Abstract
Objectives
High blood pressure variability is increasingly used as a predictor of target-organ damage and cardiovascular events. However, little is known about blood pressure variability changes with age and its possible sociodemographic, anthropometric, and genetic moderators.
Methods
Twenty-four-hour ambulatory blood pressure was measured up to 12 times over a 15-year period in 344 European Americans and 297 African–Americans with an average age of 14 years at the initial visit. Blood pressure variability was indexed by the weighted 24-h standard deviation of ambulatory blood pressure recordings.
Results
Both systolic and diastolic blood pressure variability increased with age and ambulatory blood pressure mean values. Men had higher levels of blood pressure variability (P<0.001) and showed steeper linear increase rates with age than women. African–Americans showed higher values of blood pressure variability (P<0.05) than European Americans. Body mass index and waist circumference were also associated with higher blood pressure variability levels (P< 0.001). Individuals with higher father’s education level showed lower blood pressure variability. In the full model which included all the above factors, ethnic difference in systolic blood pressure variability was no longer significant.
Conclusion
The results of the present study suggest that men and African–Americans have higher blood pressure variability than women and European Americans. Apart from these ethnicity and sex effects, blood pressure variability increases with increases in age (especially in men), ambulatory blood pressure mean values and adiposity as well as decreased socioeconomic status
Keywords: blood pressure variability, ethnicity, longitudinal study, sex, youth
Introduction
Blood pressure (BP) may vary due to short-term changes that occur during the day and night. The advent of noninvasive but accurate ambulatory blood pressure (ABP) monitor has made it possible to measure not only the BP mean but also the BP variability (BPV), which is generally estimated by the standard deviation (SD) of ABP measures over 24 representative for the wholeh. Although increased BPV has been widely shown to be associated with a greater degree of target-organ damage [1–3] and a higher rate of cardiovascular events [4,5], little is known about BPV changes with age and its possible sociodemographic, anthropometric, and genetic moderators, as assessed in a longitudinal design.
In the present longitudinal study of 344 European American and 297 African–American youths, we evaluated ABP up to 12 times over a 15-year period from childhood to early adulthood. To the best of our knowledge, this is the only cohort of youth in which 24-h ABP levels have been collected over such a long and crucial period of development. Using growth curve modeling, we aimed to examine sex and ethnic differences as well as effects of ABP level, adiposity, socioeconomic status (SES) and genetic susceptibility to essential hypertension on BPV development with age.
Methods
Patients
The patients (n= 641) were participants in an ongoing longitudinal study evaluating the development of cardiovascular risk factors in youth and young adults. It included almost equal numbers of African–American and European American youths. The data encompass a 15-year period (1989–2004), in which 12 assessments were conducted. The dataset is complicated because not all patients had the same number of visits, with patients being recruited into the study at different ages and different years. However, more than 55% of participants had at least four visits, making this data set very informative for the study of BPV changes over time.
Study design, selection criteria and the criteria to classify participants as African–Americans or European Americans for the longitudinal study have been described previously [6–8]. Briefly, recruitment and evaluation of participants began in 1989. Participants who met the following criteria were recruited: aged 7–16 years in 1989; African–Americans or European Americans; normotensive for age and sex based on BP screening, and apparently healthy based on parental reports of the child’s medical history. The annualized attrition rate has been less than 4% per year. There have been no significant differences in age, ethnicity and sex distributions between dropouts and the participants who remained in the study. During the study, 11 participants began to take antihypertensive medication, and the data obtained during this period were excluded from analyses. The Institutional Review Board at the Medical College of Georgia gave its approval for the study.
Measurements
On each laboratory visit during the 15-year period, both anthropometric and cardiovascular evaluations were conducted [7,9]. Participants’ height and weight were measured with a Healthometer medical scale that was calibrated daily. Body mass index (BMI, weight/height2) was calculated as a measure of general adiposity. Waist circumference, a measure of central adiposity, was evaluated as described elsewhere [10].
Socioeconomic status was represented by father’s education level. Because this measure remained highly stable across the years of the study, its value measured at the midpoint of the study was taken as a representative for the whole study period. Father’s education level was measured in years on a 7-point scale that ranged from less than high school to postgraduate education. Eighteen participants with missing values for father’s education level were omitted from analyses in which this variable was included.
Family history of essential hypertension was used as a proxy for genetic susceptibility of hypertension. A positive family history of essential hypertension was defined as the occurrence of essential hypertension in one or both biological parents at any visit. Diagnosis of essential hypertension was verified by the individual’s physician or medical records [9]. Seven participants with missing values for family history of essential hypertension were omitted from analyses in which this variable was included.
Ambulatory blood pressure recordings and blood pressure variability
Our procedures for ABP recordings have previously been described in detail [8,11]. Briefly, an ABP monitor (model 90207; SpaceLabs, Redmond, Washington, USA) was fitted to the nondominant arm. Measures were obtained every 20 minduring the daytime (0800 to 2200 h) and every 30 min during the night-time (12 midnight to 0600 h). Transitional periods from 0600 to 0800 h and 2200 h to midnight were not included in the analyses. Adequacy of recordings were based on acceptable readings using previously established criteria [11] for at least 14 readings over the 14 h designated as daytime and at least six readings over the 6 h designated as the night-time, as suggested by the European Society of Hypertension Working Group on Blood Pressure Monitoring [12].
In the present study, we examined the BPV trajectories based on these intermittent ABP measurements. BPV is usually indexed by the standard deviation (SD) of the ambulatory BP recordings over the entire 24 h [13]. However, BPV during a 24-h period is usually defined as circadian BP variation and mainly reflects the day– night variation. Since the magnitude of the nocturnal BP fall is positively related with 24-h BPV [14] and the clinical significance of these two parameters is opposite, with a increased BPV [1,15] and a reduced degree of nocturnal BP fall [16] both being associated with a greater degree of end-organ damage and cardiovascular events, we did not focus on the 24-h BPV in the present study. Instead, to account for the influence of the nocturnal BP fall on the 24-h BP SD and quantify 24-h BPV without including the circadian component, the weighted 24-h BP SD was used in this study, which is the mean of the daytime BPV and night-time BPV weighted for the duration of daytime and night-time subperiods [17].
Statistical analyses
Growth curve modeling
All analyses in the present study were explored by use of individual growth curve modeling within a multilevel framework [18], which is a data analysis technique particularly designed to explore longitudinal data. Longitudinal data can be considered to be clustered or hierarchical data since repeated observations (first level) are nested within patients (second level). Individual growth curve modeling accounts for the dependency of the data owing to this clustering, and fits a curve for each individual. These individual growth curves (e.g. systolic/ diastolic BPV development with age) are characterized by their intercept (or level) and slope (rate of change). Addition of independent variables to the model, such as ABP level, ethnicity and sex, is aimed at explaining between-patient variation (in level and slope) of the BPV growth curves [18,19].
Analytical strategy and software
Each of the BPV indices was regarded as a two-level hierarchy, with patients at level 2 (between-patient variation) and repeated BPV measurements at level 1 (within-patient variation).
We first specified the unconditional growth model, in which fixed and random linear and quadratic trends were fitted by adding age and age2 to the intercept-only model, respectively. Age was expressed as a deviation from its mean of 17 years. Ethnicity and sex were then separately added to the unconditional growth model to test their effects on BPV intercept and on the rate of change, the latter was modeled as interactions with age and age2. Ethnicity × sex and ethnicity × sex × age interactions were tested as well.
Next, because changes in BPV can be dependent on the progressive increment in BP values per se, ABP mean values during 24 h were added to the growth model for BPV.
In the third step, we separately added anthropometric variables (i.e. height, BMI and waist circumference), SES (father’s education level) and a measure of genetic susceptibility (family history of essential hypertension) to the model to estimate the effect of these variables on the development of BPV. The main effects of these variables and effects of their interactions with age, ethnicity and sex on BPV were tested as well. Height, BMI, waist circumference and father’s education level were centered at 167 cm, 24.0 kg/m , 80 cm and 13 years, respectively.
In the final step, all variables, including interaction terms, that had significant effects on BPV trajectories in the previous models were entered simultaneously in a full model to obtain independent effects of age, ABP, ethnicity, sex, adiposity (BMI and waist circumference were entered into the full model separately due to the high collinearity between these two variables), SES and family history of essential hypertension on BPV development.
A likelihood ratio test was used to determine the significance of the fixed and random effects that were added to the model in each of the analysis steps. This test yields the deviance of the model which is defined as −2 × log likelihood. The deviance difference (between two models) is asymptotically χ2 distributed, with the number of degrees of freedom equal to the difference in number of estimated parameters between the two models. To judge the significance of parameters in the full model, each parameter was removed from the model, and a likelihood ratio test was used to examine whether its effect was significant in the full model [6,8,18]. Multilevel modeling was conducted using the MLwiN software [19].
Results
Descriptive characteristics by ethnicity and sex at participants’ first evaluation are shown in Table 1.
Table 1.
Characteristics of patients’ first visit by ethnicity and sex
| European American |
African–American |
P |
||||
|---|---|---|---|---|---|---|
| Men | Women | Men0 | Women | Sex | Ethnicity | |
| Demographics | ||||||
| N | 179 | 165 | 133 | 164 | ||
| Age, years | 14.5±3.5 | 14.7 ±3.2 | 14.7 ±3.3 | 14.8±3.6 | NS | NS |
| Patients with ≥ 4 visits, % | 52.5% | 53.9% | 57.9% | 63.4% | NS | <0.05 |
| Father’s education level, years | 13.9 ±2.5 | 13.8 ±2.2 | 12.5 ±2.4 | 12.9±2.1 | NS | <0.001 |
| Family history of essential hypertension, % | 66.5% | 66.3% | 85.7% | 89.3% | NS | <0.001 |
| Anthropometric measures | ||||||
| Height, cm | 163.5 ±16.7 | 158.3 ± 10.8 | 163.1 ± 14.0 | 159.4 ± 10.7 | <0.001 | NS |
| Weight, kg | 59.9 ±20.4 | 57.2 ± 17.0 | 63.3 ±22.5 | 64.9 ±23.9 | NS | 0.001 |
| BMI, kg/m2 | 21.7±4.7 | 22.5 ±5.3 | 23.1 ±5.8 | 25.1 ± 7.9 | <0.01 | <0.001 |
| Waist, cm | 76.7 ±14.0 | 74.3 ± 12.9 | 77.4 ± 15.7 | 78.9 ± 17.5 | NS | <0.05 |
| Ambulatory BP, mmHg | ||||||
| 24h SBP | 115.8±8.1 | 111.5 ±6.6 | 117.9 ±9.0 | 113.5 ± 7.1 | <0.001 | 0.001 |
| 24h DBP | 66.5±5.0 | 66.0 ±5.0 | 67.9 ±6.0 | 67.9±5.3 | NS | <0.001 |
| Ambulatory BPV, mmHg | ||||||
| Weighted-24h SBP SD | 9.5 ±2.2 | 8.7 ± 1.5 | 9.3 ± 1.9 | 9.0± 1.8 | <0.001 | NS |
| Weighted-24h DBP SD | 9.2 ±1.8 | 8.5 ± 1.7 | 9.7 ±2.1 | 9.3 ± 1.9 | <0.001 | <0.001 |
Values are mean ±SD unless otherwise indicated. BMI, body mass index; BPV, blood pressure variability; DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation.
Systolic blood pressure variability growth curve
Results of growth curve modeling for systolic BPV are shown in Table 2. The unconditional growth model with fixed and random linear effects (age) and a fixed quadratic effect (age2) provided the best fit. Age showed a significant positive effect (β = 0.110, P< 0.001), which indicates that systolic BPV increased with age.
Table 2.
Results of growth curve modelling of variables associated with systolic BPV
| Model | Variables | β | P | Explained variance within patients (%) |
Explained variance between patients (%) |
|---|---|---|---|---|---|
| 1 | Age+ethnicity+sex model | 2.89% | 37.59% | ||
| Age | 0.110 | <0.001 | … | … | |
| Age2 | −0.013 | <0.001 | … | … | |
| Ethnicity | 0.271 | <0.050 | … | … | |
| sex | −0.993 | <0.001 | … | … | |
| Sex × age | −0.092 | <0.001 | … | … | |
| Sex × age2 | 0.011 | <0.050 | … | … | |
| 2 | SBP model: model 1+SBP | 3.81% | 48.76% | ||
| SBP | 0.045 | <0.001 | … | … | |
| SBP × age | −0.004 | <0.001 | … | … | |
| 3 | Height model: model 2+height | … | … | ||
| Height | −0.005 | NS | … | … | |
| 4 | BMI model: model 2+BMI | 3.94% | 52.11% | ||
| BMI | 0.029 | <0.001 | … | … | |
| 5 | Waist model: model 2+waist | 3.69% | 51.24% | ||
| Waist | 0.011 | <0.001 | … | … | |
| 6 | Age+ethnicity+sex+SBP modela | … | … | 4.06% | 49.68% |
| 7 | Father’s education model: model 6 + father’s education | 4.22% | 50.82% | ||
| Father’s education | −0.059 | <0.001 | … | … | |
| 8 | Age+ethnicity+sex+SBP modelb | … | … | 3.80% | 48.65% |
| 9 | Family history model: model 8 + family history | … | … | ||
| Family history of essential hypertension | 0.093 | NS | … | … | |
| 10 | Full model: model 7+adipositya | … | |||
| Ethnicity | 0.083 | NS | … | … | |
| Sex | −0.700 | <0.050 | … | … | |
| SBP | 0.043 | <0.050 | … | … | |
| Father’s education | −0.050 | <0.050 | … | … | |
| Adiposity | … | ||||
| (1) BMI | 0.025 | <0.050 | 4.22% | 52.96% | |
| (2) Waist | 0.009 | <0.050 | 4.10% | 52.59% |
BMI, body mass index; BPV, blood pressure variability; SBP, systolic blood pressure. Coding of ethnicity: 0 for European Americans and 1 for African–Americans; coding of sex: 0 for men and 1 for women; coding for family history of essential hypertension: 0 for negative and 1 for positive.
Smaller sample size involving 623 patients and 2593 measurements (cases missing on father’s education were excluded).
Smaller sample size involving 634 patients and 2615 measurements (cases missing on family history of essential hypertension were excluded).
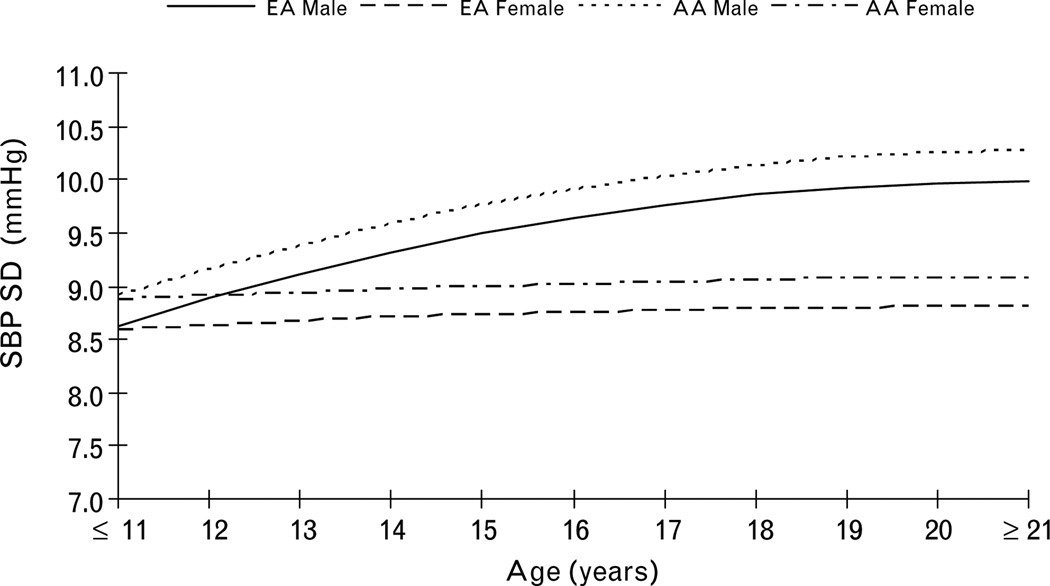
Ethnicity and sex showed a significant effect on systolic BPV levels, with African–Americans and men having higher levels than European Americans and women (Table 2, model 1). Sex also showed significant interactions with age and age2, reflecting that men had higher linear increase and a stronger leveling-off of the increase in systolic BPV over time than did women (Fig. 1). Compared with the unconditional growth model, the ethnicity and sex model explained an additional 23.2% of the between-patient variance and only 0.7% of the within-patient variance in systolic BPV.
Fig. 1.
The increase of systolic blood pressure variability with age by ethnicity and sex.
Twenty-four-hour SBP mean value had significant effect on systolic BPV levels (β = 0.045, P < 0.001), with higher SBP mean value associated with higher BPV levels. SBP mean value also showed significant interaction with age (β= −0.004, P< 0.001), which reflects that the positive effect of SBP mean value on systolic BPV levels decreased with age (Table 2, model 2). After adjusting for SBP mean value, we found the significant effect of sex on systolic BPV levels remained, but the significant effect of ethnicity disappeared (data not shown). Compared with the ethnicity and sex model, SBP mean value model explained an additional 11% of the between-patient variance and 0.9% of the within-patient variance in systolic BPV.
As shown in Table 2 (models 4 and 5), BMI and waist circumference showed significant effects on systolic BPV levels (P < 0.001), with increasing adiposity (both general and central) associated with increased BPV. However, systolic BPV levels were not affected by height (Table 2, model 3). Compared with the ABP mean value model, anthropometric models explained additional 2.6–3.8% of the between-patient variance and less than 0.2% of the within-patient variance in systolic BPV.
As an index of SES, father’s education level showed a significant negative effect systolic BPV levels (β= −0.059, P< 0.001) (Table 2, model 7), which indicates that patients with higher father’s education levels had lower systolic BPV levels. Compared with the ABP mean value model, father’s education level explained an additional 1.2% of the between-patient variance and less than 0.2% of the within-patient variance in systolic BPV. Family history of essential hypertension did not show any significant effect on systolic BPV (Table 2, model 9).
In the full model (Table 2, model 10) including age, age2, ethnicity, sex, SBP mean value, father’s education level and adiposity (BMI and waist circumference were entered into the full model separately), ethnicity was no longer significant. The full model explained in total 53.0% of the between-patient variance and 4.2% of the within-patient variance in systolic BPV.
Diastolic blood pressure variability growth curve
Results of growth curve modeling for diastolic BPV are shown in Table 3. The unconditional growth model with fixed and random linear effects (age) provided the best fit. Age showed a significant positive effect on diastolic BPV levels (β = 0.071, P < 0.001).
Table 3.
Results of growth curve modelling of variables associated with diastolic BPV
| Model | Variables | β | P | Explained variance within patients (%) |
Explained variance between patients (%) |
|---|---|---|---|---|---|
| 1 | Age+ethnicity+sex model | 3.04% | 29.14% | ||
| Age | 0.071 | <0.001 | … | … | |
| Ethnicity | 0.557 | <0.001 | … | … | |
| Sex | −0.707 | <0.001 | … | … | |
| Sex × age | −0.068 | <0.001 | … | … | |
| 2 | DBP model: model 1+DBP | 3.26% | 32.49% | ||
| DBP | 0.035 | <0.001 | … | … | |
| 3 | Height model: model 2+height | … | … | ||
| Height | 0.007 | NS | … | … | |
| 4 | BMI model: model 2+BMI | 3.15% | 42.26% | ||
| BMI | 0.064 | <0.001 | … | … | |
| 5 | Waist model: model 2+waist | 3.04% | 41.09% | ||
| Waist | 0.025 | <0.001 | … | … | |
| 6 | Age+ethnicity+sex+DBP modela | 3.45% | 33.30% | ||
| 7 | Father’s education model: model 6+ father’s education | 3.56% | 33.93% | ||
| Father’s education | −0.056 | <0.050 | … | … | |
| 8 | Age+ethnicity+sex+SBP modelb | 3.18% | 32.58% | ||
| 9 | Family history model: model 8 + family history | … | … | ||
| Family history of essential hypertension | 0.160 | NS | … | … | |
| 10 | Full model: model 7+adipositya | ||||
| Ethnicity | 0.352 | <0.050 | … | … | |
| Sex | −0.745 | <0.050 | … | … | |
| DBP | 0.045 | <0.050 | … | … | |
| Father’s education | −0.031 | NS | … | … | |
| Adiposity | |||||
| (1) BMI | 0.063 | <0.050 | 3.38% | 43.42% | |
| (2) Waist | 0.024 | <0.050 | 3.30% | 42.08% |
DBP, diastolic blood pressure; BPV, blood pressure variability; BMI, body mass index. Coding of ethnicity: 0 for European Americans and 1 for African–Americans; coding of sex: 0 for men and 1 for women; coding for family history of essential hypertension: 0 for negative and 1 for positive.
Smaller sample size involving 623 patients and 2593 measurements (cases missing on father’s education were excluded).
Smaller sample size involving 634 patients and 2615 measurements (cases missing on family history of essential hypertension were excluded).
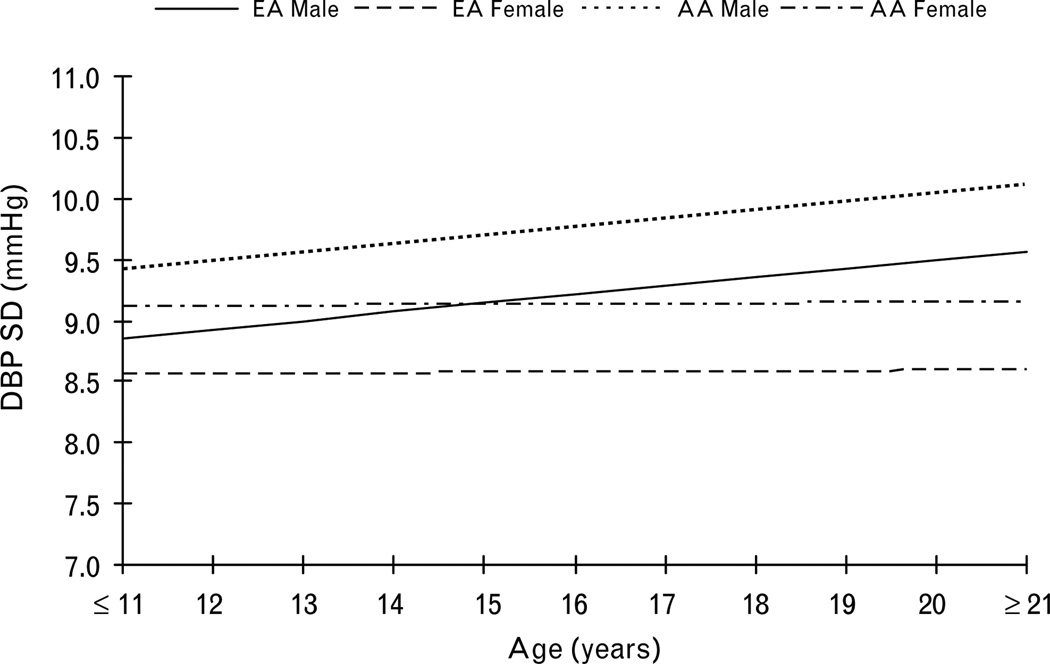
Ethnicity and sex showed significant effects on diastolic BPV levels, with African–Americans and men having higher levels than European Americans and women (Table 3, model 1). Sex also showed significant interaction with age, reflecting that men had higher linear increase of diastolic BPV over time than did women (Fig. 2). Compared with the unconditional growth model, the ethnicity and sex model explained an additional 16.2% of the between-patient variance and only 0.6% of the within-patient variance in diastolic BPV.
Fig. 2.
The increase of diastolic blood pressure variability with age by ethnicity and sex.
Twenty-four-hour DBP mean value had a significantly positive effect on diastolic BPV levels (β = 0.035, P< 0.001) (Table 3, model 2). Compared with the ethnicity and sex model, DBP mean value model explained an additional 3.4% of the between-patient variance and 0.3% of the within-patient variance in diastolic BPV levels.
As shown in Table 3 (models 4 and 5), BMI and waist circumference showed significant effects on diastolic BPV, with increasing adiposity (both general and central) associated with higher BPV levels. Compared with the ABP mean value model, anthropometric models explained additional 8.6–9.8% of the between-patient variance in diastolic BPV.
Father’s education level showed a significant effect on diastolic BPV levels (β = −0.056, P<0.05) (Table 3, model 7), with patients with higher father’s education levels having lower levels. Compared with the ABP mean value model, father’s education level explained an additional 0.6% of the between-patient variance and 0.1% of the within-patient variance in diastolic BPV. Family history of essential hypertension did not show any significant effect on diastolic BPV (Table 3, model 9).
In the full model (Table 3, model 10) including age, ethnicity, sex, DBP mean value, father’s education level and adiposity (BMI and waist circumference were entered into the full model separately), father’s education level was no longer significant. The full models explained in total 42.1–43.4% of the between-patient variance and 3.3–3.4% of the within-patient variance in diastolic BPV.
Discussion
To the best of our knowledge, the present study is the first longitudinal study to examine effects of age, ABP level, sex, ethnicity, adiposity, SES and genetic susceptibility of essential hypertension on BPV development from childhood to early adulthood. We found that both BPV measures (weighted 24-h SBP SD and weighted 24-h DBP SD) increased with age and ABP levels. As for sex and ethnic difference, we found that men and African–Americans have higher BPV than women and European Americans. BPV measures increased with adiposity. Furthermore, the present study is the first to report that lower SES associates with increased BPV.
That increased BPV is associated with aging and high ABP levels [13,14,20], although some studies did not confirm these relationships, is generally accepted [21– 23]. Fluckiger et al. [21] and Sakai et al. [22] found no significant association between age and BPV. Pickering et al. [23] found BPV was not correlated with ABP levels. In the present study, after adjusting for potential confounding effects, we found that age and ABP mean levels independently affected all BPV measures. Although precise mechanisms responsible for BPV are not fully understood, it is proposed that behavioral, neural, reflex and humoral factors all participate in this phenomenon [24]. Baroreflex sensitivity diminishes with increasing arterial stiffness due to aging and high BP, which may partly explain the positive effects of age and ABP level on BPV growth.
Existing evidence on the sex difference in BPV is limited and conflicting. In a community-based study (823 patients aged ≥20 years) in Ohasama, Japan, Imai et al. [14] found that women had significantly higher levels of 24-h SD (both SBP and DBP) than men. Watson et al. [25] did not find any sex differences in BPV in 26 patients with essential hypertension. In contrast to the two cross-sectional studies above, we found that men had significantly higher levels of all BPV measures than women, which is consistent with the prognostic value of higher BPV being associated with a greater degree of cardiovascular events, since it is generally accepted that men usually have higher morbidity and mortality of cardiovascular diseases than women. Conclusive evidence has shown that acute dynamic physical exercise may cause blood pressure fluctuations [24,26]. So we suspected that higher levels of dynamic physical exercise in men compared to women might have been one possible explanation of the sex difference in BPV. Unfortunately, we could not fully adjust for the confounding effect of daily dynamic physical exercise due to the absence of this kind of data. However, since ABP is also related to dynamic physical exercise [27] and we have adjusted for ABP mean values, we believe that we have at least partially adjusted for differences in daily physical exercise between men and women. This indicates that other intrinsic factors may be involved in the sex difference in BPV. One of these may be arterial baroreflex sensitivity, because there is evidence showing that women may have greater baroreflex sensitivity and alterations of BP are more efficiently controlled than in men [28].
On the basis of the only previously available study on ethnic difference in BPV which included only 26 patients with essential hypertension in England, Watson et al. [25] found that none of the BPV measures were related to ethnicity (6 blacks vs. 20 whites). However, the cross-sectional design and the small sample size limit the generalizability of this study. In the present study, we found that African–Americans showed higher levels of BPV than European Americans. The higher BPV level in African–Americans than European Americans is also consistent with the prognostic value of higher BPV, because substantial evidence has shown that African– Americans experience higher incidence and mortality of cardiovascular disease than European Americans. In the present study, most of ethnic differences in BPV, for example SBP SD, were explained by ethnic differences in ABP mean values per se. However, the significantly higher DBP SD in African–Americans than European Americans persisted even after adjustment for DBP mean values and other potential confounders. Therefore, additional mechanisms which may affect the ethnic difference in BPV need to be clarified in the future.
The present study further examined effects of other moderators, for example adiposity, SES and genetic susceptibility to essential hypertension, on BPV development. Consistent with previous cross-sectional studies [14,22,25], we found that adiposity was positively related to all BPV measurements. Reduced baroreflex sensitivity [29] and vagal cardiac dysfunction [30,31] have been observed in obese patients, which may explain the increased BPV in obese patients. To the best of our knowledge, we are the first to report effects of SES (father’s education level) and family history of essential hypertension on BPV growth. We found that patients with higher father’s education level showed lower BPV levels. Together with the protective effect of higher SES on other cardiovascular risk factors, for example ABP level [8] and left ventricular mass [7], our results confirmed that father’s education attainment might be a cardiovascular protective investment for their offspring. We used family history of essential hypertension as a proxy for genetic susceptibility in the present study, and failed to find any significant positive relationship between family history of essential hypertension and BPV measures. However, we must be cautious in interpreting the genetic predisposition on BPV growth, because family history of essential hypertension is only a crude measure of genetic susceptibility, and it also partly reflects familiar environment. In addition, using the same dataset, we have observed low tracking coefficients of BPV measures over time [32]. The low stability of BPV indicates that a larger sample size might have to be used to identify small effects.
Several limitations of the present study need to be recognized. Firstly, BPV indices in the present study represented 20-min (daytime)/30-min (night-time) intermittent BP variability, not beat-to-beat BP variability. Short-term BP variability, including sporadic and random variations as well as physiological variations, should be examined by beat-to-beat measurements of BP [14,33]. However, when the time period between the intermittent measurements range from 5 to 20 min, the BPV obtained by intermittent measurements was not significantly different from those from beat-to-beat measurements [33]. Secondly, ethnic and sex differences in BPV may be due to differences in pubertal maturation stage. Since we have addressed effects of body height and adiposity in our study, which have been shown to be associated with pubertal maturation stage, we have at least partly adjusted for sexual maturation through body size. Thirdly, we did not have data on physical activity and it is a possibility that physical activity may explain the differences in BPV between sex and ethnicity. We conducted the same analysis using night-time BPV and observed the same effect. That is, African–Americans and men having higher BPV than European Americans and women. This indicates that it is unlikely that ethnic and sex differences in BPV can be explained by differences in physical activity. Overall, these limitations do not diminish the value of this study. For example, we are the first longitudinal study to examine predictors of BPV development from childhood to early adulthood, and we are also the first to report the protective effect of higher SES on BPV.
Perspectives
Although BPV derived from ambulatory BP monitoring is an independent predictor of target-organ damage and cardiovascular events, longitudinal evidence about its development with age and its possible sociodemographic, anthropometric, and genetic moderators is scarce. In the present longitudinal study of 344 European Americans and 297 African–Americans with up to 12 measurements over a 15-year period from childhood to early adulthood, we discovered that men and African–Americans have higher BPV levels than women and European Americans. We also found that all BPV levels increased with increasing age, ABP level and adiposity and patients with higher father’s education level showed lower BPV. The results suggested that the higher cardiovascular morbidity and mortality in men and African–Americans may have their origin in sex and ethnic differences in BPV. Reducing and controlling BP level and adiposity could be considered to reduce the risk of developing high BPV levels.
Acknowledgements
Research was supported by HL086530 and HL69999 from the National Heart, Lung and Blood Institute as well as 0730156N from the American Heart Association.
Glossary
Abbreviations
- ABP
ambulatory blood pressure
- BP
blood pressure
- BPV
blood pressure variability
- SES
socioeconomic status
Footnotes
There are no conflicts of interest.
References
- 1.Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-h blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens. 1987;5:93–98. doi: 10.1097/00004872-198702000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Palatini P, Penzo M, Racioppa A, Zugno E, Guzzardi G, Anaclerio M, Pessina AC. Clinical relevance of nighttime blood pressure and of daytime blood pressure variability. Arch Intern Med. 1992;152:1855–1860. [PubMed] [Google Scholar]
- 3.Frattola A, Parati G, Cuspidi C, Albini F, Mancia G. Prognostic value of 24-h blood pressure variability. J Hypertens. 1993;11:1133–1137. doi: 10.1097/00004872-199310000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Verdecchia P, Angeli F, Gattobigio R, Rapicetta C, Reboldi G. Impact of blood pressure variability on cardiac and cerebrovascular complications in hypertension. Am J Hypertens. 2007;20:154–161. doi: 10.1016/j.amjhyper.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Sander D, Kukla C, Klingelhofer J, Winbeck K, Conrad B. Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: a 3-year follow-up study. Circulation. 2000;102:1536–1541. doi: 10.1161/01.cir.102.13.1536. [DOI] [PubMed] [Google Scholar]
- 6.Treiber FA, Musante L, Kapuku G, Davis C, Litaker M, Davis H. Cardiovascular (CV) responsivity and recovery to acute stress and future CV functioning in youth with family histories of CV disease: a 4-year longitudinal study. Int J Psychophysiol. 2001;41:65–74. doi: 10.1016/s0167-8760(00)00183-5. [DOI] [PubMed] [Google Scholar]
- 7.Dekkers JC, Snieder H, Van Den Oord EJ, Treiber FA. Moderators of blood pressure development from childhood to adulthood: a 10-year longitudinal study. J Pediatr. 2002;141:770–779. doi: 10.1067/mpd.2002.128113. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Poole JC, Treiber FA, Harshfield GA, Hanevold CD, Snieder H. Ethnic and gender differences in ambulatory blood pressure trajectories: results from a 15-year longitudinal study in youth and young adults. Circulation. 2006;114:2780–2787. doi: 10.1161/CIRCULATIONAHA.106.643940. [DOI] [PubMed] [Google Scholar]
- 9.Dekkers JC, Treiber FA, Kapuku G, Snieder H. Differential influence of family history of hypertension and premature myocardial infarction on systolic blood pressure and left ventricular mass trajectories in youth. Pediatrics. 2003;111:1387–1393. doi: 10.1542/peds.111.6.1387. [DOI] [PubMed] [Google Scholar]
- 10.Mensah GA, Treiber FA, Kapuku GK, Davis H, Barnes VA, Strong WB. Patterns of body fat deposition in youth and their relation to left ventricular markers of adverse cardiovascular prognosis. Am J Cardiol. 1999;84:583–588. doi: 10.1016/s0002-9149(99)00383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harshfield GA, Barbeau P, Richey PA, Alpert BS. Racial differences in the influence of body size on ambulatory blood pressure in youths. Blood Press Monit. 2000;5:59–63. [PubMed] [Google Scholar]
- 12.O’Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Macia G, et al. European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821–848. doi: 10.1097/00004872-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Ragot S, Herpin D, Siche JP, Poncelet P, Mallion JM. Relationship between short-term and long-term blood pressure variabilities in essential hypertensives. J Hum Hypertens. 2001;15:41–48. doi: 10.1038/sj.jhh.1001111. [DOI] [PubMed] [Google Scholar]
- 14.Imai Y, Aihara A, Ohkubo T, Nagai K, Tsuji I, Minami N, et al. Factors that affect blood pressure variability. A community-based study in Ohasama, Japan. Am J Hypertens. 1997;10:1281–1289. doi: 10.1016/s0895-7061(97)00277-x. [DOI] [PubMed] [Google Scholar]
- 15.Parati G, Mancia G. Blood pressure variability as a risk factor. Blood Press Monit. 2001;6:341–347. doi: 10.1097/00126097-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Verdecchia P, Schillaci G, Guerrieri M, Gatteschi C, Benemio G, Boldrini F, Porcellati C. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation. 1990;81:528–536. doi: 10.1161/01.cir.81.2.528. [DOI] [PubMed] [Google Scholar]
- 17.Parati G, Bilo G, Vettorello M, Groppelli A, Maronati A, Tortorici E, et al. Assessment of overall blood pressure variability and its different components. Blood Press Monit. 2003;8:155–159. doi: 10.1097/00126097-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Dekkers C, Treiber FA, Kapuku G, Van Den Oord EJ, Snieder H. Growth of left ventricular mass in African American and European American youth. Hypertension. 2002;39:943–951. doi: 10.1161/01.hyp.0000015612.73413.91. [DOI] [PubMed] [Google Scholar]
- 19.Rasbash J, Browne W, Goldstein H, Yang M, Plewis I, Healy M, et al. A user’s guide to MLwin. London, UK: University of London, Institute of Education; 2000. [Google Scholar]
- 20.Mancia G, Ferrari A, Gregorini L, Parati G, Pomidossi G, Bertinieri G, et al. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ Res. 1983;53:96–104. doi: 10.1161/01.res.53.1.96. [DOI] [PubMed] [Google Scholar]
- 21.Fluckiger L, Boivin JM, Quilliot D, Jeandel C, Zannad F. Differential effects of aging on heart rate variability and blood pressure variability. J Gerontol A Biol Sci Med Sci. 1999;54:B219–B224. doi: 10.1093/gerona/54.5.b219. [DOI] [PubMed] [Google Scholar]
- 22.Sakai M, Tamura K, Tanaka Y, Tsurumi Y, Okano Y, Koide Y, et al. Analysis of factors that affect short-term blood pressure variability in patients with chronic renal failure. Clin Exp Hypertens. 2005;27:139–147. [PubMed] [Google Scholar]
- 23.Pickering TG, Harshfield GA, Kleinert HD, Blank S, Laragh JH. Blood pressure during normal daily activities, sleep, and exercise. Comparison of values in normal and hypertensive subjects. JAMA. 1982;247:992–996. [PubMed] [Google Scholar]
- 24.Mancia G, Grassi G. Mechanisms and clinical implications of blood pressure variability. J Cardiovasc Pharmacol. 2000;35:S15–19. doi: 10.1097/00005344-200000004-00003. [DOI] [PubMed] [Google Scholar]
- 25.Watson RD, Stallard TJ, Flinn RM, Littler WA. Factors determining direct arterial pressure and its variability in hypertensive man. Hypertension. 1980;2:333–341. doi: 10.1161/01.hyp.2.3.333. [DOI] [PubMed] [Google Scholar]
- 26.Leary AC, Donnan PT, MacDonald TM, Murphy MB. The influence of physical activity on the variability of ambulatory blood pressure. Am J Hypertens. 2000;13:1067–1073. doi: 10.1016/s0895-7061(00)01186-9. [DOI] [PubMed] [Google Scholar]
- 27.Rowlands DB, Stallard TJ, Watson RD, Littler WA. The influence of physical activity on arterial pressure during ambulatory recordings in man. Clin Sci (Lond) 1980;58:115–117. doi: 10.1042/cs0580115. [DOI] [PubMed] [Google Scholar]
- 28.Hinojosa-Laborde C, Chapa I, Lange D, Haywood JR. Gender differences in sympathetic nervous system regulation. Clin Exp Pharmacol Physiol. 1999;26:122–126. doi: 10.1046/j.1440-1681.1999.02995.x. [DOI] [PubMed] [Google Scholar]
- 29.Skrapari I, Tentolouris N, Perrea D, Bakoyiannis C, Papazafiropoulou A, Katsilambros N. Baroreflex sensitivity in obesity: relationship with cardiac autonomic nervous system activity. Obesity (Silver Spring) 2007;15:1685–1693. doi: 10.1038/oby.2007.201. [DOI] [PubMed] [Google Scholar]
- 30.Tonhajzerova I, Javorka M, Trunkvalterova Z, Chroma O, Javorkova J, Lazarova Z, et al. Cardio-respiratory interaction and autonomic dysfunction in obesity. J Physiol Pharmacol. 2008;59:709–718. [PubMed] [Google Scholar]
- 31.Rabbia F, Silke B, Conterno A, Grosso T, De Vito B, Rabbone I, et al. Assessment of cardiac autonomic modulation during adolescent obesity. Obes Res. 2003;11:541–548. doi: 10.1038/oby.2003.76. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Snieder H, Harshfield GA, Treiber FA, Wang X. A 15-year longitudinal study on ambulatory blood pressure tracking from childhood to early adulthood. Hypertens Res. 2009;32:404–410. doi: 10.1038/hr.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.di Rienzo M, Grassi G, Pedotti A, Mancia G. Continuous vs. intermittent blood pressure measurements in estimating 24-h average blood pressure. Hypertension. 1983;5:264–269. doi: 10.1161/01.hyp.5.2.264. [DOI] [PubMed] [Google Scholar]