Abstract
In vivodelivery is a major barrier to the use of molecular tools for gene modification. Here we demonstrate site-specific gene editing of human cells in vivo in hematopoietic stem cell-engrafted NOD-scid IL2rγnull mice, using biodegradable nanoparticles loaded with triplex-forming peptide nucleic acids (PNAs) and single-stranded donor DNA molecules. In vitro screening showed greater efficacy of nanoparticles containing PNAs/DNAs together over PNA-alone or DNA-alone. Intravenous injection of particles containing PNAs/DNAs produced modification of the human CCR5 gene in hematolymphoid cells in the mice, with modification confirmed at the genomic DNA, mRNA, and functional levels. Deep sequencing revealed in vivo modification of the CCR5 gene at frequencies of 0.43% in hematopoietic cells in the spleen, and 0.05% in the bone marrow: off-target modification in the partially homologous CCR2 gene was two orders of magnitude lower. We also induced specific modification in the β-globin gene using nanoparticles carrying β-globin-targeted PNAs/DNAs, demonstrating this method’s versatility. In vivo testing in an EGFP- β-globin reporter mouse showed greater activity of nanoparticles containing PNAs/DNAs together over DNA only. Direct in vivo gene modification, such as we demonstrate here, would allow for gene therapy in systemic diseases or in cells that cannot be manipulated ex vivo.
Keywords: gene modification, peptide nucleic acid, nanoparticles, gene delivery, CCR5, triple-forming oligonucleotide
INTRODUCTION
While viral vectors or plasmid delivery vehicles have been used for many gene therapy applications in vivo, the clinical use of these methods has been limited partially because of the non-specific nature of gene editing and delivery: delivered genes are integrated into random sites, or expressed in plasmids where they are not under their normal regulation. In contrast, site-specific editing of genes at their endogenous loci could be used to treat monogenic disorders or introduce advantageous genomic changes. Genes edited in this fashion remain under their normal regulatory control, unlike genes delivered via plasmids or inserted as cDNA constructs into other genomic loci. Site-specific editing by homologous recombination does not pose the dangers of non-specific integration inherent to gene therapy dependent on delivery by viral vectors1. Despite these advantages, efficient non-toxic intracellular delivery remains a barrier to the clinical use of triplex-forming oligonucleotides (TFOs), small fragment homologous recombination (SFHR), transcription activator-like effector nucleases (TALENs), and zinc finger nucleases (ZFNs), although several delivery strategies have been developed for these systems, ranging from microinjection to direct addition of reagents to cells. The most commonly used methods are standard nucleofection or electroporation which leads to cell death when applied ex vivo2–4, and cannot readily be applied in vivo. Direct in vivo gene editing would eliminate the expense and risk of cell harvests, opening the doors for gene therapy in cells that cannot be manipulated outside of the body.
Previous studies have demonstrated site-specific editing of the mouse genome or reporter genes in vivo5–7, ex vivo editing of human cells followed by transplant into murine models3, 8, or in vivo knock-in of cDNA fragments9. SFHR has been used for site-specific editing of the human globin and cystic fibrosis transmembrane receptor genes10, 11, and TALENs have been successfully used for targeted mutagenesis in several model systems. ZFNs represent a promising technology for site-specific gene editing, with clinical trials in progress for use of this technology in the treatment of HIV-1 infection. ZFNs have been used for both ex vivo editing of CCR5 in hematopoietic cells and subsequent transplant into mouse models conferring HIV-1 resistance3, and insertion of whole cDNA fragments in vivo in mice with high efficiency using viral vectors9. However, none of the current technologies for site-specific gene editing have been used to directly edit human genes in human cells in vivo. Our study is unique in its combination of two technologies – synthetic TFOs and polymer nanoparticles – to modify human cells after systemic delivery into chimeric mice.
Triplex-forming PNAs are an effective chemical tool that can mediate the recombination of 50–60 bp donor DNA fragments with genomic DNA to introduce gene modifications12. These PNA molecules form a PNA-DNA-PNA triplex that provokes the cell’s own DNA repair machinery12, 13 and thereby stimulates recombination with donor DNAs to cause heritable changes in targeted genes. Mechanisms of DNA repair and recombination induced by triplex structures have been previously reviewed14, 15. Current evidence suggests that nucleotide excision repair (NER) plays a role in recognizing and repairing triplex-induced DNA helical alterations 12, 13, 16, 17 and that triplex structures are recognized by the XPA and RPA repair factors17. However, further work is needed to fully elucidate the mechanism of PNA triplex promotion of homologous recombination events. In earlier work, we developed PNA/DNA combinations that modify the human CCR5 gene, leading to the production of HIV resistant cells8, along with PNA/DNA molecules to correct the human β-globin gene at the location of a common thalassemia splice-site mutation13. In both of these systems, co-delivery of the PNAs and DNAs led to much higher levels of genome modification than delivery of DNAs alone. However, delivery of PNAs remains a challenge. Previous studies have shown that systemic administration of naked triplex-forming PNAs led to minimal levels of mutagenesis above background in a mouse reporter system7. Novel delivery techniques, such as conjugation with cell penetrating peptides7 or use of new delivery vehicles, such as we report here, will be needed to improve the in vivo activity of triplex-forming PNAs.
We have previously engineered poly(lactic-co-glycolic acid) (PLGA), an FDA-approved biocompatible material, to produce nanoparticles that deliver nucleic acid cargo18, 19. Most recently, we showed that PLGA nanoparticles carrying PNA/DNA can introduce site-specific genomic modifications in human hematopoietic stem and progenitor cells (HSPCs) in vitro, with higher efficiency and lower toxicity than nucleofection2. In this recent study, we found that particles containing both PNAs and DNAs were more effective than particles with DNAs alone, or than a combination of particles separately containing the two molecules. We report here that this technology can also produce in vivo gene editing in human hematopoietic cells, which was demonstrated by intravenous injection of PNA/DNA-containing nanoparticles in a humanized mouse model that is relevant to clinical medicine.
RESULTS
PLGA nanoparticles encapsulating PNAs/DNAs for the introduction of modifications in the CCR5 gene were formulated using a double emulsion solvent evaporation technique, yielding particles approximately 150 nm in diameter (Fig. 1a). Two donor DNA molecules were used (donor 1 and donor 2), each capable of introducing a different 6 bp modification that inserts an in-frame stop codon into the CCR5 gene. Nanoparticles coated with a cell-penetrating peptide derived from HIV-1 transactivating protein (TAT) or from the Drosophila antennapedia peptide (AP) were also produced, using a surface-modification approach which has been previously described20.
Figure 1. Nanoparticle formulation and characterization.

(a) A tail-clamp PNA (tcPNA-697) which forms a PNA/DNA/PNA triple helix “clamp” at positions 679–688 of CCR5 and includes a 5-bp “tail” forming a PNA/DNA duplex at positions 674–678 has been previously described. tcPNA-697 induces the recombination of donor DNA sequences that contain a 6 bp modification (indicated by circles) which includes an in-frame stop codon. This modification can then be detected by allele-specific PCR (AS-PCR) or quantitative AS-PCR (qAS-PCR). A scanning electron micrograph of PLGA nanoparticles containing the PNA and DNA molecules is also shown. (b) Loading of PLGA nanoparticles was determined by dissolving particles in dichloromethane and performing aqueous phase extractions. OD260 was measured and compared to a standard (blanking to PBS-containing nanoparticles) to determine nucleic acid loading. (c) Controlled release profile of DNA, PNA, and PNA/DNA loaded nanoparticles. Release of nucleic acid was measured when particles were suspended in PBS at 37°C.
Both unmodified (CD) and DSPE-PEG (DP) surface modified particles were formulated and tested. Addition of DSPE-PEG to nanoparticles in the aqueous phase of the second emulsion results in partitioning of the DSPE phospholipid in the polymer, with PEG being displaced on the surface of the nanoparticle along with any moiety attached to the PEG20, such as TAT or AP. This surface modification technique was chosen because of previous success in using DSPE-PEG modified particles to deliver siRNA to a cell line reporter and tumor xenografts20. Loading of nucleic acids in nanoparticles (Fig. 1b) and nucleic acid release over 48 hours (Fig. 1c) was determined for all particle types, showing efficient loading and release of nucleic acids. The biodegradation of PLGA polymer has been extensively studied in the past: hydrolysis of the polymer follows first order kinetics, with degradation occurring over the span of weeks, with initial PLGA molecular weight and pH of the incubating media both affecting the duration of breakdown21. In addition, to show degradability of our particles, we examined the loss of the PLGA nanoparticles over 10 days when incubated at 37°C, and found approximately 50% loss of material over 10 days (Supplemental Fig. 1).
In vitro nanoparticle screening was performed to determine which particles to use in the mouse model. Consistent with our previous studies22, we found that combined PNA/DNA nanoparticles had greater in vitro activity than DNA-alone nanoparticles (Fig. 2a). In addition, we found that PNAs and DNAs released from the particles during controlled release could be nucleofected into cells and still mediate modification, showing that released PNAs/DNAs are still active. Finally, released nucleic acid simply added to the cells did not lead to levels of modification as high as with nanoparticle delivery or nucleofection, confirming that the delivery step is key. As we have observed before, PNA/DNA nanoparticles were more effective than introduction of PNAs/DNAs by nucleofection. Cell survival was also greater with nanoparticle addition than with nucleofection, consistent with our previous studies22. DSPE-PEG, DSPE-PEG-TAT, and DSPE-PEG-AP nanoparticles carrying the donor DNAs alone were compared to unmodified nanoparticles (equivalent nucleic acid doses). In CD34+ HSPCs, both AP and TAT-modified particles were found to be slightly more active (Fig. 2b). Our results with TAT were most consistent and thus TAT-modified particles were tested in subsequent experiments in animals. In addition, TAT has been shown to increase uptake of payloads to HSPCs23.
Figure 2. In vitro nanoparticle screening, and in vivo experimental design.

For each treatment group, 1 million human CD34+ cells were treated with nanoparticles or nucleic acid for 24 hours in 1 mL media, after which cells were harvested, counted for live cells using a trypan blue stain, and genomic DNA harvested for AS-PCR. Released nucleic acid: cells were treated with 2 nmoles PNA and DNA which was collected after controlled release from nanoparticles incubated at 37°C in PBS for 48 hours. Nucleofected released: cells were treated by Amaxa nucleofection as previously described, using 2 nmoles of PNA and DNA which was collected after controlled release from nanoparticles incubated at 37°C in PBS for 48 hours. PNA nanoparticles: 2 nmoles of PNA encapsulated in PLGA nanoparticles (appropriate mg of particles added based on loading), added directly to cells. PNA/DNA nanoparticles: 2 nmoles of PNA and DNA encapsulated in PLGA nanoparticles, added directly to cells. DNA nanoparticles: 2 nmoles of DNA encapsulated in PLGA nanoparticles, added directly to cells. (a) Cell survival and a gel showing the site-specific modification in CCR5 by AS-PCR. (b) Particles containing donor DNA targeting the CCR5 locus show increased recombination when surface modified with TAT and AP. Results from qAS-PCR with SD (n=4). Untr = untreated. CD = unmodified CCR5 donor DNA nanoparticles. DP = DSPE-PEG-COOH surface modified donor DNA nanoparticles. AP = DSPE-PEG-antennapedia-peptide surface modified donor DNA nanoparticles. TAT = DSPE-PEG-HIV-transactivating peptide surface modified donor DNA nanoparticles. 1 million human CD34+ cells were treated for 24 hours with 0.5 nmoles total DNA encapsulated in nanoparticles, in 1 mL of media. (c) Description of experiment design. Newborn NOD-scid IL2rγnull mice were irradiated then reconstituted with untreated human CD34+ hematopoietic stem and progenitor cells by intracardiac injection. After 12 weeks to allow for reconstitution of the hematopoietic system with human cells, mice were treated with 10 mg of nanoparticles or equivalent doses of PNA/DNA in PBS (naked oligo, 5 nmole PNA + 5 nmole DNA per mouse). Ten days post-treatment, mice were sacrificed and genomic DNA was harvested from whole organs or sorted cells from bone marrow and spleen. qAS-PCR was used to determine the presence of the targeted modifications.
NOD-scid IL2rγnull mice exhibit complete reconstitution of the hematopoietic system after transplantation of human CD34+ cells into newborns, with a significant presence of human cells in peripheral blood, bone marrow, spleen, lung, liver, thymus, and small intestine3. Our basic experimental design using this mouse model is outlined in Fig. 2c. Untreated wild-type human CD34+ HSPCs were injected into NOD-scid IL2rγnull neonatal mice and reconstitution of the hematolymphoid system was confirmed 12 weeks post-transplant at normal levels (Fig. 3a). PNA/DNA-containing nanoparticles or naked PNAs/DNAs were then injected via tail vein. Ten days post treatment, mice were sacrificed and analyzed for the presence of the targeted modifications in whole organs and sorted cells.
Figure 3. Engraftment and AS-PCR controls.
Untx = untreated, np = nanoparticles. 12 mice for CCR5 studies, 9 for globin studies. (a) Engraftment of human cells in mice confirmed after 12 weeks via FACS. (b) Un-reconstituted mice (no human cells) show no amplification of either wildtype or modified CCR5, even with nanoparticle (np) treatment. Untreated mouse genomic DNA spiked with both donor oligos in excess of the primer concentration immediately prior to the PCR reactions shows no modification of either modified allele, showing that presence of donor oligos does not create any artifact for the AS-PCR reaction. Spleen and lung from nanoparticle-treated reconstituted mice shown for comparison, and treated CD34+ cell gDNA was used as a positive control.
We previously developed an allele-specific PCR (AS-PCR) to reliably detect CCR5 modifications at the DNA level: detection of gene modification by AS-PCR corresponded well to mRNA and protein expression of the modified gene 8. A different primer set can be used to separately detect each of the two introduced modifications. Quantitative AS-PCR (qAS-PCR) can be used to determine the relative levels of gene modification between treatment groups, and relative gene modification can be calculated using the 2−ΔΔCt method24. As controls, non-reconstituted mice (which lack human cells) treated with CCR5 targeted nanoparticles do not exhibit detectable gene modification, consistent with the absence of the target sequence in the mouse genome (Fig. 3b), confirming that any gene modifications observed are in the CCR5 gene in human cells. Also, genomic DNA from reconstituted NOD-scid IL2rγnull mice spiked with the CCR5 donor DNAs does not lead to spurious amplification, showing that possible persistence of donor DNA does not lead to any PCR artifact (Fig. 3b).
In the mice engrafted with human hematopoietic cells, we detected CCR5 modification in the bone marrow, spleen, thymus, small intestine, liver, and lung of nanoparticle-treated mice, with only low to negligible gene modification detected when naked oligonucleotides were used (Fig. 4a). These results suggest that PLGA nanoparticles become widely distributed throughout the mouse, where they are taken up in human cells, allowing for reliable gene modification, which was not achievable with equivalent dosages of naked oligonucleotide. These results are consistent with previous studies that demonstrate rapid clearance of free oligonucleotides after intravenous injection25.
Figure 4. Intravenous injection of human HSC engrafted mice with nanoparticles leads to modification in the human CCR5 gene in multiple organs and in clinically relevant human cell types.
Untreated n = 2, naked oligo n = 3, nanoparticles n =4, TAT nanoparticles n = 3. (a) Each data point represents either modification 1 or modification 2 from one mouse as determined by qAS-PCR, and one gel of PCR products for one CCR5 modification is shown for each organ. 2−ΔΔCT values (modified allele versus untreated, normalized to average untreated) are given. Samples for which a threshold value were not reached were assigned a threshold value of the maximum cycle number for the run. Lower limit of scale bar is 0.1, samples below this limit do not show on the graph. All organs had presence of wildtype allele (gels not shown). (b) Purity of sorted CD34+ population from bone marrow as assessed by FACS. (c) Purity of sorted T cell population from spleen as assessed by FACS. (d) Modification of CD4+ T-cells harvested and sorted from the spleen. (e) Modification of CD34+ cells harvested and sorted from bone marrow.
To show that site-specific gene modification occurs in relevant human cell populations, CD4+ human T cells and CD34+ human HSPCs were sorted from the spleens and bone marrow, respectively, of reconstituted mice that had been treated with nanoparticles or naked oligonucleotides (Fig. 4b–c). Modification was detected in sorted cells, with nanoparticle-treated mice showing significantly greater levels of gene modification than the mice injected with naked PNAs/DNAs (Fig. 4d–e). Unlike what was seen in vitro, we have not seen significant differences between unmodified nanoparticles and nanoparticles surface-modified with TAT in vivo. This may be due to reduced release kinetics of TAT-modified nanoparticles as shown in Fig. 1c. However, because of the robust activity of unmodified nanoparticles, we continued using the unmodified delivery system.
We used additional methods to confirm modification with the nanoparticles, and also tested for toxicity and effects on pluripotency. Mononuclear cells harvested from the bone marrow and spleens of the treated mice were able to form both myeloid and erythroid colonies with the same frequency and morphology as untreated controls (Fig. 5a, b). This suggests that the nanoparticle treatment is not toxic to hematopoietic progenitors and does not affect their ability to differentiate, consistent with our previous finding that nanoparticle treatment does not affect the survival or differentiation capacity of human HSPCs in vitro2. Individual colonies were picked for genomic DNA extraction and AS-PCR, and since each colony is theoretically derived from a single HSPC, we were able to estimate absolute modification frequencies, which were as high as 4% in the bone marrow and 19% in the spleen in this small sample set (Table 1). We confirmed the presence of the modification in one of these colonies by direct sequencing of genomic DNA (Fig. 5c). Gene modification was observed in both myeloid and erythroid colonies suggesting that gene modification using this method does not affect the differentiation capacity or survival of hematopoietic progenitors.
Figure 5. Nanoparticle treatment is nontoxic to bone marrow and spleen progenitors, with modification confirmed by genomic sequencing.

Untreated n = 3 mice, nanoparticles n = 3 mice. (a) Whole bone marrow or mononuclear cells from spleens harvested from the treated and untreated mice were plated for a colony-forming cell assay in methylcellulose medium with cytokines for growth of erythroid colonies (burst-forming unit (BFU) or colony forming unit erythroid (CFU-E), granulocyte/macrophage colonies (CFU-G, CFU-M, and CFU-GM), or combined colonies (CFU-GEMM, granulocyte, erythroid, monocyte/macrophage, megakaryocyte). Colonies were counted as either white (CFU-G/M/GM), red (CFU/BFU-E), or combined (CFU-GEMM). (b) Representative pictures of hematopoietic colonies. (c) Direct sequencing of genomic DNA from a harvested colony confirms the presence of the targeted modification.
Table 1.
Colonies derived from human hematopoietic progenitors harvested from treated mice show high levels of genomic modification. Individual colonies were picked for genomic DNA extraction and AS-PCR for both CCR5 modifications, and PCR for the presence of human CCR5, allowing for determination of a frequency of modification of human hematopoietic progenitors.
| Colonies with Modification 1 | Colonies with Modification 2 | Total colonies with human CCR5 | Description of modified colonies | |
|---|---|---|---|---|
| Untreated Bone Marrow | 0 | 0 | 109 | |
| Nanoparticle-treated Bone Marrow | 4 (4%) | 2 (2%) | 100 | 3 G/M/GM, 3 CFU-BFU/E |
| Untreated Spleen | 0 | 0 | 38 | |
| Nanoparticle-treated Spleen | 3 (14%) | 4 (19%) | 21 | 4 G/M/GM, 2 CFU/BFU-E, 1 GEM |
The treatment produced minimal off-target genotoxicity: a section of the CCR2 gene with significant homology to our target site was subjected to PCR and sequencing, and no mutations were detected in any of the 93 assayed colonies (Table 2). In addition, we performed deep sequencing to determine absolute frequencies of CCR5 gene modification and of off-target CCR2 effects in cell populations within the treated mice after the in vivo nanoparticle treatments. These results are shown in Table 3. In human hematopoietic cells contained within the spleen, modification of CCR5 was 0.43%, and off-target modification of CCR2 was only 0.004%. In the bone marrow, modification of CCR5 was 0.05%, and off target modification was more than 80 times less.
Table 2.
Sequencing of individual colonies to determine off-target effects in CCR2. Genomic DNA from colonies was analyzed for the presence of mutations in a region of CCR2 with homology to the donor DNA used in this study. A 400 bp region of CCR2 was amplified from genomic DNA by PCR from 93 colonies, and submitted for sequencing. None of the 93 colonies had mutations in CCR2. These were the same colonies harvested for analysis for the presence of the targeted modifications in Fig. 5 and Table 1 (bone marrow and spleen), and the percentage of the assayed colonies that had either modification are noted in the table.
| Gene | # CFCs assayed | Modified | Percent |
|---|---|---|---|
| CCR5 | 93 | 6 | 6.45% |
| CCR2 | 93 | 0 | <1.075% |
Table 3. Deep sequencing to determine absolute on and off-target modification frequencies.
100 bp regions of CCR5 and CCR2 were amplified by PCR with sample-specific barcodes, with the site of the 6 bp modification near the center of the amplicon, and set to the W.M. Keck West Campus Facility at Yale University for deep sequencing using an Illumina HiSeq sequencer. Percent modification was calculated by counting exact numbers of amplicon copies with either of the 6 bp modifications. Background non-specific mutation frequency inherent to the machine was not different between samples from treated and untreated mice.
| Sample | CCR5 Sequences assayed | CCR2 Sequences assayed | % CCR5 Modified | %CCR2 Modified |
|---|---|---|---|---|
| Nanoparticle-treated Bone Marrow | 2,038,398 | 676,575 | 0.05% | 0.0006% |
| Nanoparticle-treated Spleen | 1,870,844 | 27,295 | 0.43% | 0.004% |
We also found that nanoparticles were capable of modifying true hematopoietic stem cells, as confirmed by serial transplant. Mice were treated as described above with PNA/DNA-containing nanoparticles by tail vein injection, or left untreated, and after 8 days to allow for targeted modification, bone marrow was harvested from 6 mice (3 per group) to transplant into secondary recipients (2 per group) (Fig. 6a). Donors had engraftment of human hematopoietic cells at high levels as determined by FACS of peripheral blood (Fig. 6b). The donor mice that had been treated with PNA/DNA-containing nanoparticles showed the presence of both targeted modifications in the bone marrow, consistent with results above (Fig. 6c, lane 2). Of the recipient mice, one mouse receiving cells from the untreated mice and one mouse receiving cells from the nanoparticle-treated mice had engraftment of human cells as determined by presence of the human CCR5 gene by FACS (Fig. 6c, lane 4 and 5). The engrafted mouse receiving cells from the treated donor also showed the presence of the DNA donor 2 targeted modification in cells from the bone marrow, showing that this modification was present in the engrafting cells in the serial transplant, indicating persistence in the stem/progenitor cell compartment (Fig. 6c, modification 2 lane 5). In addition, deep sequencing of DNA from the bone marrow obtained from this recipient mouse confirmed the presence of the CCR5 modification, with 8 out of 43857 sequenced alleles showing the targeted change.
Figure 6. In vivo nanoparticles treatment results in specific modification in true human hematopoietic stem cells.
(a) Experimental design. Six newborn NOD-scid IL2rγnull mice were irradiated then reconstituted with untreated human CD34+ hematopoietic stem and progenitor cells (from the same donor) by intracardiac injection. After 12 weeks to allow for reconstitution of the hematopoietic system with human cells, mice were treated with 15 mg of nanoparticles over 5 days (three treatments of 5 mg). Eight days after the first treatment, mice were sacrificed, and bone marrow harvested. Bone marrow was pooled from three treated mice, and three untreated mice, and then depleted for mouse CD45+ cells. The harvested cells were then injected via tail vein into NSG-KH220 mice, which support human HSC engraftment in the absence of irradiation. Two secondary recipients (male and female) were injected with cells from the treated mice, and two with cells from the untreated mice. After six weeks, secondary recipients were tested for engraftment, then sacrificed to harvest gDNA from organs for AS-PCR. (b) Engraftment of NOD-scid IL2rγnull with untreated CD34+ cells. (c) Presence of the targeted modification in the bone marrow of cells from donor mice and in secondary recipients. Untx = untreated, np = nanoparticles treated. Received from untx = secondary recipients that received cells from untreated donor mice. Receiped from np = secondary recipients that received cells from treated donor mice. F = female, M = male. Plasmid controls = wildtype (left) and modification-containing (right) plasmid. CCR5 PCR confirms presence of human CCR5 sequence (to show engraftment of human cells).
Next we confirmed that nanoparticle treatment leads to CCR5 modification at the mRNA and functional levels (based on HIV-1 resistance), and does so with minimal induced inflammatory response (Fig. 7). We first confirmed the expression of sequence-modified CCR5 mRNA in blood cells of treated mice by AS-RT-PCR (Fig. 7a). Next, adult NOD-scid IL2rγnull mice were engrafted with primary human peripheral blood mononuclear cells (PBMCs) heterozygous for the Δ32 mutation in the CCR5 gene. This PBMC model was used because T cells from primary human PBMCs are activated, resulting in high levels of CCR5 expression. Also, cells heterozygous with the 32 mutation only require one more additional CCR5 allele to be modified for total CCR5 knockout and HIV-1 resistance. After engraftment, these mice were treated with PNA/DNA-containing nanoparticles or blank nanoparticles (no oligonucleotides), and challenged with HIV-1 infection. Importantly, the mice treated with the PNA/DNA nanoparticles showed some resistance to infection, with preservation of CD4+ cell counts, consistent with resistance to HIV-1 mediated cytotoxicity of CD4+ lymphocytes (Fig. 7b). As shown, mice treated with PNA/DNA nanoparticles showed preservation of CD4+ counts at levels more than twice as high as control mice that had been treated with blank nanoparticles containing no oligonucleotide therapeutic. In addition, there was no difference in the expression levels of two inflammatory cytokines after nanoparticle treatment (Fig. 7c), suggesting relatively low inflammatory response in these mice.
Figure 7. Nanoparticle treatment leads to CCR5modification at the mRNA level, with minimal inflammatory response.
(a) Reverse-transcription-AS-PCR for detection of CCR5 modification 1 in mRNA isolated from the lung of mice injected with nanoparticles. (b) Adult NOD-scid IL2rγnullmice were injected with Δ32 heterozygous human PBMCs, then nanoparticles were injected in 3 doses of 5 mg/200 μL. Mice were bled to assess the presence of T cell populations by FACS before and after infection with 30pfu HIV-1BaL. CCR5 nanoparticle-treated mice show significantly higher CD4+ T-cell levels in comparison to blank nanoparticle-treated mice, at later time-points post injection. 6 mice were in each treatment group. (c) Lungs were harvested from human HSC-reconstituted mice for RNA extraction ten days post treatment. Reverse transcription quantitative PCR was performed to determine expression of inflammatory markers TNF-alpha and IL6.
To demonstrate the flexibility of our approach, we produced nanoparticles targeting a different gene, human β-globin (Fig. 8). We formulated nanoparticles encapsulating PNA/DNA sequences designed to introduce a 6 bp modification at a site commonly associated with thalassemia in the human β-globin gene using previously designed donor DNA and PNA molecules (Fig. 8a)13. The targeted modification was only detected by AS-PCR in mice reconstituted with human cells (Fig. 8b). In addition as a control, β-globin specific particles did not mediate site-specific modification in CCR5 or vice versa (Fig. 8c). Mice sacrificed both five and ten days post treatment had detectable β-globin gene modification in human blood cells in the bone marrow, spleen, and lung (Fig. 8d).
Figure 8. Nanoparticles with PNA and DNA targeting the human β-globin gene mediate site-specific genomic modification.
(a) We have previously designed a bis-PNA with six terminal lysines that binds in position 194 of intron 2 of the human β-globin gene, that increases recombination of a donor DNA segment (circles indicating the desired modification) at the site of a common thalassemia mutation (IVS2-1). (b) AS-PCR can detect the β-globin modification and the wildtype sequence in treated reconstituted mice, but not in unreconstituted mice or reconstituted mouse genomic DNA spiked with donor oligo. (c) Mice treated with CCR5 nanoparticles do not exhibit the globin modification, and vice versa (lung gDNA). (d) Mice were treated with 10 mg of nanoparticles with PNA/DNA targeting the β-globin gene. Five or ten days post-treatment, mice were sacrificed and genomic DNA was harvested from organs for qAS-PCR. Each data point represents the level of modification from one mouse, and one gel (day 10) of PCR products is shown for each organ. Samples for which a threshold value were not reached were assigned a threshold value of the maximum cycle number for the run. (e) In vivo modification of the human β-globin gene was confirmed in a globin/EGFP transgenic reporter mouse. Blank, DNA-containing, and PNA/DNA containing nanoparticles were tested in 654-EGFP mice genetically modified to contain the human β-globin IVS2–654 site with a thalassemia-associated splice site mutation, inserted into a ubiquitously expressed EGFP. When the splice-site mutation is corrected by genome modification, EGFP is expressed. Mice were given three treatments of 5 mg nanoparticles in 200 μL PBS, and six days after the final treatment, mice were euthanized. Mononuclear cells from the bone marrow were fixed with paraformaldehyde and examined by FACS for EGFP expression (100,000 cells assayed per sample). Each data point represents one mouse.
In addition, we synthesized nanoparticles containing PNAs and DNAs targeting another common mutation in β-thalassemia, a C to T mutation in βIVS2–654. We tested these nanoparticles in transgenic mice, EGFP-65426, whose cells ubiquitously express a modified enhanced green fluorescent protein (EGFP) gene which contains the aberrantly spliced intron from human β-globin, preventing EGFP expression. Correction of the IVS-654 splice site mutation in the β-globin sequences in the fusion gene results in correct splicing and production of EGFP. After treatment with nanoparticles by tail vein injection, treated mice showed EGFP expression in cells from the bone marrow (Figure 8e), using both antisense and sense-sequence donor DNA molecules. For both antisense and sense DNA, when the nanoparticles also contained triplex-forming PNAs, there was substantially increased EGFP expression, with PNA#1 being more effective than PNA#2.
While it is known that polymer nanoparticles similar to those used here have extensive in vivo biodistribution27, 28, the mechanism of nucleic acid delivery via biodegradable nanoparticles is largely unknown. We have previously shown uptake of fluorescent nanoparticles into cells in the bone marrow after IV injection29. Here, we again found particle uptake in the bone marrow 6 hr after injection (Fig. 9), which was not detectable after 24 hr, suggesting clearance over this time period. Since nucleic acid cargo from the PNA/DNA nanoparticles is released relatively rapidly in aqueous solution2, we assume that intracellular nucleic acid delivery occurs within the first few hours after nanoparticle administration. This is consistent with previous biodistribution studies demonstrating that PLGA nanoparticles are rapidly cleared from the blood (half-life of seconds to hours), with longer persistence in bone, lung, spleen, and liver30.
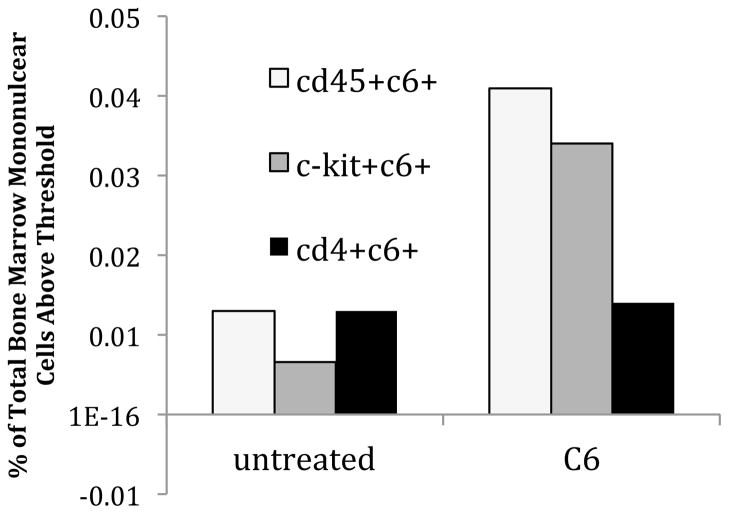
Figure 9. Distribution of fluorescently labeled nanoparticles in hematopoietic cells of mouse.
12 week old NCr nu/nu mice were injected with 4 mg of coumarin 6 (C6) fluorescent dye-loaded PLGA nanoparticles in 200 μL RPMI media (Gibco) via tail vein, or just 200 μL RPMI (untreated). Mice were then sacrificed at 6 hours to assess for nanoparticle uptake in bone marrow cells by FACS. Bone marrow was harvested by flushing of femurs and tibias with RPMI media, and red blood cells were lysed with ACK lysing buffer. Cells were then stained with APC antibodies for FACS. Shown here are the percentage of cells from total bone marrow above a C6 threshold fluorescence, that co-stain with CD45 (hematopoietic cells), c-kit (hematopoietic stem and progenitor cells), and CD4 (T cells) are given.
DISCUSSION
We report here the development of a new technology for direct in vivo site-specific gene editing for the treatment of human disease, using synthetic biodegradable nanoparticles containing triplex-forming PNAs and donor DNAs. This work provides the first demonstration of direct in vivo site-specific gene editing in human cells in a chimeric mouse, and we were able to modify difficult-to-target hematopoietic progenitor cells. We have shown that intravenous injection of PLGA nanoparticles loaded with PNAs/DNAs can produce specific gene modification in the CCR5 gene in human hematopoietic cells and in clinically relevant cell types throughout a humanized mouse, including CD34+ hematopoietic progenitors, CD4+ T cells, and engraftment-capable hematopoietic stem cells.
By deep sequencing we determined the frequency of editing after direct in vivo treatment to be at least 0.05% in the bone marrow, and 0.43% in the spleen, with off-target effects in the CCR2 gene 80 to 100 times less. In comparison, ex vivo treatment of human PBMCs with ZFNs led to much higher levels of modification, on the order of 35%, but the off-target mutagenesis in the CCR2 gene was on the order of 5%, only 7 times less than the on-target modification, ZFNs targeting CCR5 have been approved for use in clinical trials. Hence, the much lower off-target effects of triplex-mediated gene editing should not pose a barrier to possible clinical development. While deep sequencing of the 80% homologous CCR2 gene gives an upper limit of off-target (or “partial-target”) gene modification, with gene disruption31. Despite this relatively higher proportion of off-target both our technology and others, more sophisticated genome-wide screens will be necessary to determine true rates of non-specific induction of mutations.
PCR analysis of individually picked hematopoietic colonies suggested that the targeting frequencies could be at a higher level in certain cell types as modification frequencies were higher in colony forming cells from both the spleen and bone marrow than the frequencies determined by deep sequencing of DNA from whole spleen and bone marrow cell populations. Several possibilities may help to explain this discrepancy. First, the smaller sample size in the colony forming cell assay may have led to falsely inflated modification rates due to the statistical fluctuations inherent in small samples sizes. Second, it may be possible that certain cell types, such as myeloid colony forming cells, are more susceptible to modification than other cell types. Since colony forming progenitors form a small subpopulation within the bone marrow and spleen, it is feasible that modification frequencies could be higher in these cells without significantly increasing the overall modification rate. If this is the case, the mechanism of increased modification remains to be determined, and further studies in both the chimeric and transgenic model systems may help identify other cell types that may also exhibit increased susceptibility to modification. Nonetheless, the modified progenitors were able to differentiate into various hematopoietic colony types and the injected mice showed no inflammatory response in the lung, an indication that nanoparticle treatment in vivo was relatively non-toxic.
While simple unmodified PLGA nanoparticles proved to be effective, there was no additional advantage to using cell-penetrating peptides on the surface of the particles, possibly due to slower release kinetics from these nanoparticles. Multiple methods, including direct sequencing and mRNA expression (AS-RT-PCR), were used to confirm the presence of the targeted modification using the PLGA nanoparticles.
We were also able to use this technology for site-specific editing of the β-globin gene in vivo, both in human cells and in a transgenic EGFP reporter system, demonstrating the versatility of our method. The frequency of gene editing achieved in CCR5, as determined by deep sequencing, was similar to those we achieved in the EGFP reporter, as determined by quantitative flow cytometry. In the EGFP reporter system, the activity of nanoparticles containing both donor DNAs and PNAs was higher than that of nanoparticles containing donor DNA only. The increase in gene modification with PNA addition in vivo is consistent with our previous findings in vitro with both CCR5 and β-globin gene modification. However, it is important to note that both in vitro and in vivo, some gene modification occurs even without the addition of PNA molecules to the nanoparticles. This finding suggests that the nanoparticle system may be useful for delivery of DNA molecules alone for SFHR, which has been used by other groups for gene modification in several model systems including human β-globin32, or for delivery of other nucleic acid molecules such as mRNAs or plasmids expressing ZFNs or TALENs.
In addition, preliminary studies in activated-PBMC engrafted mice showed functional effects of CCR5 modification in terms of resistance to HIV-1 depletion of CD4+ T-cells, although further experiments will be necessary to fully characterize efficacy and toxicity in this system. Because of the short-lived time-span of human cell proliferation in the PBMC-engrafted mice, we were limited in the ability to perform multiple nanoparticle treatments, but our experiments here suggest that multiple treatments over a longer time-span could help develop even larger populations of cells resistant to HIV-1 infection. When our biodegradable nanoparticles are used, multiple treatments are possible, and gene editing frequencies may be cumulative. Thus, although the CCR5 modification frequencies are less than those reported for ZFNs, the simplicity and gentleness of this treatment system offer the possibility for multiple treatments to achieve more significant effects. Nonetheless, we were able to observe some preservation of CD4+ T-cell levels in the nanoparticle-treated mice in the face of HIV-1 infection, so even the low frequencies of modification produced in short-term nanoparticle treatment was seen to yield measurable functional effects in vivo.
In comparison, ex vivo treatment with ZFNs was able to achieve higher levels of CCR5 disruption, with subsequent production of HIV-resistant chimeric mice after transplant of the modified human cells3, 31. However, several advantages do exist for PNA/DNA-containing nanoparticles for gene modification, including the more favorable on-to-off target modification ratio discussed above. In addition, the nanoparticle delivery technology may be useful for diseases in which ex vivo harvest and re-transplantation of modified cells is not an option, such as in cystic fibrosis. Finally, our technology is designed for specific modification of base-pairs, rather than simple gene disruption or full-gene knock-in, for treatment of diseases in which regulation of the mutant gene is important for patient survival.
Several avenues still exist for the possible improvement of this nanoparticle delivery system. Once genome modification occurs, it will persist in the cell progeny, so we do not necessarily need sustained release of our nucleic acid cargo. However, sustained release of nucleic acid cargo may allow more opportunity for genome modification as cells go through multiple rounds of cell division. The use of new polymer blends or polymers such as PLGA with poly-L-lysine19, PBAE/PLGA blends33, or new poly-(amine-co-ester) formulations34 could help tune release kinetics of our nanoparticles. In addition, our group has developed methods for extensive surface modification which may enhance cell-specific targeting and/or uptake of nanoparticles20, 35.
In summary, we have developed a nanoparticle-mediated approach for gene editing that is virus free, non-toxic, has low genotoxicity, can be readily re-engineered to target different genes, targets cells in multiple organs with simple injection, and mediates site-specific modification rather than gene knock-in. This type of direct in vivo gene editing technology could be used to target cells that are difficult to grow ex vivo, treat diseases that involve multiple organ systems, or easily perform multiple treatments to increase overall gene modification frequency. This technology could also be expanded to deliver nucleic acid payloads such as plasmids, siRNA, or microRNAs to difficult-to-target human cells in vivo, providing a new methodology that could open up many new avenues of research in both the study and treatment of human disease.
EXPERIMENTAL METHODS
Oligonucleotides
All donor DNA are 5′ and 3′ end protected by three phosphorothioate internucleoside linkages. In the PNA, “O” represents the 8-amino-2,6-dioxaoctanoic acid linker, and “J” stands for pseudoisocytosine, a replacement for cytosine that allows pH-independent triplex formation. Donor DNA was purchased from Midland Certified Reagent Company (Midland, TX), and PNA was purchased from Bio-Synthesis (Lewisville, TX), or Panagene (Daejeon, South Korea). CCR5 and β-globin targeted PNA and DNA sequences are given in figures 1 and 8. Sequences for PNA and DNA targeting the IVS2–654 site are as follows.
Donor DNA:
5′AAAGAATAACAGTGATAATTTCTGGGTTAAGGCAATAGCAATATCTCTGCATATAAATAT3,
654PNA1: N terminus KKK-JTTTJTTTJTJT-OOO-TCTCTTTCTTTCAGGGCA-KKK C terminus
654PNA2: N terminus KKK-JJJTJJTTJT-OOO-TCTTCCTCCCACAGCTCC-KKK C terminus
Nanoparticle formulation
PLGA nanoparticles were formulated by a double-emulsion solvent evaporation technique as previously described2. 40 nmoles of PNA and 40 nmoles of DNA in 61.6 μL dH20 were added dropwise to a polymer solution of 80 mg 50:50 ester-terminated PLGA dissolved in 800 μL dichloromethane, then sonicated. This first emulsion was then added dropwise to 1.6 mL 5% polyvinyl alcohol, and sonicated again. The final mixture was added to 20 mL of 0.3% polyvinyl alcohol, stirred at room temperature for 3 hours to evaporate the dichloromethane, and then nanoparticles were collected, washed, frozen, and lyophilized. Surface modifiers were added to the second emulsion at 4 nmole DSPE-PEG-ligand/mg PLGA. While equal nmoles of DNA were delivered to each treatment group, this corresponds to different milligram quantities of nanoparticles because of variability in loading. Loading of CD particles was 0.584 +/− 0.01 nmoles/mg, loading of DP particles was 0.308 +/− 0.005 nmoles/mg, loading of AP particles was 0.307 +/− 0.005 nmoles/mg, and loading of TAT particles was 0.293 +/− 0.008 nmoles/mg.
Mouse models
Animal use was in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of Massachusetts Medical School, Yale University, and The Jackson Laboratory. NOD.Cg-PrkdcscidIL2rγtm1Wjl (abbreviated NOD-scid IL2rγnull ) mice were obtained from the research colony maintained at The Jackson Laboratory. CD34-reconstituted NOD-scid IL2rγnull mice were generated by intracardiac injection of cord-blood derived human CD34+ cells into newborn mice as previously described36. Twelve weeks post transplant, mononuclear cells from peripheral blood were harvested by eye bleeds for staining and FACS analysis for the presence of human cell markers to confirm engraftment. Five mg of nanoparticles were resuspended in 200 μL PBS with brief water sonication, and injected via tail vein. Mice were sacrificed 5 or 10 days post treatment, and organs/cells harvested and frozen for subsequent analysis.
The 654EGFP transgenic mice were obtained from the Ryszard Kole and Rudolph Juliano at the University of North Carolina, and bred and maintained at the Yale University animal facilities in the Boyer Center for Molecular Medicine according to the guidelines of the IACUC of Yale University.
Cell sorting
The Miltenyi Beads Kit catalog #130-045-702 (Miltenyi Biotec, Auburn, CA) was used to isolate human CD4+ cells from splenocytes from each homogenized mouse spleen as per manufacturer’s protocol. Human CD34+ cells were isolated from mouse bone marrow using the Miltenyi Beads Kit catalog #130-045-702 as per the manufacturer protocol. For serial transplant experiments, mouse CD45+ cells were removed from the bone marrow using Miltenyi Beads Kit catalog #130-042-301.
Genomic DNA extraction
Genomic DNA was isolated from frozen cells or organs using a phenol-chloroform extraction method. Organs/cells were digested overnight in 10 mM Tris HCl pH 8, 150 mM NaCl, 20 mM EDTA, 1% SDS, with proteinase K. Digests were subjected to extraction with phenol/chloroform/isoamyl alcohol followed by re-extraction with choloroform, precipitated with KoAc in EtOH, spun down and dried at room temperature, and resuspended in dH2O.
Quantitative AS-PCR
Equal amounts of genomic DNA from each sample were subjected to allele-specific PCR, with a gene-specific reverse primer, and an allele-specific forward primer in which the 3′ end corresponds to the 6 bp modified sequence, primers and reaction mixes are previously described (Schleifman et al8 Chin et al13). Quantitative PCR was performed using a Stratagene Mx 3000P cycler. 0.2 μM donor DNA was used in spiking experiments. PCR products were separated on a 1% agarose gel and visualized using a gel imager. Primer sequences and cycler programs available on request. Relative gene modification was calculated using the 2−ΔΔCt method, with the average of the untreated controls used as the reference groups24.
Deep Sequencing
Bar-coded PCR amplicons were generated spanning a 100 bp region of the CCR5 or CCR2 gene, with the location of the targeted modification near the center of the amplicon. Samples were ligated to adapters and sequenced on an Illumina HiSeq platform, with 75 base-pair paired-end reads, at the Yale West Campus Keck Sequencing Facility.
Reverse-transcription AS-PCR for CCR5
RNA extraction was performed using the RNAeasy Plus Qiagen kit (Gaithersburg, MD). cDNA was then made using the Invitrogen superscript III kit (Carlsbody, CA). Forward primers were designed to bind in exon 2 with allele-specific reverse primers binding in exon 3, to guarantee cDNA rather than genomic DNA amplification. PCR reactions contained cDNA, 20% Betaine, 0.2 mM dNTPS, Advantage 2 Polymerase Mix, 0.2 μM of each primer, and 2% Platinum Taq. Primer sequences and cycle programs available on request8.
Colony forming cell assay for human cells
Bone marrow or splenocytes were plated in MethoCult H4434 Classic methylcellulose medium with recombinant cytokines for human cells (Stemcell Technologies, Vancouver, Canada), which is formulated to support the growth of human hematopoietic progenitor cells into erythroid, granulocyte, macrophage, or mixed colonies. Colonies were counted and harvested after 1 and 2 weeks. All individual colonies were picked directly into lysis buffer for phenol-choloroform genomic DNA extraction and subsequent AS-PCR.
PBMC mouse model and HIV infection
5×106 Δ32 heterozygous PBMCs were injected into adult NOD-scid IL2rγnull mice by IP injection in 400–500 μL. 3, 5, and 7 days post injection of PBMCs, mice were treated with 5 mg nanoparticles (in 200 μL PBS), either with (6 mice) or without (blank, 6 mice) the CCR5-modifying PNA and DNA molecules. 10 days post injection of PBMCs, 4 mice in each treatment group were infected with 30pfu HIV-1BaL by IP injection in 100 μL volume. Blood was collected in heparin by retro-orbital bleeds at various timepoints post injection, and mononuclear cells stained for analysis of CD4 expression by FACs.
Measurement of production of inflammatory cytokines
Production of TNF-alpha and IL6 mRNA was measured from RNA extracted from lungs using the RNAeasy Plus kit as above. and cDNA synthesized with SuperScript II First-Strand Kit (Invitrogen). Quantitative PCR was performed on cDNA with 20% Betaine (Sigma), 0.2 mM dNTPS (American Bioanalytical), advantage 2 polymerase mix (Clontech), SybrGreen, ROX, and 2% Platinum Taq (Invitrogen, Calrsbad, CA). These primers have previously been used37 and their sequences are as follows. TNF-alpha: 5′GTGGAGATCTCTTCTTGCAC3′ and 3′AGTGCCCTTAACATTCTCAAG5′. IL6: 5′ACTCACCTCTTCAGAACGAA3′ and 3′GTCTCCTCATTGAATCCAGA5′. GAPDH sense 5′GAAGGTGAAGGTCGGAGT3′ and 3′GAAGATGGTGATGGGATTTC5′. The cycle conditions were 94°C 2 min, followed by 40 cycles of 94°C 30s, 50°C 30s, and 72°C 1min. The 2−ΔCt method was used to calculate relative mRNA expression, with GAPDH as the reference gene.
Graphs and Statistical Analysis
Graphs were created using Microsoft Excel 2007. Data averaged for multiple samples is given as the mean +/− standard deviation.
Supplementary Material
Acknowledgments
Supported by: NIGMS Medical Scientist Training Program T32GM07205 (to N.A.M.), the NIH Genetics Training Grant T32 GM007499 (to E.B.S.) and the National Institute of Health grants R01HL082655 (to P.M.G) and R01EB000487 (to W.M.S.). This work was also supported by F30HL110372 from the National Heart, Lung, and Blood institute (to N.A.M.). This work was also supported by National Institutes of Health research grants AI46629 (DLG, LDS, MAB), AI083911 (MAB), HL077642 (LDS), CA34196 (LDS), AI073871 (DLG, LDS), DK32520 (DLG, LDS), P30 AI042845 (MAB), and grants from the Juvenile Diabetes Foundation, International (DLG, LDS, MAB), and the Helmsley Foundation (DLG, LDS, MAB).
We thank Hanspeter Neiderstrasser and Faye Rogers for their technical assistance and helpful discussions. We also thank John Overton, Francesc Lopez, and Jennifer Yamtich for assistance with deep sequencing and analysis. This work was supported by the NIGMS Medical Scientist Training Program T32GM07205 (to N.A.M.), the NIH Genetics Training Grant T32 GM007499 (to E.B.S.) and the National Institute of Health grants R01HL082655 (to P.M.G) and R01EB000487 (to W.M.S.). This work was also supported by F30HL110372 from the National Heart, Lung, and Blood institute, and the content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI. This work was also supported by National Institutes of Health research grants AI46629 (DLG, LDS, MAB), AI083911 (MAB), HL077642 (LDS), CA34196 (LDS), AI073871 (DLG, LDS), DK32520 (DLG, LDS), P30 AI042845 (MAB), and grants from the Juvenile Diabetes Foundation, International (DLG, LDS, MAB), and the Helmsley Foundation (DLG, LDS, MAB). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
The authors report no commercial conflicts of interest.
Supplemental information is available on the journal website.
References
- 1.Woods NB, Bottero V, Schmidt M, von Kalle C, Verma IM. Gene therapy: therapeutic gene causing lymphoma. Nature. 2006;440(7088):1123. doi: 10.1038/4401123a. [DOI] [PubMed] [Google Scholar]
- 2.McNeer NA, Chin JY, Schleifman EB, Fields RJ, Glazer PM, Saltzman WM. Nanoparticles Deliver Triplex-forming PNAs for Site-specific Genomic Recombination in CD34(+) Human Hematopoietic Progenitors. Mol Ther. 2010 doi: 10.1038/mt.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holt N, Wang J, Kim K, Friedman G, Wang X, Taupin V, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol; 28(8):839–47. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maurisse R, De Semir D, Emamekhoo H, Bedayat B, Abdolmohammadi A, Parsi H, et al. Comparative transfection of DNA into primary and transformed mammalian cells from different lineages. BMC Biotechnol. 2010;10:9. doi: 10.1186/1472-6750-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasquez KM, Narayanan L, Glazer PM. Specific mutations induced by triplex-forming oligonucleotides in mice. Science. 2000;290(5491):530–3. doi: 10.1126/science.290.5491.530. [DOI] [PubMed] [Google Scholar]
- 6.Goncz KK, Colosimo A, Dallapiccola B, Gagne L, Hong K, Novelli G, et al. Expression of DeltaF508 CFTR in normal mouse lung after site-specific modification of CFTR sequences by SFHR. Gene Ther. 2001;8(12):961–5. doi: 10.1038/sj.gt.3301476. [DOI] [PubMed] [Google Scholar]
- 7.Rogers FA, Lin SS, Hegan DC, Krause DS, Glazer PM. Targeted Gene Modification of Hematopoietic Progenitor Cells in Mice Following Systemic Administration of a PNA-peptide Conjugate. Mol Ther. 2012;20(1):109–18. doi: 10.1038/mt.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schleifman EB, Bindra R, Leif J, Del Campo J, Rogers FA, Uchil P, et al. Targeted Disruption of the CCR5 Gene in Human Hematopoietic Stem Cells Stimulated by Peptide Nucleic Acids. Chem Biol. 18(9):1189–98. doi: 10.1016/j.chembiol.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Haurigot V, Doyon Y, Li T, Wong SY, Bhagwat AS, et al. In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature. 475(7355):217–21. doi: 10.1038/nature10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goncz KK, Prokopishyn NL, Chow BL, Davis BR, Gruenert DC. Application of SFHR to gene therapy of monogenic disorders. Gene Ther. 2002;9(11):691–4. doi: 10.1038/sj.gt.3301743. [DOI] [PubMed] [Google Scholar]
- 11.Goncz KK, Kunzelmann K, Xu Z, Gruenert DC. Targeted replacement of normal and mutant CFTR sequences in human airway epithelial cells using DNA fragments. Hum Mol Genet. 1998;7(12):1913–9. doi: 10.1093/hmg/7.12.1913. [DOI] [PubMed] [Google Scholar]
- 12.Rogers FA, Vasquez KM, Egholm M, Glazer PM. Site-directed recombination via bifunctional PNA-DNA conjugates. Proc Natl Acad Sci U S A. 2002;99(26):16695–700. doi: 10.1073/pnas.262556899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chin JY, Kuan JY, Lonkar PS, Krause DS, Seidman MM, Peterson KR, et al. Correction of a splice-site mutation in the beta-globin gene stimulated by triplex-forming peptide nucleic acids. Proc Natl Acad Sci U S A. 2008;105(36):13514–9. doi: 10.1073/pnas.0711793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chin JY, Schleifman EB, Glazer PM. Repair and recombination induced by triple helix DNA. Frontiers in bioscience: a journal and virtual library. 2007;12:4288–97. doi: 10.2741/2388. [DOI] [PubMed] [Google Scholar]
- 15.Chin JY, Glazer PM. Repair of DNA lesions associated with triplex-forming oligonucleotides. Mol Carcinog. 2009;48(4):389–99. doi: 10.1002/mc.20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang G, Seidman MM, Glazer PM. Mutagenesis in mammalian cells induced by triple helix formation and transcription-coupled repair. Science. 1996;271(5250):802–5. doi: 10.1126/science.271.5250.802. [DOI] [PubMed] [Google Scholar]
- 17.Vasquez KM, Christensen J, Li L, Finch RA, Glazer PM. Human XPA and RPA DNA repair proteins participate in specific recognition of triplex-induced helical distortions. Proc Natl Acad Sci U S A. 2002;99(9):5848–53. doi: 10.1073/pnas.082193799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8(6):526–33. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blum JS, Saltzman WM. High loading efficiency and tunable release of plasmid DNA encapsulated in submicron particles fabricated from PLGA conjugated with poly-L-lysine. J Control Release. 2008;129(1):66–72. doi: 10.1016/j.jconrel.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng CJ, Saltzman WM. Enhanced siRNA delivery into cells by exploiting the synergy between targeting ligands and cell-penetrating peptides. Biomaterials. 2011;32(26):6194–203. doi: 10.1016/j.biomaterials.2011.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu XS, Wang N. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers. Part II: biodegradation. J Biomater Sci Polym Ed. 2001;12(1):21–34. doi: 10.1163/156856201744425. [DOI] [PubMed] [Google Scholar]
- 22.McNeer NA, Chin JY, Schleifman EB, Fields RJ, Glazer PM, Saltzman WM. Nanoparticles deliver triplex-forming PNAs for site-specific genomic recombination in CD34+ human hematopoietic progenitors. Mol Ther. 19(1):172–80. doi: 10.1038/mt.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domashenko AD, Danet-Desnoyers G, Aron A, Carroll MP, Emerson SG. TAT-mediated transduction of NF-Ya peptide induces the ex vivo proliferation and engraftment potential of human hematopoietic progenitor cells. Blood. 2010;116(15):2676–83. doi: 10.1182/blood-2010-03-273441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal S, Temsamani J, Tang JY. Pharmacokinetics, biodistribution, and stability of oligodeoxynucleotide phosphorothioates in mice. Proc Natl Acad Sci U S A. 1991;88(17):7595–9. doi: 10.1073/pnas.88.17.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts J, Palma E, Sazani P, Orum H, Cho M, Kole R. Efficient and persistent splice switching by systemically delivered LNA oligonucleotides in mice. Mol Ther. 2006;14(4):471–5. doi: 10.1016/j.ymthe.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Park J, Fong PM, Lu J, Russell KS, Booth CJ, Saltzman WM, et al. PEGylated PLGA nanoparticles for the improved delivery of doxorubicin. Nanomedicine. 2009 doi: 10.1016/j.nano.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris TJ, Green JJ, Fung PW, Langer R, Anderson DG, Bhatia SN. Tissue-specific gene delivery via nanoparticle coating. Biomaterials. 2010;31(5):998–1006. doi: 10.1016/j.biomaterials.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNeer NA, Schleifman EB, Glazer PM, Saltzman WM. Polymer delivery systems for site-specific genome editing. J Control Release. 2011;155(2):312–6. doi: 10.1016/j.jconrel.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panagi Z, Beletsi A, Evangelatos G, Livaniou E, Ithakissios DS, Avgoustakis K. Effect of dose on the biodistribution and pharmacokinetics of PLGA and PLGA-mPEG nanoparticles. Int J Pharm. 2001;221(1–2):143–52. doi: 10.1016/s0378-5173(01)00676-7. [DOI] [PubMed] [Google Scholar]
- 31.Perez EE, Wang J, Miller JC, Jouvenot Y, Kim KA, Liu O, et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26(7):808–16. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goncz KK, Prokopishyn NL, Abdolmohammadi A, Bedayat B, Maurisse R, Davis BR, et al. Small fragment homologous replacement-mediated modification of genomic beta-globin sequences in human hematopoietic stem/progenitor cells. Oligonucleotides. 2006;16(3):213–24. doi: 10.1089/oli.2006.16.213. [DOI] [PubMed] [Google Scholar]
- 33.Little SR, Lynn DM, Ge Q, Anderson DG, Puram SV, Chen J, et al. Poly-beta amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proc Natl Acad Sci U S A. 2004;101(26):9534–9. doi: 10.1073/pnas.0403549101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou J, Liu J, Cheng CJ, Patel TR, Weller CE, Piepmeier JM, et al. Biodegradable poly(amine-co-ester) terpolymers for targeted gene delivery. Nat Mater. 11(1):2–90. doi: 10.1038/nmat3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou J, Patel TR, Fu M, Bertram JP, Saltzman WM. Octa-functional PLGA nanoparticles for targeted and efficient siRNA delivery to tumors. Biomaterials. 2012;33(2):583–91. doi: 10.1016/j.biomaterials.2011.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearson T, Greiner DL, Shultz LD. Creation of “humanized” mice to study human immunity. Curr Protoc Immunol. 2008;Chapter 15(Unit 15):21. doi: 10.1002/0471142735.im1521s81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park MC, Chung SJ, Park YB, Lee SK. Pro-inflammatory effect of leptin on peripheral blood mononuclear cells of patients with ankylosing spondylitis. Joint Bone Spine. 2009;76(2):170–5. doi: 10.1016/j.jbspin.2008.04.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.