Abstract
Background/Aims
Patients with cirrhosis are classified in a compensated and a decompensated stage. Portal hypertension is responsible for most of the complications of cirrhosis that mark the transition from compensated to decompensated cirrhosis. The objectives of this study were (a) to analyse survival of the different stages and substages of cirrhosis and (b) to examine the prognostic value of the hepatic venous pressure gradient (HVPG) at each of the stages.
Methods
A total of 729 patients with suspected cirrhosis underwent routine measurement of portal pressure and systemic haemodynamics between 11/1995 and 12/2004. The primary end-point of the study was death, collected until November 30th, 2006. Multivariable analysis was performed using two models to determine predictors of death at each stage.
Results
A total of 443 patients were included in the study. The 1-year mortality was 5.4% in compensated and 20.2% in decompensated patients. Compensated patients in stage 1 (no varices) had a longer survival than stage 2 patients (varices present) (P = 0.015). In decompensated patients, survival was not different between stage 3 (ascites, with or without varices) and stage 4 (variceal haemorrhage, with or without ascites). Age and HVPG (cut-off 10 mmHg) were independent predictors of death in compensated patients, whereas MELD was in decompensated patients.
Conclusion
Survival rates and predictors of death are different between patients with compensated and decompensated cirrhosis. Unlike the Italian cohort staging system, ascites is a better stratifying clinical event than variceal haemorrhage in patients with decompensated cirrhosis. The presence of clinically significant portal hyper-tension has prognostic value in compensated cirrhosis.
Keywords: ascites, cirrhosis, oesophageal varices, variceal haemorrhage
The natural course of cirrhosis is characterized by a compensated stage followed by a decompensated stage (1, 2). Transition to a decompensated stage is marked by the development of any of the following complications: variceal haemorrhage, ascites, encephalopathy and jaundice (1, 3). These complications result from portal hypertension and/or from liver insufficiency. The survival of both stages is markedly different with compensated patients having a median survival time of over 12 years compared to decompensated patients who survive less than 2 years (1, 3). It has also been shown that both stages have different predictors of death (1). Therefore, it was recently determined, by consensus, that compensated and decompensated cirrhosis should be considered separate disease entities (4).
Moreover, based on data from an Italian cohort of 1649 patients followed prospectively over 10 years, the 2005 Baveno consensus conference (5) and a recent published systematic review (1), two prognostic substages within compensated cirrhosis (stage 1 and 2) and decompensated cirrhosis (stages 3 and 4) have been identified. Stage 1 consists of compensated patients without varices with a low 1-year mortality of 1%. Stage 2 comprises compensated patients with varices (without variceal haemorrhage), with a low mortality of 3% per year, but significantly greater than at stage 1. Stage 3 comprises patients with ascites (with or without varices) but without variceal haemorrhage, in whom the 1-year mortality was 20%. Stage 4 was associated with the highest 1-year mortality of 57% and consists of patients presenting variceal haemorrhage (with or without ascites) (1). The Italian cohort's follow-up started 30 years ago, at a time when standards of care for variceal haemorrhage and other complications of cirrhosis were different from current standards. In a recent AASLD/EASL consensus conference, the recommendation was made to validate this substaging system of patients with cirrhosis (4).
Given that portal hypertension is one of the main factors responsible for the complications of cirrhosis, the importance of measurements of hepatic venous pressure gradient (HVPG) (an estimation of portal hypertension) at the time of diagnosis of cirrhosis in predicting mortality in cirrhosis is important and has not been sufficiently analysed, particularly in the setting of other recognized predictors of death in cirrhosis (1).
The objectives of this study were to analyse, in a current real-life setting in which patients with cirrhosis receive standard therapies for the different complications; (a) survival of the different stages of cirrhosis to validate (or not) the Italian substaging system and (b) the prognostic value of the HVPG at each stage in relationship with other recognized predictive factors, such as Child and MELD.
Methods
The study is a single centre cohort study of patients with cirrhosis. Written informed consent was given from all patients to the procedures. Patients were considered for inclusion in the study if they had cirrhosis and had measurements of portal and systemic haemodynamic (HVPG and right heart catheterization). These measurements are performed routinely in the study centre (Martin-Luther-University Halle-Wittenberg hospital) in the majority of all patients admitted with the suspected diagnosis of cirrhosis. Therefore, indications of HVPG measurement were either to establish the diagnosis of cirrhosis (based in the increased value of the HVPG or to perform a transjugular liver biopsy) or to assess baseline portal pressure, prior to pharmacological therapy or prior to placement of a transjugular intrahepatic porto-systemic shunt (TIPS). The diagnosis of cirrhosis was either biopsy-proven or clinically suspected and confirmed by the presence of an HVPG of 6 mmHg or greater.
Exclusion criteria included primary biliary cirrhosis, previous TIPS placement, HCC, splenic or portal vein thrombosis, concurrent illnesses expected to decrease life expectancy to less than 1 year.
Clinical characteristics and laboratory values were collected from the clinical record at the time of the haemodynamic evaluation.
Patients were classified into a compensated and a decompensated group according to the absence or presence of clinical complications of cirrhosis, specifically variceal haemorrhage, ascites, encephalopathy or jaundice. Patients with a previous episode of decompensation were classified as decompensated patients. Those with compensated cirrhosis were divided into patients without (Stage 1) and with (Stage 2) varices. Patients with decompensated cirrhosis were divided into those with ascites (Stage 3) and those with a history or a presence of variceal haemorrhage with or without ascites (Stage 4). Patients were treated as per evidence-based recommendations. Primary prophylaxis in patients with medium/large varices was based mostly on beta blockers (with some patients treated with EVL); active variceal haemorrhage treated with combination of vasoactive drugs and endoscopic therapy as well as antibiotics; and for secondary prophylaxis EVL or beta blockers were recommended with TIPS or surgical shunts for failures (6). First-line therapy for ascites were diuretics with large-volume paracentesis being the main therapy for refractory ascites (7).
The primary end-point was death. Data regarding death were collected until November 30th, 2006. Survival was determined using medical records and the German national death registry.
The study was approved by the local ethical committee and in accordance with German legislation on patient confidentiality.
Haemodynamic assessment
All vasoactive drugs were discontinued at least 72 h before haemodynamic assessment. Right heart catheterization was conducted using a quadruple-lumen thermodilution catheter (Abbott Lab, Abbott Park, IL, USA) inserted into the internal jugular vein under local anaesthesia with assessment of mean arterial pressure (MAP) and heart rate using automated non-invasive monitoring (Sirecust; Siemens AG, Erlangen, Germany). Standard measurements included central venous pressure (CVP), cardiac output and systemic vascular resistance. Cardiac output (CO) was determined via thermodilution and standardized for body surface area as the cardiac index (CI = CO/BSA). Systemic vascular resistance (SVR) was calculated using the standard formula: SVR = ((MAP–CVP)/CO) × 80. Portal pressure was determined by the HVPG as previously described (8). Following catheterization of a hepatic vein with a standard occlusion balloon catheter (Boston Scientific, Natick, MA, USA), free (FHVP) and wedged (WHVP) hepatic venous pressures were assessed. Measurements were repeated three times and the average was taken. Hepatic venous pressure gradient was calculated as the difference of the two measurements (HVPG = WHVP–FHVP).
Statistical analyses
Survival curves were obtained using the Kaplan–Meier method, and comparisons between subgroups were done by the log-rank and Breslow tests. Survival curves of patients with compensated cirrhosis at presentation included patients who developed decompensation during the course of their disease (i.e. they were not censored at the time of decompensation). Patients who had a liver transplant (n = 22) were censored at the time of transplant.
One-year mortality was calculated using the Trend Test (Incidence Cohort Study) by Domenech and Granero V2008.2.28 for spss (SPSS Inc., Chicago, IL, USA).
Comparisons between patients who died and those who survived were performed using univariate Cox analysis.
Multivariate analysis with backward stepwise Cox regression analysis was performed with two predetermined sets of variables (i.e. two models for each compensated and decompensated cirrhosis), both of which included HVPG. Given that the number of outcomes (death) per substage was too small, multivariable analysis was only performed to predict mortality in the compensated and decompensated stages. In compensated cirrhosis the first model included age, Child score and HVPG, whereas the second model included age, HVPG and the laboratory components of the Child score (i.e. albumin, bilirubin, INR). In decompensated cirrhosis, the first model included Child score, MELD score and HVPG, and the second included HVPG and the laboratory components of the Child and MELD scores (i.e. albumin, bilirubin, INR, creatinine).
A P value <0.05 was considered statistically significant and all data were analysed using spss (SPSS Inc.).
Results
Description of the general population
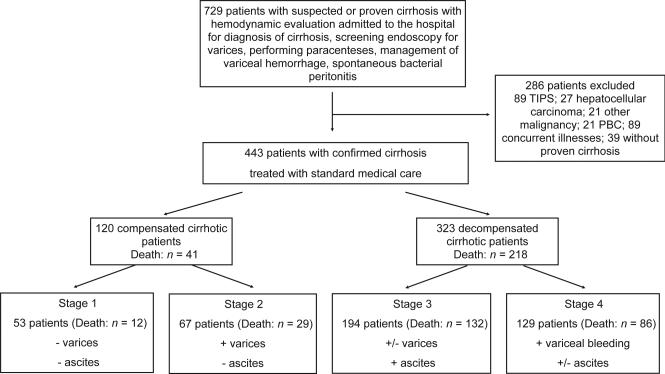
Between November 1995 and December 2004 a total of 1830 patients with suspected or previous evaluated cirrhosis were screened in the Martin-Luther University Halle-Wittenberg. Of these, 729 patients were included in the study. A total of 286 of the 729 patients were excluded (Fig. 1). Thus, 443 patients were included in the final examination. Their baseline characteristics are shown in Tables 1 and 2. As expected, liver synthetic function, Child and MELD scores are better in the compensated group compared with the decompensated group (Table 1). In the substages, liver synthetic function, Child and MELD scores were best in stage 1 patients, more impaired in stage 2 and the most impaired in stage 3, whereas stage 4 was intermediate between stage 2 and 3 (Table 2). During a median follow-up time of 47.0 months (range 0.13–131.8), 259 patients died (41 compensated and 218 decompensated) (Fig. 1; Tables 1 and 2).
Fig. 1.
Flow chart of the patients.
Table 1.
Descriptive parameters of all patients with cirrhosis and its two main stages: compensated and decompensated. Results are expressed as median; 95% confidence interval. HPVG, hepatic venous pressure gradient
| All patients | Compensated | Decompensated | |
|---|---|---|---|
| N | 443 | 120 | 323 |
| Age, years | 53.7; 35.1–71.4 | 57.7; 35.1–71.5 | 52.4; 34.9–71.3 |
| Aetiology, n (alcohol/viral/autoimmune/others) | 309/48/26/60 | 51/24/11/34 | 258/24/15/26 |
| Male gender, n (%) | 286 (64.6) | 68 (56.7) | 218 (67.5) |
| Follow-up, months | 46.8; 1.0–119.8 | 78.7; 16.7–127.7 | 29.5; 0.7–109.9 |
| Death, n | 259 | 41 | 218 |
| Alcoholic hepatitis, n (%) | 54 (12.2) | 3 (2.5) | 51 (15.8)* |
| Albumin, g/L | 31.0; 19.0–47.0 | 38.0; 26.0–50.6 | 29.0; 18.0–44.0* |
| Bilirubin, mg/dl | 1.8; 0.6–17.1 | 1.2; 0.5–5.3 | 2.2; 0.7–19.0* |
| Creatinine, mg/dl | 0.87; 0.55–2.20 | 0.80; 0.54–1.37 | 0.90; 0.56–2.71* |
| INR | 1.3; 1.0–2.1 | 1.2; 0.9–1.6 | 1.4; 1.0–2.2* |
| Platelet count | 117; 44–292 | 132; 58–299 | 115; 35–292 |
| AST, nmol/L | 898; 363–3723 | 869; 343–5738 | 928; 372–3038 |
| ALT, nmol/L | 557; 253–2585 | 654; 300–6307 | 525; 231–2106* |
| Cardiac index, L/min/m2 | 3.8; 2.4–6.2 | 3.7; 2.4–5.8 | 3.8; 2.4–6.2 |
| Systemic vascular resistance, dyn/s/cm5 | 923; 473–1638 | 1014; 576–1616 | 902; 464–1671* |
| Child A/B/C, n | 105/185/130 | 76/31/0 | 29**/154/130 |
| Child-Pugh score | 8; 5–12 | 6; 5–8 | 9;6–12* |
| MELD score | 13.0; 7.0–27.0 | 9.4; 6.4–15.9 | 14.8; 7.8–28.9* |
| HVPG, mmHg | 16.0; 6.0–25.0 | 11.0; 4.0–20.0 | 17.0; 9.0–25.0* |
P < 0.01.
patients mostly with variceal haemorrhage.
Table 2.
Descriptive parameters of patients according to the different substages. Results are expressed as median; 95% confidence interval. HPVG, hepatic venous pressure gradient
| Compensated patients |
Decompensated patients |
|||
|---|---|---|---|---|
| Stage 1 (no varices) | Stage 2 (varices present) | Stage 3 (ascites, with or without varices) | Stage 4 (variceal haemorrhage, with or without ascites) | |
| N | 53 | 67 | 194 | 129 |
| Age ,years | 56.7; 31.0–69.6 | 57.7; 35.8–73.2 | 52.6; 35.3–71.9 | 51.4; 34.1–71.0 |
| Aetiology, n (alcohol/hepatitis/Autoimmune/others) | 18/12/6/17 | 33/12/5/17 | 156/15/9/14 | 102/9/6/12 |
| Male gender, n (%) | 30 (56.6) | 38 (56.7) | 126 (64.9) | 92 (71.3) |
| Follow-up, month | 85.3; 9.9–128.2 | 70.3; 17.5–127.3 | 24.0; 0.6–105.5 | 35.7; 0.8–119.6 |
| Death, n | 12 | 29 | 132 | 86 |
| Alcoholic hepatits, n (%) | 2 (3.8) | 1 (1.5) | 37 (19.1) | 14 (10.9) |
| Albumin, g/L | 41.0; 29.3–50.7 | 37.0; 25.0–51.4 | 28.0; 17.0–41.0 | 30.0; 20.3–45.0 |
| Bilirubin, mg/dl | 1.1; 0.5–7.8 | 1.3; 0.5–4.6 | 3.0; 0.7–20.9 | 1.7; 0.6–15.4 |
| Creatinine, mg/dl | 0.8; 0.5–1.2 | 0.8; 0.5–1.5 | 0.9; 0.6–4.0 | 0.8; 0.5–1.8 |
| INR | 1.1; 0.9–1.7 | 1.2; 1.0–1.6 | 1.4; 1.0–2.2 | 1.3; 1.1–2.2 |
| Platelet count | 143; 59–456 | 112; 56–269 | 129; 44–303 | 100; 34–275 |
| AST, nmol/L | 1032; 342–10 431 | 768; 339–2526 | 1127; 395–3696 | 745; 358–2471 |
| ALT, nmol/L | 838; 326–10 823 | 559; 294–2468 | 574; 230–2374 | 498; 230–1097 |
| Cardiac index, L/min/m2 | 3.4; 2.4–5.4 | 3.8; 2.4–6.2 | 3.9; 2.3–6.4 | 3.6; 2.4–6.0 |
| Systemic vascular resistance, dyn/s/cm5 | 1116; 711–1798 | 927; 473–1481 | 865; 428–1624 | 938; 506–1744 |
| Child A/B/C | 39/6/0 | 37/25/0 | 5/89/95 | 24/65/35 |
| Pugh score | 5; 5–7 | 6;5–8 | 10; 7–13 | 8; 5–12 |
| MELD score | 8.5; 6.4–16.9 | 10.0; 6.4–16.0 | 16.0; 7.9–32.7 | 13.1; 7.6–24.4 |
| HVPG, mmHg | 10.0; 3.7–20.0 | 12.0; 6.4–20.0 | 17.0; 8.0–25.0 | 18.0; 9.5–26.0 |
Survival comparison between compensated and decompensated patients
Median survival time was significantly longer in compensated patients (78.7 months [95% CI: 16.7–127.7]) compared with decompensated patients (29.5 months [95% CI: 0.7–109.9]; P < 0.001). The calculated 1-year mortality was 5.4% (95% CI: 3.9–7.4) in compensated patients and 20.2% (95% CI: 17.6–23.1) in decompensated patients. These correspond to a 3.7-fold (95% CI: 2.7–5.2) higher risk of 1-year mortality in decompensated patients.
Survival comparison among the four different stages
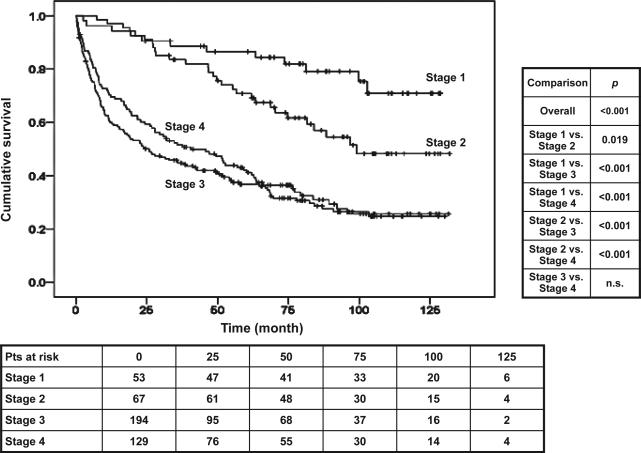
Compensated patients in stage 1 (no varices) had a significantly longer survival than compensated patients in stage 2 (varices present) (P = 0.015; Fig. 2 and Table 2). One-year mortality in stage 1 was 3.4% (95% CI: 1.8–5.9), whereas it was 7.3% (95% CI: 4.9–10.4) in stage 2. This corresponds to a 2.1 (95% CI: 1.1–4.2) higher risk of mortality in stage 2 compared with stage 1.
Fig. 2.
Kaplan–Meier survival curves according to the different stages (stage 1 = compensated, no varices; stage 2 = compensated, varices present; stage 3 = ascites/with or without varices; stage 4 = variceal haemorrhage/with or without ascites).
In decompensated patients there were no significant differences in survival between substages 3 and 4 (Fig. 2 and Table 2). One-year mortality in stage 3 was 21.9% (95% CI: 18.3–26.0) and in stage 4 was 18.1% (95% CI: 14.5–22.3) (P = n.s.).
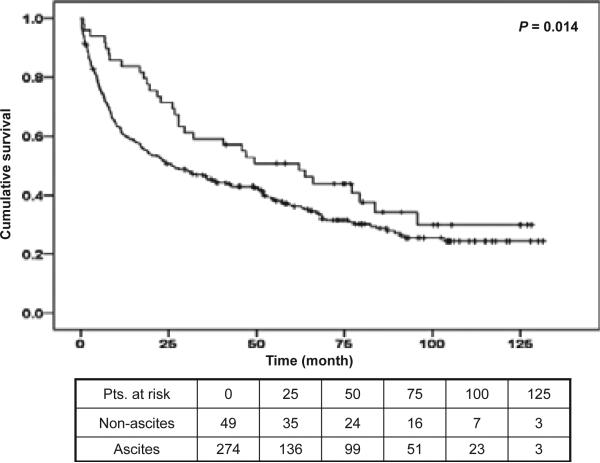
Clinical experience would indicate that patients with ascites have a poorer prognosis than patients with variceal haemorrhage, who do not have ascites. We therefore decided to re-analyse the decompensated group by dividing them in two subgroups based on the presence (or absence) of ascites (Fig. 3).
Fig. 3.
Kaplan–Meier survival curves of decompensated patients separated by the presence of ascites. The upper curve belongs to decompensated patients with variceal haemorrhage without ascites, the lower curve to decompensated patients with ascites with or without variceal haemorrhage.
Patients without ascites (but with variceal haemorrhage as decompensating event) had a 1-year mortality of 14.1% (95% CI: 9.5–20.0) compared with 21.5% (95% CI: 16.2–28.0; P = 0.058) in patients with ascites (with or without variceal haemorrhage). This corresponds to a 1.5 (95% CI: 1.0–2.4) higher risk of death in the latter group and the comparison between the survival curves was significantly different (P = 0.014, Fig. 3).
As shown in Table 1, the majority of the patients had alcoholic cirrhosis and 12.2% had a diagnosis of alcoholic hepatitis. When the survival analyses described above were recalculated excluding patients with alcoholic hepatitis, the results were unchanged.
Predictors of death in compensated patients
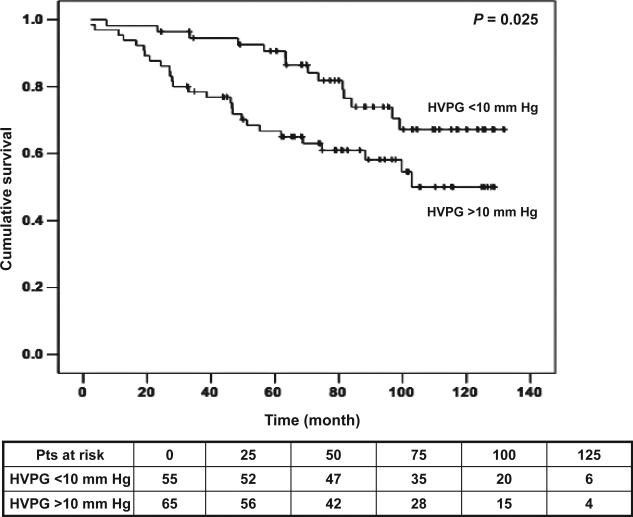
Regarding our main parameter of comparison, the HVPG, AUC under the ROC curve for prediction of death in compensated patients was 0.76 (1-year survival; P = 0.07) and 0.71 (2-year survival; P = 0.03). The best cut-off value in the prediction of death obtained from these ROC curves was a HVPG of 10 mmHg (1-year: sensitivity 0.70, specificity 0.47). Based on this cut-off value Kaplan–Meier survival curves were constructed dividing compensated patients in two groups according to the HVPG of 10 mmHg (P = 0.025; Fig. 4). Multivariate analyses in the first model (in which age, CPS and HVPG were introduced) revealed age (1.04 [1.01–1.07]; P < 0.011) as the only predictor of survival in the second model (in which age, bilirubin, albumin, INR and HVPG were entered), age (1.04 [1.01–1.07]; P = 0.008) and INR (15.18 [3.49–66.08]; P < 0.001) remained as significant variables. Although, HVPG introduced in the model as continuous variable was not significant, when introduced as a categorical value (cutoff 10 mmHg) it was an independent predictor of death in both models with a hazard ratio of 2.2 [1.09–4.40]; P = 0.027) (Fig. 4).
Fig. 4.
Kaplan–Meier survival curves of compensated patients divided by hepatic venous pressure gradient at a cut-off of 10 mmHg.
Predictors of death in decompensated patients
The AUC under the ROC curve for prediction of death by HVPG was 0.58 (1-year survival; P = 0.02) and 0.58 (2-year survival; P = 0.02). The best cut-off value revealed from these ROC curves was a HVPG of 16.5 mmHg (1-year: sensitivity 0.64, specificity: 0.47; 2-year: sensitivity 0.63, specificity 0.48). Based on this cut-off value Kaplan–Meier survival curves were constructed dividing decompensated patients into two groups according to the HVPG of 16 mmHg. However, differences between groups were not significant.
Multivariate analyses in the first model that included Child-Pugh score, MELD score and HVPG showed MELD score (1.04 [1.02–1.06] as the only independent predictor of death; P < 0.001). In the second model that included only the components of CPS and MELD score, serum bilirubin (1.03 [1.01–1.05]; P = 0.002) was the only significant predictor of death. HVPG was not significant in either model, whether it was introduced as a continuous or a categorical (cut-off 16.5 mmHg) variable.
Discussion
In this study, performed in an era when different therapies are recommended for patients with compensated and decompensated cirrhosis, we confirm that, as previously proposed (1), patients with compensated and decompensated cirrhosis have significant differences in survival and different predictors of death.
We also confirm that, within the group of patients with compensated cirrhosis, those with varices (‘stage 2’) have a significantly lower survival than those without varices (‘stage 1’). This substantiates the substratification of patients with compensated cirrhosis, and proposes that studies (therapeutic, prognostic or other) involving patients with compensated cirrhosis should stratify patients by the presence or absence of varices (9). On the other hand, we were unable to confirm a difference in survival rates between the previously proposed stages 3 (ascites) and 4 (variceal haemorrhage) (1). This may be because of more recent advances in the therapy of variceal haemorrhage that have led to an improved survival (10), whereas therapies for ascites have not led to significant improvements in survival (11). The Italian cohort was accrued starting in 1974 (more than 35 years ago) (12), whereas this study enrolled patients between 1995 and 2004, and in this period the standard of care for variceal haemorrhage in our institution included antibiotic prophylaxis and variceal ligation in the majority of patients, factors that have been related to the improvement in survival (8). In recent randomized trials of treatment of variceal haemorrhage, mortality has been shown to be essentially nil in Child A patients (practically all of whom would not have ascites) (13–16). Our survival rates in patients with variceal haemorrhage and in those with ascites are in accordance with a recent Danish cohort study (17).
On the basis of our results we propose two new stages for decompensated patients. The redefined stage 3 would be composed of patients with variceal haemorrhage without ascites and the redefined stage 4 would be composed of patients with ascites with or without variceal haemorrhage. This proposed substaging system should be confirmed in prospective studies as, by only including patients who had haemodynamic measurements performed, our study could have excluded patients with variceal haemorrhage with a complicated course and/or a poor outcome who would not have been referred for haemodynamic measurements.
Regarding the second objective of the study, determining the prognostic value of HVPG performed at the time of diagnosis of cirrhosis, we found that in compensated patients an HVPG >10 mmHg was an independent predictor of death. Other predictive factors were age and INR. Interestingly, an HVPG >10 mmHg has been designated as ‘clinically significant portal hypertension’ because it has been found to be predictive of the development of varices, clinical decompensation and hepatocellular carcinoma in patients with stage 1 cirrhosis (18,19). This cut-off HVPG should thus be used in the risk stratification of patients with compensated cirrhosis.
However, in decompensated cirrhosis HVPG was not predictive of death and the only independent predictors were MELD score (in one model) and serum bilirubin (in the other where only components were entered). The fact that bilirubin is a component of both the Child and MELD scores could have accounted for the latter finding. This is not surprising as at advanced stages of cirrhosis, it is liver insufficiency and haemodynamic factors leading to renal dysfunction rather than portal pressure that determine patient survival (2). In addition, portal pressure in this stage may have been altered by the subsequent use of different portal pressure-reducing therapies or subsequent complications that may have further increased portal pressure.
The majority of patients included in our study had alcoholic cirrhosis and therefore results apply mostly to this patient population and will need to be confirmed in patients with other aetiologies. Because alcoholic hepatitis is a unique entity that will impact the natural history of alcoholic cirrhosis, we analysed the survival rates and the predictors of survival excluding these patients and were gratified to find out that results were the same.
In conclusion, this study confirms different survival rates and different predictors of death between patients with compensated and decompensated cirrhosis and supports the AASLD/EASL consensus recommendation that compensated and decompensated cirrhosis should be considered two separate entities both in clinical practice and in clinical research and that studies in cirrhosis should consider and analyse these entities separately (4).
We also confirm that compensated cirrhosis can be substratified in two prognostic groups based on the presence or absence of varices and that in this stage HVPG should be used to stratify patients at a cut-off level of 10 mmHg.
In decompensated cirrhosis, and contrary to findings from the Italian cohort, we found that variceal haemorrhage is not an ideal substratifying clinical event and that the presence (or absence) of ascites may be better. In this stage, the MELD score (as a marker of liver and circulatory dysfunction) is a stronger predictor of death than portal pressure and single measurements of HVPG would thus not seem to be warranted.
Acknowledgements
Authors contribution: A. Zipprich: study concept and design, acquisition of data, analysis and interpretation of data and drafting of the manuscript. G. Garcia-Tsao: study concept and design, analysis and interpretation of data and drafting of the manuscript. S. Rogowski: acquisition of data. W. E. Fleig: critical revision of the manuscript for important intellectual content. T. Seufferlein: critical revision of the manuscript for important intellectual content. M. M. Dollinger: critical revision of the manuscript for important intellectual content.
Footnotes
Financial disclosure: No conflicts of interest exist.
References
- 1.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–31. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: in search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445–9. doi: 10.1002/hep.23478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gines P, Quintero E, Arroyo V, et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7:122–8. doi: 10.1002/hep.1840070124. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Tsao G, Bosch J, Groszmann RJ. Portal hypertension and variceal bleeding–unresolved issues. Summary of an American Association for the study of liver diseases and European Association for the study of the liver single-topic conference. Hepatology. 2008;47:1764–72. doi: 10.1002/hep.22273. [DOI] [PubMed] [Google Scholar]
- 5.de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167–76. doi: 10.1016/j.jhep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 6.de Franchis R. Updating consensus in portal hypertension: report of the Baveno III Consensus Workshop on definitions, methodology and therapeutic strategies in portal hypertension. J Hepatol. 2000;33:846–52. doi: 10.1016/s0168-8278(00)80320-7. [DOI] [PubMed] [Google Scholar]
- 7.Runyon BA. Management of adult patients with ascites due to cirrhosis. Hepatology. 2004;39:841–56. doi: 10.1002/hep.20066. [DOI] [PubMed] [Google Scholar]
- 8.Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology. 2004;39:280–2. doi: 10.1002/hep.20062. [DOI] [PubMed] [Google Scholar]
- 9.Jensen DM. Endoscopic screening for varices in cirrhosis: findings, implications, and outcomes. Gastroenterology. 2002;122:1620–30. doi: 10.1053/gast.2002.33419. [DOI] [PubMed] [Google Scholar]
- 10.Carbonell N, Pauwels A, Serfaty L, et al. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;40:652–9. doi: 10.1002/hep.20339. [DOI] [PubMed] [Google Scholar]
- 11.Cardenas A, Gines P. Management of complications of cirrhosis in patients awaiting liver transplantation. J Hepatol. 2005;42(Suppl.):S124–33. doi: 10.1016/j.jhep.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 12.D'Amico G, Morabito A, Pagliaro L, Marubini E. Survival and prognostic indicators in compensated and decompen-sated cirrhosis. Dig Dis Sci. 1986;31:468–75. doi: 10.1007/BF01320309. [DOI] [PubMed] [Google Scholar]
- 13.Bosch J, Thabut D, Albillos A, et al. Recombinant factor VIIa for variceal bleeding in patients with advanced cirrhosis: a randomized, controlled trial. Hepatology. 2008;47:1604–14. doi: 10.1002/hep.22216. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Pagan JC, Villanueva C, Albillos A, et al. Nadolol plus isosorbide mononitrate alone or associated with band ligation in the prevention of recurrent bleeding: a multicentre randomised controlled trial. Gut. 2009;58:1144–50. doi: 10.1136/gut.2008.171207. [DOI] [PubMed] [Google Scholar]
- 15.Abraldes JG, Villanueva C, Banares R, et al. Hepatic venous pressure gradient and prognosis in patients with acute variceal bleeding treated with pharmacologic and endoscopic therapy. J Hepatol. 2008;48:229–36. doi: 10.1016/j.jhep.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Villanueva C, Piqueras M, Aracil C, et al. A randomized controlled trial comparing ligation and sclerotherapy as emergency endoscopic treatment added to somatostatin in acute variceal bleeding. J Hepatol. 2006;45:560–7. doi: 10.1016/j.jhep.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Jepsen P, Ott P, Andersen PK, Sorensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51:1675–82. doi: 10.1002/hep.23500. [DOI] [PubMed] [Google Scholar]
- 18.Ripoll C, Groszmann R, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–8. doi: 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 19.Ripoll C, Groszmann RJ, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol. 2009;50:923–8. doi: 10.1016/j.jhep.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]