saturation). Ligands that come in close contact with the target receive part of this saturation via intermolecular 1H-1H cross relaxation pathways, which results in a decrease in the ligand’s line intensity. An apparent decrease in the one-dimensional signal intensity of the ligand-resonances upon subtraction of a reference spectrum (lacking target saturation, and as such, magnetization transfer) accurately indicates binding of the ligand to the protein target.21 STD studies often employ extremely low, undetectable concentrations of the target, and ideally the resulting difference spectrum contains only ligand signals corresponding to protons making NOE contacts with the protein; however, for studies requiring high concentration of the protein target, relaxation filters may be applied to suppress the signals from the protein.3,54 Differences in the observed signal intensities of the resonances from atoms in the ligand indicate which moieties are embedded in the protein-ligand interface. Because the magnetization transfer depends on the distance between the ligand and protein target, proximal interaction leads to more efficient transfer of saturation, which in turn leads to a greater loss of signal, allowing identification of the ligand’s binding epitope with the difference spectrum.3 This method was used to characterize the binding epitope of the cyclic RGD peptides that inhibit fibrinogen binding to integrins to prevent platelet aggregation and to permit optimization of these peptides as potential drug candidates. Conformational changes of the RGD peptide significantly impact selectivity for specific integrins involved in clot formation, and the appropriate conformation was identified to selectively target these peptides to the correct integrin type.88 When used with isotopic labeling strategies (See Advances in NMR; Isotopic Labeling), STD NMR can identify the ligand binding epitope and the amino acid composition of the ligand-binding site simultaneously. This setup forms the basis of the SOS-NMR experiment (Structural information using Overhauser effects and Selective labeling). SOS utilizes STD NMR to examine a ligand complexed to a series of selectively protonated protein target samples to characterize the amino acid composition of the ligand-binding site, while concomitantly identifying the ligand binding epitope.89
The STD experiment offers more sensitivity than relaxation, diffusion, and transfer NOE experiments.52 The increased sensitivity depends on the ability to selectively pulse the protons of the protein target and the ability of the pulse to saturate all of its spins. Typically, the radio-frequency pulse used to saturate the protein resonances occurs at ppm values that lack ligand signals in the outermost region of the spectrum (typically below −2 or above 10 ppm). Because the pulse only perturbs selective protons of the protein target, the saturation must transfer to the rest of the protein signals via dipolar interactions and, as such, depends on the protein’s ability to transfer energy. STD NMR is ideally suited for protein targets of 30,000 molecular weight or greater, because the saturation disperses more quickly to the other protons of the protein target that were not directly perturbed by the selective radio-frequency pulse. The larger the protein, the more efficient transfer of magnetization is to other nuclei, including the bound ligand, making this technique an extremely useful alternative to protein target detected methods for species of large molecular weight.21,23,87 Additionally, the better sensitivity of the STD experiment means that these experiments require even smaller concentrations of the protein target; however, optimal setup, use, and interpretation of the STD experiments require some familiarity with the exchange processes at work, and as such, some prior knowledge the protein target’s structure or function.
Ligands with KD values in the range of 10−8-10−3 are ideally suited for STD methods. For extremely weak binders, too many protein target molecules will lack a ligand in the binding site to achieve detectable signal by STD. For tight binders, the exchange occurs so infrequently that the pool of free-ligand is barely influenced by its visit to the saturated protein target and relaxes back to equilibrium before it is released from the protein. The population of saturated ligands will not influence the total ligand magnetization to produce a measurable STD signal.21 Despite these limitations, a broad range of binding constants can be evaluated. By expressing the signal intensity of the STD spectrum as a fraction of the intensity of an unsaturated reference spectrum [(I0-Isat)/I0], the binding affinity can be calculated. In the preceding equation, the intensity of the reference peak is I0 and the intensity of the peak after saturation is Isat. A value of 0.5 corresponds to 50% saturation of the ligands (equating to 50% of the ligands in exchange with the protein target).86 This value is then multiplied by the ligand:protein ratio to generate the STD factor. For a series of ligand concentrations, plotting the STD factor versus ligand concentration generates a curve from which the KD value is determined. The ability to accurately quantify binding constants is a unique advantage of this NMR method.
An important adaptation of the STD method is the waterLOGSY (water-ligand observed via gradient spectroscopy) experiment.90,91 The aim of both methods is to transfer magnetization to the bound ligand and detect differences in the spectrum of the free ligand. Where the STD method achieves this by directly saturating the protein, the waterLOGSY method accomplishes transfer indirectly through excitation of the bulk water magnetization. The excited water interacts with the protein-ligand complex transferring the magnetization, which is subsequently retained by the ligand after it dissociates. Bound ligand directly interacts with bound water molecules within the binding site via cross-relaxation mechanisms. Alternatively, labile protons (NH and OH groups) in the protein target exchange with bulk water and acquire the saturated magnetization previously applied. Then the magnetization is propagated to the free ligand when the water molecules interact directly with the bound ligand at the protein-ligand interface via the same types of intermolecular cross-relaxation mechanisms.21 In both of these cases, the magnetization transfer pathway conserves the sign of the magnetization from the bulk water and, as such, non-binding compounds appear with opposite sign compared to the water signal. Nonbinders and binders are easily discriminated from each other in a mixture because they give waterLOGSY signals of opposite sign.21,91 False positives may be observed if magnetization is transferred directly to the free ligand via chemical exchange of bulk water with exchangeable ligand protons. The exchangeable proton resonances, when visible, may complicate the interpretation of waterLOGSY data. To overcome this problem, a waterLOGSY spectrum for the free ligands without protein target can be collected as a control.21 Like all ligand-based screening methods, the waterLOGSY experiment is limited to the detection of weakly binding ligands in the low micromolar range. Ligands with a tighter affinity will stay bound to the protein too long and relax back to their original state before dissociating. An important advantage of the waterLOGSY method is that it is much more sensitive than other ligand-detected methods. This may be attributed to the large excess of solvent available and the large number of exchangeable protons in a protein-ligand complex. The exceptional sensitivity of this method permits the use of nanomolar concentrations of unlabeled protein target, making it a powerful method for primary screening of compounds that bind to therapeutically relevant protein targets.91
Advances in NMR
Many recent advances in solution NMR have served as tools to overcome the limitations encountered with each screening methodology discussed. Advances in instrumentation and innovative experimental protocols (pulse sequences) have been utilized to overcome the problems of fast relaxing signals and lack of resolution with higher molecular weight proteins. Isotopic labeling strategies have facilitated the study of higher molecular weight protein targets (up to 900,000 kDa) by decreasing the number of observable signals and reducing spectral crowding. In addition, computational methods have improved the amount of information gleaned from limited NMR data sets. The development of competition experiments has expanded the range of binding affinities that can be evaluated by ligand-detected screening methods. These advances have contributed to a more extensive use of NMR as a high-throughput screening tool.
Instrumentation Advances
Instrumentation development has rapidly improved the quality of data available from NMR experiments, especially the development of higher frequency spectrometers. Increasing the magnetic field strength simplifies complex or overlapped spectra by generating more dispersion along the observed chemical shift axis, because the chemical shift dispersion increases linearly with field strength, while the line widths of aliphatic protons and carbons remain unchanged.12 Higher field strength not only resolves overlapping signals but also increases the NMR sensitivity because stronger magnets achieve better signal-to-noise, increasing roughly as the 3/2 power of the magnetic field.39 For example, moving from a 400 MHz spectrometer to an 800 MHz spectrometer increases the sensitivity by a factor of approximately three and resolution by a factor of two. Because of this, field strengths of 14.1 Tesla (600 MHz) and above are routinely utilized for drug screening and development. Additionally, magnets with proton resonance frequencies up to 1 GHz are becoming available and are employed currently for the study of higher molecular weight protein targets.92 Because of the increase in signal-to-noise, better spectra can be recorded in less time using less material. This has greatly improved the quality, speed, and resolution of data acquired for protein target structure determination and ligand interactions.16
Increasing the sensitivity of the NMR signals observed may be accomplished by either an increase in the signals or decrease in noise. Increasing the sample concentration or improving the field strength can enhance the signal-to-noise by directly increasing the intensity of the resonances. Cryogenically cooled probes (probes which have had their electronics cooled to 20–25 K) greatly diminish the level of thermal noise, increasing the signal to noise ratio by a factor of three or more and, consequently, improving the sensitivity of the NMR experiment.92 This is important because a three-fold enhancement in sensitivity corresponds to approximately a 10-fold shorter experiment time or a three-fold decrease in the protein concentration needed to achieve the same signal-to-noise with a room temperature probe.3 A cryogenic probe can effectively convert a lower field instrument (500 MHz) to the equivalent of a higher field instrument (800 MHz) at a greatly reduced cost compared to obtaining a higher field spectrometer. It should be noted that the signal-to-noise in a cryogenic probe depends strongly on the conductivity and dielectric losses of the solution, such that an increase in ionic strength corresponds to an increase in noise. At approximately 100 millimolar salt concentration in a standard 5 mm tube, the signal-to-noise advantage over a conventional room temperature probe begins to diminish.93–96 This can be overcome by using a narrow diameter tube, which positions the ions in a region with the lowest electric field; however, the sample concentration must be increased to account for the decrease in volume being analyzed. Using a cryogenic probe with low ionic strength solutions, 2D 1H-15N HSQC spectra can be obtained in a few hours on protein samples as low as 50 micromolar. This advance has facilitated high-throughput analysis of binding interactions, because minimizing the protein concentration permits a greater number of compounds to be screened simultaneously: 100 compounds at 50 micromolar each may be used while keeping the total concentration of added components to 5 millimolar. This increases the throughput of the NMR screening assay by 10-fold, because earlier studies used concentrations of one millimolar protein target and were limited to ten compounds in each mixture, allowing a maximum throughput of 1000 compounds per day. Using this strategy, more than 10,000 compounds can be screened in one day, greatly facilitating the drug discovery process.16,92,97 This can be an advantage when examining systems with very low hit rates.41
Pulse Sequence Improvements
The greatest limitation of early protein target observed NMR screening was the inability to observe higher molecular weight protein targets because the relaxation mechanisms of the larger proteins cause lines to broaden and signals to disappear; however, the introduction of Tansverse Relaxation Optimized SpectroscopY (TROSY) has supported the investigation of many protein targets up to a molecular weight of 100 kDa.98,99 Protein target NMR screening studies require well-resolved, high-quality spectra to determine the involvement of specific residues in the binding reaction. The slower tumbling of larger proteins results in very efficient spin-spin relaxation between protons and primarily affects transverse relaxation processes. This effect is reflected in the line widths of the observed resonances, making these basic spectral requirements harder to fulfill.92 The TROSY technique reduces signal loss due to increased relaxation rates and consequent line broadening by exploiting the differential relaxation effects arising from chemical shift anisotropy (CSA) and dipole-dipole couplings, which are quite significant at higher magnetic fields (greater than 500 MHz). The measurement of these two phenomena using an uncoupled 1H-15N HSQC spectrum results in four differently shaped peaks, only one of which is sharp and narrow. The TROSY protocol detects only the sharp, narrow peak resulting in a better-resolved NMR spectrum for higher molecular weight protein targets.16,22,98 Dipole-dipole couplings arise in proteins independent of the field strength, while CSA increases at higher fields. Consequently, an optimal field strength exists at which the rate in transverse relaxation in TROSY experiments approaches zero (1 GHz).9 As such, the increase in sensitivity due to the TROSY pulse sequence is more pronounced at higher field,3,16 further illustrating the importance of higher field instruments. With TROSY, the molecular size of proteins accessible for detailed NMR investigations has been extended several fold. The approach may be applied to a variety of NMR experiments, including two-and three-dimensional experiments used for sequential assignments to identify important residues of the protein target involved in binding.13 To fully benefit from the line narrowing effects of TROSY, other relaxation mechanisms should be suppressed. These mechanisms include proton-proton dipolar relaxation between NH and CH protons and intermediate exchange broadening due to segmental motion. Deuteration of the amino acid side chains may be performed to facilitate the suppression of these relaxation mechanisms.
Cross Relaxation-Enhance Polarization Transfer (CRINEPT) and Cross Relaxation-Induced Polarization Transfer (CRIPT) experiments can yield up to a three-fold signal-to-noise enhancement for amide groups and have been applied to a 110 kDa protein100 and 900 kDa protein complex.101 In a correlation experiment such as the 1H-15N HSQC, the pulse sequence includes a step in which transfer of the magnetization of a sensitive nucleus (1H) to an insensitive nucleus (15N) via spin-spin couplings takes place (Insensitive Nuclei Enhanced by Polarization Transfer; INEPT). This key step has been incorporated into many of the multidimensional NMR experiments used for sequential assignments and structure determination. The efficiency of the INEPT sequences depends on the strength of the magnetization of the sensitive nuclei, and as such, also depends on the transverse relaxation rate (an increase in R2 causes rapid deterioration of the magnetization). For protein targets with molecular weights beyond 100 kDa, transverse relaxation during the transfer time may become a limiting factor because the magnetization of a fast relaxing 1H signal is relatively short lived and cannot transfer its magnetization to the bonded 15N. CRINEPT and CRIPT experiments utilize cross-relaxation pathways (NOEs) to transfer the magnetization to the 15N nucleus. An increase in molecular weight increases the efficiency of cross-relaxation pathways, increasing the sensitivity of the experiment for larger protein targets. The CRINEPT and CRIPT experiments permit high-resolution analysis of membrane bound protein targets and protein targets that oligomerize in solution.7,100
While TROSY based experiments address the relaxation and resolution difficulties of higher molecular weight proteins, the problem of resonance overlap due to a large number of amino acids and corresponding backbone amides remains a major obstacle for resonance assignment and chemical shift perturbation studies. The Solvent Exposed Amide-TROSY (SEA-TROSY) experiment provides a modification of the 1H-15N correlation experiments to reduce the problem of resonance overlap in very large proteins. This type of pulse sequence assumes that only the amides exposed to the solvent contribute to the intermolecular interactions involved in binding, so that those buried in the core of the protein may be eliminated and ignored. After a partially or completely deuterated protein sample (see next section) is dissolved in water, the labile deuterons near the surface of the protein will exchange rapidly with protons from the aqueous bulk solution. The pulse sequence manipulates the rf-energy in such a way as to eliminate any magnetization generated from amide protons, without modifying the water signal. The water magnetization exchanges with exposed amide protons, which can be detected with a TROSY-type correlation experiment. This eliminates any signals that may result from amides in the core of the protein. The resulting spectrum contains fewer resonances with peaks for only the amides near the surface of the protein, which then may be monitored upon the addition of small molecule ligands to examine interactions between ligands and surface residues.102
Isotopic Labeling Strategies
Many newly emerging isotope-labeling techniques address the limitations of examining large protein targets by solution NMR. Specifically, the overlap of NMR signals from large protein targets precludes full interpretation of the spectral data, and selective isotopic labeling strategies can permit observation of only specific moieties, reducing the number of signals and decreasing spectral crowding.103 For example, the use of deuteration at non-exchangeable sites (aliphatic sites) in combination with uniformly 13C- and 15N-labeling dramatically improves the quality of NMR spectra of larger proteins. Expressing the protein in partially or completely deuterated growth media followed by purification in protonated buffers causes most of the deuterated amides at the surface of the protein to be replaced by protons, permitting the 1H-15N HSQC to be utilized for screening.22 Replacing protons in the core of a large protein with deuterons not only improves spectral overlap by decreasing the number of observable signals but also results in a narrowing of the remaining proton signals,22 increasing the overall quality of the spectra obtained. This effect occurs because deuteration results in a considerable reduction in the proton spin density, which decreases proton spin-spin interactions and, therefore, the overall relaxation rate of the protein target.104 Additionally, the inclusion of specifically protonated amino acids or amino acid precursors in D2O growth media permits selective retention of protons at aliphatic resiudes (Ala, Val, Leu, Ile) or aromatic side chains (Phe, Tyr), which yields important structural information about protein target hydrophobic cores.105–107 Phe and Tyr side chains are often located at binding interfaces as well,103 and as such, protonation at these specific residues may be used to detect direct binding of a ligand.
Selective 13C-labeling of Val, Leu and Ile methyl groups and subsequent 1H-13C HSQC chemical shift perturbation assays results in a three-fold increase in sensitivity compared to the 1H-15N experiment.36 This labeling strategy, of course, improves the molecular weight limit of the protein target and has been used to study proteins with as many as 723 amino acids.108 More importantly, 13C-methyl labeling greatly simplifies the 1H-13C HSQC spectrum of the protein target and makes it amenable to implementation in high-throughput protocols. Additionally, the increase in signal-to-noise of the 1H-13C cross peaks shortens the required acquisition time and permits a lower concentration of protein to be used, facilitating the screening of many ligand mixtures in one day.7 13C-methyl labeling utilizes 13C-labeled amino acid precursors instead of 13C-labeled glucose, making the cost of protein preparation comparable to uniform 15N-labeling.36
With prior knowledge of the structure or function of a protein, a variety of 15N- and 13C-labeling techniques can be used to simplify the spectrum observed upon addition of ligand to provide information about a specific region of interest in the protein target. Selective enrichment, applicable to many naturally occurring amino acids, is accomplished using supplemented media with one or more specific 15N-amino acids.109 For example, the specific 15N labeling of active site residues simplifies the 1H-15N HSQC perturbation assay. With knowledge of the protein target’s function unique amino acid sequences can be selectively labeled and used to detect binding using a simple approach. 13C and 15N labeling of two consecutive amino acids respectively (13C-X, 15N-Y) in the active site of the protein target will yield only one signal or a limited number of signals in a 13C-15N-1H three-dimensional correlation experiment (HNCO),38 provided that only one or a limited number of the chosen amino acid pairs occur in the protein sequence. This particular labeling strategy facilitates the identification of ligand binding to a selective site without the need for sequence specific assignments because only adjoined 13C-15N labeled nuclei are detected with the HNCO experiment (see Figure 3).110 In another application, prior knowledge of the protein’s structure or function can guide segmental 13C and 15N-labeling of multidomain protein targets via trans-splicing111,112 or chemical ligation techniques.113 This reduces the number of observed signals in the correlation experiments, simplifies the analysis of chemical shifts upon titration of ligand and permits the evaluation of multidomain proteins in their native form. Selective 15N-labeling of specific amino acids is an additional approach that facilitates evaluation of larger molecular weight proteins as well as multidomain proteins. This type of labeling scheme also permits structural investigations of proteins that undergo allosteric regulation. For example, induced conformational changes of the acetylcholine binding protein (AChBP) pentamer were studied by selectively labeling Cys residues in the protein with 15N. Conformational changes in the Cys-loop distant from the binding site were observed upon the addition of ACh, which confirmed the important regulatory role of the Cys-loop to AChBP function.114
Computational Methodologies
When full structure determination by NMR is not practical, computational strategies can be used to generate structural information about the protein-ligand complex to guide drug development using a limited amount of NMR data. NMR-DOC115 (Nuclear Magnetic Resonance DOcking of Compounds) can be used to develop models of the ligand bound to the protein target once the residues involved in binding have been identified. To determine important residues involved in binding, selective isotopic labeling is performed to observe a small number of key residues in the binding site, eliminating the need for complete sequential assignments during the screening process. Using a previously determined x-ray or solution structure of the protein as a starting point and experimentally measured intermolecular NOEs that arise between the ligand and the selectively labeled protein, models are developed that dock the ligand in the binding site. Selective labeling simplifies interpretation of the NOE spectra because fewer signals are present. This method has great utility because it can be used to examine higher molecular weight proteins with only a few NMR experiments and limited data analysis.
NMR-SOLVE115 (Structurally Oriented Library Valency Engineering) is a very useful alternative to the SAR by NMR method of drug screening because it is applied to different protein targets that belong to a class of proteins related by binding site properties (pharmacofamily), as opposed to one protein target at a time. Proteins are classified into families based on common structural and functional features. Those from the same family typically share similar attributes, which may be exploited to narrow the search space to more rapidly identify high-affinity lead compounds. With NMR-SOLVE, the development of high-affinity ligands is tailored to the family of protein targets related by the presence of a common binding site adjacent to a variable binding site.116 A known binder (often a cofactor) and its interactions with the common binding site are first studied by protein-detected NMR to identify important residues involved in binding. Common ligand mimics that bind with low affinity to a model member of the protein-family are then investigated by the same means. Intermolecular NOEs that develop between the ligand and the common and variable binding sites are used to develop NMR-DOC models based on the previously determined structure of the reference family member. The model of the low affinity ligand bound to the common binding site is then used to design a linker from which various molecular fragments can be added. Because the protein family selected has the same geometric relationship between the common and variable sites, the addition of various molecular fragments to the linker yields a compound library tailored to a given protein family without specific knowledge of their three-dimensional structure.115 Once the compound library has been constructed, screening of additional family members may be performed using other methods with a higher probability of finding a high-affinity (nanomolar) hit. This approach may prove to be an important drug design strategy in the post-genomics era.9
The protein-ligand NOE matching117 method was developed in order to bypass the requirement of backbone resonance assignments of a protein’s binding site in order to determine the location, orientation, and internal conformation (binding pose) of a ligand bound to the protein. The binding pose of the complex can be determined at high-resolution using isotope-filtered NMR experiments118 but may be difficult to accomplish in a reasonable time frame, as the time-consuming task of assigning binding site residues for each protein-ligand complex studied does not support repetitive cycles of structure-based ligand design. In this method, trial binding poses are scored based on matching an experimental pattern of intermolecular protein-ligand NOEs to predicted NOE patterns based on theoretical calculations. Trial protein-ligand binding poses are generated by any suitable computational docking method and are based on a previously determined structure (either X-ray or NMR) of a protein target of interest. For each binding pose, the intermolecular NOE pattern is predicted and matched to the experimental patterns. The scoring is based on a fast, deterministic algorithm, making it suitable for scoring a large number of trials in a short amount of time. Only the proton assignments for the bound ligand are required, making this a useful alternative to traditional methods when a structure of the unbound protein is available.117
Competition Experiments
While it is possible to examine a wide range of binding affinities by NMR, ligand-detected NMR screening has been expanded further to detect strongly binding ligands with slow dissociation rates by the use of competition assays. The inability to observe tightly bound ligands directly results from the need to distinguish the ligand and target signals by exploiting size-dependent effects. Ligand-based screening methods detect only the free ligand signals and require rapid exchange between free and bound forms. To detect high-affinity ligands, competition-based experiments have been developed that rely on 1H or 19F relaxation and the STD NMR or waterLOGSY experiments because these methods are amenable to high-throughput protocols in which mixtures of compounds can be screened simultaneously.58,119–120,121
Competition-based experiments utilize a reporter ligand that is in rapid exchange between free and bound forms with the protein target of interest.21 Tight binding by another compound results in displacement of the reporter.4 Displacement is manifested as a shift of the reporter compound’s NMR parameters toward those of the free state.21 Fluorine moieties on the reporter molecule are especially useful because they facilitate the rapid high-throughput screening of large chemical mixtures against the protein target of interest because of the absence of overlap with signals from the mixtures to be screened.58 Competition experiments permit the detection of high-affinity molecules, significantly expanding the KD range amenable to ligand-based NMR screening to include high-affinity ligands.
Conclusions
High-field NMR is a powerful tool for the investigation of transiently forming complexes, and has become increasingly useful as the methodologies available to study these complexes expand. Methodologies are available to assess protein-ligand interactions from either the perspective of the protein target or the ligand molecule. They take advantage of the differences in the fundamental properties of small and large molecules as well as the presence of unique connectivities or atoms within the molecules to selectively detect binding. Advances in NMR technology and experimentation have greatly increased the size of molecules that can be analyzed, as well as increasing the throughput, sensitivity and resolution of the technique. As a result, high-field solution NMR plays a significant role in the identification of binding partners and the physicochemical characterization of protein-ligand interactions and has been applied successfully to advance pharmaceutical research. NMR’s unique ability to examine weak interactions and to structurally characterize binding events has substantially improved rational drug design and development and the analysis of potential therapeutics to protein targets. Given the versatility of high-field NMR and the continual innovation of the drug evaluation process, NMR is likely to find further applications in various aspects of drug development programs.
Acknowledgements
The authors would like to thank Dr. Russ Middaugh, Loren Schieber and Dr. Klaas Hallenga for their discussion of the manuscript. This publication was made possible by NIH Grant Number P20 RR-17708 from the National Center for Research Resources and the Kansas University Center for Research. This work additionally was supported by fellowships for Andria Skinner from Amgen and the Edith and Eleta Ernst Cancer Research Fellowship.
References
- 1.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1(9):727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 2.Mulder FA, Hon B, Muhandiram DR, Dahlquist FW, Kay LE. Flexibitility and ligand exchange in a buried cavity mutant of T4 lysozyme studied by multinuclear NMR. Biochem. 2000;39:12614–12622. doi: 10.1021/bi001351t. [DOI] [PubMed] [Google Scholar]
- 3.Wyss DF, McCoy MA, Senior MM. NMR-Based Approaches for Lead Discovery. Curr Opin Drug Discov Devel. 2002;5:630–647. [PubMed] [Google Scholar]
- 4.Coles M, Heller M, Kessler H. NMR-based Screening Technologies. Drug Discov Today. 2003;8:803–810. doi: 10.1016/s1359-6446(03)02796-x. [DOI] [PubMed] [Google Scholar]
- 5.Jahnke W. Spin labels as a tool to identify and characterize protein-ligand interactions by NMR spectroscopy. Chembiochem. 2002;3(2–3):167–173. doi: 10.1002/1439-7633(20020301)3:2/3<167::AID-CBIC167>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 6.Diercks T, Coles M, Kessler H. Applications of NMR in drug discovery. Curr Opin Chem Biol. 2001;5(3):285–291. doi: 10.1016/s1367-5931(00)00204-0. [DOI] [PubMed] [Google Scholar]
- 7.Salvatella X, Giralt E. NMR-based methods and strategies for drug discovery. Chem Soc Rev. 2003;32(6):365–372. doi: 10.1039/b210047a. [DOI] [PubMed] [Google Scholar]
- 8.Stockman BJ. NMR Spectroscopy as a tool for structure-based drug design. Prog Nucl Magn Reson Spectrosc. 1998;33:109–151. [Google Scholar]
- 9.Pellecchia M, Sem DS, Wuthrich K. NMR in drug discovery. Nat Rev Drug Discov. 2002;1(3):211–219. doi: 10.1038/nrd748. [DOI] [PubMed] [Google Scholar]
- 10.Sanders JKM, Hunter BK. Modern NMR Spectroscopy: A Guide for Chemists. 1st ed. Oxford: Oxford University Press; 1987. p. 308. [Google Scholar]
- 11.Primrose WU. Sample Preparaion. In: Roberts GCK, editor. NMR of Macromolecules. New York: Oxford University Press; 1993. pp. 7–34. [Google Scholar]
- 12.Evans JNS. Biomolecular NMR Spectroscopy. 1st ed. Oxford: Oxford University Press; 1995. p. 444. [Google Scholar]
- 13.Wider G. Structure determination of biological macromolecules in solution using NMR spectroscopy. BioTechniques. 2000;29:1278–1294. doi: 10.2144/00296ra01. [DOI] [PubMed] [Google Scholar]
- 14.Levy GC, Craik DJ. Recent Developments in Nuclear Magnetic Resonance Spectroscopy. Science. 1981;214(4518):291–299. doi: 10.1126/science.6169151. [DOI] [PubMed] [Google Scholar]
- 15.Roberts GC. Applications of NMR in drug discovery. Drug Discov Today. 2000;5(6):230–240. doi: 10.1016/s1359-6446(00)01479-3. [DOI] [PubMed] [Google Scholar]
- 16.Wishart D. NMR spectroscopy and protein structure determination: applications to drug discovery and development. Curr Pharm Biotechnol. 2005;6(2):105–120. doi: 10.2174/1389201053642367. [DOI] [PubMed] [Google Scholar]
- 17.Fielding L. Determination of association constants (Ka) from solution NMR data. Tetrahedron. 2000;56:6151–6170. [Google Scholar]
- 18.Fielding L. NMR methods for the determination of protein-ligand dissociation constants. Curr Top Med Chem. 2003;3:39–53. doi: 10.2174/1568026033392705. [DOI] [PubMed] [Google Scholar]
- 19.Fielding L. NMR methods for the determination of protein-ligand dissociation constants. Prog Nucl Magn Reson Spectrosc. 2007;51(4):219–242. [Google Scholar]
- 20.Medek A, Hajduk P, Mack J, Fesik SW. The use of differential chemical shifts for determination of the binding site location and orientation of the protein-bound ligands. J Am Chem Soc. 2000;122:1241–1242. [Google Scholar]
- 21.Lepre CA, Moore JM, Peng JW. Theory and applications of NMR-based screening in pharmaceutical research. Chem Rev. 2004;104(8):3641–3676. doi: 10.1021/cr030409h. [DOI] [PubMed] [Google Scholar]
- 22.Pochapsky SS, Pochapsky TC. Nuclear magnetic resonance as a tool in drug discovery, metabolism and disposition. Curr Top Med Chem. 2001;1(5):427–441. doi: 10.2174/1568026013394967. [DOI] [PubMed] [Google Scholar]
- 23.Carlomagno T. Ligand-target interactions: what can we learn from NMR? Annu Rev Biophys Biomol Struct. 2005;34:245–266. doi: 10.1146/annurev.biophys.34.040204.144419. [DOI] [PubMed] [Google Scholar]
- 24.Reibarkh M, Malia TJ, Wagner G. NMR distinction of single- and multiple-mode binding of small-molecule protein ligands. J Am Chem Soc. 2006;128:2160–2161. doi: 10.1021/ja055971z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shuker SB, Hajduk PJ, Meadows RP, Fesik SW. Discovering high-affinity ligands for proteins: SAR by NMR. Science. 1996;274(5292):1531–1534. doi: 10.1126/science.274.5292.1531. [DOI] [PubMed] [Google Scholar]
- 26.Zartler ER, Hanson J, Jones BE, Kline AD, Martin G, Mo H, Shapiro MJ, Wang R, Wu H, Yan J. RAMPED-UP NMR: multiplexed NMR-based screening for drug discovery. J Am Chem Soc. 2003;125(36):10941–10946. doi: 10.1021/ja0348593. [DOI] [PubMed] [Google Scholar]
- 27.Kallen J, Spitzfaden C, Zurini MGM, Wider G, Widmer H, Wuthrich K, Walkinshaw M. Structure of human cyclophilin and its binding site for cyclosporin A determined by X-ray crysallography and NMR spectroscopy. Nature. 1991;353:276–279. doi: 10.1038/353276a0. [DOI] [PubMed] [Google Scholar]
- 28.Clubb RT, Omichinski JG, Clore GM, Gronenborn AM. Mapping the binding surface of interleukin-8 complexed with an N-terminal fragment of type 1 human interleukin-8 receptor. FEBS Lett. 1994;338:93–97. doi: 10.1016/0014-5793(94)80123-1. [DOI] [PubMed] [Google Scholar]
- 29.Lian L-Y, Barsukov IL, Derrick JP, Robers GCK. Mapping the interactions between streptococcal protein G and the Fab fragment of IgG in solution. Nat Struct Biol. 1994;1(6):355–357. doi: 10.1038/nsb0694-355. [DOI] [PubMed] [Google Scholar]
- 30.Farmer BT, Constantine KL, Goldfarb V, Friedrichs MS, Wittekind M, Yanchunas JJ, Robertson JG, Mueller L. Localizing the NADP+ binding site on the MurB enzyme by NMR. Nat Struct Biol. 1996;3(12):995–997. doi: 10.1038/nsb1296-995. [DOI] [PubMed] [Google Scholar]
- 31.Hajduk PJ, Boyd S, Netesheim D, Nienaber V, Severin J, Smith R, Davidson D, Rockway T, Fesik SW. Identification of novel inhibitors of urokinase via NMR-based screening. J Med Chem. 2000;43:3862–3866. doi: 10.1021/jm0002228. [DOI] [PubMed] [Google Scholar]
- 32.Hajduk P, Sheppard G, Nettesheim DG, Olejniczak ET, Shuker SB, Meadows RP, Steinman DH, Carrera GMJ, Marcotte PA, Severin J, Walter K, Smith H, Gubbins E, Simmer R, Holzman TF, Morgan DW, Davidsen SK, Summers JB, Fesik SW. Discovery of potent nonpeptide inhibitors of stromelysin using SAR by NMR. J Am Chem Soc. 1997;119:5818–5827. [Google Scholar]
- 33.Hajduk PJ, Dinges J, Miknis GF, Merlock M, Middleton T, Kempf DJ, Egan DA, Walter KA, Robins TS, Shuker SB, Holzman TF, Fesik SW. NMR-based discovery of lead inhibitors that block DNA binding of human papillomavirus E2 protein. J Med Chem. 1997;40(20):3144–3150. doi: 10.1021/jm9703404. [DOI] [PubMed] [Google Scholar]
- 34.Hajduk PJ, Dinges J, Schkeryantz JM, Janowick D, Kaminiski M, Tufano M, Augeri DJ, Petros A, Nienaber V, Zhong P, et al. Novel inhibitors of Erm methyltransferases from NMR and parallel synthesis. J Med Chem. 1999;42:3852–3859. doi: 10.1021/jm990293a. [DOI] [PubMed] [Google Scholar]
- 35.Lugovskoy AA, Degterev AI, Fahmy AF, Zhou P, Gross JD, Yuan J, Wagner G. A novel approach for characterizing protein ligand complexes: molecular basis for specificity of small-molecule Bcl-2 inhibitors. J Am Chem Soc. 2002;124(7):1234–1240. doi: 10.1021/ja011239y. [DOI] [PubMed] [Google Scholar]
- 36.Hajduk PJ, Augeri DJ, Mack J, Mendoza R, Yang J, Betz SF, Fesik SW. NMR-based screening of proteins containing 13C-labeled methyl groups. J Am Chem Soc. 2000;122:7898–7904. [Google Scholar]
- 37.Reid DJ. Protein NMR Techniques. 1st ed. Humana Press; 1997. p. 419. [Google Scholar]
- 38.Sattler M, Schleucher J, Griesinger C. Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog Nucl Magn Reson Spectrosc. 1999;34:93–158. [Google Scholar]
- 39.Claridge TDW. High Resolution NMR Techniques in Organic Chemistry. 1st ed. Kidlington: Elsevier Science; 1999. p. 382. [Google Scholar]
- 40.Jahnke W, Widmer H. Protein NMR in Biomedical Research. Cell Mol Life Sci. 2004;61:580–599. doi: 10.1007/s00018-003-3382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mercier KA, Powers R. Determining the optimal size of small molecule mixtures for high throughput NMR screening. J Biomol NMR. 2005;31:243–258. doi: 10.1007/s10858-005-0948-4. [DOI] [PubMed] [Google Scholar]
- 42.Ross A, Schlotterbeck G, Klaus W, Senn H. Automation of NMR measurements and data evaluation for sytemically screening interactions of small molecules with target proteins. J Biomol NMR. 2000;16:139–146. doi: 10.1023/a:1008394910612. [DOI] [PubMed] [Google Scholar]
- 43.Ross A, Senn H. Automation of Measurements and Data Evaluation in Biomolecular NMR Screening. Drug Discov Today. 2001;6:583–593. doi: 10.1016/s1359-6446(01)01780-9. [DOI] [PubMed] [Google Scholar]
- 44.Frieden C, Hoeltzli SD, Bann JG. The preparation of 19F-labeled proteins for NMR studies. Methods Enzymol. 2004;380:400–415. doi: 10.1016/S0076-6879(04)80018-1. [DOI] [PubMed] [Google Scholar]
- 45.Yu L, Hajduk PJ, Mack J, Olejniczak E. Structural Studies of Bcl-xL/ligand Complexes using 19F NMR. J Biomol NMR. 2006;34(4):221–227. doi: 10.1007/s10858-006-0005-y. [DOI] [PubMed] [Google Scholar]
- 46.Danielson MA, Falke JJ. Use of 19F NMR to probe protein structure and conformational changes. Annu Rev Biophys Biomol Struct. 1996;25:163–195. doi: 10.1146/annurev.bb.25.060196.001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerig JT. 19F NMR of proteins. Prog Nucl Magn Reson Spectrosc. 1994;26:293. [Google Scholar]
- 48.Leone M, Rodriguez-Mias RA, Pellecchia M. Selective incorporation of 19F-labeled Trp side chains for NMR-spectroscopy-based ligand-protein interaction studies. Chembiochem. 2003;4:649–650. doi: 10.1002/cbic.200300597. [DOI] [PubMed] [Google Scholar]
- 49.Huang Y, Rich RL, Myszka DG, Wu H. Requirement of both the second and third BIR domains for the relief of X-linked inhibitor of apoptosis protein (XIAP)-mediated caspase inhibition by Smac. J Biol Chem. 2003;278(49):49517–49522. doi: 10.1074/jbc.M310061200. [DOI] [PubMed] [Google Scholar]
- 50.Van Dongen M, Weigelt J, Uppenberg J, Schultz J, Wikstrom M. Structure-based screening and design in drug discovery. Drug Discov Today. 2002;7:471–478. doi: 10.1016/s1359-6446(02)02233-x. [DOI] [PubMed] [Google Scholar]
- 51.Cavanagh J, Fairbrother WJ, Palmer AG, III, Rance M, Skelton N. 2nd ed. Boston: Academic Press; 2007. Protein NMR Spectroscopy: Principles and Practice. [Google Scholar]
- 52.Stockman BJ, Dalvit C. NMR Screening Techniques in Drug Discovery and Drug Design. Prog Nucl Magn Reson Spectrosc. 2002;41:187–231. [Google Scholar]
- 53.Fejzo J, Lepre CA, Peng JW, Bemis GW, Ajay, Murcko MA, Moore JM. The SHAPES strategy: an NMR-based approach for lead generation in drug discovery. Chem Biol. 1999;6(10):755–769. doi: 10.1016/s1074-5521(00)80022-8. [DOI] [PubMed] [Google Scholar]
- 54.Peng JW, Lepre CA, Fejzo J, Abdul-Manan N, Moore JM. Nuclear magnetic resonance-based approaches for lead generation in drug discovery. Methods Enzymol. 2001;338:202–230. doi: 10.1016/s0076-6879(02)38221-1. [DOI] [PubMed] [Google Scholar]
- 55.Fejzo J, Lepre CA, Peng JW, Su MS-S, Thomson JA, Moore JM. Dynamic NMR studies of ligand-receptor interactions: Design and analysis of a rapidly exchanging complex of FKBP-12/FK506 with a 24 kDa clacineurin fragment. Protein Science. 1996;5:1917–1921. doi: 10.1002/pro.5560050918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng JW. Cross-correlated 19F relaxation measurements for the study of fluorinated ligand-receptor interactions. J Magn Reson. 2001;153:32–47. doi: 10.1006/jmre.2001.2422. [DOI] [PubMed] [Google Scholar]
- 57.Huth JR, Sun C, Sauer DR, Hajduk PJ. Utilization of NMR-derived fragment leads in drug design. Methods Enzymol. 2005;394:549–571. doi: 10.1016/S0076-6879(05)94023-8. [DOI] [PubMed] [Google Scholar]
- 58.Dalvit C, Flocco M, Veronesi M, Stockman BJ. Fluorine-NMR competition binding experiments for high-throughput screening of large compound mixtures. Comb Chem High Throughput Screen. 2002;5(8):605–611. doi: 10.2174/1386207023329923. [DOI] [PubMed] [Google Scholar]
- 59.Shimizu T, Hatano M. Interaction of trifluoperazine with porcine calmodulin. 19F NMR and induced CD spectral studies. FEBS Lett. 1983;160(1–2):182–186. doi: 10.1016/0014-5793(83)80962-4. [DOI] [PubMed] [Google Scholar]
- 60.Shimizu T, Hatano M, Muto Y, Nozawa Y. Interaction of trifluoperazine with Tetrahymena calmodulin. A 19F NMR study. FEBS Lett. 1984;166(2):373–377. doi: 10.1016/0014-5793(84)80115-5. [DOI] [PubMed] [Google Scholar]
- 61.Jenkins BG, Lauffer RB. Detection of site-specific binding and co-binding of ligands to human serum albumin using 19F NMR. Mol Pharmacol. 1990;37(111–118) [PubMed] [Google Scholar]
- 62.Kitamura K, Omran AA, Takegami S, Tanaka R, Kitade T. 19F NMR spectroscopic characterization of the interaction of niflumic acid with human serum albumin. Anal Bioanal Chem. 2007;387(8):2843–2848. doi: 10.1007/s00216-007-1162-x. [DOI] [PubMed] [Google Scholar]
- 63.Crull GB, Nardo JV, Dawson JH. Direct observation of substrate binding to ferrous-CO cytochrome P-450-CAM using 19F NMR. FEBS Lett. 1989;254(1–2):39–42. [Google Scholar]
- 64.Crull GB, Kennington JW, Garber AR, Ellis PD, Dawson JH. 19F Nuclear magnetic resonance as a probe of the spatial relationship between the heme iron of cytochrome P-450 and its substrate. J Biol Chem. 1989;264(5):2649–2655. [PubMed] [Google Scholar]
- 65.Dalvit C, Ardini E, Flocco M, Fogliatto GP, Mongelli N, Veronesi M. A general NMR method for rapid, efficient, and reliable biochemical screening. J Am Chem Soc. 2003;125:14620–14625. doi: 10.1021/ja038128e. [DOI] [PubMed] [Google Scholar]
- 66.Dalvit C, Ardini E, Fogliatto GP, Mongelli N, Veronesi M. Reliable high-throughput functional screening with 3-FABS. Drug Discov Today. 2004;9(14):595–602. doi: 10.1016/S1359-6446(04)03161-7. [DOI] [PubMed] [Google Scholar]
- 67.Fernandez C, Jahnke W. New approaches for NMR screening in drug discovery. Drug Discov Today: Technol. 2004;1(3):277–283. doi: 10.1016/j.ddtec.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Dalvit C. Ligand- and substrate-based 19F NMR screening: principles and applications to drug discovery. Prog Nucl Magn Reson Spectrosc. 2007;51(4):243–271. [Google Scholar]
- 69.Spoerner M, Graf T, Burkhard K, Kalbitzer HR. A novel mechanism for the modulation of the Ras-effector interaction by small molecules. Biochem Biophys Res Commun. 2005;334:709–713. doi: 10.1016/j.bbrc.2005.06.144. [DOI] [PubMed] [Google Scholar]
- 70.Lin MF, Shapiro MJ. Mixture Analysis in combinatorial chemistry. Application of diffusion-resolved NMR spectroscopy. J Org Chem. 1996;61:7617–7619. doi: 10.1021/jo961315t. [DOI] [PubMed] [Google Scholar]
- 71.Lin M, Shapiro MJ, Wareing JR. Diffusion-edited NMR affinity for direct observation of molecular interactions. J Am Chem Soc. 1997;119:5249–5250. [Google Scholar]
- 72.Lin MG, Shapiro MJ. Mixture analysis by NMR spectroscopy. Anal Chem. 1997;69:4731–4733. [Google Scholar]
- 73.Altieri AS, Hinton DP, Byrd RA. Association of biomolecular systems via pulsed field gradient NMR self-diffusion measurements. J Am Chem Soc. 1995;117:7566–7567. [Google Scholar]
- 74.Hajduk PJ, Olejniczak ET, Fesik SW. One-dimensional relaxation and diffusion-edited NMR methods for screening compounds that bind to macromolecules. J Am Chem Soc. 1997;119:12257–12261. [Google Scholar]
- 75.Morris KF, Johnson CS., Jr Diffusion-ordered two-dimensional nuclear magnetic resonance spectroscopy. J Am Chem Soc. 1992;114(8):3139–3141. [Google Scholar]
- 76.Morris KF, Johnson CS., Jr Resolution of discrete and continuous molecular size distributions by means of diffusion-ordered 2D NMR spectroscopy. J Am Chem Soc. 1993;115(10):4291–4299. [Google Scholar]
- 77.Bleicher K, Lin MF, Shapiro MJ, Wareing JR. Diffusion edited NMR: screening compound mixtures by affinity NMR to detect binding ligands to vancomycin. J Org Chem. 1998;63:8486–8490. [Google Scholar]
- 78.Zartler ER, Yan J, Mo H, Kline AD, Shapiro MJ. 1D NMR Methods in Ligand-Receptor Interactions. Curr Top Med Chem. 2003;3:25–37. doi: 10.2174/1568026033392750. [DOI] [PubMed] [Google Scholar]
- 79.Post CB. Exchange-transferred NOE spectroscopy and bound ligand structure determination. Curr Opin Struct Biol. 2003;13(5):581–588. doi: 10.1016/j.sbi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 80.Leone M, Freeze HH, Chan CS, Pellecchia M. The nuclear overhauser effect in the lead identification process. Curr Drug Discov Technol. 2006;3:91–100. doi: 10.2174/157016306778108884. [DOI] [PubMed] [Google Scholar]
- 81.Moller H, Serttas N, Paulsen H, Burcell JM, Taylor-Papadimitriou J, Meyer B. NMR-based determination of the binding epitope and conformational analysis of MUC-1 glycopeptides and peptides bound to the breast cancer-selective monoclonal antibody SM3. Eur J Biochem. 2002;269(5):1444–1455. doi: 10.1046/j.1432-1033.2002.02787.x. [DOI] [PubMed] [Google Scholar]
- 82.Sanchez-Pedregal VM, Reese M, Meiler J, Blommers MJJ, Griesinger C, Carlomagno T. The INPHARMA method: protein-mediated interligand NOEs for pharmacophore mapping. An. 2005 doi: 10.1002/anie.200500503. [DOI] [PubMed] [Google Scholar]
- 83.Becattini B, Pellecchia M. SAR by ILOEs: an NMR-based approach to reverse chemical genetics. Chemistry. 2006;12(10):2658–2662. doi: 10.1002/chem.200500636. [DOI] [PubMed] [Google Scholar]
- 84.London RE, Perlman ME, Davis DG. Relaxation-matrix analysis of the transferred nuclear Overhauser effect for finite exchange rates. J Magn Reson. 1992;97:79–98. [Google Scholar]
- 85.Hajduk P, Meadows RP, Fesik SW. NMR-based Screening in Drug Discovery. Q Rev Biophys. 1999;32:211–240. doi: 10.1017/s0033583500003528. [DOI] [PubMed] [Google Scholar]
- 86.Mayer M, Meyer B. Group epitope mapping by saturation transfer difference NMR to identify segments of a ligand in direct contact with a protein receptor. J Am Chem Soc. 2001;123:6108–6117. doi: 10.1021/ja0100120. [DOI] [PubMed] [Google Scholar]
- 87.Mayer M, Meyer B. Characterization of ligand binding by saturation transfer difference NMR spectroscopy. Angew Chem Int Ed Engl. 1999;38(12):1784–1788. doi: 10.1002/(SICI)1521-3773(19990614)38:12<1784::AID-ANIE1784>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 88.Meinecke R, Meyer B. Determination of the binding specificity of an integral membrane protein by saturation transfer difference NMR: RGD peptide ligands binding to integrin alphaIIbBeta3. J Med Chem. 2001;44:3059–3065. doi: 10.1021/jm0109154. [DOI] [PubMed] [Google Scholar]
- 89.Hajduk PJ, Mack JC, Olejniczak ET, Park C, Dandliker PJ, Beutel BA. SOS-NMR: a saturation transfer NMR-based method for determining the structures of protein-ligand complexes. J Am Chem Soc. 2004;126(8):2390–2398. doi: 10.1021/ja039480v. [DOI] [PubMed] [Google Scholar]
- 90.Dalvit C, Pevarello P, Tato M, Veronesis M, Vulpetti A, Sundstrom M. Identification of compounds with binding affinity to proteins via magnetization transfer from bulk water. J Biomol NMR. 2000;18(1):65–68. doi: 10.1023/a:1008354229396. [DOI] [PubMed] [Google Scholar]
- 91.Dalvit C, Fogliatto G, Stewart A, Veronesi M, Stockman BJ. WaterLOGSY as a method for primary NMR screening: practical aspects and range of applicability. J Biomol NMR. 2001;21(4):349–359. doi: 10.1023/a:1013302231549. [DOI] [PubMed] [Google Scholar]
- 92.Keifer PA. NMR spectroscopy in drug discovery: tools for combinatorial chemistry, natural products, and metabolism research. Prog Drug Res. 2000;55:137–211. doi: 10.1007/978-3-0348-8385-6_5. [DOI] [PubMed] [Google Scholar]
- 93.Hallenga K, Tonelli M, Westler WM, Markley JL, Takeda M, Shigezane M, Kainosho M. Experimental Nuclear Magnetic Resonance Conference, ed. Pacific Grove, California: 2006. Cryogenic probe sensitivity as a function of tube geometry, sample conductivity and dielectric permittivity of the solven. [Google Scholar]
- 94.de Swiet TM. Optimal electric fields for different sample shapes in high resolution NMR spectroscopy. J Magn Reson. 2005;174:331–334. doi: 10.1016/j.jmr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 95.Flynn PF, Mattiello DL, Hill HDW, Wand AJ. Optimal use of cryogenic probe technology in NMR studies of proteins. J Am Chem Soc. 2000;122:4823–4824. [Google Scholar]
- 96.Voehler MW, Collier G, Young JK, Stone MP, Germann MW. Performance of cryogenic probes as function of ionic strength and sample tube geometry. J Magn Reson. 2006;183:102–109. doi: 10.1016/j.jmr.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hajduk PJ, Gerfin T, Boehlen JM, Haberli M, Marek D, Fesik SW. High-throughput nuclear magnetic resonance-based screening. J Med Chem. 1999;42(13):2315–2317. doi: 10.1021/jm9901475. [DOI] [PubMed] [Google Scholar]
- 98.Pervushin K, Riek R, Wider G, Wuthrich K. Attenuated T-2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci USA. 1997;94:12366–12371. doi: 10.1073/pnas.94.23.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salzmann M, Pervushin K, Wider G, Senn H, Wuthrich K. NMR assignments and secondary structure determination of an octameric 110 kDa protein using TROSY in triple resonance experiments. J Am Chem Soc. 2000;122:7543–7548. [Google Scholar]
- 100.Riek R, Wider G, Pervushin K, Wuthrich K. Polarization transfer by cross-correlated relaxation in solution NMR with very large molecules. Proc Natl Acad Sci USA. 1999;96(9):4918–4923. doi: 10.1073/pnas.96.9.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fiaux J, Bertelsen EB, Horwich AL, Wuthrich K. NMR analysis of a 900K GroEL-GroES complex. Nature. 2002;418:207–211. doi: 10.1038/nature00860. [DOI] [PubMed] [Google Scholar]
- 102.Pellecchia M, Meininger D, Shen AL, Jack R, Kasper CB, Sem DS. SEA-TROSY (Solvent Exposed Amides with TROSY): A method to resolve the problem of spectral overlap in very large proteins. J Am Chem Soc. 2001;123:4633–4634. doi: 10.1021/ja005850t. [DOI] [PubMed] [Google Scholar]
- 103.Goto NK, Kay LE. New developments in isotope labelling strategies for protein solution NMR spectrscopy. Curr Opin Struct Biol. 2000;10:585–592. doi: 10.1016/s0959-440x(00)00135-4. [DOI] [PubMed] [Google Scholar]
- 104.Sattler M, Fesik SW. Use of deuterium labeling in NMR: overcoming a sizeable problem. Structure. 1996;4:1245–1249. doi: 10.1016/s0969-2126(96)00133-5. [DOI] [PubMed] [Google Scholar]
- 105.Venters RA, Huang CC, Farmer BT, 2nd, Trolard R, Spicer LD, Fierke CA. High-level 2H/13C/15N labeling of proteins for NMR studies. J Biomol NMR. 1995;5(4):339–344. doi: 10.1007/BF00182275. [DOI] [PubMed] [Google Scholar]
- 106.Farmer BT, 2nd, Venters RA. Assignment of aliphatic side-chain 1HN/15N resonances in perdeuterated proteins. J Biomol NMR. 1996;7(1):59–71. doi: 10.1007/BF00190457. [DOI] [PubMed] [Google Scholar]
- 107.Venters RA, Farmer BT, 2nd, Fierke CA, Spicer LD. Characterizing the use of perdeuteration in NMR studies of large proteins: 13C, 15N and 1H assignments of human carbonic anhydrase II. J Mol Biol. 1996;264(5):1101–1116. doi: 10.1006/jmbi.1996.0699. [DOI] [PubMed] [Google Scholar]
- 108.Tugarinov V, Kay LE. Ile, Leu, and Val methyl assignments of the 723 residue malate synthase G using a new labeling strategy and novel NMR methods. J Am Chem Soc. 2003;125(45):12868–12878. doi: 10.1021/ja030345s. [DOI] [PubMed] [Google Scholar]
- 109.Waugh DS. Genetic tools for selective labeling of proteins with alpha-15N-amino acids. J Biomol NMR. 1996;8(2):184–192. doi: 10.1007/BF00211164. [DOI] [PubMed] [Google Scholar]
- 110.Weigelt J, et al. Site-selective screening by NMR spectroscopy with labeled amino acid pairs. J Am Chem Soc. 2002;124:2446–2447. doi: 10.1021/ja0178261. [DOI] [PubMed] [Google Scholar]
- 111.Yamazaki T, Otomo T, Oda N, Kyogoku Y, Uegaki K, Ito N, Ishino Y, Nakamura H. Segmental isotope labeling for protein NMR using peptide splicing. J Am Chem Soc. 1998;120:5591–5592. [Google Scholar]
- 112.Otomo T, Teruya K, Uegaki K, Yamazaki T, Kyogoku Y. Improved segmental isotope labeling of proteins and application to a larger protein. J Biomol NMR. 1999;14:105–114. doi: 10.1023/a:1008308128050. [DOI] [PubMed] [Google Scholar]
- 113.Xu R, Ayers B, Cowburn D, Muir TW. Chemical ligation of folded recombinant proteins: segmental isotope labeling of domains for NMR studies. Proc Natl Acad Sci USA. 1999;96:388–393. doi: 10.1073/pnas.96.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gao F, Mer G, Tonelli M, Hansen SB, Burghardt TP, Taylor P, Sine SM. Solution NMR of acetylcholine binding protein reveals agonish-mediated conformational change of the C-loop. Mol Pharmacol. 2006;70(4):1230–1235. doi: 10.1124/mol.106.027185. [DOI] [PubMed] [Google Scholar]
- 115.Pellecchia M, Meininger D, Dong Q, Chang E, Jack R, Sam DS. NMR-based structural characterization of large protein-ligand interactions. J Biomol NMR. 2002;22(2):165–173. doi: 10.1023/a:1014256707875. [DOI] [PubMed] [Google Scholar]
- 116.Sem DS, Yu L, Coutts SM, Jack R. Object-oriented approach to drug design enabled by NMR SOLVE: first real-time structural tool for characterizing protein-ligand interactions. J Cell Biochem. 2001;84(S37):99–105. doi: 10.1002/jcb.10070. [DOI] [PubMed] [Google Scholar]
- 117.Constantine KL, Davis ME, Metzler WJ, Mueller L, Claus B. Protein-ligand NOE matching: a high throughput method for binding pose evaluation that does not require protein NMR resonance assignments. J Am Chem Soc. 2006;128:7252–7263. doi: 10.1021/ja060356w. [DOI] [PubMed] [Google Scholar]
- 118.Breeze AL. Isotope-filtered NMR methods for the study of biomolecular structure and interactions. Prog Nucl Magn Reson Spectrosc. 2000;36(4):323–372. [Google Scholar]
- 119.Dalvit C, Flocco M, Knapp S, Mostardini M, Perego R, Stockman BJ, Veronesi M, Varasi M. High-throughput NMR-based screening with competition binding experiments. J Am Chem Soc. 2002;124:7702–7709. doi: 10.1021/ja020174b. [DOI] [PubMed] [Google Scholar]
- 120.Wang YS, Liu D, Wyss DF. Competition STD NMR for the detection of high-affinity ligands and NMR-based screening. Magn Reson Chem. 2004;42(6):485–489. doi: 10.1002/mrc.1381. [DOI] [PubMed] [Google Scholar]
- 121.Dalvit C, Fasolini M, Flocco M, Knapp S, Pevarello P, Veronesi M. NMR-Based Screening with Competition Water-Ligand Observed via Gradient Spectroscopy Experiments: Detection of High-Affinity Ligands. Journal of Medicinal Chemistry. 2002;45(12):2610–2614. doi: 10.1021/jm011122k. [DOI] [PubMed] [Google Scholar]