Abstract
Background
Data on resting energy expenditure (REE) and oxygen consumption (VO2) after pediatric cardiopulmonary bypass (CPB) will facilitate optimal nutrient prescription.
Methods
The authors measured continuous REE and VO2, using an in-line indirect calorimetery (IC) in 30 consecutive children with single-ventricle physiology immediately after Fontan surgery. REE during steady state at 8 hours after surgery was compared with standard equation-estimated energy expenditure (EEE). Patients were classified into 3 groups: hypermetabolic (measured REE [MREE]/EEE ratio >1.2), hypometabolic (MREE/EEE ratio <0.8), and normometabolic (MREE/EEE ratio 0.8–1.2). Demographic, anthropometric, and perioperative clinical characteristics were examined for their correlation with metabolic status.
Results
In 26 of 30 patients with completed IC, mean REE at 8 hours after surgery was 57 ± 20 kcal/kg/d, and mean VO2 was 110 ± 35 mL/min. Mean values of VO2 and REE did not change within the first 24 hours after surgery. There was poor correlation between MREE at 8 hours and the EEE using the World Health Organization equation (r = 0.32, P = .11). Most patients (n = 19, 73%) were either normometabolic or hypometabolic. Lack of hypermetabolism was significantly associated with higher intraoperative serum lactate level and positive fluid balance compared with the rest of the group.
Conclusions
The authors report a low prevalence of hypermetabolism in children with single-ventricle defects after Fontan surgery. Measured REE had poor correlation with equation-estimated energy expenditure in a majority of the cohort. The absence of increased energy expenditure after CPB will influence energy prescription in this group.
Keywords: resting energy expenditure (REE), hypermetabolism, hypometabolism, indirect calorimetry, cardiopulmonary bypass, congenital heart disease, Fontan
Introduction
The metabolic response to injury or surgery is characterized by increased serum levels of counterregulatory hormones and proinflammatory cytokines.1 This adaptive response triggers muscle-protein catabolism, providing a large amount of circulating amino acids for anti-inflammatory activity and tissue repair. The response is historically thought to be a hypermetabolic phenomenon with increased energy expenditure. For example, adult studies have shown an increased oxygen consumption (VO2) and high resting energy expenditure (REE) during the hypermetabolic inflammatory phase after cardiopulmonary bypass (CPB).2,3
Less is known about the response to injury or surgery in children. The postinjury or postoperative phase after noncardiac surgery in newborns is also characterized by hypometabolism.4-6 Similarly, using IC measurements, we have recently demonstrated a predominance of hypometabolism in older critically ill children admitted to an ICU for medical conditions or following noncardiac surgery.7 Limited metabolic data exist following cardiac surgery in children. In 1 study, infants manifested a minor increase in REE after CPB, with a return to baseline within 12 hours.8 Marginally elevated VO2 was observed in some studies during the immediate postoperative period in children after cardiac surgery but was related to rewarming in the first 4–8 hours after CPB and returned to baseline levels subsequently.9,10 Hence, the REE patterns in children following cardiac surgery with CPB are distinct from adults and variable among different cardiac conditions.9,11
Further clarification of the metabolic response to CPB in children is needed. The hypometabolic states that exist in some critically ill pediatric populations increase the potential for overfeeding, which may prolong the duration of mechanical ventilation.5,7,12 Measured REE is recommended as a guide to optimal energy intake in critically ill infants, as currently available equation estimates are generally inaccurate in this population.13 Postoperative REE and VO2 after Fontan surgery in children have not been previously described.
We conducted a prospective cohort study to characterize the metabolic profile in the early postoperative period after CPB in children undergoing Fontan surgery. We hypothesized that patients would be either normometabolic or hypometabolic at 8 hours after Fontan surgery and that the metabolic state would be correlated with perioperative variables, including CPB time, cross-clamp time, peak serum lactate, temperature, and fluid balance and postoperative inflammation assessed by serum C-reactive protein (CRP).
Methods
In this prospective, observational, single-center study, 30 consecutive children with single-ventricle heart defects admitted to the cardiac intensive care unit (CICU) at Children's Hospital Boston after Fontan surgery with CPB were enrolled. These patients were a subset enrolled in an unrelated clinical trial (ClinicalTrials.gov identifier NCT00543309) examining the effect of nesiritide and milrinone on early postoperative outcomes. Patients were excluded if they were extubated within 6 hours after surgery, required fractional inspired oxygen concentrations greater than 60% beyond the first 4 hours after surgery, if a chest tube was placed for air leak, or if there was a system leak in the ventilator circuit of >15%. Indirect calorimetery (IC) measurements were continued until the patient was deemed ready for extubation, after which the changes in ventilator settings and suctioning would potentially affect the ability to reach steady state and obtain accurate REE values. The study was approved by the institutional review board, and written informed consent was obtained from parents or guardian.
Study patients had all previously undergone prior cardiac surgical procedures intended to palliate children with single-ventricle heart defects. Initial neonatal palliation (eg, stage 1 Norwood procedure or Blalock-Taussig shunt placement) was typically followed by connection of the superior vena cava to the pulmonary artery at approximately 4–6 months of age (stage 2, bidirectional Glenn procedure). Completion of the cavopulmonary connection with the modified fenestrated Fontan procedure (stage 3) is typically undertaken at approximately 2–3 years of age. Following the Fontan operation, the systemic venous return was baffled directly to the pulmonary circulation without the benefit of a pulmonary ventricle. All except 4 patients had a fenestration created at the atrial level. Details of the Fontan surgical procedure, CPB, and anesthetic techniques have been previously described.14 In the CICU, patients were sedated using morphine and midazolam infusions to achieve comfort, anxiolysis, and sedation. Nurses titrated infusions and provided additional boluses as needed. Muscle-relaxant infusion was used in some cases to facilitate synchronous mechanical ventilatory support.
Anthropometric data, including weight, length, and head circumference, were recorded prior to surgery. Weight was measured on a digital scale accurate to 10 g. Recumbent length to the nearest 0.1 cm was measured with a nonstretchable standard measuring tape. Weight-for-age z scores (WAZ) were calculated based on World Health Organization (WHO) growth charts. As early extubation and initiation of enteral nutrition (EN) are expected, parenteral nutrition (PN) was not started on any of the patients.
Continuous VO2 and carbon dioxide elimination (VCO2) were measured, using an in-line indirect calorimeter, Vmax Encore (Viasys, Yorba Linda, CA), for the first 24 postoperative hours beginning immediately upon CICU admission after surgery. Patients received continuous maintenance intravenous (IV) dextrose-containing fluids. Hence, the metabolic measurements were obtained in a modified fasted state (ie, ≥12 hours without any oral or enteral intake). All patients were mechanically ventilated in the supine position during the study period. The flow sensor adaptor of the calorimetry device was connected to the expiratory outlet (exhaust) of the ventilator. A modified water trap was introduced in the system to drain water accumulated over time in the humidified ventilator circuit. Each measurement was preceded by calibration with a gas mixture of known composition according to the manufacturer's instructions. The indirect calorimeter measured the inspiratory concentration of oxygen (FiO2) and the difference between FiO2 and expiratory concentrations of oxygen (FeO2) using an electrochemical cell. The expiratory carbon dioxide (FeCO2) was measured continuously with a nondispersive infrared thermopile, and inspiratory CO2 concentration (FiCO2) was measured from room air. VO2 and VCO2 were calculated using these values each minute and then converted to standard temperature and pressure using dry gas equations. Respiratory quotient (RQ) and REE were calculated from measured variables during steady state using the modified Weir equation.15 These metabolic variables were obtained continuously until extubation, or until 24 hours in patients requiring longer ventilatory support.
Estimated energy expenditure (EEE) was calculated for each patient using the WHO equation.16 Metabolic state of individual patients was described based on the ratio of measured to equation-estimated energy expenditure at 8 hours after CPB, and patients were classified into 3 groups: hypermetabolic (measured REE [MREE]/EEE ratio >1.2), hypometabolic (MREE/EEE ratio <0.8), and normometabolic (MREE/EEE ratio 0.8–1.2). Clinical and demographic characteristics were compared between hypermetabolic and normometabolic/hypometabolic patients.
Statistical Analysis
Data were filtered prior to analysis using 3 steps: (1) removal of the first 3 hours of postsurgery measurements due to elevated and fluctuating FiO2, (2) exclusion of patients who had less than 3 hours of total data recorded, (3) exclusion of data points with the RQ outside the physiologic range of 0.4–1.6, and (4) exclusion of data associated with interventions such as suctioning, endotracheal tube disconnection, and travel outside the unit. Clinical and perioperative variables were compared between hypermetabolic and the normometabolic/hypometabolic groups at 8 hours after CPB using the Student t test, Fisher exact test, or Mann-Whitney U test. Mean changes in REE and VO2 every 4 hours post-CPB within the first 24 hours were assessed by repeated-measures analysis of variance (ANOVA) using the Greenhouse-Geisser F test following Mauchly's confirmation of the assumption of sphericity. Pairwise comparisons of CRP levels at hours 0, 8, and 24 were assessed by repeated-measures ANOVA with Bonferroni correction. Statistical analysis was performed using the SPSS statistical package (Version 19.0; SPSS, Inc, an IBM Company, Chicago, IL). Two-tailed P values <.05 were considered statistically significant. Sample size for this pilot study was determined using repeated-measures ANOVA with a 2-sided α level of 0.05 (Version 7.0; nQuery Advisor, Statistical Solutions, Saugus, MA).17
Results
Continuous IC measurements were performed in 30 mechanically ventilated children recovering from Fontan surgery with CPB. Four patients were excluded due to either extubation within the first 6 hours (n = 2) or technical failure to obtain IC data (n = 2). Data from 26 children, with a mean age of 3.6 ± 2.6 years, were included in the analysis. Average WAZ was −0.94. Mean durations of CBP and aortic cross clamp were 115 and 54 minutes, respectively; circulatory arrest was employed in 4 (15%) patients. There were no complications related to the IC measurements during this study.
Following data cleaning, a total of 23,624 data points (minute-to-minute REE, VO2, and VCO2) were analyzed. Approximately 5% of the data points were excluded from analyses, as determined a priori by study design. A majority of these data points represented the transition phase after admission from the operating room to the pediatric intensive care unit (PICU). Mean REE at 8 hours after surgery was 57 ± 20 kcal/kg/d, and mean VO2 was 110 ± 35 mL/min (Figure 1A,B). Mean values of VO2 and REE declined over the first 24 hours, although these changes over time did not reach significance (P = .67 and P = .41, respectively). Concentrations of serum CRP showed a significant increase between baseline and 24 hours postoperatively (P < .001; Figure 2A–C) but did not correlate with REE at 8 hours (r = 0.22, P = .29). There was poor correlation between the measured REE at 8 hours and the energy expenditure estimated using the WHO equation (r = 0.32, P = .11).
Figure 1.
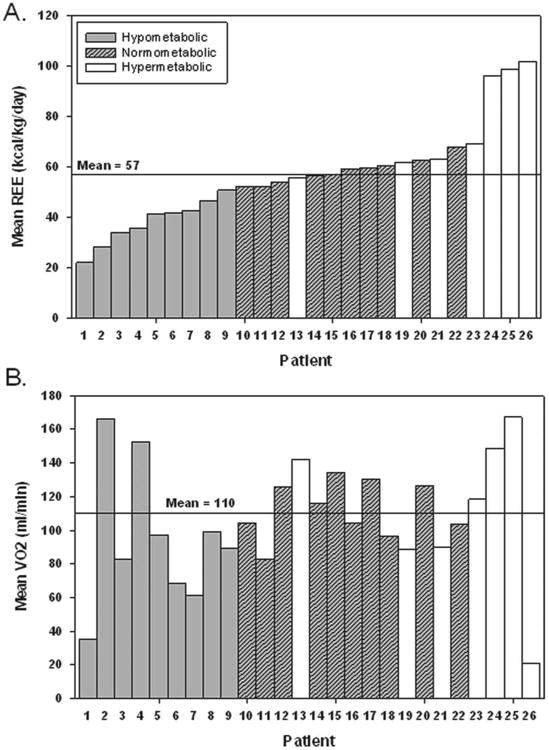
(A) Mean resting energy expenditure (REE) in kcal/kg/d for each patient at 8 hours after Fontan surgery. (B) Mean VO2 (of 30-minute data) in mL/min for each patient at 8 hours after Fontan surgery. The bars are shaded based on metabolic state determined by the ratio of measured to World Health Organization (WHO) equation-estimated energy expenditure. Each patient number is unique across both figures.
Figure 2.

(A) Resting energy expenditure (kcal/kg/d), (B) mean C-reactive protein (CRP) levels (mg/dL), (C) VO2 (mL/min), and (D) VCO2 (mL/min) every fourth hour ± 30 minutes at 0, 1, 8, and 24 hours following cardiopulmonary bypass (n = 26). CRP increased significantly over time (P < .001).
On the basis of the relationship between measured and equation-estimated REE at hour 8 after CPB, most patients (n = 19, 73%) were either normometabolic (n = 9) or hypometabolic (n = 10), whereas hypermetabolism was recorded in 7 (27%) patients. Table 1 depicts baseline patient characteristics in relation to their metabolic state. Patients with hypermetabolism (relatively higher REE) had significantly lower intraoperative peak serum lactate levels (P = .004) and less positive intraoperative fluid balance (P = 0.01) compared with those with normometabolism or hypometabolism. This subgroup of patients was younger and had shorter CPB times, although differences in these variables were not statistically significant. We did not observe any significant differences between the hypermetabolic patients and the rest in terms of demographics, anthropometry, duration of anesthesia, cross-clamp time, lowest intraoperative temperature, peak postoperative serum lactate, body temperature at 8 hours postoperatively, amount of postoperative sedation and analgesia administered, serum creatinine, postoperative serum CRP levels, or duration of mechanical ventilation, intensive care, or hospitalization.
Table 1. Baseline Clinical Variables in 26 Children Undergoing Fontan Surgery, Stratified by Metabolic Statea.
| Characteristic | Metabolic State | P Value | ||
|---|---|---|---|---|
|
| ||||
| Hypermetabolic (n = 7) | Normo- or Hypometabolic (n = 19) | All Patients (n = 26) | ||
| Age, y | 2.3 ± 0.4 | 4.1 ± 2.9 | 3.6 ± 2.6 | .11 |
| Male, No. (%) | 5 (71) | 11 (57) | 16 (62) | .67 |
| Weight, kg | 12 ± 3 | 15 ± 7 | 14 ± 6 | .26 |
| Weight-for-age z score | −0.95 ± 1.62 | −0.96 ± 1.11 | −0.96 ± 1.24 | .99 |
| Body mass index, kg/m2 | 17 ± 3 | 15 ± 1 | 16 ± 2 | .12 |
| Anesthesia time, h | 5 ± 1 | 6 ± 1 | 6 ± 1 | .15 |
| Cardiopulmonary bypass time, min | 96 ± 26 | 122 ± 31 | 115 ± 32 | .06 |
| Cross-clamp time, min | 37 ± 37 | 57 ± 36 | 52 ± 37 | .22 |
| Circulatory arrest, No. (%) | 0 | 2 (11) | 2 (8) | .99 |
| Lowest intraoperative temperature, °C | 30 ± 3 | 28 ± 4 | 29 ± 3 | .33 |
| Lowest intraoperative rectal temperature, °C | 31 ± 2 | 29 ± 3 | 29 ± 3 | .09 |
| 8-h postoperative temperature, °C | 37 ± 1 | 37 ± 1 | 37 ± 1 | .19 |
| Intraoperative fluid balance, mL, median (IQR) | 44 (−252 to 687) | 115 (−20 to 814) | 86 (−117 to 710) | .013 |
| Intraoperative serum lactate, mg/dL | 2.0 ± 0.2 | 3.1 ± 1.7 | 2.8 ± 1.5 | .004 |
| Peak 24-h serum lactate, mg/dL | 2.5 ± 0.7 | 5.3 ± 3.6 | 4.6 ± 3.4 | .51 |
| Time to peak lactate, h, median (IQR) | 14 (10–25) | 13 (8–18) | 13 (8–19) | .47 |
| CICU length of stay, d, median (IQR) | 4 (3–7) | 4 (3–7) | 4 (3–7) | .58 |
| Hospital length of stay, d, median (IQR) | 11 (8–14) | 9 (7–15) | 9 (8–14) | .93 |
| Duration of mechanical ventilation, d, median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) | .90 |
| C-reactive protein at hour 8, mg/L | 105 ± 152 | 105 ± 16 | 106 ± 16 | .99 |
| Postoperative serum creatinine, mg/dL | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | .09 |
| 24-hour morphine, per kg, median (IQR) | 0.7 (0.4–0.9) | 0.7 (0.5–1.5) | 0.7 (0.5–1.5) | .58 |
| 24-hour midazolam, per kg, median (IQR) | 0.3 (0.1–0.4) | 0.4 (0.2–0.9) | 0.4 (0.2–0.9) | .63 |
| Use of postoperative muscle relaxant therapy, No. (%) | 1 (14) | 6 (32) | 7 (27) | .63 |
Values presented as mean ± SD unless indicated otherwise. P values reflect comparison of hypermetabolic group vs normo-/hypometabolic group. Hypermetabolic (MREE/EEE ratio >1.2), hypometabolic (MREE/EEE ratio <0.8), and normometabolic (MREE/EEE ratio 0.8–1.2). CICU, cardiac intensive care unit; EEE, estimated energy expenditure; IQR, interquartile range; MREE, measured resting energy expenditure; REE, resting energy expenditure.
Metabolic state was based on the measured REE (MREE) at 8 hours after cardiopulmonary bypass, in relation to the age/sex-specific equation-estimated REE.
Discussion
We observed stable REE and VO2 during the first 24 hours after Fontan surgery. There was poor correlation between the average 8-hour postoperative REE values and the WHO equation-estimated energy expenditure, and indeed REE was lower than that estimated by the equation in a majority of the cohort. Of note, we measured continuous REE for 24 hours, which represents total daily energy expenditure. Because most patients were sedated during mechanical ventilation and in a fasting state, the measurement is likely to be close to true REE. The absence of hypermetabolism in the postoperative period was significantly associated with higher peak serum lactate levels, more positive intraoperative positive fluid balance, and a trend towards longer CPB time. To our knowledge, this is the first study to describe continuous IC for REE and VO2 measurements in children following Fontan surgery.
The use of CPB for adult cardiac surgery has been reported to elicit a hypermetabolic response.2,3 However, in children undergoing cardiac surgery, postoperative REE has been reported either at or below the preoperative level.11,13,18 Table 2 summarizes the results from studies that have reported REE after CPB in children. In a heterogeneous group of children with cyanotic and acyanotic heart disease, Avitzur and colleagues13 reported no significant difference in REE and VO2 before and after cardiac surgery. Mitchell and colleagues18 measured total energy expenditure using doubly labeled water in 18 children before and after cardiac surgery. Postoperative energy expenditure was significantly lower compared with the preoperative levels in their cohort. In neonates with hypoplastic left heart syndrome undergoing the Norwood procedure, average REE was 46 kcal/kg/d, and mean VO2 was 6.1 mL/kg/min during the first 24 hours following CPB.8 On the other hand, De Wit et al19 reported higher REE after open heart surgery, especially in children with preoperative malnutrition. The higher incidence of malnutrition in their cohort and differences in sedative usage may be responsible for the marginally higher postoperative REE in their cohort compared with the rest of the studies in Table 2. Thus, most of these studies that include a wide range of ages and cardiac conditions have demonstrated a hypometabolic response after CPB. Similarly, our data suggest a muted postoperative REE and VO2 in children with single-ventricle heart defects who underwent Fontan surgery with CPB. A growing body of literature describes a lack of anticipated hypermetabolic response following major general surgical procedures or extracorporeal life support therapy in infants and children.5,20,21
Table 2. Prior Studies of Resting Energy Expenditures After Pediatric Cardiopulmonary Bypass.
| Reference | No. of Patients | Age, mo | Clinical Diagnoses | Postoperative Day for Study | Mean (SD) REE, kcal/kg/d |
|---|---|---|---|---|---|
| Mitchell et al, 199418 | 14 | 4–33 | CHD, after surgery (with CPB) | 1 | 72 ± 20 |
| Avitzur et al, 200313 | 17 | 0.1–30 | CHD, after surgery | 5 | 62 ± 10 |
| Li et al, 20088 | 17 | 0.1–3.1 | Single ventricle, after Norwood surgery (with CPB) | 1 | 43 ± 11 |
| Li et al, 20088 | 17 | 0.1–3.1 | Single ventricle, after Norwood surgery (with CPB) | 3 | 39 ± 7 |
| De Wit et al, 201019 | 13 | 0.1–40 | CHD, after surgery (with CPB) | 1–3 | 73 ± 14 |
| Mehta et al (current study) | 26 | 12–84 | Single ventricle, after Fontan surgery (with CPB) | 1 | 57 ± 20 |
CHD, congenital heart disease; CPB, cardiopulmonary bypass. Day 1 = the day of surgery (first 24 hours after arrival in the pediatric intensive care unit).
The etiology of this muted metabolic response following major surgical procedures is likely multifactorial. Factors that influence the metabolic response after surgery include age, type of surgery, nutrition status, preoperative fasting period, anesthetic technique, and perioperative thermoregulation.22 We observed higher intraoperative peak serum lactate and more positive fluid balance in patients who were normometabolic or hypometabolic postoperatively. This relationship has not been described before. Increased intra-operative lactate levels reflect hypoperfusion and/or inadequate oxygen delivery during the surgical procedure.23,24 Serial lactate levels in the postoperative period have been used to indicate severity of postoperative illness and are correlated with morbidity after cardiac surgery.25 Another important intraoperative variable is the duration of CPB and the cross clamp. Despite a trend toward lower REE in children with longer CPB times in our cohort, this correlation was not statistically significant. Longer duration of CPB and hypothermic circulatory arrest may contribute to hypoperfusion, resulting in high lactate from anaerobic metabolism. Prolonged CPB and circulatory arrest have been shown to increase intraoperative and postoperative lactate in children undergoing cardiac surgery.26 Our findings of a lower prevalence of hypermetabolism among patients with higher lactate levels and more positive fluid balance might be explained by longer duration or depth of anesthesia and hypothermia, which would lead to an attenuated metabolic response in the immediate postoperative period. The anesthesia time and perioperative use of sedation, analgesia, and neuromuscular blockade (NMB) were similar between groups. Perioperative temperature, including degree of intraoperative hypothermia, was not predictive of metabolic state in our cohort. We did not observe a correlation between nutrition status (as measured by WAZ) and metabolic state in our cohort.
Exposure of blood to the extracorporeal circuit has been implicated in the whole-body inflammatory response.27,28 Inflammation is associated with increased VO2 and energy expenditure as seen in healthy volunteers following endotoxin administration or patients with sepsis and systemic inflammatory response syndrome.29,30 A variety of perioperative strategies such as reduction in time spent on CPB, decreased use of hypothermic circulatory arrest, prudent hypothermia, corticosteroids, aprotinin, and ultrafiltration have been employed in the intraoperative period to limit the effect of the systemic inflammatory response to CPB. CRP, an acute phase protein, is a sensitive marker of inflammation and tissue injury.31 In a previous study of children after surgical procedures, higher CRP levels were associated with significantly higher REE values in the postoperative period.32 CRP concentrations continued to rise 24 hours after CPB in our study, despite a relatively stable REE. On the basis of our observations, CRP may not be a sensitive indicator of metabolic state in children soon after CPB. The relationship between CRP and REE needs further investigation over a longer postoperative period.
Accurate assessment of energy expenditure allows optimal energy intake during the postoperative period. Our study was not designed to compare energy intake with expenditure in this population. Patients received IV crystalloid with 5% dextrose in the first 24 hours after surgery. The lack of meaningful macronutrient intake within the first 12–24 hours after cardiac surgery has been reported in other studies.8,19,33 The impact of postoperative fasting on REE in our cohort will need to be clarified in future studies. Children with single-ventricle physiology are at a risk of nutrition deterioration from inability to match energy intake to energy expenditure in the postoperative period.34 On the other hand, the delivery of calories in excess of requirement has been shown to impose a ventilatory burden due to carbon dioxide production.35,36 The likelihood of overfeeding with increased CO2 burden and liver injury has been recognized in other pediatric groups.37,38 Li et al8 demonstrated the initial lack of energy adequacy following Norwood surgery, but intake rapidly increased and by day 3 surpassed REE in their cohort. The measured REE in our patients did not correlate with estimates by the WHO equation. In the absence of IC availability, energy prescriptions based on an equation estimate of energy expenditure will further increase the risk of overfeeding.
There are several limitations to this study. The average duration of mechanical ventilation in children undergoing Fontan surgery at our institution is under 24 hours. Hence, REE data were not recorded beyond this period. Because of technical limitations of the IC device with high inspired oxygen concentrations, we excluded REE data from the first 3 hours of postoperative intensive care unit admission. High FiO2 requirement and clinical interventions result in fluctuating VO2 and VCO2, which impede the accurate gas exchange measurement during this period. It is possible that a transient elevation of REE during the initial 3 postoperative hours, as reported in infants after a general surgical procedure or Norwood surgery, might have been missed in our study.8,39 Because of uniform postoperative care, we were unable to compare the effect of variable sedation states on REE in a systematic manner. Transient postextubation elevations in REE may be expected, especially in patients from centers that routinely fast-track the postoperative extubation after Fontan surgery. Large differences in physical activity or respiratory effort may influence REE. However, the current study focused on energy expenditure in a modified resting state where patients were sedated and fasted. Finally, the effect of the primary study drugs, nesiritide and milrinone, on REE and VO2 are unknown. Although it is unlikely that the afterload-reducing drugs will have differential effects on these parameters, this effect cannot be excluded.
In conclusion, using continuous minute-to-minute REE and VO2 measurements from IC, we have demonstrated a low prevalence of hypermetabolism in children with single-ventricle defects during the first 24 hours after Fontan surgery with CPB. Measured REE poorly correlated with and was lower than the equation-estimated energy expenditure in a majority of the cohort. The metabolic state at 8 hours after surgery was significantly associated with positive intraoperative fluid balance and peak lactate. CRP levels did not correlate with REE during the early postoperative phase. Energy intake may be excessive if hypermetabolic state and high REE are presumed in children following Fontan surgery.
Clinical Relevancy Statement.
The metabolic profile of children with a single-ventricle heart defect in the first 24 hours after Fontan surgery with cardiopulmonary bypass (CPB) has not been previously described. These data enhance our understanding of the metabolic stress response to CPB in children, which appears to lack the hypermetabolic response that was historically expected. Furthermore, the standard equation failed to reliably estimate the measured resting energy expenditure in our study, and its use could increase the risk of overfeeding with undesirable side effects in this vulnerable group.
Footnotes
Financial disclosure: C.D. was supported in part by K24HD058795.
References
- 1.de Groof F, Joosten KF, Janssen JA, et al. Acute stress response in children with meningococcal sepsis: important differences in the growth hormone/insulin-like growth factor I axis between nonsurvivors and survivors. J Clin Endocrinol Metab. 2002;87(7):3118–3124. doi: 10.1210/jcem.87.7.8605. [DOI] [PubMed] [Google Scholar]
- 2.Oudemans-van Straaten HM, Jansen PG, te Velthuis H, et al. Increased oxygen consumption after cardiac surgery is associated with the inflammatory response to endotoxemia. Intensive Care Med. 1996;22(4):294–300. doi: 10.1007/BF01700449. [DOI] [PubMed] [Google Scholar]
- 3.Jakob SM, Ensinger H, Takala J. Metabolic changes after cardiac surgery. Curr Opin Clin Nutr Metab Care. 2001;4(2):149–155. doi: 10.1097/00075197-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Chwals WJ. The metabolic response to surgery in neonates. Curr Opin Pediatr. 1994;6(3):334–340. doi: 10.1097/00008480-199406000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Jaksic T, Shew SB, Keshen TH, Dzakovic A, Jahoor F. Do critically ill surgical neonates have increased energy expenditure? J Pediatr Surg. 2001;36(1):63–67. doi: 10.1053/jpsu.2001.20007. [DOI] [PubMed] [Google Scholar]
- 6.Mehta NM, Bechard LJ, Leavitt K, Duggan C. Cumulative energy imbalance in the pediatric intensive care unit: role of targeted indirect calorimetry. JPEN J Parenter Enteral Nutr. 2009;33(3):336–344. doi: 10.1177/0148607108325249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta NM, Bechard LJ, Dolan M, Ariagno K, Jiang H, Duggan C. Energy imbalance and the risk of overfeeding in critically ill children. Pediatr Crit Care Med. 2011;12(4):398–405. doi: 10.1097/PCC.0b013e3181fe279c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Zhang G, Herridge J, et al. Energy expenditure and caloric and protein intake in infants following the Norwood procedure. Pediatr Crit Care Med. 2008;9:55–61. doi: 10.1097/01.PCC.0000298756.82286.23. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Schulze-Neick I, Lincoln C, et al. Oxygen consumption after cardiopulmonary bypass surgery in children: determinants and implications. J Thorac Cardiovasc Surg. 2000;119(3):525–533. doi: 10.1016/s0022-5223(00)70132-2. [DOI] [PubMed] [Google Scholar]
- 10.Puhakka K, Rasanen J, Leijala M, Peltola K. Oxygen consumption following pediatric cardiac surgery. J Cardiothorac Vasc Anesth. 1994;8(6):642–648. doi: 10.1016/1053-0770(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 11.Gebara BM, Gelmini M, Sarnaik A. Oxygen consumption, energy expenditure, and substrate utilization after cardiac surgery in children. Crit Care Med. 1992;20(11):1550–1554. doi: 10.1097/00003246-199211000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Chwals WJ. Overfeeding the critically ill child: fact or fantasy? New Horiz. 1994;2(2):147–155. [PubMed] [Google Scholar]
- 13.Avitzur Y, Singer P, Dagan O, et al. Resting energy expenditure in children with cyanotic and noncyanotic congenital heart disease before and after open heart surgery. JPEN J Parenter Enteral Nutr. 2003;27(1):47–51. doi: 10.1177/014860710302700147. [DOI] [PubMed] [Google Scholar]
- 14.Jonas RA. Comprehensive Surgical Management of Congenital Heart Disease. London: Arnold (Hodder Education); 2004. [Google Scholar]
- 15.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109(1-2):1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization (WHO) Energy and protein requirements. Report of a joint FAO/WHO/UNU Expert Consultation. World Health Organ Tech Rep Ser. 1985;724:1–206. [PubMed] [Google Scholar]
- 17.Muller KE, Barton CN. Approximate power for repeated measures ANOVA lacking sphericity. J Am Stat Assoc. 1989;84:549–555. [Google Scholar]
- 18.Mitchell IM, Davies PS, Day JM, Pollock JC, Jamieson MP. Energy expenditure in children with congenital heart disease, before and after cardiac surgery. J Thorac Cardiovasc Surg. 1994;107:374–380. [PubMed] [Google Scholar]
- 19.De Wit B, Meyer R, Desai A, Macrae D, Pathan N. Challenge of predicting resting energy expenditure in children undergoing surgery for congenital heart disease. Pediatr Crit Care Med. 2010;11(4):496–501. doi: 10.1097/PCC.0b013e3181ce7465. [DOI] [PubMed] [Google Scholar]
- 20.Groner JI, Brown MF, Stallings VA, Ziegler MM, O'Neill JA., Jr Resting energy expenditure in children following major operative procedures. J Pediatr Surg. 1989;24:825–827. doi: 10.1016/s0022-3468(89)80546-9. [DOI] [PubMed] [Google Scholar]
- 21.Keshen TH, Miller RG, Jahoor F, Jaksic T. Stable isotopic quantitation of protein metabolism and energy expenditure in neonates on- and post-extracorporeal life support. J Pediatr Surg. 1997;32(7):958–962. doi: 10.1016/s0022-3468(97)90377-8. discussion 962-963. [DOI] [PubMed] [Google Scholar]
- 22.McHoney M, Eaton S, Pierro A. Metabolic response to surgery in infants and children. Eur J Pediatr Surg. 2009;19(5):275–285. doi: 10.1055/s-0029-1241192. [DOI] [PubMed] [Google Scholar]
- 23.Basaran M, Sever K, Kafali E, et al. Serum lactate level has prognostic significance after pediatric cardiac surgery. J Cardiothorac Vasc Anesth. 2006;20(1):43–47. doi: 10.1053/j.jvca.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Charpie JR, Dekeon MK, Goldberg CS, Mosca RS, Bove EL, Kulik TJ. Serial blood lactate measurements predict early outcome after neonatal repair or palliation for complex congenital heart disease. J Thorac Cardiovasc Surg. 2000;120(1):73–80. doi: 10.1067/mtc.2000.106838. [DOI] [PubMed] [Google Scholar]
- 25.Molina Hazan V, Gonen Y, Vardi A, et al. Blood lactate levels differ significantly between surviving and nonsurviving patients within the same risk-adjusted Classification for Congenital Heart Surgery (RACHS-1) group after pediatric cardiac surgery. Pediatr Cardiol. 2010;31(7):952–960. doi: 10.1007/s00246-010-9724-7. [DOI] [PubMed] [Google Scholar]
- 26.Munoz R, Laussen PC, Palacio G, Zienko L, Piercey G, Wessel DL. Changes in whole blood lactate levels during cardiopulmonary bypass for surgery for congenital cardiac disease: an early indicator of morbidity and mortality. J Thorac Cardiovasc Surg. 2000;119(1):155–162. doi: 10.1016/s0022-5223(00)70231-5. [DOI] [PubMed] [Google Scholar]
- 27.Westaby S. Organ dysfunction after cardiopulmonary bypass: a systemic inflammatory reaction initiated by the extracorporeal circuit. Intensive Care Med. 1987;13(2):89–95. doi: 10.1007/BF00254791. [DOI] [PubMed] [Google Scholar]
- 28.Chenoweth DE, Cooper SW, Hugli TE, Stewart RW, Blackstone EH, Kirklin JW. Complement activation during cardiopulmonary bypass: evidence for generation of C3a and C5a anaphylatoxins. N Engl J Med. 1981;304(9):497–503. doi: 10.1056/NEJM198102263040901. [DOI] [PubMed] [Google Scholar]
- 29.Fong YM, Marano MA, Moldawer LL. The acute splanchnic and peripheral tissue metabolic response to endotoxin in humans. J Clin Invest. 1990;85(6):1896–1904. doi: 10.1172/JCI114651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moriyama S, Okamoto K, Tabira Y, et al. Evaluation of oxygen consumption and resting energy expenditure in critically ill patients with systemic inflammatory response syndrome. Crit Care Med. 1999;27(10):2133–2136. doi: 10.1097/00003246-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- 32.Chwals WJ, Letton RW, Jamie A, Charles B. Stratification of injury severity using energy expenditure response in surgical infants. J Pediatr Surg. 1995;30(8):1161–1164. doi: 10.1016/0022-3468(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 33.Rogers EJ, Gilbertson HR, Heine RG, Henning R. Barriers to adequate nutrition in critically ill children. Nutrition. 2003;19(10):865–868. doi: 10.1016/s0899-9007(03)00170-9. [DOI] [PubMed] [Google Scholar]
- 34.Kelleher DK, Laussen P, Teixeira-Pinto A, Duggan C. Growth and correlates of nutritional status among infants with hypoplastic left heart syndrome (HLHS) after stage 1 Norwood procedure. Nutrition. 2006;22(3):237–244. doi: 10.1016/j.nut.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Lo HC, Lin CH, Tsai LJ. Effects of hypercaloric feeding on nutrition status and carbon dioxide production in patients with long-term mechanical ventilation. JPEN J Parenter Enteral Nutr. 2005;29(5):380–387. doi: 10.1177/0148607105029005380. [DOI] [PubMed] [Google Scholar]
- 36.Liposky JM, Nelson LD. Ventilatory response to high caloric loads in critically ill patients. Crit Care Med. 1994;22(5):796–802. doi: 10.1097/00003246-199405000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Letton RW, Chwals WJ, Jamie A, Charles B. Early postoperative alterations in infant energy use increase the risk of overfeeding. J Pediatr Surg. 1995;30(7):988–992. doi: 10.1016/0022-3468(95)90327-5. discussion 992-993. [DOI] [PubMed] [Google Scholar]
- 38.Mehta NM, Bechard LJ, Dolan M, Ariagno K, Jiang H, Duggan C. Energy imbalance and the risk of overfeeding in critically ill children. Pediatr Crit Care Med. 2011;12(4):398–405. doi: 10.1097/PCC.0b013e3181fe279c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones MO, Pierro A, Hammond P, Lloyd DA. The metabolic response to operative stress in infants. J Pediatr Surg. 1993;28:1258–1262. doi: 10.1016/s0022-3468(05)80309-4. [DOI] [PubMed] [Google Scholar]


