Abstract
Objective
To examine whether the genetic influences on blood pressure (BP) during night-time are different from those during daytime and the extent to which they depend on ethnicity or sex.
Methods
Ambulatory BP was measured in 240 European–American and 190 African–American twins (mean ± SD age, 17.2 ± 3.4). Individuals with night-time BP falls more than 10% of the daytime values were defined as dippers. A bivariate analysis of the daytime and the night-time BP levels, as well as a liability-threshold model of dippers vs. nondippers were used.
Results
Bivariate model fitting showed no ethnic or sex differences for any of the measures, with heritabilities of 0.70 and 0.68 for SBP and 0.70 and 0.64 for DBP at daytime and at night-time. The genetic influences on daytime and night-time were not significantly different for SBP or DBP. The bivariate analysis also indicated that about 56 and 33% of the heritabilities of night-time SBP and DBP could be attributed to genes that also influenced daytime levels. The specific heritabilities due to genetic effects only influencing night-time values were 0.30 for SBP and 0.43 for DBP. The heritabilities of systolic and diastolic dipping were 0.59 and 0.81, respectively.
Conclusion
Independent of ethnicity and sex, an overlap exists between genes that influence daytime and night-time BP, as well as a significant genetic component that is specific to the night-time BP. These findings suggest that different genes or sets of genes contribute to BP regulation at daytime and night-time.
Keywords: ambulatory blood pressure monitoring, blacks, dipping, heritability, twin study
Introduction
Ambulatory blood pressure (ABP) monitoring offers advantages over casual blood pressure (BP) readings, including the ability to track BP at night and to study circadian BP patterns [1]. There is increasing evidence from prospective studies that night-time BP is superior to daytime BP as a predictor of cardiac mortality [2,3]. In addition, studies [4,5] reported that individuals with a blunted nocturnal decline in BP (the so-called nondipping) display the highest risk because this pattern exposes these individuals to a greater cardiovascular load each day.
Despite the importance of night-time BP and nocturnal BP fall, little is known about their heritabilities. To date, only two twin studies [6,7] and three family studies [8–10] reported heritability estimates for night-time BP. Estimates ranged from 29 to 72% for SBP and from 31 to 51% for DBP. So far, only one study [11] explored the genetic influence on nocturnal BP fall and observed a heritability of 38% for systolic dipping and 9% for diastolic dipping. Another question of great interest that has not been addressed is whether the genetic influences on nighttime BP are different from those on daytime BP. Although two previous studies [9,10] observed a higher heritability for night-time BP in comparison with daytime BP, univariate models were used in these two studies, which are unable to address the following two important questions. First, does the magnitude of the genetic influence on BP differ significantly during night-time and daytime? Second, are the genes influencing night-time BP the same or different from those for daytime BP? Furthermore, although studies in both adult and pediatric populations have shown a higher night-time BP and a higher prevalence of nondipping in African–Americans compared with European–Americans [12–14], no studies have compared the relative influence of genetic factors on these two traits between African–Americans and European Americans.
In this study, which includes a large number of European Americans and African–Americans adolescent and young adult twin pairs who had both daytime and night-time BP measured, we aim to determine whether the genetic influences on BP during night-time are different from those during daytime using a multivariate model. We further examined whether dipping, as a categorical phenotype, is heritable using a liability threshold model. Finally, we investigated the extent to which genetic estimates on these traits depend on ethnicity or sex.
Methods
Participants
The present study comprised of participants from the Georgia Cardiovascular Twin Study, which was established in 1996 [15,16]. It included roughly equal numbers of African–Americans and European Americans (>500 pairs), with the purpose to explore the change in relative influence of genetic and environmental factors on the development of cardiovascular risk factors. All twin pairs were reared together, and zygosity was determined using five standard microsatellite markers in DNA collected with buccal swabs [17]. Participants were recruited from the Southeastern United States and were overtly healthy and free of any acute or chronic illness based on parental report. Study design, selection criteria and the criteria to classify participants as African–Americans or European Americans for this twin study have been described previously [15,16,18].
For the current study, data were available from 240 European Americans (105 pairs and 30 singletons) and 190 African–Americans (82 pairs and 26 singletons) twins (mean ± SD age, 17.2 ± 3.4; range, 11.9–30.0), who had 24-h ABP measured from 2000 to 2002 during a routine scheduled examination. The Institutional Review Board at the Medical College of Georgia had given approval for this study. Written informed consent was provided by all participants and by parents if participants were less than 18 years.
Measurements
Our procedures for ABP recordings have previously been described in detail [19]. Briefly, an ABP monitor was fitted to the nondominant arm (model 90207; SpaceLabs, Redmond, Washington, USA). Measures were obtained for every 20 min during the daytime (0800–2200 h) and for every 30 min during the night-time (12 midnight–0600 h) (the narrow fixed time definition of awake–asleep). Transitional periods from 0600 to 0800 h and 2200 to midnight were not included in the analyses. Acceptable readings were defined according to the following criteria: SBP of at least 70 mmHg and of 180 mmHg or less; DBP of at least 40 mmHg and of 140 mmHg or less; pulse pressure of at least 20 mmHg and of 140 mmHg or less; and heart rate of at least 40 beats/min and of 180 beats/min or less [19]. Adequacy of recordings was defined as at least 14 readings over the 14 h designated as daytime and at least six readings over the 6 h designated as the night-time, as suggested by the European Society of Hypertension Working Group on Blood Pressure Monitoring [1]. Individuals with nighttime BP falls more than 10% of the daytime values were defined as dippers.
Anthropometric measures including height, weight and waist were recorded using established protocols. BMI was calculated as a measure of general adiposity. Casual SBP and DBP were measured with the Dinamap Vital Signs Monitor (model 1864 SX; Criticon Incorporated, Tampa, Florida, USA). BP measurements were taken at 11, 13 and 15 min during a 15-min supine relaxation period. The average of the last two readings was used to represent casual SBP and DBP values [20].
Analytical approach
The purposes of our analyses were: to test whether the genetic influences on night-time BP are different from those on daytime BP using a bivariate model, to determine whether nocturnal BP fall as a categorical trait (dippers vs. nondippers) is heritable using a liability-threshold model and to assess the dependency of these genetic influences on ethnicity and sex.
Genetic modeling using twin data
Twin methodology makes use of the fact that monozygotic twins share identical genotypes, whereas dizygotic twins share on average 50% of their genes. It is assumed that both types of twins share their common family environment to the same extent so that any greater similarity between monozygotic compared with dizygotic twins reflects genetic influences. In this study, structural equation modeling (SEM) was used. SEM is based on the comparison of the variance–covariance matrices in monozygotic and dizygotic twin pairs and allows separation of the observed phenotypic variance into its genetic and environmental components: additive (A) genetic, common (C) and unique (E) environmental components. Dividing each of these components by the total variance yields the different standardized components of variance, for example, the heritability (h2) can be defined as the proportion of the total variance attributable to additive genetic variation [21].
Bivariate model
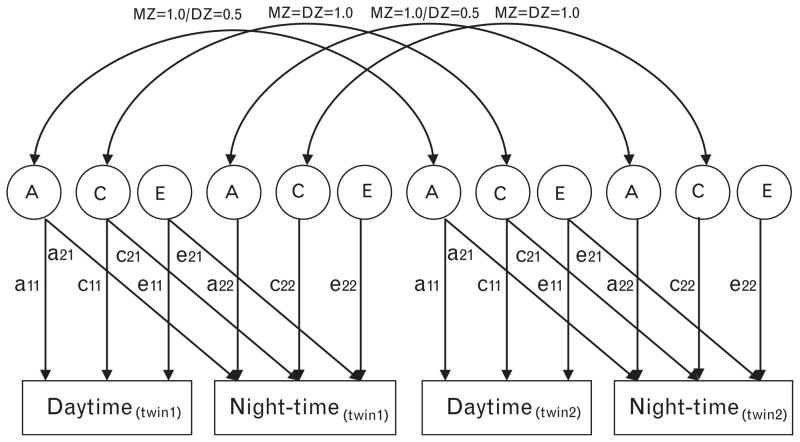
For the first purpose of the current study, a bivariate path model, which is shown in Fig. 1, was used. With this model, making use of the ‘Cholesky decomposition’, we can not only estimate the heritability of daytime and night-time but also can test whether the magnitude of the genetic influence differs in daytime and night-time conditions (i.e. ?). We can further test whether the genes influencing night-time BP are the same (i.e. a22 = 0?), partly the same (i.e. a21 ≠ 0 and a22 ≠ 0?) or entirely different (i.e. a21 = 0?) from daytime BP. If they are partly the same, this bivariate model allows further determination of the amount of overlap between genes influencing BP of daytime and night-time by calculating the genetic correlation between the two BP levels [rg = COVA(day and night)/√(VA day * VA-A night)]. Shared and unique environmental correlations can be calculated in a similar fashion [22].
Fig. 1.
Path diagram for a bivariate model. Observed variables (for twin 1 and twin 2) are shown in rectangles. Latent variables (or factors) are shown in circles. A single-headed arrow indicates a direct influence of one variable on another, its value represented by a path coefficient (comparable to a factor loading). Double-headed arrows between two variables indicate a correlation without any assumed direct relationship. A, genetic variance components; C, common environmental variance components; E, nonshared environmental variance components; a11 through a22, genetic path coefficients (or factor loadings) of which a22 represents novel genetic influences on BP of night-time; c11 through c22, shared environmental path coefficients (or factor loadings) of which c22 represents novel shared environmental influences on BP of night-time; e11 through e22, nonshared environmental path coefficients (or factor loadings) of which e22 represents novel environmental influences on BP of night-time. The correlation of A between twin 1 and twin 2 is 1.0 for monozygotic and 0.5 for dizygotic twins. The correlation of C between twin 1 and twin 2 is 1.0 for both monozygotic and dizygotic twins. Formulas for the different heritability estimates are as follows: .
Liability-threshold model
The heritability of nocturnal BP fall was assessed using a liability-threshold model. This model assumes a latent, normally distributed liability to the trait that is manifest as a categorical phenotype. For nocturnal BP fall, the underlying distribution was modeled to have one threshold, which allows for two categories, dippers and nondippers. Similar to a quantitative trait, sources of variation in nocturnal BP fall liability considered in the modeling were additive (A) genetic, common (C) and unique (E) environmental factors [21].
Sex differences
Sex differences were examined by comparing a full model, in which parameter estimates are allowed to differ in magnitude between males and females, with a reduced model, in which parameter estimates are constrained to be equal across the sexes. In addition to those models, a scalar model was tested. In a scalar model, heritabilities are constrained to be equal across sexes, but total variances may be different [21].
Ethnic differences
Ethnic differences were, just like sex differences, examined by comparing a full model, in which parameter estimates are allowed to differ in magnitude between African–Americans and European Americans, with a reduced model, in which parameter estimates are constrained to be equal across ethnicity. In addition to those models, a scalar model was tested in a similar fashion as done for sex [21].
Model-fitting procedure
Prior to analysis, effect of age was regressed out for all variables before using the residuals in model fitting. As our sample includes adolescents whose BP levels are also dependent on height, we further regressed out the effect of height for all variables and redid the analysis. However, this further adjustment of height did not change the results, so only the results with the adjustment of age were reported here.
The significance of variance components a and c was assessed by testing the deterioration in model fit after each component was dropped from the full model. e is always retained in these models, as it also includes measurement error. Standard hierarchic χ2 tests were used to select the best fitting models in combination with Akaike’s information criterion (AIC = χ2 − 2 DF). The model with the lowest AIC reflects the best balance of goodness of fit and parsimony [21]. The analyses were based on the raw data instead of variance–covariance matrices. This allows for inclusion of singletons who contribute to estimates of means and variances (but not to cross-twin correlations).
Statistical software
Prior to analysis, both daytime and night-time BP values were log-transformed to obtain a better approximation of the normal distribution. The ethnic and sex difference in the general characteristics of the twins was tested by generalized estimating equations (GEEs). GEE is a multiple regression technique that allows for nonindependence of twin or family data yielding unbiased standard errors and P values. This analysis was done using STATA version 8 (StataCorp, College Station, Texas). Genetic modeling was carried out with Mx, a computer program specifically designed for the analysis of twin and family data [23].
Results
Table 1 presents the general characteristics of the twins by ethnicity and sex. Men were taller and had higher casual SBP but lower DBP levels than women. African–Americans had higher BMI, weight and causal BP levels than European Americans. For the 24-h ABP measures, African–Americans had higher night-time SBP and DBP despite similar daytime levels to European Americans, which resulted in a higher proportion of nondippers in African–Americans. Higher daytime and night-time SBP levels were also observed in men compared with women.
Table 1.
General characteristics of study participants by ethnicity and sex
| Characteristics | European Americans*
|
African–Americans†
|
P
|
|||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Ethnicity | Sex | |
| n | 117 | 123 | 83 | 107 | ||
| Age (years) | 17.1 ± 3.6 | 17.1 ± 3.0 | 17.1 ± 3.2 | 17.5 ± 3.8 | 0.75 | 0.94 |
| BMI (kg/m2)‡ | 23.2 ± 6.0 | 22.3 ± 4.6 | 23.6 ± 5.4 | 24.5 ± 5.6 | <0.05 | 0.54 |
| Height (cm)‡ | 171.7 ± 12.2 | 161.7 ± 7.4 | 172.5 ± 10.2 | 162.6 ± 6.0 | 0.56 | <0.001 |
| Casual SBP (mmHg)‡ | 113 ± 11 | 106 ± 9 | 118 ± 11 | 111 ± 11 | <0.001 | <0.001 |
| Casual DBP (mmHg)‡ | 57 ± 7 | 58 ± 6 | 60 ± 7 | 62 ± 8 | <0.001 | <0.01 |
| Daytime SBP (mmHg)‡ | 122 ± 9 | 114 ± 7 | 122 ± 7 | 117 ± 9 | 0.19 | <0.001 |
| Daytime DBP (mmHg)‡ | 70 ± 7 | 70 ± 6 | 70 ± 6 | 71 ± 8 | 0.68 | 0.79 |
| Night-time SBP (mmHg)‡ | 109 ± 9 | 103 ± 8 | 114 ± 10 | 107 ± 9 | <0.001 | <0.001 |
| Night-time DBP (mmHg)‡ | 57 ± 7 | 57 ± 6 | 60 ± 7 | 60 ± 8 | <0.001 | 0.84 |
| Nondippers for SBP (%)‡ | 41.0 | 52.8 | 66.3 | 63.6 | <0.01 | 0.30 |
| Nondippers for DBP (%)‡ | 14.5 | 11.4 | 31.3 | 21.5 | <0.01 | 0.16 |
Data are mean ± SD.
Including 51 monozygotic and 54 dizygotic pairs as well as 30 singletons.
Including 36 monozygotic and 46 dizygotic pairs as well as 26 singletons.
Age was adjusted for the evaluation of ethnic and sex effects.
Table 2 presents twin correlations for daytime and nighttime BP for each ethnic and zygosity group. In both ethnic groups, twin correlations in monozygotic twin pairs were larger than those in dizygotic twin pairs, indicating genetic influences. We present the correlations collapsed over sex, because models that best explained the variance and covariance of these variables did not show any sex differences (see below).
Table 2.
Twin correlations (and their standard error) of daytime and night-time blood pressure by ethnicity and zygosity
| European Americans
|
African–Americans
|
|||
|---|---|---|---|---|
| Monozygotic | Dizygotic | Monozygotic | Dizygotic | |
| Daytime | ||||
| n | 51 | 54 | 36 | 46 |
| SBP | 0.68 (0.07)* | 0.27 (0.10)* | 0.60 (0.08)* | 0.49 (0.08)* |
| DBP | 0.75 (0.06)* | 0.22 (0.09) | 0.69 (0.07)* | 0.57 (0.08)* |
| Night-time | ||||
| n | 51 | 53 | 33 | 44 |
| SBP | 0.73 (0.06)* | 0.20 (0.09) | 0.55 (0.08)* | 0.27 (0.09) |
| DBP | 0.74 (0.06)* | 0.27 (0.09)* | 0.57 (0.07)* | 0.13 (0.09) |
P < 0.05.
Strong positive correlations between daytime and nighttime BP values were observed for both SBP (r = 0.64) and DBP (r = 0.48). Table 3 shows the results from bivariate testing, using the model depicted in Fig. 1. Models including only an additive genetic and a unique environmental component (AE model) gave the overall best fit to the data for both SBP (ACE vs. AE model, , P = 0.057; ACE vs. CE model, , P < 0.001) and DBP (ACE vs. AE model, , P = 0.74; ACE vs. CE model, , P < 0.001). For SBP, a significant scalar effect for sex was found, indicating that males show a larger variability in SBP than do females, whereas for DBP, a significant scalar effect for ethnicity was found, indicating that African–Americans show a large variability in DBP than do European Americans. However, for both SBP and DBP, the best fitting model showed no significant differences in genetic and environmental variance component estimates between African–Americans and European Americans or between men and women, indicating that African–Americans and men show similar heritabilities to European Americans and women.
Table 3.
Heritability estimates of best-fitting bivariate models for daytime and night-time blood pressure
| SBP | DBP | ||
|---|---|---|---|
| Day | h2 (CI) | 0.70 (0.59–0.78) | 0.70 (0.60–0.78) |
| e2 (CI) | 0.30 (0.22–0.41) | 0.30 (0.22–0.40) | |
| Night | h2 (CI) | 0.68 (0.56–0.77) | 0.64 (0.51–0.73) |
| e2 (CI) | 0.32 (0.23–0.44) | 0.36 (0.27–0.49) | |
| Day–night | rg (CI) | 0.75 (0.65–0.83) | 0.56 (0.42–0.69) |
| re (CI) | 0.46 (0.29–0.60) | 0.29 (0.10–0.46) | |
| Night-specific | h2 (CI) | 0.30 (0.21–0.39) | 0.43 (0.31–0.54) |
| e2 (CI) | 0.25 (0.19–0.34) | 0.33 (0.25–0.45) |
CI, 95% confidence interval; e2, variance explained by unique environment; h2, variance explained by additive genetics (heritability); re, unique environment correlations; rg, genetic correlations.
As shown in Table 3, significant heritability was found for both daytime and night-time SBP and DBP. There was a slight decrease in heritability of SBP (from 0.70 to 0.68) and DBP (from 0.70 to 0.64) from daytime to night-time, but this decrease in heritability did not reach statistical significance (P = 0.76 for SBP and P = 0.31 for DBP). Furthermore, neither a21 nor a22 can be set to 0 (a21 ≠ 0 model vs. a21 = 0 model, , P < 0.001 for SBP and , P < 0.001 for DBP; a22 ≠ 0 model vs. a22 = 0 model, , P < 0.001 for SBP and , P < 0.001 for DBP). This indicates that the genes influencing BP at night-time are partly shared with those at daytime. Genetic correlations were 0.75 [95% confidence interval (CI) 0.65–0.83] between daytime and night-time SBP, and 0.56 (95% CI 0.42–0.69) between daytime and night-time DBP. As shown in Table 3, 56% of night-time SBP heritability [(0.68–0.30)/0.68] and 33% of night-time DBP heritability [(0.64–0.43)/0.64] were attributed to genes that also influenced their daytime levels. The specific heritability due to novel genetic effects emerging during night-time was 0.30 (95% CI 0.21–0.39) for SBP and 0.43 (95% CI 0.31–0.54) for DBP.
Table 4 shows the heritability of nocturnal BP fall. For both SBP and DBP, the liability threshold was higher for European Americans compared with African–Americans (P < 0.001 for SBP and P < 0.05 for DBP). For SBP, no statistically significant distinction could be made between a model that attributed familial resemblance solely to shared environment or genetic factors (ACE vs. AE model, , P = 0.64; ACE vs. CE model, , P = 0.27), but given the AICs of the AE (AIC = −263.235) and CE (AIC = −262.186) models, it is most likely that the AE model was the preferred model.
Table 4.
Heritability estimates for dipping
| h2 (CI) | e2 (CI) | |
|---|---|---|
| SBP | 0.59 (0.32–0.78) | 0.41 (0.22–0.68) |
| DBP | 0.81 (0.54–0.95) | 0.19 (0.05–0.46) |
CI, 95% confidence interval; e2, variance explained by unique environment; h2, variance explained by additive genetics (heritability).
As shown in Table 4, in the AE model, variance in SBP dipping was for 59% explained by genetic influences. For DBP dipping, the AE model was the best fitting model (ACE vs. AE model, , P = 1.0; ACE vs. CE model, , P < 0.05) with a heritability of 81%. For both SBP and DBP dipping, the best fitting model showed no significant differences in genetic and environmental variance component estimates between African–Americans and European Americans or between men and women.
Discussion
The important findings in this study are that both nighttime BP and nocturnal BP fall are substantially heritable. There is an overlap between genes that influences daytime and night-time BP, but a significant genetic component that is specific to the night-time BP also exists. This suggests that different genes or sets of genes contribute to BP regulation at daytime and night-time. Heritability estimates do not show any differences between African–Americans and European Americans or men and women.
With the finding that night-time BP is a better predictor of cardiovascular events [2,3] and the reasonable assumption that environmental factors might be less prevalent during the night, it is of great interest to know whether night-time BP will be a better phenotype than daytime BP for finding genes influencing BP regulation. In the previous five twin and family studies [6–10], which measured both daytime and night-time BP, a higher night-time than daytime BP heritability was observed in two studies, of which Fava et al. [9] reported an increase from daytime to night-time of 33–37% for SBP and of 22–32% for DBP in Swedish families, and Kotchen et al. [10] reported an increase from 33 to 69% for SBP and from 25 to 51% for DBP in black sibpairs with essential hypertension. On the basis of these two studies, it is tempting to conclude that genetic studies using nighttime BP as the phenotype are likely to be more powerful than those using daytime BP. However, there are two caveats. First, it was not tested whether the increase in night-time BP heritability was statistically significant in these two studies. According to the study by Kotchen et al. [10], which provided the standard error of the estimated heritabilities, we found a large overlap between the 95% CI of daytime and night-time BP heritabilites, which indicates that the increase in nighttime BP heritability did not reach statistical significance. Second, an increase in heritability does not necessarily mean an increase in statistical power to find genes because daytime and night-time BP regulation may be influenced by partly different genes. That is, the genetic correlation between daytime and night-time BP levels might be less than 1. In the current study, we not only confirmed the nonsignificant difference between daytime and night-time BP heritabilities but also showed for the first time that the actual genes responsible for daytime and night-time BP regulation differ largely. Only 56% of night-time SBP heritability and 33% of night-time DBP heritability can be attributed to genes that also influenced their daytime levels. Our findings raise the possibility that the underlying mechanisms for BP regulation might change with day–night shift, either via a change in the expression of relevant genes or via a change in their penetrance. It may also imply a change from daytime to night-time in the environmental determinants of BP regulation or in the susceptibility to relevant environmental agents (gene–environment interaction).
Nocturnal BP fall is another interesting feature revealed by ABP. Blunted nocturnal dipping has been associated with greater left ventricular mass and decreased renal function in youth [24]. However, to date, only one study [11] explored the genetic influence on nocturnal BP fall and observed a heritability of 38% for systolic dipping and 9% for diastolic dipping in 104 adult Swedish sibships. In that study, a narrow fixed time definition of awake–asleep was used, and nocturnal BP fall was indexed by a continuous variable, the night-to-day ratio. The recent study by Henskens et al. [25] shows that the narrow fixed time definition yields proportions of dippers and non-dippers comparable with the definition by diary but overestimates actual values of night-to-day ratio. Therefore, in the current study, which also used the narrow fixed time method to define day and night, a liability-threshold model was used to determine the heritability of dipping profile as a categorical phenotype. Another reason that we use dippers vs. nondippers is because previous study showed that dichotomous dipping was a good predictor of cardiovascular mortality [4]. We observed a heritability of 59% for SBP dipping and 81% for DBP dipping. So far, few studies have explored the physiological determinants of nocturnal BP fall. The identified factors included fitness [26], body size [19] and the capacity to excrete sodium during daytime [27]. The high heritability of nocturnal BP fall identified in this study implicates that genes also play an important role in the dipping pattern.
Ethnic difference in ABP pattern has been noted with African–Americans having higher night-time BP values and higher prevalence of nondippers than European Americans [12–14]. We confirmed these observations in the current study. It is noteworthy that our study is the first twin study to include both African–Americans and European Americans and found that African–Americans show similar heritabilities of night-time BP and nocturnal BP fall to European Americans. The classic twin study is established as the ideal study design to estimate the relative importance of genetic and environmental factors to the variance of traits and diseases in human populations, but without actual measurement of specific genes or environments, it cannot attribute the ethnic difference in mean values to either of these factors [28]. However, our study does show that the observed difference in mean values did not translate to many differences in genetic and environmental variability within each ethnic group. The fact that a similar amount of variation is explained by genetic factors within different ethnic groups does not exclude the possibility, however, that the actual genes or their number responsible for these effects may differ between ethnic groups.
We need to be cautious in the interpretation of our findings because this is the first study to examine similarities and differences in the genetic influence on daytime and nighttime BP, and the results need to be replicated in other samples. Several limitations of the present study also need to be recognized. First, as the Georgia Cardiovascular Twin Study is comprised of youth and young adults, the generalizability of these results to other adult populations remains to be determined. Second, our overall sample size was substantial for twin ABP studies but might not have enough power to detect small ethnic or sex difference in the heritability of the studied BP traits. Further twin studies with large sample sizes involving multiethnic groups are warranted. Third, the daytime BP, night-time BP and the classification of dippers and nondippers were based on a single ABP measurement. Although previous studies including our own [29] have shown that the reproducibility and long-term tracking stability of daytime and night-time BP from childhood to early adulthood are relatively high, the reproducibility of dipper patterns over time on the basis of a single measurement is limited. Continued follow-up of this twin cohort including ABP measurements at multiple time points will provide more solid evidence on the genetic and environmental sources of dipping patterns [30].
It is crucial to identify the potential mechanisms that underlie BP regulation at night because night-time is superior to daytime BP as a predictor of target organ damage and cardiovascular events [31]. In this study, we demonstrated that, in addition to the genes that also influence daytime BP, night-time BP has its own specific genetic determinants. This observation has important implications for gene finding studies. For example, impaired renal capacity to excrete sodium was recently hypothesized to be a cause for higher night-time BP levels [27]. On the basis of the fact that renal sodium excretion is critically dependent upon the activity of sodium channels and transporters expressed in the nephron, genetic variants in the genes coding for these channels and transporters such as epithelial sodium channels (ENaC) seem to be plausible candidates for nighttime BP regulation. On the contrary, the recent hypothesis-free genome-wide association approach also provides us the opportunity to supersede the limitations imposed by candidate gene studies and search the whole genome in an unbiased manner. For example, common variants in 14 genes have recently been identified for office BP in two large-scale genome-wide association meta-analyses from the Global BPgen and the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium [32,33]. These newly discovered genes could be specifically tested for their effects on daytime vs. night-time BP. Identification of genes specifically influencing night-time BP will provide new insights into the mechanisms of BP regulation at night. It may also help us to understand the pathophysiology and clinical consequences of night-time BP and to develop more accurate prediction of individuals at risk for cardiovascular disease or new therapeutic strategies to normalize the dipping profile.
Acknowledgments
Research was supported by HL086530 and HL69999 from the National Heart, Lung and Blood Institute, as well as 0730156N from the American Heart Association.
Abbreviations
- ABP
ambulatory blood pressure
- AIC
Akaike’s Information Criterion
- BP
blood pressure
- CI
confidence interval
- GEEs
generalized estimating equations
- SEM
structural equation modeling
Footnotes
There are no conflicts of interest.
References
- 1.O’Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821–848. doi: 10.1097/00004872-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Kikuya M, Ohkubo T, Asayama K, Metoki H, Obara T, Saito S, et al. Ambulatory blood pressure and 10-year risk of cardiovascular and noncardiovascular mortality: the Ohasama study. Hypertension. 2005;45:240–245. doi: 10.1161/01.HYP.0000152079.04553.2c. [DOI] [PubMed] [Google Scholar]
- 3.Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–161. doi: 10.1161/01.HYP.0000170138.56903.7a. [DOI] [PubMed] [Google Scholar]
- 4.Ohkubo T, Imai Y, Tsuji I, Nagai K, Watanabe N, Minami N, et al. Relation between nocturnal decline in blood pressure and mortality. The Ohasama Study. Am J Hypertens. 1997;10:1201–1207. doi: 10.1016/s0895-7061(97)00274-4. [DOI] [PubMed] [Google Scholar]
- 5.Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–2189. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Fagard R, Brguljan J, Staessen J, Thijs L, Derom C, Thomis M, Vlietinck R. Heritability of conventional and ambulatory blood pressures. A study in twins. Hypertension. 1995;26 (6 Pt 1):919–924. doi: 10.1161/01.hyp.26.6.919. [DOI] [PubMed] [Google Scholar]
- 7.Vinck WJ, Fagard RH, Loos R, Vlietinck R. The impact of genetic and environmental influences on blood pressure variance across age-groups. J Hypertens. 2001;19:1007–1013. doi: 10.1097/00004872-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Bochud M, Bovet P, Elston RC, Paccaud F, Falconnet C, Maillard M, et al. High heritability of ambulatory blood pressure in families of East African descent. Hypertension. 2005;45:445–450. doi: 10.1161/01.HYP.0000156538.59873.86. [DOI] [PubMed] [Google Scholar]
- 9.Fava C, Burri P, Almgren P, Groop L, Hulthen UL, Melander O. Heritability of ambulatory and office blood pressure phenotypes in Swedish families. J Hypertens. 2004;22:1717–1721. doi: 10.1097/00004872-200409000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Kotchen TA, Kotchen JM, Grim CE, George V, Kaldunski ML, Cowley AW, et al. Genetic determinants of hypertension: identification of candidate phenotypes. Hypertension. 2000;36:7–13. doi: 10.1161/01.hyp.36.1.7. [DOI] [PubMed] [Google Scholar]
- 11.Fava C, Burri P, Almgren P, Arcaro G, Groop L, Lennart Hulthen U, Melander O. Dipping and variability of blood pressure and heart rate at night are heritable traits. Am J Hypertens. 2005;18:1402–1407. doi: 10.1016/j.amjhyper.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Profant J, Dimsdale JE. Race and diurnal blood pressure patterns. A review and meta-analysis. Hypertension. 1999;33:1099–1104. doi: 10.1161/01.hyp.33.5.1099. [DOI] [PubMed] [Google Scholar]
- 13.Harshfield GA, Alpert BS, Willey ES, Somes GW, Murphy JK, Dupaul LM. Race and gender influence ambulatory blood pressure patterns of adolescents. Hypertension. 1989;14:598–603. doi: 10.1161/01.hyp.14.6.598. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Poole JC, Treiber FA, Harshfield GA, Hanevold CD, Snieder H. Ethnic and gender differences in ambulatory blood pressure trajectories: results from a 15-year longitudinal study in youth and young adults. Circulation. 2006;114:2780–2787. doi: 10.1161/CIRCULATIONAHA.106.643940. [DOI] [PubMed] [Google Scholar]
- 15.Snieder H, Treiber FA. The Georgia Cardiovascular Twin Study. Twin Res. 2002;5:497–498. doi: 10.1375/136905202320906354. [DOI] [PubMed] [Google Scholar]
- 16.Ge D, Dong Y, Wang X, Treiber FA, Snieder H. The Georgia Cardiovascular Twin Study: influence of genetic predisposition and chronic stress on risk for cardiovascular disease and type 2 diabetes. Twin Res Hum Genet. 2006;9:965–970. doi: 10.1375/183242706779462877. [DOI] [PubMed] [Google Scholar]
- 17.Jackson RW, Snieder H, Davis H, Treiber FA. Determination of twin zygosity: a comparison of DNA with various questionnaire indices. Twin Res. 2001;4:12–18. doi: 10.1375/1369052012092. [DOI] [PubMed] [Google Scholar]
- 18.Snieder H, Dong Y, Barbeau P, Harshfield GA, Dalageogou C, Zhu H, et al. Beta2-adrenergic receptor gene and resting hemodynamics in European and African American youth. Am J Hypertens. 2002;15:973–979. doi: 10.1016/s0895-7061(02)02991-6. [DOI] [PubMed] [Google Scholar]
- 19.Harshfield GA, Barbeau P, Richey PA, Alpert BS. Racial differences in the influence of body size on ambulatory blood pressure in youths. Blood Press Monit. 2000;5:59–63. [PubMed] [Google Scholar]
- 20.Snieder H, Harshfield GA, Treiber FA. Heritability of blood pressure and hemodynamics in African- and European-American youth. Hypertension. 2003;41:1196–1201. doi: 10.1161/01.HYP.0000072269.19820.0D. [DOI] [PubMed] [Google Scholar]
- 21.Neale MC, Cardon LR. Methodologies for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- 22.De Geus EJ, Kupper N, Boomsma DI, Snieder H. Bivariate genetic modeling of cardiovascular stress reactivity: does stress uncover genetic variance? Psychosom Med. 2007;69:356–364. doi: 10.1097/PSY.0b013e318049cc2d. [DOI] [PubMed] [Google Scholar]
- 23.Neale MC, Boker SM, Xie G, Maes HH. Mx: statistical modeling. Richmond, Virginia: Department of Psychiatry, Virginia Commonwealth University; 1999. [Google Scholar]
- 24.Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, et al. Ambulatory blood pressure monitoring in children and adolescents: recommendations for standard assessment: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension. 2008;52:433–451. doi: 10.1161/HYPERTENSIONAHA.108.190329. [DOI] [PubMed] [Google Scholar]
- 25.Henskens LH, Kroon AA, van Oostenbrugge RJ, Haest RJ, Lodder J, de Leeuw PW. Different classifications of nocturnal blood pressure dipping affect the prevalence of dippers and nondippers and the relation with target-organ damage. J Hypertens. 2008;26:691–698. doi: 10.1097/HJH.0b013e3282f4225f. [DOI] [PubMed] [Google Scholar]
- 26.Harshfield GA, Dupaul LM, Alpert BS, Christman JV, Willey ES, Murphy JK, Somes GW. Aerobic fitness and the diurnal rhythm of blood pressure in adolescents. Hypertension. 1990;15 (6 Pt 2):810–814. doi: 10.1161/01.hyp.15.6.810. [DOI] [PubMed] [Google Scholar]
- 27.Bankir L, Bochud M, Maillard M, Bovet P, Gabriel A, Burnier M. Night-time blood pressure and nocturnal dipping are associated with daytime urinary sodium excretion in African subjects. Hypertension. 2008;51:891–898. doi: 10.1161/HYPERTENSIONAHA.107.105510. [DOI] [PubMed] [Google Scholar]
- 28.Snieder H, MacGregor AJ. Twin methodology. In: Cooper DN, editor. Encyclopedia of the human genome. London: Nature Publishing Group; 2003. [Google Scholar]
- 29.Li Z, Snieder H, Harshfield GA, Treiber FA, Wang X. A 15-year longitudinal study on ambulatory blood pressure tracking from childhood to early adulthood. Hypertens Res. 2009;32:404–410. doi: 10.1038/hr.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuspidi C, Macca G, Sampieri L, Fusi V, Severgnini B, Michev I, et al. Target organ damage and nondipping pattern defined by two sessions of ambulatory blood pressure monitoring in recently diagnosed essential hypertensive patients. J Hypertens. 2001;19:1539–1545. doi: 10.1097/00004872-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Daytime and night-time blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51:55–61. doi: 10.1161/HYPERTENSIONAHA.107.100727. [DOI] [PubMed] [Google Scholar]
- 32.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]