Abstract
Aim
Determine the effect of residual leaning force on intrathoracic pressure (ITP) in healthy children receiving mechanical ventilation. We hypothesized that application of significant residual leaning force (2.5 kg or 20% of subject body weight) would be associated with a clinically important change in ITP.
Methods
IRB-approved pilot study of healthy, anesthetized, paralyzed mechanically ventilated children (6 months to 7 years). Peak endotracheal pressure (ETP), a surrogate of ITP, was continuously measured before and during serial incremental increases in sternal force from 10% to 25% of the subject’s body weight. A delta ETP of ≥2.0 cmH2O was considered clinically significant.
Results
13 healthy, anesthetized, paralyzed mechanically ventilated children (age: 26 ± 24 m, range: 6.5–87 m; weight: 13 ± 5 kg, range: 7.4–24.8 kg) were enrolled. Peak ETP increased from baseline for all force applications (10% body weight: mean difference of 0.8 cmH2O, p < 0.01; 15% body weight: mean difference of 1.1 cmH2O, p < 0.01; 20% body weight: mean difference of 1.5 cmH2O, p < 0.01; 25% body weight: mean difference of 1.89 cmH2O, p < 0.01). Residual leaning force of ≥2.5 kg was associated with a 2.0 cmH2O change in peak ETP (odds ratio 7.5; CI95 1.5–37.7; p = 0.014) while sternal force ≥20% body weight was not (odds ratio 2.4; CI95 0.6–9.2; p = 0.2).
Conclusion
In healthy anesthetized children, changes in ETP were detectable at residual leaning forces as low as 10% of subject body weight. Residual leaning force of 2.5 kg was associated with increases in ETP ≥2.0 cmH2O.
Keywords: Pediatric, Cardiopulmonary resuscitation, Chest
1. Introduction
The quality of cardiopulmonary resuscitation (CPR) performed during resuscitation attempts is critically important and related to short and long term survival.1–3 Unfortunately, most CPR delivered by both laypersons and health care professionals is not of high quality.1,2,4–8 While incorrect chest compression rates and depths, and excessive ventilation have been documented, inadequate chest recoil (leaning) between compressions is particularly common.9–12
In both animal and adult cardiac arrest studies, incomplete recoil of the chest caused by “leaning” on the sternum (i.e., residual sternal leaning force) during the decompression (release) phase of CPR adversely affects hemodynamics, presumably by increasing intrathoracic pressure (ITP) and decreasing venous blood return to the heart.10–15 Using surrogates for ITP, such as endotracheal pressure (ETP)/right atrial pressure (RAP), these studies have established that even small changes in ITP can be clinically significant, adversely affecting blood flow to critical tissues and survival. Leaning forces of 1.8–3.6 kg (>15–30% body weight) during a pediatric swine model of CPR had substantial effects on coronary perfusion pressure and cardiac output (i.e., had clinically significant effects).13
Our overall goal of this line of research is to determine the residual leaning force threshold above which hemodynamics during actual pediatric CPR may be affected. In order to initially address this issue, the objective of this pilot study was to observe, measure, and report the effect of small, incremental increases in sternal force on measured ETP in sedated and paralyzed, mechanically ventilated children (a model designed to mimic the relaxed chest of the pediatric cardiac arrest victim). We hypothesized that small applications of sternal force (10% of subject body weight) would impart detectable changes in ETP. Moreover, we hypothesized that the application of residual leaning forces of ≥2.5 kg or ≥20% body weight, would be significantly associated with changes in ETP ≥2 cmH2O (a change presumed to be clinically significant based on animal and human studies10,12–15).
2. Methods
This prospective interventional pilot study including consent procedures was approved by the Institutional Review Board at the Children’s Hospital of Philadelphia. Data collection procedures were completed in compliance with the guidelines of the Health Insurance Portability and Accountability Act (HIPAA) to ensure subject confidentiality. Parental/guardian informed consent was obtained from all subjects.
Children ages 6 months to 7 years undergoing an elective urological procedure in the Operating Room (OR) receiving volume-limited conventional mechanical ventilation were screened for inclusion. Exclusion criteria included body weight less than 4.8 kg, presence of a thoracostomy tube or obvious chest wall deformity, and recent thoracic surgery or asthma exacerbation.
3. Data collection
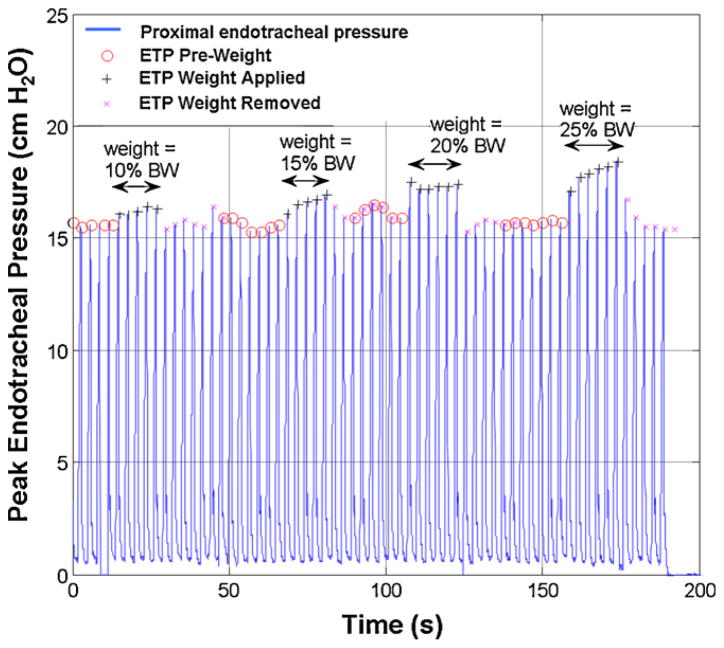
Per standard OR procedure, each subject was sedated, anesthetized, paralyzed, (oral premedication with midazolam 0.5 mg/kg (max 10 mg); anesthetic induction and maintenance with sevoflurane ± switch to desflurane; paralysis with vecuronium 0.1 mg/kg) and intubated with an appropriately sized cuffed endotracheal tube by the attending anesthesiologist. Subsequently, the cuff of the tracheal tube was inflated to eliminate air leak so that the inhaled delivered tidal volume equaled the exhaled tidal volume recorded by the monitor. All subjects were ventilated using standard mechanical ventilation in volume controlled mode (target tidal volume of 6–8 mL/kg body weight). Peak endotracheal pressure at the proximal tip of the tracheal tube was monitored and recorded by a pneumotachometer monitoring system (Novametrix CO2SMO Plus, Novametrix Medical Systems, Inc., Wallingford, CT). The CO2SMO Plus was then interfaced to a local laptop computer using Analysis Plus! (version 5.0, Novametrix Medical Systems, Inc., Wallingford, CT) for continuous recording of ventilation data. Incremental sternal forces from 10% up to 25% of the subject’s body weight (to a maximum of 5 kg) was serially placed on a force transducer (FT) developed by the Laerdal Medical Corporation (Stavanger, Norway) measuring 127 mm length × 62 mm width × 24 mm height and weighing 250 g. The sensor was positioned on the mid-sternum of the subject for 5 consecutive assisted ventilations. The average change in peak ETP over these 5 consecutive ventilations was noted for each incremental weight placement. Prior to each successive sternal force placement, peak ETP was documented to have returned to the initial baseline value (i.e., peak ETP recorded prior to first weight placement). Please see Fig. 1 for a sample recording of peak endotracheal pressure as a function of time.
Fig. 1.
Sample subject recording. Peak endotracheal pressure (ETP) vs. time (s).
4. Data analysis
Standard descriptive summaries were used for baseline demographic data. Univariate analysis compared ETP measured pre vs. post sternal force application using a paired t-test. In accordance with previous animal and adult investigations, we prospectively designated an increase in peak ETP of ≥2 cmH2O as a minimal clinically important difference.10,12–15 Moreover, using the findings of Tomlinson et al.,16 and Maltese et al.,17 that demonstrated average chest compression forces of approximately 25–30 kg during adolescent and adult resuscitation, and Zuercher et al.,13 who found in a piglet model of cardiac arrest that clinically important hemodynamic effects were seen with leaning ≥10% of the overall compression force, significant sternal force was defined as ≥2.5 kg (i.e., 10% of 25 kg). Also based upon the findings of this pediatric model of cardiac arrest, in an attempt to investigate a cutoff standardized to bodyweight for pediatric subjects, we defined a leaning force of ≥20% of the subject’s body weight as significant as well. Univariate analysis by Student t-test was performed comparing sternal force (expressed both as total kilograms applied and percent body weight) in epochs with ≥2 cmH2O change in peak ETP to those with <2 cmH2O change. Generalized estimating equations,18 with a working independence correlation structure using the Huber-White sandwich variance estimator to appropriately adjust the standard errors for within-subject correlation, were used to determine: 1) the association between application of ≥2.5 kg of sternal force with ≥2 cmH2O change in peak ETP; 2) the association between application of sternal force ≥20% of the subject’s body weight with a ≥2 cmH2O change in peak ETP; and 3) the association between the sternal force applied as a function of percent body weight and the change in peak ETP as a continuous variable. P values less than 0.05 were considered significant. Statistical analysis was completed using the Stata-IC statistical package (Version 10.0, StataCorp, College Station, TX).
5. Results
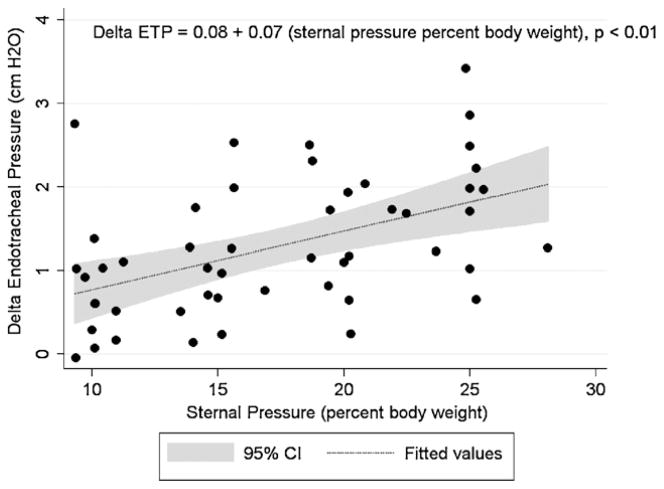
From October 2007 to June 2008 a total of 13 healthy children at the Children’s Hospital of Philadelphia met eligibility criteria and were enrolled (age: 26 ± 24 months, range: 6.5–87 months; weight: 13 ± 5 kg, range: 7.4–24.8 kg). Please see Table 1 for the demographic data of each subject. Fifty evaluable sessions with full pre–post sternal force application data sets were collected. No adverse events were observed with the application of the sternal forces during the investigation. Placement of the force sensor (250 g) did not cause measurable increases in peak ETP for any subject. Peak ETP increased significantly from baseline for all categories of sternal force applied (10% body weight: mean difference of 0.8 cmH2O, p < 0.01; 15% body weight: mean difference of 1.1 cmH2O, p < 0.01; 20% body weight: mean difference of 1.5 cmH2O, p < 0.01; 25% body weight: mean difference of 1.89 cmH2O, p < 0.01). The mean sternal force associated with a ≥2 cmH2O peak ETP change was 2.8 ± 0.9 kg compared to a mean sternal force of 2.0 ± 1.0 kg associated a <2 cmH2O peak ETP change, p = 0.04. Similarly, the mean force expressed as percent body weight of the subject associated with ≥2.0 cmH2O peak ETP change was 20.4 ± 5.4 compared with the mean percent body weight associated with <2.0 cmH2O peak ETP change of 16.6 ± 5.6%, p = 0.07. Application of 2.5 kg of sternal force was significantly associated with a 2.0 cmH2O change in peak ETP (OR 7.5; CI95: 1.5–37.7; p = 0.014) while application of sternal force ≥20% of subject body weight was not (OR: 2.4; CI95: 0.6–9.2; p = 0.2). For each 10% of body weight applied, there was a corresponding 0.7 cmH2O increase in peak ETP (CI95: 0.43–1.0, p < 0.01; Fig. 2).
Table 1.
Demographic data of subjects.
| Subject # | Age (m) | Weight (kg) | Gender | Chest depth (cm) |
|---|---|---|---|---|
| 1 | 22 | 13.7 | M | 12.5 |
| 2 | 8 | 9.9 | M | 9.5 |
| 3 | 6.5 | 8.9 | M | 10 |
| 4 | 16 | 10 | M | 12 |
| 5 | 24 | 12 | M | 13 |
| 6 | 60 | 18 | M | 15 |
| 7 | 40 | 16.1 | M | 15 |
| 8 | 87 | 24.8 | M | 15 |
| 9 | 7.8 | 8 | M | 10.5 |
| 10 | 9.8 | 9.9 | M | 10.5 |
| 11 | 12 | 10.7 | M | 11.5 |
| 12 | 7.9 | 7.4 | M | 11 |
| 13 | 35 | 16 | F | 13 |
| Summary | 26 ± 24* | 13 ± 5* | 92% Male | 12 ± 2* |
Mean ± SD.
Fig. 2.
Delta endotracheal pressure (ETP) vs. sternal pressure applied (percent body weight of subject).
6. Discussion
This is the first study to evaluate the effect of residual sternal force (leaning) on endotracheal pressure in healthy, anesthetized, paralyzed children on mechanical ventilation. This study demonstrates that sternal force equal to as little as 10% of the body weight of the subject can cause measurable changes in ETP. Moreover, the application of ≥2.5 kg (significant residual sternal force as defined by previous animal and human CPR studies13,16,17) is associated with ≥2 cmH2O increases in ETP (consistent with the predetermined minimal clinically important difference in ETP10,12–15).
The critical importance of positive and negative intrathoracic pressures during cardiopulmonary resuscitation has been recently demonstrated. Excessive positive intrathoracic pressure caused by overzealous ventilation or incomplete chest wall decompression during CPR impedes venous return to the heart, thereby decreasing coronary perfusion pressure, and is associated with worse survival rates.10–12 Published studies in animals and adult humans suggest that incomplete recoil of the chest caused by “leaning” on the sternum (e.g., residual sternal force) during the decompression (release) phase of CPR can result in high residual intrathoracic pressure and resultant decrease of venous blood return to the thorax and heart. During CPR, ITP increases caused by “leaning” adversely affects hemodynamics in both experimental laboratory animals and humans.10,12–15
Extrapolation of animal and adult data to the pediatric subject is fraught with difficulty, specifically in cardiac arrest models. Children differ from the adult not only geometrically, but materially and structurally, and these differences likely influence the mechanical response of the child to sternal force and impact.19 These fundamental differences in chest wall mechanics highlight the significance of our investigation that has collected data from actual children. From a flow dynamics perspective, while our model may differ from a fully arrested child, our findings are similar to those of Zuercher et al., who recently demonstrated in a pediatric swine model that leaning forces of 1.8–3.6 kg (15–30% body weight) caused substantial changes in coronary perfusion pressure, cardiac index and myocardial blood flow.13 Because the hemodynamic effects of 1.8 and 3.6 kg leaning forces were not significantly different, that data supports the notion that full chest recoil during pediatric CPR is important.
The value of determining pediatric leaning thresholds is highlighted in the recent findings that automated CPR feedback devices have improved quantitative measures of CPR quality and short term clinical outcomes.2,5 Therefore, incorporation of leaning threshold triggers formulated from sound scientific evidence is warranted for pediatric CPR feedback devices. In this investigation, we chose leaning thresholds extrapolated from animal and adult data (2.5 kg and 20% body weight). Interestingly, our findings seem to support the argument to use an absolute trigger of sternal forces ≥2.5 kg rather than a relative threshold such as 20% of subject body weight, because the former was associated with ≥2 cmH2O differences in ETP, whereas the latter was not. Moreover, the ability to simplify a feedback trigger, that would be independent of the age or weight of the subject, is attractive for automated devices aimed at improving CPR quality.
There were several limitations during this study. First, while we have defined significant leaning forces (≥2.5 kg) and important changes in ETP (≥2 cmH2O) based upon previous animal and human adult data, the clinical significance of the study findings as they pertain to the actual pediatric arrest victim is not yet established. To adequately answer this question, future studies should ideally use invasive and extensive measures of intrathoracic pressure including pleural pressure, esophageal balloon pressure, intravascular pressure, and invasive catheterization of the heart to quantify venous blood return to the right atrium to determine the clinical effect of leaning force. While these techniques were carefully considered in order to maximize the information about the impact of incremental sternal force, after much thought and consideration for safety, cost, and minimal risk, the investigators decided to use a very simple and safe estimation of intrathoracic pressure that could be measured directly from the mechanical ventilator monitoring system itself; therefore, endotracheal pressure was used as a surrogate measure. Since the patients did not have an esophageal balloon in place, the ETP was measured indirectly via CO2SMO Plus® sensor. This minimized risk and simplified the experiment in the pilot phase. During breathing, ETP differs from alveolar pressure by an amount determined primarily by resistance of the airway and flow rate, while alveolar pressure differs from intrathoracic pressure by an amount determined primarily by lung elastic recoil pressure and lung volume.20 While lung volume and elastic recoil may have been lower, and airway resistance higher, during successively increasing weight applied to the sternum, these changes should both have lowered the intrapleural pressure transmitted to the ETT. Thus, our findings of increased ETT pressure at higher sternal weights are presumably an underestimate of changes in intrapleural pressure. It is also important to note that the subjects in this investigation were healthy and had spontaneous circulation during ETP monitoring. As such, while we have demonstrated modest changes in ETP, it is possible that the model used in this investigation differs from the arrested child in both chest wall compliance and thoracic muscular tone. As a result, the relationship between sternal force and ETP found in this investigation may be different during actual cardiac arrest and CPR. Finally, we did not measure the effects of ETP changes on blood flow; however, such changes in ETP have been associated with important changes in blood flow during CPR.13,14
7. Conclusions
In healthy anesthetized and paralyzed children, changes in ETP were detectable at sternal wall force applications as low as 10% of the body weight of the subject. Application of 2.5 kg of sternal force was significantly associated with ≥2.0 cmH2O changes in ETP. Future studies using more extensive and elegant measures of hemodynamic recordings while applying incremental sternal forces may identify the “tipping” point that imparts hemodynamic effects.
Acknowledgments
This study was supported by a Laerdal Medical Foundation Center of Excellence Grant and the Endowed Chair of Pediatric Critical Care Medicine at the Children’s Hospital of Philadelphia. We wish to thank Stephanie Tuttle MBA, Kathryn Roberts RN, Sujatha Devale MBBS, Lisa Tyler RRT, Raymond Matthews RRT, Shawn Colborn RRT, and the staff of the Pediatric ICU and Perioperative Complex for their support and contributions to this study.
Abbreviations
- ITP
intrathoracic pressure
- CPR
cardiopulmonary resuscitation
- ETP
endotracheal pressure
Footnotes
A Spanish translated version of the abstract of this article appears as Appendix in the final online version at doi:10.1016/j.resuscitation.2010.03.015.
Conflicts of interest
The authors acknowledge the following potential conflicts of interest. Jon Nysaether and Mette Stavland were employees of Laerdal Medical Corporation at this time of this work. Vinay Nadkarni, Dana Niles, and Robert Sutton receive unrestricted research grant support from the Laerdal Foundation for Acute Care Medicine.
References
- 1.Abella BS, Sandbo N, Vassilatos P, et al. Chest compression rates during cardiopulmonary resuscitation are suboptimal: a prospective study during inhospital cardiac arrest. Circulation. 2005;111:428–34. doi: 10.1161/01.CIR.0000153811.84257.59. [DOI] [PubMed] [Google Scholar]
- 2.Kramer-Johansen J, Myklebust H, Wik L, et al. Quality of out-of-hospital cardiopulmonary resuscitation with real time automated feedback: a prospective interventional study. Resuscitation. 2006;71:283–92. doi: 10.1016/j.resuscitation.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Edelson DP, Abella BS, Kramer-Johansen J, et al. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation. 2006;71:137–45. doi: 10.1016/j.resuscitation.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Abella BS, Alvarado JP, Myklebust H, et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005;293:305–10. doi: 10.1001/jama.293.3.305. [DOI] [PubMed] [Google Scholar]
- 5.Abella BS, Edelson DP, Kim S, et al. CPR quality improvement during in-hospital cardiac arrest using a real-time audiovisual feedback system. Resuscitation. 2007;73:54–61. doi: 10.1016/j.resuscitation.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 6.Edelson DP, Litzinger B, Arora V, et al. Improving in-hospital cardiac arrest process and outcomes with performance debriefing. Arch Intern Med. 2008;168:1063–9. doi: 10.1001/archinte.168.10.1063. [DOI] [PubMed] [Google Scholar]
- 7.Sutton RM, Niles D, Nysaether J, et al. Quantitative analysis of CPR quality during in-hospital resuscitation of older children and adolescents. Pediatrics. 2009;124:494–9. doi: 10.1542/peds.2008-1930. [DOI] [PubMed] [Google Scholar]
- 8.Wik L, Kramer-Johansen J, Myklebust H, et al. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA. 2005;293:299–304. doi: 10.1001/jama.293.3.299. [DOI] [PubMed] [Google Scholar]
- 9.Niles D, Nysaether J, Sutton R, et al. Leaning is common during in-hospital pediatric CPR, and decreased with automated corrective feedback. Resuscitation. 2009;80:553–7. doi: 10.1016/j.resuscitation.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Aufderheide TP, Lurie KG. Death by hyperventilation: a common and life-threatening problem during cardiopulmonary resuscitation. Crit Care Med. 2004;32:S345–51. doi: 10.1097/01.ccm.0000134335.46859.09. [DOI] [PubMed] [Google Scholar]
- 11.Aufderheide TP, Pirrallo RG, Yannopoulos D, et al. Incomplete chest wall decompression: a clinical evaluation of CPR performance by EMS personnel and assessment of alternative manual chest compression–decompression techniques. Resuscitation. 2005;64:353–62. doi: 10.1016/j.resuscitation.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Aufderheide TP, Sigurdsson G, Pirrallo RG, et al. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109:1960–5. doi: 10.1161/01.CIR.0000126594.79136.61. [DOI] [PubMed] [Google Scholar]
- 13.Zuercher M, Hilwig RW, Ranger-Moore J, et al. Leaning during chest compressions impairs cardiac output and left ventricular myocardial blood flow in piglet cardiac arrest. Crit Care Med. 2010 doi: 10.1097/CCM.0b013e3181ce1fe2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yannopoulos D, McKnite S, Aufderheide TP, et al. Effects of incomplete chest wall decompression during cardiopulmonary resuscitation on coronary and cerebral perfusion pressures in a porcine model of cardiac arrest. Resuscitation. 2005;64:363–72. doi: 10.1016/j.resuscitation.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Yannopoulos D, Tang W, Roussos C, Aufderheide TP, Idris AH, Lurie KG. Reducing ventilation frequency during cardiopulmonary resuscitation in a porcine model of cardiac arrest. Respir Care. 2005;50:628–35. [PubMed] [Google Scholar]
- 16.Tomlinson AE, Nysaether J, Kramer-Johansen J, Steen PA, Dorph E. Compression force-depth relationship during out-of-hospital cardiopulmonary resuscitation. Resuscitation. 2007;72:364–70. doi: 10.1016/j.resuscitation.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Maltese M, Castner T, Niles D, et al. Methods for determining pediatric thoracic force-deflection characteristics from cardiopulmonary resuscitation. Stapp Car Crash J. 2008;52:83–105. doi: 10.4271/2008-22-0004. [DOI] [PubMed] [Google Scholar]
- 18.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 19.Papastamelos C, Panitch HB, England SE, Allen JL. Developmental changes in chest wall compliance in infancy and early childhood. J Appl Physiol. 1995;78:179–84. doi: 10.1152/jappl.1995.78.1.179. [DOI] [PubMed] [Google Scholar]
- 20.Mead J. Expiratory flow limitation: a physiologist’s point of view. Fed Proc. 1980;39:2771–5. [PubMed] [Google Scholar]