Abstract
To determine to what extent the genetic influences on blood pressure (BP) measured in the office, under psychologically stressful conditions in the laboratory and during real life are different from each other. Office BP, BP during a video game challenge and a social stressor interview, and 24-h ambulatory BP were measured in 238 European American and 186 African American twins. BP values across the two tasks were averaged to represent stress levels. Genetic model fitting showed no ethnic or gender differences for any of the measures. The model fitting resulted in heritability estimates of 63, 75 and 71% for office, stress and 24-h systolic BP (SBP) and 59, 67 and 69% for diastolic BP (DBP), respectively. Up to 81% of the heritability of office SBP and 71% of office DBP were attributed to genes that also influenced stress BP. However, only 45% of the heritability of 24-h SBP and 49% of 24-h DBP were attributed to genes that also influence office BP. Similarly, about 39% of the heritability of 24-h SBP and 42% of 24-h DBP were attributed to genes that also influence stress BP. Substantial overlap exists between genes that influence BP measured in the office, under laboratory stress and during real life. However, significant genetic components specific to each BP measurement also exist. These findings suggest that partly different genes or sets of genes contribute to BP regulation in different conditions.
Keywords: African American, ambulatory blood pressure, heritability, stress, twin
INTRODUCTION
To date, studies reporting on the impact of genetic factors on blood pressure (BP) have almost exclusively been based on conventional office measurements.1 In psychophysiological studies, BP is also measured under standardized laboratory challenges. Prospective studies have shown that individual differences in BP reactivity to such laboratory stressors predict future hypertension.2–4 Furthermore, in the past two decades ambulatory BP (ABP) monitoring has evolved from an innovative tool in fundamental research to a widely used method in fundamental as well as clinical and applied research settings. BP data is acquired in subjects who are free to go about their normal daily activities, outside the confines of the hospital or laboratory environment. In comparison with BP measured in the office or laboratory, real-world recordings are of fundamental importance, for if certain responses do have a role in the etiology of cardiovascular disease, it is in the arena of real-world behavioral challenge and everyday psychosocial interactions that they will take their toll.5 The added value of ABP measurements has indeed been illustrated by studies showing that ABP is a better predictor of target organ damage and cardiovascular morbidity and mortality than BP measured in the clinic.6–8
The underlying physiological mechanisms of BP regulation (and their genetic and environmental influences) may be partly different in these different settings. However, as mentioned above, most studies investigating genetic influences on BP have been based on office measurements.1 In their seminal 1992 review of twin studies that explored the genetic and environmental origins of individual differences in BP reactivity to psychological challenge, Turner and Hewitt suggested that future studies should examine the relationship between BP measured under different settings using multivariate approaches. Twin studies of this kind are crucial to furthering our understanding of the physiological mechanisms underlying BP regulation.5
In this study, which includes a large number of European American (EA) and African American (AA) adolescent, and young adult twin pairs who had BP measured in the office, under two psychologically stressful conditions in the laboratory, and by 24-h ABP monitoring, we, for the first time, examined to what extent the genetic influences on BP assessed under these three conditions are different from each other.
METHODS
Subjects
This study comprised subjects from the Georgia Cardiovascular Twin Study, which was established in 1996.9,10 All twin pairs were reared together and zygosity was determined using five standard microsatellite markers in DNA collected with buccal swabs.11 Subjects were recruited from the southeastern United States and were overtly healthy and free of any acute or chronic illness based on parental report. Study design, selection criteria and the criteria to classify subjects as AA or EA for this twin study have been described previously.9,10,12
For this study, data were available from 238 EA (104 pairs and 30 singletons) and 186 AA (78 pairs and 30 singletons) twins (mean ± s.d. age: 17.1 ± 3.4; range: 11.9–30.0), who had BP measured under these three conditions from 2000 to 2002 during a routine scheduled examination. The Institutional Review Board at the Medical College of Georgia had given approval for this study. Written informed consent was provided by all subjects and by parents if subjects were <18 years.
Office BP recordings
On each laboratory visit, anthropometrics, including height and weight, and resting hemodynamic evaluations were conducted as described elsewhere.13 Body mass index was calculated as a measure of general adiposity. Office systolic BP (SBP) and diastolic BP (DBP) were measured with the Dinamap Vital Signs Monitor (model 1864 SX; Criticon Incorporated, Tampa, FL, USA). BP measurements were taken at the 11, 13 and 15th min during a 15-min supine relaxation period. The average of the last two readings was used to represent office SBP and DBP values.12
ABP recordings
Our procedures for ABP recordings have previously been described in detail.14,15 Briefly, an ABP monitor was fitted to the non-dominant arm (model 90207, SpaceLabs, Redmond, WA, USA). Measures were obtained every 20 min during the daytime (08:00 to 22:00 hours) and every 30 min during the nighttime (00:00 to 06:00 hours). Transitional periods from 06:00 to 08:00 hours and 22:00 hours to midnight were not included in daytime and nighttime period. Adequacy of recordings were based on acceptable readings using previously established criteria14 for ≥14 readings over the 14 h designated as daytime and ≥6 readings over the 6 h designated as the nighttime, as suggested by the European Society of Hypertension Working Group on Blood Pressure Monitoring.16 For the calculation of 24-h mean values (for which transition periods were included), 1-h mean values were first calculated. Subsequently these 1-h mean values were averaged. We also computed 24-h mean values with weights according to the time interval between successive readings. As the correlation between these two calculations is 0.996 for SBP and 0.992 for DBP, and both calculations gave virtually the same results, only the results from the first calculation were reported here.
BP recordings during laboratory challenges
The subject engaged in two 10-min laboratory stressors (the virtual reality car driving and the social competence interview) using standardized protocols. These two stressors have been successfully used in our laboratory studies for over 10 years.17–19
The virtual reality car driving stressor was administered using a protocol developed in our laboratory. Briefly, the subject wore a Kaiser-Optic Visual Immersion Monitor (VIM-500, Kaiser Aerospace and Electronics, Carlsbad, CA, USA) fitted on his/her head. The VIM 500 was interfaced with a Panasonic Real 3DO Interactive Multiplayer System (Model FZ-1, Matsushita Electric of America, Secaucus, NJ, USA). The subject played ‘Need for Speed’ under the condition of challenge (that is, money incentive) without harassment for 10min.
The social competence interview was administered using an established protocol.20 Briefly, subjects discussed a recent interpersonal interaction, which resulted in significant anger and/or frustration. A 10-min structured interview was used to guide the subject in describing the event, including his/her affective and behavioral responses and summarization of outcome of the event.
During each 10-min stressor, BP was recorded once every 2min by the Dinamap Vital Signs Monitor (model 1864 SX). The average of the five BP readings during each stressor was calculated. As aggregation over multiple tasks has been shown to enhance reliability because of its ability to reduce the relative influence of unique situational variance,21,22 the aggregated stress score, that is, the average BP value across the two tasks, was used as the stress level.
Analytical approach
The purposes of our analyses were (1) to test whether the genetic influences on these three BP measurements (24-h ABP, office BP and BP under stress) are different from each other using a multivariate biometric model, and (2) to assess the dependency of these genetic influences on age, ethnicity and gender.
Genetic modeling using twin data
Twin methodology makes use of the fact that monozygotic (MZ) twins share identical genotypes, whereas dizygotic (DZ) twins share on average 50% of their genes. It is assumed that both types of twins share their common family environment to the same extent so that any greater similarity between MZ compared with DZ twins reflects genetic influences. In this study, structural equation modeling was used. Structural equation modeling is based on the comparison of the variance–covariance matrices in MZ and DZ twin pairs, and allows separation of the observed phenotypic variance into its genetic and environmental components: additive (A) genetic, common (C) and unique (E) environmental components. E also contains measurement error. Dividing each of these components by the total variance yields the different standardized components of variance, for example the heritability (h2) can be defined as the proportion of the total variance attributable to additive genetic variation.23
Multivariate biometric models
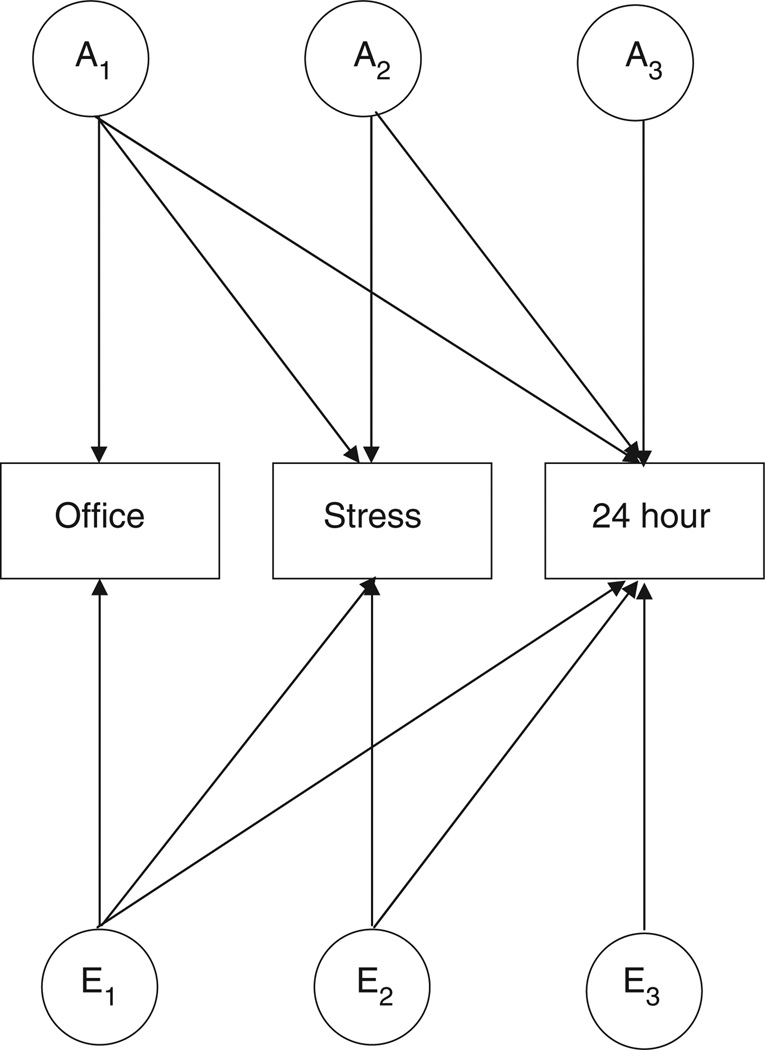
For the purpose of this study, a full trivariate ACE Cholesky decomposition was tested against the nested more parsimonious AE, CE or E models (Figure 1). The Cholesky model represents the most general model, without specific hypotheses regarding the variance–covariance structure being tested. This analysis estimates the proportion of phenotypic variance of individual phenotypes due to genetic and environmental variances, as well as the genetic and environmental covariation between phenotypes. Significance tests of the individual path coefficients (A, C or E) were carried out by constraining paths to zero and applying likelihood ratio tests.23
Figure 1.
Cholesky decomposition of the genetic and environmental factors among three BP measures. An represents the additive genetic effect on the subset of measures, for example, A1 represents the genetic influences on office BP, which are partly shared with stress and 24-h BP. A2 represents the genetic influences on stress BP, which are partly shared with 24-h BP, but unshared with office BP. A3 represents the remaining genetic influences on 24-h BP that are unshared with office and stress BP. Notation for the unique environmental factors (E1–E3) follows analogous reasoning. For clarity only genetic and unique environmental factors are illustrated.
Age differences
Age differences were examined by comparing a full model, in which parameter estimates are allowed to differ in magnitude between twins younger than 18 years old and twins equal or older than 18 years old, with a reduced model, in which parameter estimates are constrained to be equal across the age group. In addition to those models a scalar model was tested. In a scalar model heritabilities are constrained to be equal across age groups, but total variances may be different.23
Gender differences
Gender differences were examined by comparing a full model, in which parameter estimates are allowed to differ in magnitude between males and females, with a reduced model, in which parameter estimates are constrained to be equal across the genders. In addition to those models, a scalar model was tested in a similar manner as carried out for the age group.23
Ethnic differences
Ethnic differences were, similar to gender differences, examined by comparing a full model, in which parameter estimates are allowed to differ in magnitude between AAs and EAs, with a reduced model, in which parameter estimates are constrained to be equal across ethnicity. In addition to those models, a scalar model was tested in a similar manner as carried out for gender.23
Statistical software
The ethnic and gender difference in the general characteristics of the twins was tested by generalized estimating equations. Generalized estimating equation is a multiple regression technique that allows for non-independence of twin or family data yielding unbiased standard errors and P-values. These analyses were done using STATA 8 (StataCorp, College Station, TX, USA). Genetic modeling was carried out with Mx, a computer program specifically designed for the analysis of twin and family data.24 Effects of age (a), sex (s), ethnicity (e) and their interactions (a*s, a*e, e*s, e*a*s) were regressed out for all variables before using the residuals in model fitting. The analyses were based on the raw data instead of variance–covariance matrices. This allows inclusion of singletons who contribute to estimates of means and variances (but not to cross-twin correlations).
RESULTS
Table 1 presents the general characteristics of the twins by ethnicity and gender. Males were taller than females and AAs were more obese (higher body mass index) than EAs. Males had higher SBP levels under different conditions, but lower office DBP than females. At all conditions, AAs had both higher SBP and DBP levels than EAs.
Table 1.
General characteristics of study subjects by ethnicity and gender
| EA |
AA |
P-value |
||||
|---|---|---|---|---|---|---|
| Characteristics | Male | Female | Male | Female | Ethnicity | Gender |
| N | 113 | 122 | 79 | 106 | ||
| Age (years) | 17.1 ± 3.4 | 17.0 ± 3.0 | 16.7 ± 2.8 | 17.6 ± 3.8 | NS | NS |
| BMI (kg m−2)a | 23.2 ± 5.9 | 22.3 ± 4.6 | 23.1 ± 4.7 | 24.5 ± 5.6 | 0.028 | NS |
| Height (cm)a | 172.0 ± 12.1 | 161.8 ± 7.4 | 172.1 ± 10.2 | 162.7 ± 5.9 | NS | <0.001 |
| 24-h SBP (mm Hg)a | 117.7 ± 8.1 | 110.2 ± 6.5 | 119.1 ± 7.0 | 113.6 ± 8.5 | 0.003 | <0.001 |
| 24-h DBP (mm Hg)a | 65.71 ± 6.01 | 65.0 ± 5.1 | 66.3 ± 5.3 | 67.3 ± 7.4 | 0.036 | NS |
| Office SBP (mm Hg) a | 113.3 ± 10.3 | 106.1 ± 8.5 | 117.2 ± 10.9 | 111.1 ± 11.5 | <0.001 | <0.001 |
| Office DBP (mm Hg)a | 56.8 ± 6.5 | 58.1 ± 5.6 | 60.4 ± 7.1 | 62.5 ± 8.2 | <0.001 | 0.002 |
| Stress SBP (mm Hg)a | 127.3 ± 13.4 | 115.6 ± 9.9 | 128.7 ± 12.9 | 119.1 ± 13.0 | 0.049 | <0.001 |
| Stress DBP (mm Hg)a | 67.7 ± 7.7 | 67.2 ± 6.0 | 69.16 ± 7.34 | 70.7 ± 8.9 | 0.003 | NS |
Abbreviations: AA, African American; BMI, body mass index; DBP, diastolic blood pressure; EA, European American; NS, non-significant; SBP, systolic blood pressure.
Data are mean ± s.d.
Age was adjusted for the evaluation of ethnic and gender effects.
Table 2 presents twin correlations for 24-h, office and stress BP for each ethnic and zygosity group. In both ethnic groups, twin correlations in MZ twin pairs were larger than those in DZ twin pairs, indicating genetic influences. We present the correlations collapsed over gender and age groups, because models that best explained the variance and covariance of these variables did not show any gender or age differences (see below).
Table 2.
Twin correlations of 24-h, office and stress BP by ethnicity and zygosity
| EA |
AA |
|||
|---|---|---|---|---|
| MZ | DZ | MZ | DZ | |
| Number of twins | 49 | 52 | 34 | 44 |
| 24 h | ||||
| SBP | 0.78 | 0.23 | 0.49 | 0.43 |
| DBP | 0.83 | 0.15 | 0.58 | 0.52 |
| Office | ||||
| SBP | 0.61 | 0.30 | 0.61 | 0.33 |
| DBP | 0.65 | 0.35 | 0.63 | 0.47 |
| Stress | ||||
| SBP | 0.78 | 0.43 | 0.76 | 0.34 |
| DBP | 0.71 | 0.40 | 0.77 | 0.29 |
Abbreviations: AA, African American; BP, blood pressure; DBP, diastolic BP; DZ, diazygotic twins; EA, European Americans; MZ, monozygotic twins; SBP, systolic BP.
For both SBP and DBP, multivariate model fitting resulted in the preference for a model without shared environmental factors (AE model) over the full model (ACE model; for SBP, ACE vs. AE model, χ2(6)=1.61, P=0.95; ACE vs. CE model, χ2(6)=33.9, P<0.001; for DBP, ACE vs. AE model, χ2(6)=5.06, P=0.54; ACE vs. CE model, χ2(6)=19.9, P<0.001). For both SBP and DBP, a significant scalar effect for ethnicity was found, indicating that AAs show large variability in SBP and DBP then do EAs. However, for both SBP and DBP, the best fitting model showed no significant differences in genetic and environmental variance components estimates between AAs and EAs or between males and females or between different age groups, indicating that AAs, males and adolescents show heritabilities similar to EAs, females and early adults. As shown in Table 3, this best fitting model resulted in heritability estimates of 63% (95% confidence interval: 50–72%), 75% (66–82%) and 71% (60–79%) for office, stress and 24-h SBP and 59% (47–68%), 67% (56–75%) and 69% (59–77%) for DBP, respectively. The increase in heritability of stress SBP in comparison with office BP reached statistical significance (P=0.012).
Table 3.
Heritability estimates for office, 24-h and stress BP from the best fitting model
| SBP | DBP | |
|---|---|---|
| 24 h | ||
| h2, total (95% CI) | 0.71 (0.60–0.79) | 0.69 (0.59–0.77) |
| e2, total (95% CI) | 0.29 (0.21–0.40) | 0.31 (0.23–0.41) |
| Office | ||
| h2, total (95% CI) | 0.63 (0.50–0.72) | 0.59 (0.47–0.68) |
| e2, total (95% CI) | 0.37 (0.28–0.50) | 0.41 (0.32–0.53) |
| Stress | ||
| h2, total (95% CI) | 0.75 (0.66–0.82) | 0.67 (0.56–0.75) |
| e2, total (95% CI) | 0.25 (0.18–0.34) | 0.33 (0.25–0.44) |
Abbreviations: BP, blood pressure; CI, confidence interval; DBP, diastolic BP; e2, unique environmental contribution to the variance; h2, genetic contribution to the variance (also known as heritability); SBP, systolic BP.
As shown in Table 4, phenotypic correlations were very high for office and stress BP (0.79 for SBP and 0.72 for DBP) and moderately high for office and 24-h BP (0.59 for both SBP and DBP) as well as 24-h and stress BP (0.56 for SBP and 0.52 for DBP). The genetic correlations estimated from the above best fitting model had similar patterns to the phenotypic correlations. This suggests that common genetic factors contribute to each measurement. Nonetheless, none of the genetic correlations reached 1, which also indicates the existence of genetic influences that are specific to each BP measurement.
Table 4.
Phenotypic, genetic and unique environmental correlations among office, 24-h and stress BP
| r | rg (95% CI) | re (95% CI) | |
|---|---|---|---|
| SBP | |||
| 24 h vs. office | 0.59 | 0.67 (0.55–0.78) | 0.41 (0.23–0.57) |
| 24 h vs. stress | 0.56 | 0.63 (0.51–0.73) | 0.36 (0.17–0.52) |
| Stress vs. office | 0.79 | 0.90 (0.84–0.96) | 0.55 (0.41–0.68) |
| DBP | |||
| 24 h vs. office | 0.59 | 0.70 (0.58–0.81) | 0.37 (0.20–0.52) |
| 24 h vs. stress | 0.52 | 0.64 (0.52–0.76) | 0.20 (0.01–0.38) |
| Stress vs. office | 0.72 | 0.85 (0.76–0.94) | 0.49 (0.33–0.62) |
Abbreviations: BP, blood pressure; CI, confidence interval; DBP, diastolic BP; r, phenotypic correlation coefficient; re, unique environmental correlation; rg, genetic correlation; SBP, systolic BP.
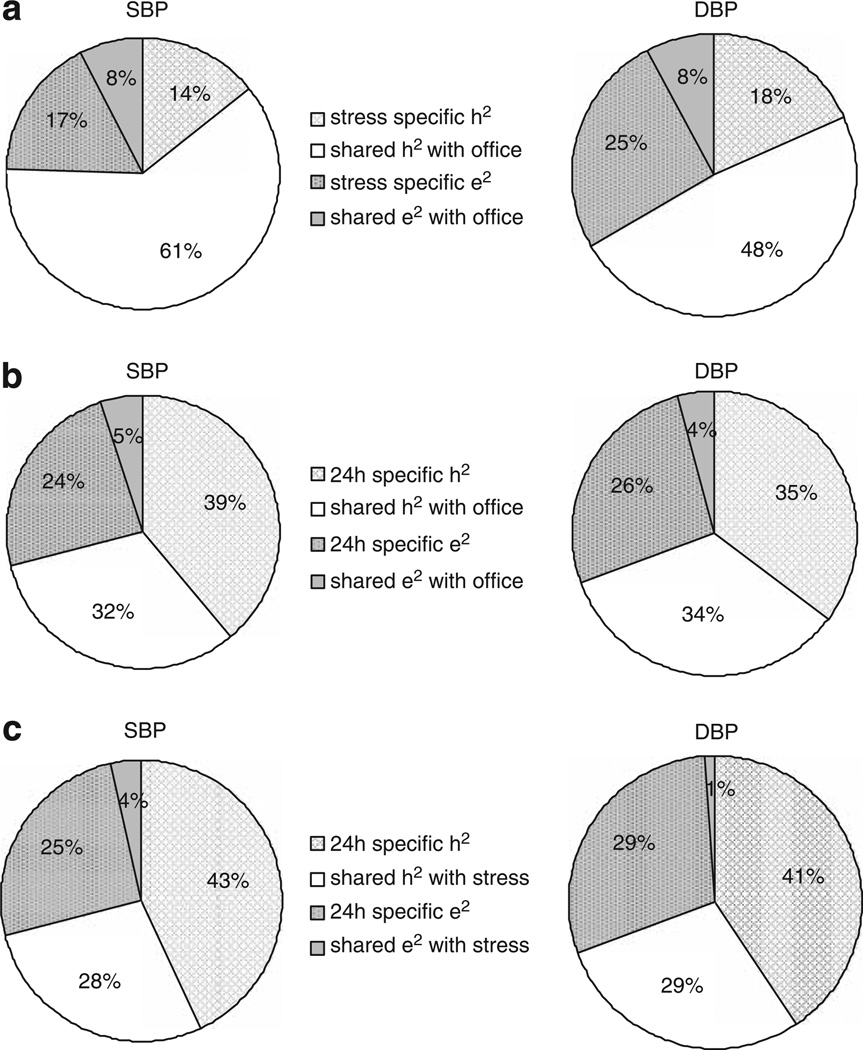
On the basis of best fitting model, we further assessed to what extent genetic and environmental influences were shared between office and stress BP, 24-h and office BP as well as 24-h and stress BP. As shown in Figure 2a, up to 81% of the heritability of office SBP (0.61/0.75) and 71% of office DBP were attributed to genes that also influenced stress BP. On the contrary, only 45% of the heritability of 24-h SBP and 49% of 24-h DBP were attributed to genes that also influence office BP (Figure 2b). Similarly, about 39% of the heritability of 24-h SBP and 42% of 24-h DBP were attributed to genes that also influence stress BP (Figure 2c).
Figure 2.
(a) Sources of variance in stress BP in comparison with office BP. (b) Sources of variance in 24-h BP in comparison with office BP. (c) Sources of variance in 24-h BP in comparison with stress BP. BP, blood pressure; DBP, diastolic BP; SBP, systolic BP.
DISCUSSION
The important findings in this study are that BP levels measured in the office, under laboratory stress and during real life are substantially heritable. There is some overlap between genes that influence BP at these three conditions, but a significant genetic component that is specific to each BP measurement also exists. This suggests that different genes or sets of genes contribute to BP regulation at different conditions. Heritability estimates do not show any differences between AAs and EAs or males and females or different age groups.
The substantial heritability of office BP has motivated many large-scale efforts to identify hypertension predisposing genes through linkage and association approaches. Because ABP is a better predictor of target organ damage, cardiovascular morbidity and mortality than office BP,6–8 it is of great interest to know whether 24-h BP will be a better phenotype than office BP to find genes for EH. To date, four twin studies25–28 and three family studies29–31 have reported heritability estimates for 24-h ABP recording. However, only three studies25,30,31 reported the heritability estimates both for office and 24-h BP, with two family studies30,31 observing higher heritability and one twin study observing similar heritability for 24-h BP.25 For example, in the study by Fava et al.,31 which included 260 siblings from 118 Swedish families, the heritability was 30% for 24-h SBP and 29% for 24-h DBP, whereas the heritability estimates for office BP were not significantly different from zero. In the study by Bochud et al.,30 which included 314 subjects from 76 AA pedigrees, the heritability was 40% for 24-h SBP and 28% for 24-h DBP, whereas it was only 20% for office SBP and 5% for office DBP. In the study by Fagard et al.,25 which included 26MZ and 27 DZ pairs, the heritability was 70% for 24-h SBP and 73% for 24-h DBP, which was similar to the results for the office BP (64 and 73%, respectively). On the basis of two family studies, it is tempting to conclude that genetic studies using 24-h BP as the phenotype are likely to be more powerful than those using office BP. However, there are three caveats. First, these two family studies30,31 might be underpowered to detect the heritability of office BP. Previous family and twin studies have established that office BP is heritable,1 whereas both of these two studies failed to detect a significant heritability for office BP. Second, it was not tested whether the increase in 24-h BP heritability was statistically significant in these two studies. According to the study by Bochud et al.,30 which provided the standard error of the estimated heritabilities, we found a large overlap between the 95%confidence interval of 24-h and office SBP heritabilities, which indicates that the increase in 24-h SBP heritability does not reach statistical significance. Third, an increase in heritability does not necessarily mean an increase in statistical power to find genes, because 24-h and office BP may be influenced by partly different genes. That is, the genetic correlation between 24-h and office BP might be <1. In this study, we did observe an increase in heritability for 24-h SBP (71 vs. 63%) and DBP (69 vs. 59%) in comparison with office BP. However, the increase in heritability did not reach statistical significance. We also showed for the first time that the actual genes responsible for 24-h and office BP differ largely. Only 45% of 24-h SBP heritability and 49% of 24-h DBP heritability can be attributed to genes that also influenced their office levels. Our findings raise the possibility that different genetic pathways may affect BP assessed in the office compared to BP recorded over prolonged periods of time in real life settings.
Data regarding the heritability of BP measured during stress are also of interest because an enhanced cardiovascular response to stress may be an early predictor for the development of essential hypertension.32–34 Previous studies have shown that BP measured under laboratory challenges may be more heritable than its office measures.35,36 For example, in a recent study by De Geus et al.,35 in which BP response to a choice reaction time and a mental arithmetic test was measured in 160 adolescent and 212 middle-aged Dutch twin pairs, the heritability of SBP significantly increased from 59% at rest to 72% under stress in the adolescent cohort. In this study, which includes subjects of the same age range as the Dutch adolescent cohort, we also observed a significant increase in heritability for SBP under stress (from 63 to 75%) in comparison with office BP. Moreover, a stress-specific genetic component for SBP was identified in both the Dutch adolescent cohort (16%) and this study (14%). This has clear implications for gene finding studies. The genetic variation that emerges exclusively during stress can only be found in studies that have attempted to measure the stress levels of BP.
According to the cardiovascular reactivity hypothesis, high reactors during laboratory challenges may experience larger physiological responses to daily demands compared with their less reactive counterparts, with the cumulative impact of these responses acting to enhance the development of disease.37 Although ABP monitoring has been used as a means of assessing the generalizability of laboratory-based BP cardiovascular reactivity to actual real life situations,38–40 this is the first study to assess whether the sources of individual difference in BP regulation under standardized laboratory conditions are different from BP regulation in real life settings. We observed that only 39% of the heritability of 24-h SBP and 42% of 24-h DBP were attributed to genes that also influence stress BP. This might be one of the reasons that the generalization of cardiovascular reactivity from standardized laboratory situations to actual real life situations is only moderate at best.41,42
Ethnic difference in BP levels has been noted with AAs having higher BP than EAs. We confirmed this observation in this study.43,44 It is noteworthy that our study is the first twin study to include both AAs and EAs, and found that AAs show similar heritabilities of 24-h BP and stress BP to EAs. The classic twin study is established as the ideal study design to estimate the relative importance of genetic and environmental factors to the variance of traits and diseases in human populations, but without actual measurement of specific genes or environments, it cannot attribute the ethnic difference in mean values to either of these factors.45 However, our study does show that the observed difference in mean values did not translate to many differences in genetic and environmental variability within each ethnic group. The fact that a similar amount of variation is explained by genetic factors within different ethnic groups does not exclude the possibility, however, that the actual genes or their number responsible for these effects may differ between ethnic groups.
Several limitations to this study need to be recognized. First, as the Georgia Cardiovascular Twin Study is comprised of youth and young adults, the generalizability of these results to other adult populations remains to be determined. However, the focus on healthy youth and young adults avoids the confounding by diseases and the use of medication. Second, the current sample size only has sufficient power (β=0.83) to detect a heritability increase of 30% in the studied BP traits in AAs or males in comparison with EAs or females (assuming the heritability of BP traits in the EAs and females is 60%). Further twin studies with large sample sizes involving multi-ethnic groups are warranted. Third, BP was recorded by different devices, this is, office and stress BP was recorded by Dinamap and 24-h BP was recorded by SpaceLabs. Although both devices use the same methodology (oscillometry) to measure BP, the use of Dinamap to measure office BP has been criticized.46 However, other studies, including our own, also confirmed the strong temporal stability of BP measurements using Dinamap. For example, in our recent study on BP tracking from childhood into early adulthood, we demonstrated that office SBP measured by Dinamap tracked equally well as the 24-h SBP measured by Spacelabs.47 Another piece of evidence is from the recent genome wide association study on BP by the GLOBALBPGEN consortium.48 Out of the six replication cohorts, the one with BP measured by Dinamap showed consistently lower standard errors (and P-values) for the effects of eight newly identified BP loci, suggesting that BP measured by Dinamap is accurate and reliable. Fourth, diary records during ABP monitoring were not collected, whereas recent efforts to characterize laboratory-to-life generalizability have been using the diary records to identify periods of psychosocial demand.49 ABP readings during these periods may be most relevant for comparison with laboratory measures. During the next visit of our twin cohort, subjects will be asked to use PDAs (personal digital assistants) to keep diaries of their activities during ABP monitoring, which will provide more solid evidence on the similarities and differences in genetic and environmental sources of BP measured under laboratory stress and BP measured in the real life setting.
An overall summary of our findings is that, some overlap exists between genes that influence BP measured in the office, under laboratory stress and during real life. A significant specific genetic component influenced BP measured at each condition. The genetic variation that emerges during stress and in real life setting can only be found in gene finding studies that have attempted to measure stress and 24-h BP levels. The identification of these genes will provide new insights into the mechanisms of BP regulation at different conditions. It may also help us to understand the pathophysiology of BP regulation and to develop more accurate prediction of individuals at risk for cardiovascular disease.
References
- 1.Wang X, Snieder H. Familiar aggregation of blood pressure. In: Portman RJ, Sorof JM, Ingelfinger JR, editors. Pediatric Hypertension. 2nd edn. Totowa: Humana Press Inc; 2009. [Google Scholar]
- 2.Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110:74–78. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- 3.Light KC, Girdler SS, Sherwood A, Bragdon EE, Brownley KA, West SG, Hinderliter AL. High stress responsivity predicts later blood pressure only in combination with positive family history and high life stress. Hypertension. 1999;33:1458–1464. doi: 10.1161/01.hyp.33.6.1458. [DOI] [PubMed] [Google Scholar]
- 4.Newman JD, McGarvey ST, Steele MS. Longitudinal association of cardiovascular reactivity and blood pressure in Samoan adolescents. Psychosom Med. 1999;61:243–249. doi: 10.1097/00006842-199903000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Turner JR, Hewitt JK. Twin studies of cardiovascular response to psychological challenge: a review and suggested future directions. Ann Behav Med. 1992;14:12–20. [Google Scholar]
- 6.Verdecchia P. Prognostic value of ambulatory blood pressure : current evidence and clinical implications. Hypertension. 2000;35:844–851. doi: 10.1161/01.hyp.35.3.844. [DOI] [PubMed] [Google Scholar]
- 7.Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA, O’Brien E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–161. doi: 10.1161/01.HYP.0000170138.56903.7a. [DOI] [PubMed] [Google Scholar]
- 8.Kikuya M, Ohkubo T, Asayama K, Metoki H, Obara T, Saito S, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. Ambulatory blood pressure and 10-year risk of cardiovascular and noncardiovascular mortality: the Ohasama study. Hypertension. 2005;45:240–245. doi: 10.1161/01.HYP.0000152079.04553.2c. [DOI] [PubMed] [Google Scholar]
- 9.Snieder H, Treiber FA. The Georgia Cardiovascular Twin Study. Twin Res. 2002;5:497–498. doi: 10.1375/136905202320906354. [DOI] [PubMed] [Google Scholar]
- 10.Ge D, Dong Y, Wang X, Treiber FA, Snieder H. The Georgia Cardiovascular Twin Study: influence of genetic predisposition and chronic stress on risk for cardiovascular disease and type 2 diabetes. Twin Res Hum Genet. 2006;9:965–970. doi: 10.1375/183242706779462877. [DOI] [PubMed] [Google Scholar]
- 11.Jackson RW, Snieder H, Davis H, Treiber FA. Determination of twin zygosity: a comparison of DNA with various questionnaire indices. Twin Res. 2001;4:12–18. doi: 10.1375/1369052012092. [DOI] [PubMed] [Google Scholar]
- 12.Snieder H, Dong Y, Barbeau P, Harshfield GA, Dalageogou C, Zhu H, Carter ND, Treiber FA. Beta2-adrenergic receptor gene and resting hemodynamics in European and African American youth. Am J Hypertens. 2002;15:973–979. doi: 10.1016/s0895-7061(02)02991-6. [DOI] [PubMed] [Google Scholar]
- 13.Snieder H, Harshfield GA, Treiber FA. Heritability of blood pressure and hemodynamics in African- and European-American youth. Hypertension. 2003;41:1196–1201. doi: 10.1161/01.HYP.0000072269.19820.0D. [DOI] [PubMed] [Google Scholar]
- 14.Harshfield GA, Barbeau P, Richey PA, Alpert BS. Racial differences in the influence of body size on ambulatory blood pressure in youths. Blood Press Monit. 2000;5:59–63. [PubMed] [Google Scholar]
- 15.Wang X, Poole JC, Treiber FA, Harshfield GA, Hanevold CD, Snieder H. Ethnic and gender differences in ambulatory blood pressure trajectories: results from a 15-year longitudinal study in youth and young adults. Circulation. 2006;114:2780–2787. doi: 10.1161/CIRCULATIONAHA.106.643940. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, Parati G, Pickering T, Redon J, Staessen J, Stergiou G, Verdecchia P. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821–848. doi: 10.1097/00004872-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Jackson RW, Treiber FA, Turner JR, Davis H, Strong WB. Effects of race, sex, and socioeconomic status upon cardiovascular stress responsivity and recovery in youth. Int J Psychophysiol. 1999;31:111–119. doi: 10.1016/s0167-8760(98)00044-0. [DOI] [PubMed] [Google Scholar]
- 18.Malpass D, Treiber FA, Turner JR, Davis H, Thompson W, Levy M, Strong WB. Relationships between children’s cardiovascular stress responses and resting cardiovascular functioning 1 year later. Int J Psychophysiol. 1997;25:139–144. doi: 10.1016/s0167-8760(96)00736-2. [DOI] [PubMed] [Google Scholar]
- 19.Treiber FA, Turner JR, Davis H, Thompson W, Levy M, Strong WB. Young children’s cardiovascular stress responses predict resting cardiovascular functioning 2 1/2 years later. J Cardiovasc Risk. 1996;3:95–100. [PubMed] [Google Scholar]
- 20.Ewart CK, Kolodner KB. Social competence interview for assessing physiological reactivity in adolescents. Psychosom Med. 1991;53:289–304. doi: 10.1097/00006842-199105000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Kamarck TW, Jennings JR, Pogue-Geile M, Manuck SB. A multidimensional measurement model for cardiovascular reactivity: stability and cross-validation in two adult samples. Health Psychol. 1994;13:471–478. doi: 10.1037//0278-6133.13.6.471. [DOI] [PubMed] [Google Scholar]
- 22.Kamarck TW, Lovallo WR. Cardiovascular reactivity to psychological challenge: conceptual and measurement considerations. Psychosom Med. 2003;65:9–21. doi: 10.1097/01.psy.0000030390.34416.3e. [DOI] [PubMed] [Google Scholar]
- 23.Neale MC, Cardon LR. Methodologies for Genetic Studies of Twins and Families. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- 24.Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. Richmond, VA: Department of Psychiatry, Virginia Commonwealth University; 1999. [Google Scholar]
- 25.Fagard R, Brguljan J, Staessen J, Thijs L, Derom C, Thomis M, Vlietinck R. Heritability of conventional and ambulatory blood pressures. A study in twins. Hypertension. 1995;26(6 Part 1):919–924. doi: 10.1161/01.hyp.26.6.919. [DOI] [PubMed] [Google Scholar]
- 26.Somes GW, Harshfield GA, Alpert BS, Goble MM, Schieken RM. Genetic influences on ambulatory blood pressure patterns. The Medical College of Virginia Twin Study. Am J Hypertens. 1995;8(5 Part 1):474–478. doi: 10.1016/0895-7061(95)00017-j. [DOI] [PubMed] [Google Scholar]
- 27.Vinck WJ, Fagard RH, Loos R, Vlietinck R. The impact of genetic and environmental influences on blood pressure variance across age-groups. J Hypertens. 2001;19:1007–1013. doi: 10.1097/00004872-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Fagard RH, Loos RJ, Beunen G, Derom C, Vlietinck R. Influence of chorionicity on the heritability estimates of blood pressure: a study in twins. J Hyperten. 2003;21:1313–1318. doi: 10.1097/00004872-200307000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Kotchen TA, Kotchen JM, Grim CE, George V, Kaldunski ML, Cowley AW, Hamet P, Chelius TH. Genetic determinants of hypertension: identification of candidate phenotypes. Hypertension. 2000;36:7–13. doi: 10.1161/01.hyp.36.1.7. [DOI] [PubMed] [Google Scholar]
- 30.Bochud M, Bovet P, Elston RC, Paccaud F, Falconnet C, Maillard M, Shamlaye C, Burnier M. High heritability of ambulatory blood pressure in families of east African descent. Hypertension. 2005;45:445–450. doi: 10.1161/01.HYP.0000156538.59873.86. [DOI] [PubMed] [Google Scholar]
- 31.Fava C, Burri P, Almgren P, Groop L, Hulthen UL, Melander O. Heritability of ambulatory and office blood pressure phenotypes in Swedish families. J Hypertens. 2004;22:1717–1721. doi: 10.1097/00004872-200409000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Falkner B, Kushner H, Onesti G, Angelakos ET. Cardiovascular characteristics in adolescents who develop essential hypertension. Hypertension. 1981;3:521–527. doi: 10.1161/01.hyp.3.5.521. [DOI] [PubMed] [Google Scholar]
- 33.Manuck SB. Cardiovascular reactivity in cardiovascular disease: ‘once more unto the breach’. Int J Behav Med. 1994;1:4–31. doi: 10.1207/s15327558ijbm0101_2. [DOI] [PubMed] [Google Scholar]
- 34.Light KC, Sherwood A, Turner JR. Turner JR, Sherwood A, Light KC. Individual Differences in Cardiovascular Response to Stress. New York: Plenum Press; 1992. High cardiovascular reactivity to stress; pp. 281–293. [Google Scholar]
- 35.De Geus EJ, Kupper N, Boomsma DI, Snieder H. Bivariate genetic modeling of cardiovascular stress reactivity: does stress uncover genetic variance? Psychosom Med. 2007;69:356–364. doi: 10.1097/PSY.0b013e318049cc2d. [DOI] [PubMed] [Google Scholar]
- 36.Boomsma DI, Snieder H, de Geus EJ, van Doornen LJ. Heritability of blood pressure increases during mental stress. Twin Res. 1998;1:15–24. doi: 10.1375/136905298320566447. [DOI] [PubMed] [Google Scholar]
- 37.Manuck SB, Kasprowicz AL, Muldoon MR. Behaviorally evoked cardiovascular reactivity and hypertension: conceptual issues and potential associations. Ann Behav Med. 1990;12:17–29. [Google Scholar]
- 38.Ewart CK, Kolodner KB. Predicting ambulatory blood pressure during school: effectiveness of social and nonsocial reactivity tasks in black and white adolescents. Psychophysiology. 1993;30:30–38. doi: 10.1111/j.1469-8986.1993.tb03202.x. [DOI] [PubMed] [Google Scholar]
- 39.Light KC, Turner JR, Hinderliter AL, Sherwood A. Race and gender comparisons: II. Predictions of work blood pressure from laboratory baseline and cardiovascular reactivity measures. Health Psychol. 1993;12:366–375. doi: 10.1037//0278-6133.12.5.366. [DOI] [PubMed] [Google Scholar]
- 40.Fredrikson M, Blumenthal JA, Evans DD, Sherwood A, Light KC. Cardiovascular responses in the laboratory and in the natural environment: is blood pressure reactivity to laboratory-induced mental stress related to ambulatory blood pressure during everyday life? J Psychosom Res. 1989;33:753–762. doi: 10.1016/0022-3999(89)90091-3. [DOI] [PubMed] [Google Scholar]
- 41.Kamarck TW, Schwartz JE, Janicki DL, Shiffman S, Raynor DA. Correspondence between laboratory and ambulatory measures of cardiovascular reactivity: a multilevel modeling approach. Psychophysiology. 2003;40:675–683. doi: 10.1111/1469-8986.00069. [DOI] [PubMed] [Google Scholar]
- 42.Gerin W, Rosofsky M, Pieper C, Pickering TG. A test of generalizability of cardiovascular reactivity using a controlled ambulatory procedure. Psychosom Med. 1994;56:360–368. doi: 10.1097/00006842-199407000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Profant J, Dimsdale JE. Race and diurnal blood pressure patterns. A review and metaanalysis hypertension. Hypertension. 1999;33:1099–1104. doi: 10.1161/01.hyp.33.5.1099. [DOI] [PubMed] [Google Scholar]
- 44.Dekkers JC, Snieder H, Van Den Oord EJ, Treiber FA. Moderators of blood pressure development from childhood to adulthood: a 10-year longitudinal study. J Pediatr. 2002;141:770–779. doi: 10.1067/mpd.2002.128113. [DOI] [PubMed] [Google Scholar]
- 45.Snieder H, MacGregor AJ. Twin methodology. In: Cooper DN, editor. Encyclopedia of the Human Genome. London: Nature Publishing Group; 2003. [Google Scholar]
- 46.O’Brien E, Waeber B, Parati G, Staessen J, Myers MG. Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ (clinical research ed) 2001;322:531–536. doi: 10.1136/bmj.322.7285.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z, Snieder H, Harshfield GA, Treiber FA, Wang X. A 15-year longitudinal study on ambulatory blood pressure tracking from childhood to early adulthood. Hypertens Res. 2009;32:404–410. doi: 10.1038/hr.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, Papadakis K, Voight BF, Scott LJ, Zhang F, Farrall M, Tanaka T, Wallace C, Chambers JC, Khaw KT, Nilsson P, van der Harst P, Polidoro S, Grobbee DE, Onland-Moret NC, Bots ML, Wain LV, Elliott KS, Teumer A, Luan J, Lucas G, Kuusisto J, Burton PR, Hadley D, McArdle WL, Brown M, Dominiczak A, Newhouse SJ, Samani NJ, Webster J, Zeggini E, Beckmann JS, Bergmann S, Lim N, Song K, Vollenweider P, Waeber G, Waterworth DM, Yuan X, Groop L, Orho-Melander M, Allione A, Di Gregorio A, Guarrera S, Panico S, Ricceri F, Romanazzi V, Sacerdote C, Vineis P, Barroso I, Sandhu MS, Luben RN, Crawford GJ, Jousilahti P, Perola M, Boehnke M, Bonnycastle LL, Collins FS, Jackson AU, Mohlke KL, Stringham HM, Valle TT, Willer CJ, Bergman RN, Morken MA, Doring A, Gieger C, Illig T, Meitinger T, Org E, Pfeufer A, Wichmann HE, Kathiresan S, Marrugat J, O’Donnell CJ, Schwartz SM, Siscovick DS, Subirana I, Freimer NB, Hartikainen AL, McCarthy MI, O’Reilly PF, Peltonen L, Pouta A, de Jong PE, Snieder H, van Gilst WH, Clarke R, Goel A, Hamsten A, Peden JF, Seedorf U, Syvanen AC, Tognoni G, Lakatta EG, Sanna S, Scheet P, Schlessinger D, Scuteri A, Dorr M, Ernst F, Felix SB, Homuth G, Lorbeer R, Reffelmann T, Rettig R, Volker U, Galan P, Gut IG, Hercberg S, Lathrop GM, Zelenika D, Deloukas P, Soranzo N, Williams FM, Zhai G, Salomaa V, Laakso M, Elosua R, Forouhi NG, Volzke H, Uiterwaal CS, van der Schouw YT, Numans ME, Matullo G, Navis G, Berglund G, Bingham SA, Kooner JS, Connell JM, Bandinelli S, Ferrucci L, Watkins H, Spector TD, Tuomilehto J, Altshuler D, Strachan DP, Laan M, Meneton P, Wareham NJ, Uda M, Jarvelin MR, Mooser V, Melander O, Loos RJ, Elliott P, Abecasis GR, Caulfield M, Munroe PB. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steptoe A, Cropley M, Joekes K. Task demands and the pressures of everyday life: associations between cardiovascular reactivity and work blood pressure and heart rate. Health Psychol. 2000;19:46–54. doi: 10.1037//0278-6133.19.1.46. [DOI] [PubMed] [Google Scholar]