Abstract
Adoptive transfer of primary T cells genetically modified to have desired specificity can exert an anti-tumor response in some patients. To improve our understanding of their therapeutic potential we have developed a clinically-appealing approach to reveal their in vivo biodistribution using nanoparticles that serve as a radiotracer for imaging by positron emission tomography (PET). T cells electroporated with DNA plasmids from the Sleeping Beauty transposon/transposase system to co-express chimeric antigen receptor (CAR) specific for CD19 and Firefly luciferase (ffLuc) were propagated on CD19+ K562-derived artificial antigen presenting cells. The approach to generating our clinical-grade CAR+ T cells was adapted to electro-transfer of gold nanoparticles (GNP) functionalized with 64Cu2+ using the macrocyclic chelator (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid, DOTA) and polyethyleneglycol (GNP-64Cu/PEG2000). MicroPET/CT was used to visualize CAR+EGFPffLucHyTK+GNP-64Cu/PEG2000+ T cells and correlated with bioluminescence imaging and assessment of radioactivity. These data demonstrate that GNPs conjugated with 64Cu2+ can be prepared as radiotracer for PET and used to image T cells using an approach that has translational implications.
INTRODUCTION
Adoptive cell therapy infusing primary T cells genetically modified to express a chimeric antigen receptor (CAR) specific for a tumor-associated antigen has been shown to be effective against hematologic malignancies and solid tumors.1 The genetic manipulation of primary T cells can improve potency through the engineering of CAR2 to impart a fully-competent activation signal as measured in part by the persistence and homing after infusion. To assess biodistribution of systemically-administered CAR+ T cells, investigators typically undertake quantitative PCR and flow cytometry using CAR-specific probes from serially sampled tissues and peripheral blood.3 However, this is invasive and does not provide real-time whole-body spatio-temporal distribution of infused T cells. Longitudinal non-invasive imaging can be undertaken on T cells genetically modified to enforce expression of reporter genes, such as Firefly luciferase (ffLuc) for bioluminescent imaging (BLI)4 in animal models and thymidine kinase (TK) and associated muteins from herpes simplex virus-1 for positron emission tomography (PET).5 Locally administered human TK+ T cells and intravenously-infused macaque T cells have been imaged by PET.6 Additional studies are needed regarding improving sensitivity and reducing immunogenicity before systemically administered TK+ T cells can be reproducibly imaged by PET in clinical trials. Compounding the difficulties associated with human application of this approach to PET is that 18F-based probes requires enzymatic trapping of the radiotracer in the cytoplasm by recombinant TK and the infused non-metabolized 18F creates a background signal from pools within tissues and undermines sensitivity.3, 6b, 7 The short radioactive half-life (t1/2= 109.8 min) of 18F also imposes practical limitations and requires an on-site cyclotron or expedited delivery of up to 500 mCi 18F-based probes as starting material for single infusion of 10 mCi per patient. 64Cu conjugated to lipophilic chelator pyruvaldehyde-bis(N4-methylthiosemicarbazone) for PET tracking has been proposed as an alternative and used to track C6 rat glioma cells in mice for up to 20 hours, although leakage of 64Cu from cells was observed.8 Gold nanoparticles, however, have been investigated for their uptake and have been found appropriate for intracellular retention.9 Therefore, as an alternative to in vivo labeling we developed an ex vivo approach to radiolabel primary T cells with gold nanoparticle (GNPs) conjugated to 64Cu (GNP-64Cu/PEG2000) using electroporation that renders T cells capable of being imaged by PET.
Engineering of immunotherapies is a burgeoning field with active contribution from physical technologies10 and can be used to address and important challenge and an unmet clinical need for the in vivo tracking of tumor-targeting T cells.11 Assessments of T-cell trafficking kinetics to tumor locations have been made.12 A recent study by Koya et al.12b using signal correlation from PET and BLI reporter genes showed that T cells home within 2 to 5 days leading to reduction in tumor sizes. We demonstrated that cultured CAR+ T cells pre-labeled with 64Cu before infusion could be tracked in vivo using μPET/CT. Although room for improvement still remain, (e.g. towards the impact of electroporation process on T-cell death and contribution of free GNP-64Cu/PEG2000 released from necrotic cells) our work has translational implications as we use an approach that can be undertaken in compliance with current good manufacturing practice for Phase I/II trials.
RESULTS & DISCUSSION
We have developed a strategy to genetically modify primary peripheral blood mononuclear cells (PBMC) and propagate CD19-specific CAR+ T cells that have application in clinical trials for patients with B-cell malignancies (INDs# 14193, 14577, and 14739). Our approach uses the Sleeping Beauty (SB) transposon/transposase system,13 a non-viral gene delivery method based on electro-transfer of DNA plasmids, to introduce the CAR and designer artificial antigen presenting cells (aAPC) to retrieve and numerically expand genetically modified primary T cells.14 We adapted the electroporation process to co-express a 2nd generation CAR (that signals through CD28 and CD3-ζ) and ffLuc. This was achieved using a process we dubbed “double transposition” (Figure 1A).13–14 Thawed PBMC isolated from the peripheral blood of healthy volunteer donors were electroporated with three SB DNA supercoiled plasmids expressing three codon optimized15 (CoOp) genes: (i) CAR transposon (Figure 1B), (ii) fusion of enhanced Green Fluorescent Protein (EGFP), ffLuc, hygromycin phosphotransferase (Hy), thymidine kinase (TK) to create the EGFPffLucHyTK transposon (Figure 1C), and (iii) SB11 transposase (Figure 1D). The genetically modified T cells (designated CAR+EGFPffLucHyTK+) were selectively propagated on γirradiated designer aAPC in the presence of recombinant human cytokines IL-2 and IL-21 in the presence of cytocidal concentration of hygromycin B. The aAPC (clone #4)16 are derived from K562 and genetically modified to express truncated CD19 along with desired co-stimulatory molecules (CD64, CD86, CD137L, and membrane bound IL-15 (mIL-15) co-expressed with EGFP). The electroporated and propagated T cells homogeneously expressed CD3 with 12% co-expressing CD4 and 84% co-expressing CD8. Flow cytometry also revealed 68% expression of CAR and 78% expression of EGFP (Figure S1). To track the T cells in vivo we modified the electroporation procedure to load the genetically modified T cells with GNPs modified to function as a reporter for PET. 64Cu2+ was chosen as a radioisotope for PET based on longer half-life of 64Cu (t1/2= 12.7 hr) compared to 18F, and absence of high-energy γ-emission that could otherwise lead to DNA damage in the cells.17 64Cu2+ was conjugated to 7 nm GNPs using the macrocyclic chelator, 1,4,7,10-tetraazacyclododecane-1,4,7,10- tetraacetic acid (DOTA)18 and coated with long chain polyethylene glycol to make it biocompatible (GNP-64Cu/PEG2000) for the final size of 35 nm (Figure 1E).19 Although, endocytosis has been investigated as a usual mode for internalization of GNP, it requires extended periods of time,20 and is a practical limitation with radioactive material which have short half life. Therefore, we developed electro-transfer as a method to label T cells with GNP-64Cu/PEG2000 due to its ability to instantly label the cells.
Figure 1.
(A) Schematic of the processes for the generation of CAR+EGFPffLucHyTK+GNP-64Cu/PEG2000+ T cells. Components used include (B) CAR transposon (CD19RCD28mZ(CoOp)/pSBSO). (C) SB11 transposon (pKan-CMV-SB11) (D) ffLuc transposon (EGFPffLucHyTK) (E) GNP-64Cu/PEG2000; Synthesis of DOTA-TA). 7 nm GNP (shown) was compared with 15 nm GNP. All other parameters stayed the same during this comparison. TEA, triethylamine; DCM, dichloromethane.
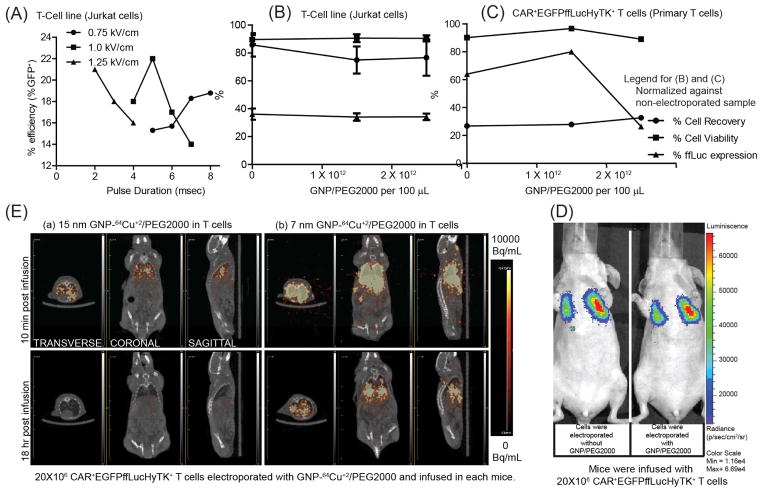
In vitro testing of electroporated and propagated CAR+EGFPffLucHyTK+ primary T cells revealed ffLuc activity at 2.17 ± 0.08 CPM/cell upon administration of D-Luciferin, compared with 0.011 ± 0.001 CPM/cell when no D-Luciferin was administered (data not shown). The ffLuc activity is a measure of T-cell viability as this enzyme requires ATP as a co-factor which is present only in live cells. Electroporation parameters were adjusted to improve uptake of GNPs. Initially, acute T-cell leukemia cell line (Jurkat cells) were used to assess the efficiency of electro-transfer by EGFP expression using control DNA plasmid (designated as pmaxGFP). The highest transfection efficiency, as measured by transient GFP expression 24 hours after electroporation was observed at the field strength of 1 kV/cm (200 V across 2 mm electrode spacing) applied for 5 msec using square-wave pulse generator (Figure 2A). (This finding was supported by repeating the experiment (Figure S2). Although, conditions for electroporation may differ for cell types, 1 kV/cm has been previously reported in literature as an appropriate value for transfection in mammalian cells and was therefore adopted for future experiments.21 GNP/PEG2000 (synthesized in a similar fashion as GNP-64Cu/PEG2000 but without 64Cu2+ addition) was used to determine the concentration of GNP-64Cu/PEG2000 to be used in the electroporation reaction. Using the conditions determined for electro-transfer of DNA plasmid into T-cell line (Jurkat cells), we first investigated the effect of GNP/PEG2000 concentration and its electro-transfer into 5x106 GFP+ffLuc+ T-cell lines (Jurkat cells) (Figure 2B). Since primary cell response differ from donor to donor, we repeated this investigation on 20x106 CAR+EGFPffLucHyTK+ T cells using the cells from the donor that was used in vivo (Figure 2C). GNP/PEG2000 concentration of 1.5×1012 particles in 100 μL of electroporation reaction was considered as appropriate for electro-transfer into 20x106 CAR+EGFPffLucHyTK+ primary T cells in context of cell recovery, ffLuc expression and cell viability determined by trypan blue exclusion method. To assess the impact of electro-transferred GNPs on BLI, we electroporated CAR+EGFPffLucHyTK+ T cells with 1.5×1012 GNP/PEG2000 using 1 kV/cm for 5 msec and intravenously injected 20x106 T cells in a mouse. There was no significant difference in average radiance from mouse infused with T cells that did (1.33e4 p/s/cm2/sr) and did not (1.12e4 p/s/cm2/sr) carry the GNPs (Figure 2D) which supports the premise that although the process of electroporation may have the potential to affect cell viability, the presence of GNP/PEG2000 in the cells did not detract from their ability to affect ffLuc activity.
Figure 2.
(A) Effect of electric field intensity and pulse duration on efficiency of electro-transfer of DNA plasmid (pmaxGFP) into T-cell lines (Jurkat cells). (B,C) Cell recovery, cell viability and ffLuc expression after electroporation of (B) GFP+ffLuc+ T-cell lines (Jurkat cells) and (C) CAR+EGFPffLucHyTK+ T cells with GNP/PEG2000 (normalized against non-electroporated sample) as a function of GNP/PEG2000 concentration. (D) Effect of intracellular GNP/PEG2000 on in vivo BLI signal from ffLuc. (E) Transverse, coronal, and sagittal planes of mice infused with T cells labeled with 15 nm and 7 nm GNP-64Cu/PEG2000, respectively, imaged after 10 min and 18 hours by μPET/CT.
To assess the impact of size of GNPs to cross pores in the T-cell membrane introduced by electroporation, we electro-transferred 15 nm and 7 nm GNPs conjugated with 64Cu2+ and PEG2000 into 20x106 CAR+EGFPffLucHyTK+ primary T cells which resulted in 1 μCi and 5.8 μCi of radioactivity for the two sets of cells. T cells were injected into two mice and μPET imaging of transverse, coronal, and sagittal planes was undertaken beginning at 10 minutes post infusion (Figure 2E). Although initial PET signal was observed from both mice, longer term in vivo PET signal was observed only from mice that received T cells bearing 7 nm GNP-64Cu/PEG2000. We hypothesize that 15 nm GNP-64Cu/PEG2000 were less efficiently electro-transferred into T cells due to the increased size of the GNP.
The 7 nm GNP-64Cu/PEG2000 was then used to label genetically modified CAR+EGFPffLucHyTK+ T cells using the established electroporation conditions (20x106 T cells, 1.5×1012 GNP-64Cu/PEG2000, 100 μL, 1 kV/cm, 5 msec) to deliver 3 μCi. 20x106 radiolabeled primary T cells were intravenously injected and imaged using μPET/CT and BLI. The PET signals from transverse, coronal, and sagittal planes of the mouse co-localized with the BLI signal (Figure 3A). As the BLI signal indicates the presence of live cells, the co-localized PET/CT and BLI data supports the hypothesis that viable (metabolically active) genetically modified T cells can be tracked in vivo using positron emitter, 64Cu, and imaged by μPET/CT scanner.
Figure 3.
(A) PET images (transverse, coronals and sagittal planes) from CAR+EGFPffLucHyTK+GNP-64Cu/PEG2000+ T cells infused in a mouse correlated with BLI signal. (B) Comparison of post-mortem biodistribution of the GNP-64Cu/PEG2000 when enveloped inside the T cells compared to when infused directly, 14 hours after intravenous infusion. (C) Lung-to-liver and lung-to-spleen ratios of GNP-64Cu/PEG2000 when enveloped inside the T cells compared to direct injection, 14 hours after intravenous injection.
GNPs loaded within primary T cells are expected to bypass clearance by the liver and spleen which are part of the reticuloendothelial system (RES). This was assessed by comparing the biodistribution of 20x106 T cells radiolabeled with GNP-64Cu/PEG2000 compared with free GNP-64Cu/PEG2000 (90 μCi) also administered via tail vein. The post-mortem biodistribution of the radioactivity, 14 hours after infusion, revealed that the T cells preferentially carried the GNP-64Cu/PEG2000 to the lungs. Whereas, when free GNP-64Cu/PEG2000 were administered, comparatively larger fraction was found outside the pulmonary system (Figure 3B) and into major RES organs (liver and spleen)22. Kennedy et al.23 also reported four-fold more efficient delivery of GNP to the tumor site when transported within T cells to the tumor locations, compared to accumulation via enhanced permeability and retention effect. The loading of GNP-64Cu/PEG2000 in T cells resulted in less uptake by RES and desired delivery to the lungs. Cell-free GNP-64Cu/PEG2000 was taken up 17.5-fold more in liver and 25-fold more in spleen compared to when packaged in T cells (Figure 3C). This supports the premise that T cells can act as delivery vehicles for nanostructures without being sequestered by the RES.23–24
In this report, as another example of contribution of engineering approaches to immunotherapy,10 we show that 64Cu conjugated to GNP and coated with PEG could be used to image primary T cells in vivo. A barrier to successful implementation of cell-based therapies is determination of optimal dosage and schedule for administering the biologic and ascertaining that these cells reach their desired target locations.25 As a result, non-invasive real-time monitoring of infused cells has been an area of active research as it can inform on the dosage needed to achieve therapeutically effective numbers of infused cells within desired tissues. Various methods under consideration for tracking include ex vivo and in vivo labeling of cells for imaging and each has pros and cons.11 An advantage of our approach using GNPs is that the conjugation to 64Cu as well as the electro-transfer into T cells can be undertaken using methods in compliance with current good manufacturing practice for Phase I/II trials.
MATERIALS AND METHODS
DNA plasmids
Three DNA plasmids expressing kanamycin resistance gene were constructed coding for three codon optimized (CoOp) transgenes flanked by IR/DR sequences and used to generate CAR+EGFPffLucHyTK+ T cells. (1) CD19-specific CAR expressed under human elongation factor 1-α (hEF-1α) promoter (CD19RCD28mZ(CoOp)/pSBSO) (Figure 1B),26 (2) multi-function protein fusing ffLuc downstream of EGFP and upstream of hygromycin phosphotransferase (Hy) and thymidine kinase (TK, HyTK) expressed under hEF-1α promoter (EGFPffLucHyTK) (Figure 1C), (3) SB11 transposase expressed under cytomegalovirus (CMV) promoter (pKan-CMV-SB11) (Figure 1D).26 Supercoiled DNA plasmid pmaxGFP, coding for EGFP, was used to assess the efficiency of electro-transfer by measuring fluorescence.
GNP-64Cu/PEG2000
GNPs with average diameter of 7 nm were purchased from Nanopartz, Inc, CO. 64CuCl2 was produced on a CS-15 biomedical cyclotron at Washington University School of Medicine. DOTA-TA was synthesized by linking p-NH2- Bn-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (Macrocyclics Inc., TX, USA) and thioctic acid (TA) (Acros, NJ, USA) with a di-amine linker.27 GNPs were functionalized by forming gold-thiol bond with DOTA-TA (GNP-DOTA).27 GNP-DOTA were incubated with 64Cu2+ at pH 6.5 at 50°C followed by PEGylation using thiolated PEG (Item# MPEG-SH-2000; Laysan Bio, Inc, AL, USA) (GNP-64Cu/PEG2000)28 (Figure 1E). Radiolabeled GNP-64Cu/PEG2000 was measured 35 nm in diameter by dynamic light scattering and the labeling efficiency was > 95% determined by radio thin layer chromatography.
Artificial antigen presenting cells (aAPC)
K562-derived aAPCs (referred to as clone #4) weretransduced with lentivirus to co-express CD19, CD64, CD86, CD137L (CD137L), and membrane-bound (m) interleukin (IL)-15 (mIL-15, co-expressed with EGFP, Figure 1A) and used to support the ex vivo numeric expansion of CD19-specific CAR+EGFPffLucHyTK+ T cells.16
Peripheral Blood Mononuclear Cells (PBMC)
PBMC were isolated from blood by Ficoll- Paque density gradient centrifugation (Cat # 17-1440-02, GE Healthcare Bio-sciences AB, Sweden), re-suspended in freezing medium (40% RPMI-1640, 50% fetal bovine serum (FBS), 10% DMSO) at the density of 20x106 cells/mL/vial, and stored in liquid nitrogen.14a
Generation of EGFP+ acute T-cell leukemia cell line (Jurkat cells)
On the day of electroporation (Day 0), 106 Jurkat cells were re-suspended in 100 μL of phosphate buffered saline (PBS) (Cat# D8537, Sigma-Aldrich, Inc., MO, USA) along with pmaxGFP (2 μg supercoiled DNA, Lonza Cologne GmbH, Germany), transferred to a single cuvette, and electroporated with specific pulse duration and amplitude using ECM 830 BTX electroporation apparatus (BTX Instrument Division, Harvard Apparatus, Inc., MA, USA). The cells were cultured for 24 hours at 37°C in phenol-free RPMI-1640 containing 10% FBS and 2 mM L-alanyl-L-glutamine (GlutaMAX™, Cat # 35050, Invitrogen Corp., CA, USA) before analyzing for EGFP expression.
Generation of GFP+ffLuc+ acute T-cell leukemia cell line (Jurkat cells)
Jurkat cells were cultured in RPMI-1640 containing 10% FBS and 2 mM L-alanyl-L-glutamine (GlutaMAX™, Cat # 35050, Invitrogen Corp., CA, USA) and transduced with lentiviral particles carrying ffLuc-F2A-GFP reporter gene expressed under blasticidin resistance gene (Cat# LVP323, GenTarget Inc., San Diego, CA, USA) using manufacturer’s protocol. Briefly, lentiviral particles were thawed at room temperature and 50 μL of virus was added to 0.5 mL cells (diluted to 106 cells/mL). Blasticidin S (Cat # ant-bl-5, InvivoGen, CA, USA) was added to the culture beginning 4 days after transduction and maintained at a concentration of 0.1 mg/mL until cryopreservation or use.
Generation and analysis of CAR+EGFPffLucHyTK+ T cells
CD19-specific CAR+EGFPffLucHyTK+ T cells were propagated from PBMC after SB transposition as previously described14a and depicted in Figure 1A. On the day of electroporation (Day 0), 20x106 PBMC were thawed at 37°C in a water bath and re-suspended in 100 μL of Amaxa Nucleofector solution (Human T cell Kit, Cat # VPA-1002) along with CAR transposon (CD19RCD28mZ(CoOp)/pSBSO, 7.5 μg supercoiled DNA, Figure 1B), ffLuc transposon (EGFPffLucHyTK, 7.5 μg supercoiled DNA, Figure 1C), and SB transposase (pKan-CMV-SB11, 5 μg supercoiled DNA, Figure 1D) and transferred to a single cuvette and electroporated (Program U-14) using a Nucleofector II (Lonza Cologne GmbH, Germany). The cells were rested for 2 to 3 hours at 37°C in incomplete phenol-free RPMI-1640 and subsequently cultured overnight in phenol-free RPMI-1640 containing 10% FBS and 2 mM L-alanyl-L-glutamine (GlutaMAX™, Cat # 35050, Invitrogen Corp., CA, USA). The following day γ-irradiated (100 Gy) K562-aAPC (clone #4) were added at a 1:2 T cell/aAPC ratio. Hygromycin B (HygroGold™, Cat # ant-hg-1, InvivoGen, CA, USA) was added to the co-culture beginning 5 days after electroporation and maintained at a concentration of 0.2 mg/mL, as suggested by the manufacturer, until cryopreservation of T cells. Additional γ-irradiated aAPC were added at 1:2 T cell/aAPC on days 7, 14, 21, 28, and 35 after electroporation. Soluble recombinant human IL-21 (Cat # 34-8219-85, eBioscience, Inc., CA, USA) was added at a concentration of 30 ng/mL beginning the day after electroporation, and soluble recombinant human IL-2 (Chiron Corp (Novartis V&D), CA, USA) was added to the cultures at 50 U/mL beginning 7 days after electroporation. These exogenous cytokines (IL-2, IL-21) continued to be supplemented on a Monday-Wednesday-Friday schedule. The cultures were monitored by flow cytometry for the unwanted presence of a CD3neg CD56+ cell population and if the percentage exceeded 10% of the total population (which usually occurred between day 10 and 14) a depletion for CD3negCD56+ NK cells was accomplished using CD56 beads (Cat # 130-050-401, Miltenyi Biotech Inc., CA, USA) on a LS column (Cat # 130-042-401, Miltenyi Biotech Inc., CA, USA) according to the manufacturer’s instructions. T cells were enumerated every 7 days and viable cells counted based on trypan blue exclusion using Cellometer automated cell counter (Auto T4 Cell Counter, Nexcelom Bioscience, MA, USA). 35 days after electro-transfer of SB plasmids the CAR+EGFPffLucHyTK+ cells were re-suspended in freezing medium at 20x106 cells/mL/ vial and stored in liquid nitrogen.
Generation of CAR+EGFPffLucHyTK+ T cells loaded with GNP-64Cu/PEG2000
20x106 CD19-specific CAR+EGFPffLucHyTK+ T cells were thawed and stimulated with γ-irradiated aAPC (clone #4) in presence of IL-2/21 and hygromycin B as described above.14a 37 days later the cells were centrifuged and 20x106 T cells were re-suspended in 100 μL in presence of 1X1012 GNP-64Cu/PEG2000 (Figure 1E) in PBS (Cat# D8537, Sigma-Aldrich, Inc., MO, USA) and transferred to a single cuvette and electroporated at 200 V with a 5 msec pulse using ECM 830 BTX electroporation apparatus (BTX Instrument Division, Harvard Apparatus, Inc., MA, USA).
In vivo measurement of PET signal from CAR+EGFPffLucHyTK+GNP-64Cu/PEG2000+ T cells
Micro-PET/CT imaging was acquired by Inveon Preclinical Multimodel SPECT/PET/CT System (Siemens AG, USA). Immunocompromised (nu/nu) mice were ordered from Charles River Laboratories International Inc., MA. CAR+EGFPffLucHyTK+GNP-64Cu/PEG2000+ T cells were intravenously injected (20x106 cells/mouse). All mice imaging was performed at The Methodist Hospital in accordance with guidelines from Animal Care and Use Committee. Mice were anesthetized, placed on an imaging bed attached with heating pad, laser-aligned to the center of the scanner field-of-view and serially imaged by μPET/CT scan. Images were acquired at 10 min after injection of T cells. CT image reconstruction was achieved using a Common Cone-Beam Reconstruction (COBRA) method (Siemens) and PET images were reconstructed by 2D Ordered Subset Expectation Maximization (OSEM2D) algorithm. The PET and CT images were co-registered and viewed using Inveon Research Workplace software (Siemens).
In vivo measurement of BLI signal from CAR+EGFPffLucHyTK+GNP-64Cu/PEG2000+ T cells
After PET imaging, mice were injected with 150 mg/kg D-Luciferin (XenoLight™, Cat # 122796, Caliper Life Sciences, MA, USA) and BLI was obtained using an IVIS-200 optical imaging system (Caliper Life Sciences, MA, USA). BLI were acquired using 1 minute exposure time and displayed in the same scale of intensity.
Biodistribution studies
Two mice were injected intravenously with CAR+EGFPffLucHyTK+GNP-64Cu/PEG2000+ T cells and GNP-64Cu/PEG2000. Mice were euthanized at 14 hours post injection. Blood, heart, liver, spleen, kidney, lung, stomach, intestine, muscle were harvested and weighed. Radioactivity of different organs was measured with the gamma counter (Perkin Elmer, MA). Uptake of radiolabeled T cells in tissues was calculated as the percentage of the injected dose per gram of tissue (%ID/g).
Supplementary Material
Figure S1. Flow cytometry analyses of CD19-specific CAR+EGFPffLucHyTK+ T cells before cryopreservation 35 days after electro-transfer of SB plasmids. 106 T cells in 100 μL volume were stained with fluorochrome-conjugated to antibody [phycoerythrin (PE), peridinin chlorophyll protein conjugated to cyanine dye (PerCPCy5.5), allophycocyanin (APC)]: anti–CD3 PE (Cat. # 347347, 2.5 μL, BD Biosciences), anti–CD4 APC (Cat. # 555349, 2.5 μL, BD Biosciences), anti–CD8 PerCPCy5.5 (Cat. # 341051, 4 μL, BD Biosciences), and PE-conjugated F(ab′)2 fragment of goat anti-human Fcγ (Cat. # H10104, 2.5 μL, Invitrogen), used to detect cell surface expression of the CAR. EGFP expression was analyzed and used as marker for estimating ffLuc expression. Blocking of nonspecific antibody binding was achieved using wash buffer (2% FBS and 0.1% Sodium azide in PBS). Data acquisition was on a FACSCalibur (BD Biosciences) using CellQuest version 3.3 (BD Biosciences).
Figure S2. Repeat of Figure 2A: Effect of electric field intensity and pulse duration on efficiency of electro-transfer of DNA plasmid (pmaxGFP) into T-cell lines (Jurkat cells).
INSIGHT, INNOVATION, INTEGRATION.
Clinical-grade primary T cells can be used to prevent and treat malignancies. Ex vivo manipulation of T cells improves in vivo effector functions. The link between what can be achieved in the manufacturing suites and what is achieved after infusion is compromised by an inability to assess T-cell spatio-temporal distribution. A clinically-appealing approach is needed to assess the biodistribution of T cells. Therefore, we developed gold nanoparticles (GNPs) for positron emission tomography (PET) by coupling to 64Cu. Since we have adapted electroporation to genetically modify T cells for human application, we electro-transferred GNPs into T cells. These T cells could report their distribution in vivo by PET and represents a step towards developing GNPs as radiolabels for cell-based therapies.
Acknowledgments
We thank Dr. Carl June at the University of Pennsylvania for help generating and providing the aAPC (clone #4), Dr. Perry Hackett at the University of Minnesota for help with the SB system, and the flow cytometry core at MDACC.
GRANT SUPPORT
This work was supported by funding from: Cancer Center Core Grant (CA16672); RO1 (CA124782, CA120956, CA141303); R33 (CA116127); P01 (CA148600); Albert J Ward Foundation; Alliance for NanoHealth (W81XWH-09-02-0139, W81XWH-10-02-0125); Cancer Prevention and Research Institute of Texas; Center for Transport Oncophysics – Physical Science Oncology Center at The Methodist Hospital Research Institute (U54CA 143837); Department of Defense; Burroughs Wellcome Fund; CLL Global Research Foundation; Gillson Longenbaugh Foundation; Harry T. Mangurian, Jr., Fund for Leukemia Immunotherapy; Institute of Personalized Cancer Therapy; Leukemia and Lymphoma Society; Lymphoma Research Foundation; Miller Foundation; Mr. and Mrs. Joe H. Scales; Mr. Thomas Scott; National Foundation for Cancer Research; Pediatric Cancer Research Foundation; Production Assistance for Cellular Therapies; Sister Institution Network Fund; William Lawrence and Blanche Hughes Children’s Foundation.
Footnotes
Disclosure of Potential Conflicts of Interest
None
References
- 1.(a) Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nature reviews Immunology. 2012;12:269–81. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rosenberg SA. Cell transfer immunotherapy for metastatic solid cancer-what clinicians need to know. Nat Rev Clin Oncol. 2011;8:577–85. doi: 10.1038/nrclinonc.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) S. A. Grupp, C. H. June, in Cancer Immunology and Immunotherapy, ed. G. Dranoff. 2011, vol. 344, pp 149–172; (d) Bonini C, Brenner MK, Heslop HE, Morgan RA. Genetic Modification of T Cells. Biology of Blood and Marrow Transplantation. 2011;17:S15–S20. doi: 10.1016/j.bbmt.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Jena B, Dotti G, Cooper LJN. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116:1035–1044. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Gattinoni L, Powell DJ, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohn DB, Dotti G, Brentjens R, Savoldo B, Jensen M, Cooper LJN, June CH, Rosenberg S, Sadelain M, Heslop HE. CARs on Track in the Clinic. Molecular Therapy. 2011;19:432–438. doi: 10.1038/mt.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair-Gill ED, Shu CJ, Radu CG, Witte ON. Non-invasive imaging of adaptive immunity using positron emission tomography. Immunological Reviews. 2008;221:214–228. doi: 10.1111/j.1600-065X.2008.00585.x. [DOI] [PubMed] [Google Scholar]
- 4.Rabinovich BA, Ye Y, Etto T, Chen JQ, Levitsky HI, Overwijk WW, Cooper LJN, Gelovani J, Hwu P. Visualizing fewer than 10 mouse T cells with an enhanced firefly luciferase in immunocompetent mouse models of cancer. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14342–14346. doi: 10.1073/pnas.0804105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh H, Najjar AM, Olivares S, Nishii R, Mukhopadhyay U, Alauddin M, Manuri PR, Huls H, Lee DA, Dotti G, Bollard C, Simmons PJ, Shpall EJ, Champlin RE, Gelovani JG, Cooper LJN. PET imaging of T cells derived from umbilical cord blood. Leukemia. 2009;23:620–622. doi: 10.1038/leu.2008.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Dotti G, Tian M, Savoldo B, Najjar A, Cooper LJN, Jackson J, Smith A, Mawlawi O, Uthamanthil R, Borne A, Brammer D, Paolillo V, Alauddin M, Gonzalez C, Steiner D, Decker WK, Marini F, Kornblau S, Bollard CM, Shpall EJ, Gelovani JG. Repetitive Noninvasive Monitoring of HSV1-tk-Expressing T Cells Intravenously Infused into Nonhuman Primates Using Positron Emission Tomography and Computed Tomography with F-18-FEAU. Molecular Imaging. 2009;8:230–237. doi: 10.2310/7290.2009.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yaghoubi SS, Jensen MC, Satyamurthy N, Budhiraja S, Paik D, Czernin J, Gambhir SS. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat Clin Pract Oncol. 2009;6:53–8. doi: 10.1038/ncponc1278. ncponc1278 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Dobrenkov K, Olszewska M, Likar Y, Shenker L, Gunset G, Cai S, Pillarsetty N, Hricak H, Sadelain M, Ponomarev V. Monitoring the efficacy of adoptively transferred prostate cancer-targeted human T lymphocytes with PET and bioluminescence imaging. Journal of Nuclear Medicine. 2008;49:1162–1170. doi: 10.2967/jnumed.107.047324. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Collins SA, Hiraoka K, Inagaki A, Kasahara N, Tangney M. PET Imaging for Gene & Cell Therapy. Curr Gene Ther. 2012;12:20–32. doi: 10.2174/156652312799789271. [DOI] [PubMed] [Google Scholar]
- 8.Adonai N, Nguyen KN, Walsh J, Iyer M, Toyokuni T, Phelps ME, McCarthy T, McCarthy DW, Gambhir SS. Ex vivo cell labeling with Cu-64-pyruvaldehyde-bis(N-4- methylthiosemicarbazone) for imaging cell trafficking in mice with positron-emission tomography. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3030–3035. doi: 10.1073/pnas.052709599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Oh E, Delehanty JB, Sapsford KE, Susumu K, Goswami R, Blanco-Canosa JB, Dawson PE, Granek J, Shoff M, Zhang Q, Goering PL, Huston A, Medintz IL. Cellular Uptake and Fate of PEGylated Gold Nanoparticles Is Dependent on Both Cell-Penetration Peptides and Particle Size. ACS Nano. 2011;5:6434–6448. doi: 10.1021/nn201624c. [DOI] [PubMed] [Google Scholar]; (b) Chithrani DB. Intracellular uptake, transport, and processing of gold nanostructures. Molecular Membrane Biology. 2010;27:299–311. doi: 10.3109/09687688.2010.507787. [DOI] [PubMed] [Google Scholar]; (c) Nativo P, Prior IA, Brust M. Uptake and intracellular fate of surface-modified gold nanoparticles. ACS Nano. 2008;2:1639–1644. doi: 10.1021/nn800330a. [DOI] [PubMed] [Google Scholar]
- 10.Swartz MA, Hirosue S, Hubbell JA. Engineering approaches to immunotherapy. Sci Transl Med. 2012;4:148rv9. doi: 10.1126/scitranslmed.3003763. [DOI] [PubMed] [Google Scholar]
- 11.Kircher MF, Gambhir SS, Grimm J. Noninvasive cell-tracking methods. Nat Rev Clin Oncol. 2011;8:677–688. doi: 10.1038/nrclinonc.2011.141. [DOI] [PubMed] [Google Scholar]
- 12.(a) Parente-Pereira AC, Burnet J, Ellison D, Foster J, Davies DM, van der Stegen S, Burbridge S, Chiapero-Stanke L, Wilkie S, Mather S, Maher J. Trafficking of CAR- Engineered Human T Cells Following Regional or Systemic Adoptive Transfer in SCID Beige Mice. Journal of Clinical Immunology. 2011;31:710–718. doi: 10.1007/s10875-011-9532-8. [DOI] [PubMed] [Google Scholar]; (b) Koya RC, Mok S, Comin-Anduix B, Chodon T, Radu CG, Nishimura MI, Witte ON, Ribas A. Kinetic phases of distribution and tumor targeting by T cell receptor engineered lymphocytes inducing robust antitumor responses. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14286–14291. doi: 10.1073/pnas.1008300107. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Matsui K, Wang Z, McCarthy TJ, Allen PM, Reichert DE. Quantitation and visualization of tumor-specific T cells in the secondary lymphoid organs during and after tumor elimination by PET. Nuclear Medicine and Biology. 2004;31:1021–1031. doi: 10.1016/j.nucmedbio.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Hackett PB, Largaespada DA, Cooper LJN. A Transposon and Transposase System for Human Application. Molecular Therapy. 2010;18:674–683. doi: 10.1038/mt.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Singh H, Figliola MJ, Dawson MJ, Huls H, Olivares S, Switzer K, Mi TJ, Maiti S, Kebriaei P, Lee DA, Champlin RE, Cooper LJN. Reprogramming CD19-Specific T Cells with IL-21 Signaling Can Improve Adoptive Immunotherapy of B-Lineage Malignancies. Cancer Research. 2011;71:3516–3527. doi: 10.1158/0008-5472.Can-10-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Singh H, Manuri PR, Olivares S, Dara N, Dawson MJ, Huls H, Hackett PB, Kohn DB, Shpall EJ, Champlin RE, Cooper LJN. Redirecting specificity of T-cell Populations for CD19 using the Sleeping beauty system. Cancer Research. 2008;68:2961–2971. doi: 10.1158/0008-5472.Can-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Cid-Arregui A, Juarez V, zur Hausen H. A synthetic E7 gene of human papillomavirus type 16 that yields enhanced expression of the protein in mammalian cells and is useful for DNA immunization studies. J Virol. 2003;77:4928–4937. doi: 10.1128/jvi.77.8.4928-4937.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Patterson SS, Dionisi HM, Gupta RK, Sayler GS. Codon optimization of bacterial luciferase (lux) for expression in mammalian cells. J Ind Microbiol Biotechnol. 2005;32:115–123. doi: 10.1007/s10295-005-0211-8. [DOI] [PubMed] [Google Scholar]
- 16.Manuri PVR, Wilson MH, Maiti SN, Mi TJ, Singh H, Olivares S, Dawson MJ, Huls H, Lee DA, Rao PH, Kaminski JM, Nakazawa Y, Gottschalk S, Kebriaei P, Shpall EJ, Champlin RE, Cooper LJN. piggyBac Transposon/Transposase System to Generate CD19-Specific T Cells for the Treatment of B-Lineage Malignancies. Human Gene Therapy. 2010;21:427–437. doi: 10.1089/hum.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nayak TK, Brechbiel MW. Radioimmunoimaging with Longer-Lived Positron-Emitting Radionuclides: Potentials and Challenges. Bioconjugate Chem. 2009;20:825–841. doi: 10.1021/bc800299f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shokeen M, Anderson CJ. Molecular imaging of cancer with copper-64 radiopharmaceuticals and positron emission tomography (PET) Acc Chem Res. 2009;42:832–41. doi: 10.1021/ar800255q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–70. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Letters. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 21.(a) Chen C, Smye SW, Robinson MP, Evans JA. Membrane electroporation theories: a review. Med Biol Eng Comput. 2006;44:5–14. doi: 10.1007/s11517-005-0020-2. [DOI] [PubMed] [Google Scholar]; (b) Favard C, Dean DS, Rols MP. Electrotransfer as a non viral method of gene delivery. Curr Gene Ther. 2007;7:67–77. doi: 10.2174/156652307779940207. [DOI] [PubMed] [Google Scholar]
- 22.(a) James WD, Hirsch LR, West JL, O’Neal PD, Payne JD. Application of INAA to the build-up and clearance of gold nanoshells in clinical studies in mice. J Radioanal Nucl Ch. 2007;271:455–459. doi: 10.1007/s10967-007-0230-1. [DOI] [Google Scholar]; (b) Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T, Katayama Y, Niidome Y. PEG-modified gold nanorods with a stealth character for in vivo applications. J Control Release. 2006;114:343–347. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy LC, Bear AS, Young JK, Lewinski NA, Kim J, Foster AE, Drezek RA. T cells enhance gold nanoparticle delivery to tumors in vivo. Nanoscale Res Lett. 2011;6:283. doi: 10.1186/1556-276X-6-283. 1556–276X-6–283 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(a) Choi MR, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, Badve S, Sturgis J, Robinson JP, Bashir R, Halas NJ, Clare SE. A cellular Trojan horse for delivery of therapeutic nanoparticles into tumors. Nano Letters. 2007;7:3759–3765. doi: 10.1021/nl072209h. [DOI] [PubMed] [Google Scholar]; (b) Harrington K, Alvarez-Vallina L, Crittenden M, Gough M, Chong H, Diaz RM, Vassaux G, Lemoine N, Vile R. Cells as vehicles for cancer gene therapy: The missing link between targeted vectors and systemic delivery? Human Gene Therapy. 2002;13:1263–1280. doi: 10.1089/104303402760128504. [DOI] [PubMed] [Google Scholar]
- 25.Lesterhuis WJ, Haanen J, Punt CJA. Cancer immunotherapy - revisited. Nat Rev Drug Discov. 2011;10:591–600. doi: 10.1038/nrd3500. [DOI] [PubMed] [Google Scholar]
- 26.Davies JK, Singh H, Huls H, Yuk D, Lee DA, Kebriaei P, Champlin RE, Nadler LM, Guinan EC, Cooper LJN. Combining CD19 Redirection and Alloanergization to Generate Tumor-Specific Human T Cells for Allogeneic Cell Therapy of B-Cell Malignancies. Cancer Research. 2010;70:3915–3924. doi: 10.1158/0008-5472.can-09-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang GD, Yang Z, Lu W, Zhang R, Huang Q, Tian M, Li L, Liang D, Li C. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials. 2009;30:1928–1936. doi: 10.1016/j.biomaterials.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li ZB, Cai WB, Cao QZ, Chen K, Wu ZH, He LN, Chen XY. 64Cu-Labeled “Tetrameric and octameric RGD peptides for small-animal PET of Tumor alpha(v)beta(3) integrin expression. Journal of Nuclear Medicine. 2007;48:1162–1171. doi: 10.2967/jnumed.107.039859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow cytometry analyses of CD19-specific CAR+EGFPffLucHyTK+ T cells before cryopreservation 35 days after electro-transfer of SB plasmids. 106 T cells in 100 μL volume were stained with fluorochrome-conjugated to antibody [phycoerythrin (PE), peridinin chlorophyll protein conjugated to cyanine dye (PerCPCy5.5), allophycocyanin (APC)]: anti–CD3 PE (Cat. # 347347, 2.5 μL, BD Biosciences), anti–CD4 APC (Cat. # 555349, 2.5 μL, BD Biosciences), anti–CD8 PerCPCy5.5 (Cat. # 341051, 4 μL, BD Biosciences), and PE-conjugated F(ab′)2 fragment of goat anti-human Fcγ (Cat. # H10104, 2.5 μL, Invitrogen), used to detect cell surface expression of the CAR. EGFP expression was analyzed and used as marker for estimating ffLuc expression. Blocking of nonspecific antibody binding was achieved using wash buffer (2% FBS and 0.1% Sodium azide in PBS). Data acquisition was on a FACSCalibur (BD Biosciences) using CellQuest version 3.3 (BD Biosciences).
Figure S2. Repeat of Figure 2A: Effect of electric field intensity and pulse duration on efficiency of electro-transfer of DNA plasmid (pmaxGFP) into T-cell lines (Jurkat cells).