Abstract
The multivalent vaccine BmHAT, consisting of the Brugia malayi infective larval (L3) antigens heat shock protein12.6 (HSP12.6), abundant larval transcript-2 (ALT-2) and tetraspanin large extra cellular loop (TSP-LEL), was shown to be protective in rodent models from our laboratory. We hypothesize that since these antigens were identified using protective antibodies from immune endemic normal individuals, the multivalent vaccine can be augmented by natural L3 infections providing protection to the vaccinated host. This hypothesis was tested using single dose of DNA and Protein or Protein alone of the BmHAT vaccination in gerbils followed by live trickle L3 infection as booster dose. Vaccine-induced protection in gerbils was determined by worm establishment, micropore chamber assay and by antibody dependant cell cytotoxicity (ADCC) assay. Results were compared with the traditional prime-boost vaccination regimen. Gerbils vaccinated with BmHAT and boosted with L3 trickle infection were protected 51% (BmHAT DNA-Protein) and 48% (BmHAT Protein) respectively. BmHAT vaccination plus L3 trickle booster generated significant titer of antigen-specific IgG antibodies comparable to the traditional prime boost vaccination approach. BmHAT vaccination plus L3 trickle booster also generated antigen-specific cells in the spleen of vaccinated animals and these cells secreted predominantly IFN-γ and IL-4 in response to the vaccine antigens. These studies thus show that single dose of BmHAT multivalent vaccination followed by L3 trickle booster infection can confer significant protection against lymphatic filariasis.
Keywords: Lymphatic filariasis, Brugia malayi, BmHAT multivalent vaccine, L3 trickle booster
Introduction
Lymphatic filariasis is a chronic infection caused by the nematode parasites Wuchereria bancrofti, Brugia malayi and B. timori (1). People living in areas endemic for this disease are continuously exposed to infective third stage larvae (L3) during mosquito bites and usually test positive for antibodies against filarial antigens. Among these a small percentage of population known as endemic normal, remain truly immune to the disease (2) and carry protective antibodies against L3 in their circulation (3). This led to the identification and successful testing of several vaccine candidates against lymphatic filariasis (4–8).
Single or subunit recombinant vaccine candidates have failed to deliver a high degree of protection, unlike attenuated L3 or its fractions (4,5). Abundant larval transcript (ALT-2) of the lymphatic filarial parasites is the most promising vaccine candidate till date (6–12). ALT-2 in combination with other potential antigens such as thioredoxin peroxidase-1 (6), vespid allergen homologue-1 (13) and small heat shock protein (HSP) 12.6 (14), can confer higher level of protection in experimental animals compared to either of the antigens alone. These findings showed that combining more than one vaccine candidate into a multivalent formulation can increase protection due to synergistic action. Recently we showed that a multivalent fusion (BmHAT) of three antigens [HSP12.6, ALT-2 and tetraspanin large extra cellular loop (TSP-LEL)] synergistically conferred significant protection (15).
Filarial infections are endemic in the developing nations such as Africa and Asia, where subject compliance to the vaccination remains a major concern especially when multiple booster doses are required for effective prevention of the disease. Despite extensive vector control measures, significant natural infection is present in mosquitoes in these countries. Therefore, we hypothesized that natural infections with L3 could boost single vaccination dose. To test this hypothesis, we used trickle infections with live B. malayi L3 as booster doses following vaccination with BmHAT in gerbil models and compared the protection and immune correlates with the traditional four dose BmHAT prime-boost regimen.
Materials and methods
2.1 Animals and parasites
Humane use of gerbils (Meriones unguiculatus) and Balb/c mice (Charles River laboratories, Wilmington, MA) in this study were approved by the IACUC committee of the University of Illinois college of Medicine at Rockford. B. malayi third stage infective parasites (L3) were obtained from NIH/NIAID Filariasis reagent repository center, University of Georgia, Athens, GA.
2.2 Preparation of vaccine DNA and protein antigens
The pVAX-BmHAT plasmid used in DNA vaccinations was constructed as described previously (15). Recombinant BmHSP12.6 (rBmHSP), rBmALT-2 and rBmTSP were prepared as reported earlier (7, 16, 17). rBmHAT protein was purified using Hispur™ Cobalt resin (ThermoFisher Scientific, Rockford, IL) and passed through Detoxi-Gel™ Endotoxin Removal Gel (ThermoFisher Scientific). Endotoxin levels were <1 EU/mg as determined by LAL assay (Genscript, Piscataway, NJ).
2.4 Antibody responses against BmHAT in Balb/c mice
Balb/c mice were divided into four groups of five animals each. Group 1 received 15µg of rBmHAT protein suspended in alum (Imject alum, ThermoFisher Scientific) subcutaneously followed by 100µg of pVAX-BmHAT DNA given intradermally on the same day. Group 2 received 15µg of rBmHAT protein suspended in alum. Group 3 received two priming doses of pVAX-BmHAT DNA vaccine (100µg/animal) intradermally followed by two booster doses of rBmHAT protein suspended in alum (15µg/animal) subcutaneously at two weeks interval. Group 4 served as negative controls receiving alum and pVAX given at the same schedule as group three. Blood was collected from each mouse two weeks after the last injection and sera separated. Titer of antigen-specific IgG antibodies were estimated in the sera samples using an indirect ELISA as described previously (15)
2.5 Vaccination trials in gerbils
Gerbils were divided into 5 groups consisting of 5 animals each. Group 1 (BmHAT prime-boost) received two doses of 100µg of pVAX-BmHAT DNA intradermally followed by two booster doses of 15µg rBmHAT plus alum subcutaneously at two weeks interval. Group 2 (BmHAT DNA-protein L3 trickle) were immunized simultaneously on day 0 with 100µg of pVAX-BmHAT DNA intradermally and 15µg of rBmHAT subcutaneously as a priming dose (14, 15). This was followed by booster infections with three doses of 10 B. malayi L3s injected subcutaneously at two weeks apart to mimic natural infections (trickle infection) (18, 19). Using a simple logarithmic regression model, Snow and Mitchel (20) performed a meta-analysis on published studies and show that the maximum number of L3 a mosquito can carry is between 6.6 and 10. Based on this and other (21) reports we used 10 L3 as the trickle infection dose. Group 3 (BmHAT protein L3 trickle) received 15µg rBmHAT with alum subcutaneously on day 0 as the priming dose followed by three booster trickle infections. Group 4 received only three doses of trickle infection (L3 trickle only). Group 5 (Negative controls) received two injections of 100µg of pVAX blank vector intradermally followed by two injections of alum adjuvant subcutaneously at two weeks interval.
2.6 Parasite challenge studies using micropore chambers
All gerbils were challenged 75 days after the last vaccination or after L3 trickle infection using a micropore chamber challenge method (15). This technique allowed us to differentiate the parasites recovered following challenge infection versus those developed following trickle L3 infections. Briefly, 20 B. malayi L3 placed in micropore chambers were surgically implanted into the peritoneal cavity of gerbils. 48 hours after implantation chambers were removed and parasites observed under a microscope. Larva was considered dead if it was not motile, limpid with several cells adhered on the surface. Percentage larval death was determined in control and vaccinated animals (12,15). The heart, lungs, testes and lymph nodes of gerbils that received L3 trickle infections were checked for the presence of adult worms as described earlier (22).
2.7 In vitro antibody-dependent cellular cytotoxicity (ADCC) assay
To determine the larval cytotoxic function of anti-BmHAT antibodies in the sera of vaccinated gerbils, we performed an in vitro ADCC assay (15,23). Briefly, 10 B. malayi L3 were incubated (for 48 h at 37°C and 5% CO2) with 0.5×105 peritoneal exudates cells from normal gerbils and 50 µl of the sera samples. B. malayi L3 incubated with cells alone; sera alone or medium alone were used as controls. After incubation, larval viability was determined under a light microscope. Parasites that were limpid and straight with no movements were counted as non-viable and if they were still limpid and straight for the next 8 hours at 37°C, they were counted as dead. Live larvae remain active even after 56 hrs. Results were expressed as percent of immobile or dead parasites to the total number of parasites in each well (12,15).
2.8 Antibody responses
Titer of antigen-specific anti-BmHAT, BmHSP12.6, BmALT-2 and BmTSP-LEL IgG antibodies were determined in the sera of gerbils using an indirect ELISA (15). Briefly, wells were coated with 100ng rBmHAT, rBmHSP12.6, rBmALT-2 or rBmTSP-LEL. After blocking non-specific sites with 3% BSA, sera samples (diluted two fold serially from 1:100) were added to triplicate wells. After washing the plates, anti-mouse IgG conjugated with HRP (Thermo Fisher Scientific) was added at 1:2500 dilution (anti-gerbil antibody are not commercially available, previous studies show that anti-mouse IgG antibodies cross react with gerbil IgG). O-phenylenediamine dihydrochloride substrate was added and OD read at 492nm.
2.9 Proliferative responses
Antigen-specific proliferation of spleen cells was determined using the Kit purchased from Dojindo (Rockville, MD). Briefly, 0.5×106 spleen cells (100µl/well) were stimulated with rBmHAT (1µg/ml) for 72hrs at 37°C. Concavalin A was used as positive control, while unstimulated wells or cells stimulated with a non-specific recombinant protein (Schistosoma mansoni G-binding factor, rSmGBF) served as negative controls. Results are expressed as stimulation index (SI) = OD of stimulated cultures/OD of unstimulated cultures.
2.10 Cytokine responses
Expression of cytokine mRNA transcripts in rBmHAT (1µg/ml) stimulated gerbil spleen cells (0.5×106 cells/ml in 24 well plates) were measured by qPCR (Applied Biosystems, Carsbad, CA). Primers used are given in Table 1. Total RNA was extracted using TRI reagent (Sigma, St. Louis, MO) and cDNA prepared using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). PCR conditions used for amplifying the cDNA were: initial denaturation at 95°C for 10min, 35 cycles of denaturation at 95°C followed by annealing and extension at 60°C. Data were analyzed using SDS RQ study software (Applied Biosystems) and the results expressed as mean fold change.
Table 1.
Oligonucleotide primers used for the detection of transcripts encoding gerbil cytokines; all oligonucleotides are shown 5′ to 3′
| Gene | Forward primer | Reverse primer |
|---|---|---|
| IFN-γ | GCCTCCTGGCGATTTCTGGCT | CGCCATCCTCTTGCCAGTTCCT |
| IL-4 | AGCTACCAGGGTGCTCCGCA | TGGGCGTGCTCACACTACAGC |
| IL-5 | GCTGTCCACTCACCGGGCTC | GTTTCCACGGCACCCCCACG |
| IL-10 | GCCCAGCTCGGCACTGCTAT | ACCTGGCTGAAGGCAGCTCG |
| GAPDH | CAGGAGCGAGATCCCGCCAAC | TCGGCGGAAGGGGCAGAGAT |
2.11. Statistical analysis
All data are presented as Mean±SD and statistical analyses were performed using Graphpad® Prism-5. Multiple comparisons between groups were made using one-way ANOVA analyses with Tukey’s test or two-way ANOVA with Bonferroni’s test. Probability values (P) of <0.05 were considered statistically significant.
Results
3.1 Single dose vaccination elicited significantly high titer of antigen-specific IgG antibodies in the mice
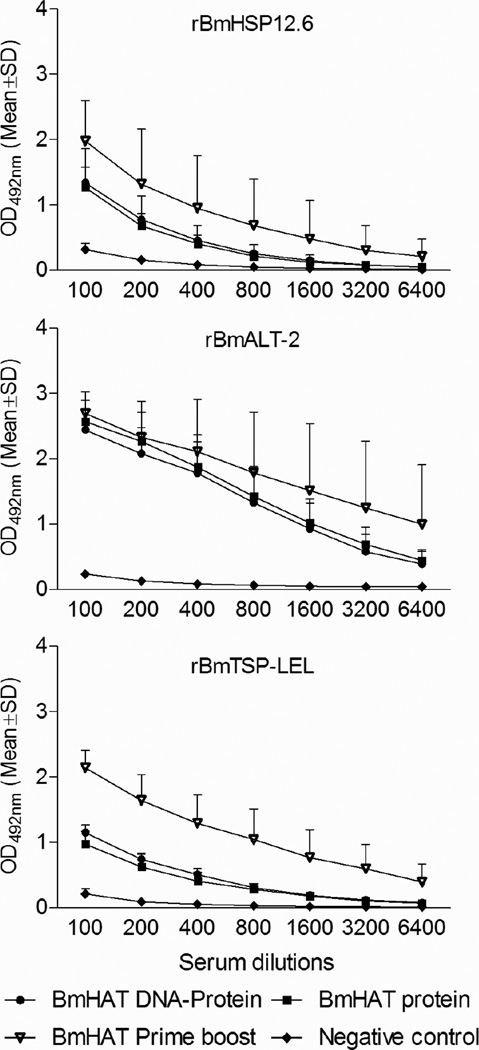
Antigen-specific IgG responses were determined against rBmHSP12.6, rBmALT-2 and rBmTSP-LEL in mice that received (a) same day vaccination with pVAXBmHAT DNA plus rBmHAT Protein or (b) single immunization with rBmHAT protein or (c) pVAXBmHAT DNA and rBmHAT protein given as a prime boost vaccination regimen at 2 weeks apart. Significant antigen-specific IgG antibodies were elicited in the prime-boost group (Fig.1). IgG antibody responses following a single immunization with rBmHAT protein was comparable to a single injection with BmHAT DNA plus rBmHAT protein. Interestingly, all three vaccination regimens induced comparable anti-BmALT-2 IgG antibody responses in vaccinated animals suggesting that BmALT-2 is a potent immunogen.
Figure 1.
Specific IgG responses against rBmHSP12.6, rBmALT-2 and rBmTSP-LEL in Balb/c mice given single priming dose of 100 µg pVAX-1 BmHAT DNA plus 15 µg rBmHAT protein same day or a single priming dose of 15 µg rBmHAT protein. Values were compared with the traditional two doses of 100 µg pVAX-1 BmHAT DNA prime followed by 2 doses of 15 µg rBmHAT boost regimen. Titers of antigen-specific IgG responses were determined by an indirect ELISA using serum from mice 14 days after the last immunization. Values presented are average of three similar experiments using five animals per group. P<0.01 at all dilutions compared to pVAX-1 and alum negative control group.
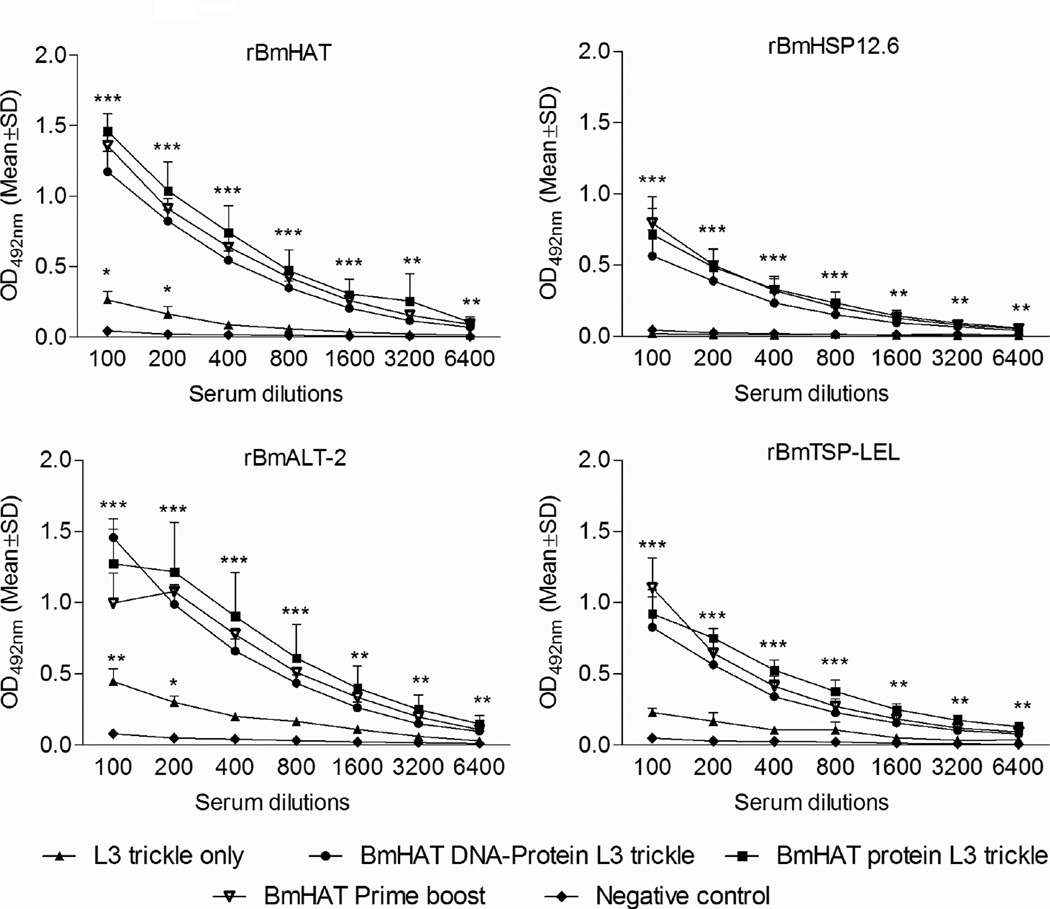
3.2 Single dose vaccination followed by trickle infection induced significantly high titer of antigen-specific IgG antibodies in gerbils
Antigen-specific IgG antibody titer (Fig.2) against rBmHAT, rBmHSP12.6, rBmALT-2 or rBmTSP-LEL were significantly higher (P<0.001-P<0.01) in the BmHAT DNA-protein L3 trickle group and in the BmHAT protein L3 trickle group compared to L3 trickle only group and negative control group. The titer of IgG antibodies against all four antigens were nearly the same in the three vaccinated groups (Fig.2) suggesting that the single day vaccination plus trickle infection induced antibody titer comparable to the traditional prime boost vaccination regimen (two doses of pVAXBmHAT DNA prime plus two doses of rBmHAT protein boost immunization). Antigen-specific IgG titer against rBmHAT and rBmALT-2 was significantly higher (P<0.05) in the trickle infection only group compared to the negative control group suggesting that low level multiple infection can generate anti-BmALT-2 IgG antibodies. Antibody titer against rBmHSP12.6 and rBmTSP were not statistically significant from negative controls.
Figure 2.
Titer of antigen specific IgG responses against rBmHAT, rBmHSP12.6, rBmALT-2 and rBmTSP-LEL were determined in the sera of (a) gerbils immunized with a 100 µg of pVAX-1 BmHAT DNA plus 15 µg of rBmHAT protein on the same day followed by L3 trickle infection (BmHAT DNA-protein L3 trickle), (b) gerbils immunized with 15 µg of rBmHAT protein followed by L3 trickle infection (BmHAT protein L3 trickle) and (c) control animals that received only the L3 trickle infection (L3 trickle only). Serum values from gerbils vaccinated with the traditional pVAX-1 BmHAT DNA prime followed by rBmHAT protein boost (BmHAT prime boost) was used as the positive controls and gerbils that received adjuvant alone remained as negative controls. Data is from one of two similar experiments. *P<0.05, **P<0.01 and ***P<0.001 compared to negative controls.
3.3 L3 Single dose of vaccination followed by trickle infection conferred significant protection against challenge infection in gerbils
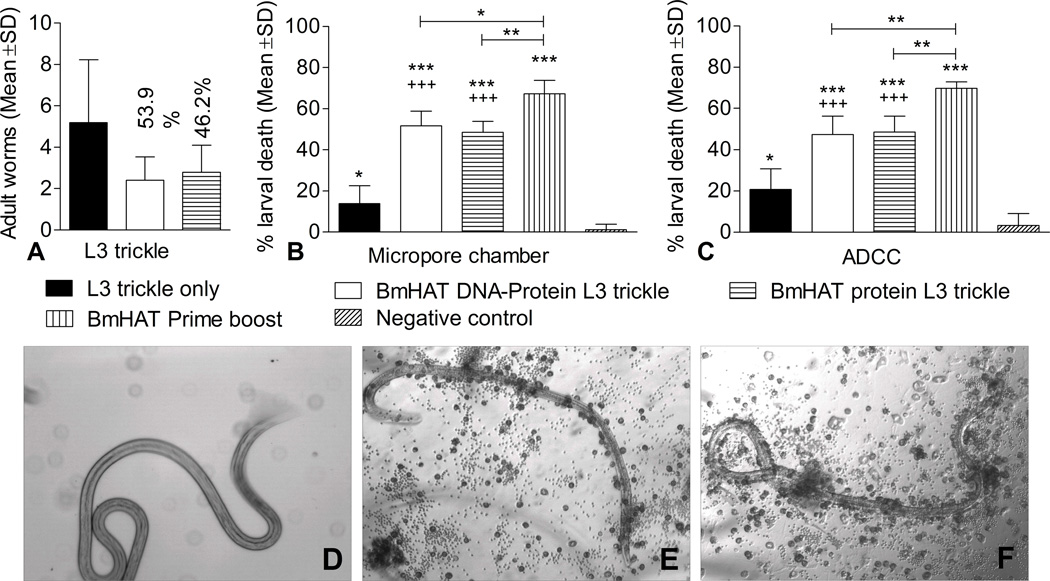
There was 53.9% reduction in adult worm establishment in gerbils vaccinated simultaneously with BmHAT DNA and rBmHAT protein followed by L3 trickle infection. In gerbils that were vaccinated with rBmHAT protein followed by trickle infection, the reduction was 46.2% compared to the L3 trickle only group (Fig.3A). Despite the reduction in worm establishment in vaccinated animals, the values were not statistically significant because of the high standard error in trickle infection only group.
Figure 3.
Vaccine-induced protection in gerbils immunized with BmHAT followed by three trickle booster infections with B malayi L3 were determined by three different approaches. (A) Adult worms recovered from the organs of vaccinated gerbils were counted to determine the percent protection compared to trickle infection only group (B) Protective responses in vaccinated gerbils were assessed by a micropore chamber challenge method (C) Presence of protective antibodies in the serum of vaccinated gerbils were determined by evaluating the ability of these antibodies to participate in the killing of B malayi L3 in an ADCC assay. BmHAT prime boost group was used as the positive control and pVAX-1/adjuvant controls were used as the negative controls. *P<0.05, **P<0.01 and ***P<0.001 compared to negative controls and +++P<0.001 compared to L3 trickle infection only group. (D–F) B. malayi L3 were observed under the microscope 48 hours after the ADCC assay. Larvae present in wells containing control gerbil serum were coiled and moving without any cells adhered to them (D), while those L3s from the wells that contained serum from vaccinated gerbils were straight and dead with cells adhered to the larval surface (E&F). Photographs taken at 100X magnification.
The micropore chamber L3 challenge experiment (Fig.3B) showed that gerbils vaccinated with BmHAT prime boost gave around 70% protection compared to negative control and was significantly high compared to the other vaccinated groups (P<0.001-P<0.05). Gerbils vaccinated with pVAXBmHAT DNA plus rBmHAT protein followed by booster trickle infection (BmHAT DNA-protein L3 trickle) gave 51% protection while gerbils vaccinated with rBmHAT followed by booster trickle infection resulted only in 48% protection compared to negative controls. Interestingly, animals that received only L3 trickle infection also showed 14% protection compared to negative controls.
We also performed an ADCC assay to determine the protective nature of the anti-BmHAT antibodies in the sera of vaccinated animals. Results showed that 47% L3 death occurred when sera from BmHAT DNA-protein L3 trickle group of animals were used (Fig.3C). Similarly, 48% L3 death occurred when sera from BmHAT protein L3 trickle group were used. 70% L3 killing occurred when sera from BmHAT prime boost vaccinated gerbils were used. Sera samples from L3 trickle infection only group also showed some protective ability similar to that observed in our micropore chamber challenge experiment. The dead larvae were straight and non-motile with cells adhered to the surface (Fig.3E & F), while live larvae were curled and motile with no cells adhered (Fig.3D). These findings showed that boosting with L3 trickle infection can provide significant protection in gerbils after a single BmHAT priming.
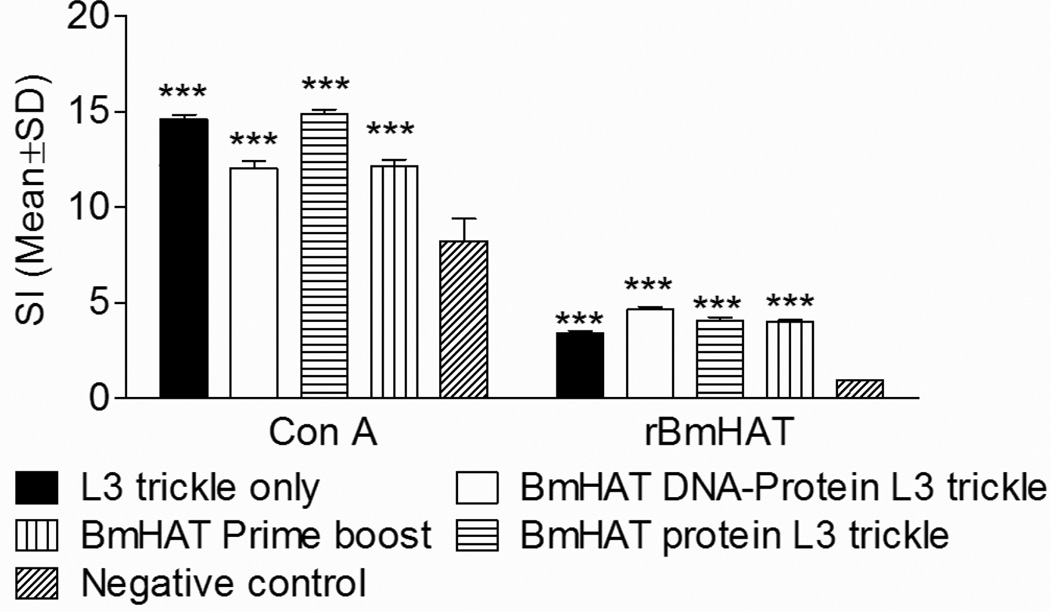
3.4 Spleen cells from vaccinated animals proliferated in response to rBmHAT
Spleen cells from vaccinated animals proliferated significantly (P<0.001) when incubated with rBmHAT compared to negative controls (Fig.4) suggesting the presence of antigen-specific cells in the spleens of vaccinated animals. ConA was used as a non-specific positive stimulus (Fig.4).
Figure 4.
Proliferative responses of gerbil spleen cells (1 × 105) stimulated with 1µg/ml of rBmHAT for 72 hrs and cell proliferation determined using a colorimetric assay. Results are expressed as stimulation index. **P<0.01 and ***P<0.001 compared to negative controls.
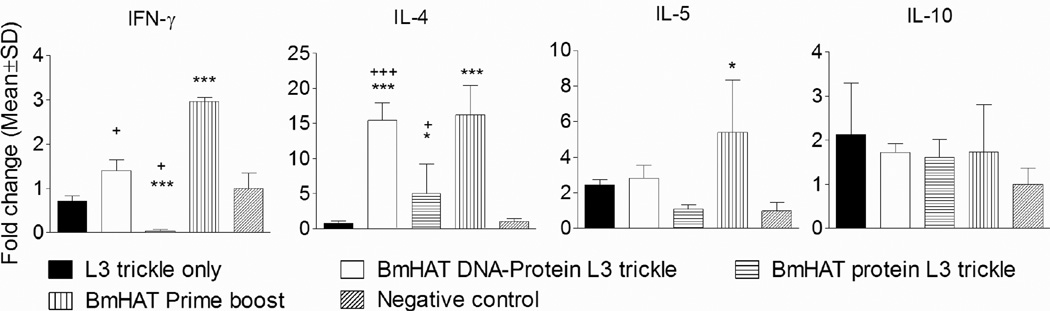
3.5 Single dose vaccination plus trickle infection generated antigen-specific cells that are capable of secreting IFN-γ and IL-4
IL-4 transcripts were predominant in rBmHAT responding spleen cells from gerbils that received BmHAT DNA-protein plus L3 trickle infection (P<0.001) and from gerbils that received multiple BmHAT prime boost immunizations compared to controls (Fig.5). IFN-y transcripts were significantly high in the spleen cells from BmHAT DNA-protein L3 trickle and multiple vaccinated BmHAT prime boost group (P<0.05). However, IFN-y transcripts were undetectable in the BmHAT protein L3 trickle group. IL-5 transcripts were significantly high in the multiple vaccinated BmHAT prime boost group compared to the negative controls (P<0.05). Levels of IL-10 transcripts did not show any significant differences between the groups (Fig.5).
Figure 5.
Cytokine mRNA in the spleen cells of gerbils stimulated with 1 µg/ml of rBmHAT for 72 hrs was quantitated using real time PCR. The results are expressed as mean fold change compared to the negative controls. *P<0.05 and ***P<0.001 compared to negative controls and +P<0.05 and +++P<0.001 compared to L3 trickle infection only group.
Discussion
Presence of protective antibodies in endemic normal individuals provided the basis for understanding immunity against lymphatic filariasis and the basis for developing an effective vaccine against this infection (2). Subsequent characterization of each potential vaccine candidates led to the development of a multivalent vaccine, rBmHAT, consisting of three antigens of B. malayi L3; HSP12.6, ALT-2 and TSP-LEL (15). This vaccine confers close to 95% protection when given as a DNA prime protein boost vaccination regimen and requires four doses of the vaccine. In the clinical setting in an endemic area, it is often difficult to achieve four immunizations to get the maximum desired vaccine-induced immunity. Endemic normal subjects are probably immune because they are constantly exposed to several low doses of the infection in the nature. Therefore, we hypothesized that exposure to low dose of infection with L3 (trickle infection) can naturally boost the immunity induced following a single dose of the multivalent BmHAT vaccine either as a protein or as a prime boost (DNA plus protein) regimen. Results presented in this study show that trickle infection can substantially boost the protective immune responses generated following a single immunization with BmHAT. This finding highlights the significance of natural boosting of immune responses in vaccinated subjects in the endemic areas where infected mosquito vectors are prevalent.
Gerbils that received repeated low dose infections of Acanthocheilonema viteae were shown to produce significant protective antibody titers especially during L3-L4 molts against L3 antigens (18). Our mouse studies show that significant levels of anti-BmHSP12.6, anti-BmALT-2 and anti-BmTSP-LEL IgG antibodies were elicited after single vaccination with BmHAT protein or protein plus DNA. However, these responses were substantially low compared to the four doses of traditional BmHAT DNA prime protein boost regimen suggesting that booster doses are critical. In subsequent studies, we used trickle infections as the booster dose after a single day vaccination. This resulted in significant titers of IgG antibodies comparable to the traditional BmHAT prime boost vaccination regimen. This suggested that multiple trickle infections with L3 can boost vaccine-induced antibody responses. People living in endemic areas are exposed to multiple mosquito bites consequently resulting in greater chance of multiple exposures to L3, which will be much more than the three doses that we evaluated in our experimental setting. Roughly 1 out of 50 mosquitoes that bite in an endemic area are infected with lymphatic filariasis (24). This can lead to continuous natural booster of the immune responses (2,25). Using a simple logarithmic regression model, Snow and Mitchel (20) performed a meta-analysis on published studies and showed that on an average infected mosquito can carry 6.6 L3. Based on this report we used 10 L3 as the trickle infection dose. The next question we asked is what is the minimum number of trickle infection dose required to obtain similar level of IgG titer as the prime-boost regimen? We found that just three trickle doses were sufficient to boost the vaccine-induced antigen-specific IgG titer to the prime-boost regimen level (Fig.2). These findings suggested that trickle infection can boost vaccine-induced immune response.
Since its discovery, BmALT-2 remained as one of the most effective vaccine candidates against lymphatic filariasis conferring around 76% protection in mouse, gerbil or mastomys models (3, 6, 26). When BmALT-2 was combined with BmHSP12.6 (14) and BmTSP-LEL as a multivalent fusion vaccine, close to 95% protection were achieved in mouse and gerbil models (15). Gene polymorphism has been reported for TSP-2 from S. japonicum (27,28). However, to date there are no reports on the gene polymorphism of B. malayi or W. bancrofti TSP-2. Previously we showed that antibodies against all three antigens in the sera of putatively immune individuals (3) can participate in the ADCC-mediated killing of B. malayi L3 in vitro (7,12,15). Depletion of these antibodies from the sera abrogated the killing responses (15). In rodent models, in vivo protective responses after vaccination restricted the migration of L3 into subcutaneous spaces where they were trapped and killed by eosinophil-rich granulomas (29,30). A single vaccination with rBmHAT followed by L3 trickle infection generated protective antibodies that can participate in the killing of approximately 47% L3s suggesting that significant prophylaxis can be achieved after a single vaccination followed by L3 booster infection.
A strong Th2 polarized responses occurred following vaccination with irradiated larvae (9,31), whereas, parasite fractions and recombinant products mainly elicited a mixed Th1/Th2 responses especially following a prime-boost approach (13,32). In this study we show that spleen cells from vaccinated gerbils that were boosted with trickle infections predominantly secreted IL-4 in response to rBmHAT stimulation suggesting a polarized Th2 responses similar to that observed with irradiated larval vaccination (31,32). However, BmHAT DNA prime protein boost vaccination followed by trickle infection resulted in a mixed Th1/Th2 responses similar to that described previously (12). Proliferative responses of the spleen cells to rBmHAT in vaccinated gerbils suggest the presence of memory T and B cells following vaccination and trickle booster (33).
In conclusion, we show that trickle infections with B. malayi L3 can boost vaccine-induced immunity following immunization with BmHAT and confer significant protection against challenge. Extending this finding to endemic populations who are continuously exposed to infective mosquito bites, we suggest that a single BmHAT multivalent vaccination may be effective as these individuals could be naturally boosted by the infection.
The highlights of the present work.
Small dose of repeated (trickle) infection can boost vaccine induced immunity.
Single dose of the vaccine plus trickle infection can confer significant protection.
BmHAT is an excellent vaccine candidate against lymphatic filariasis.
Vaccinated and trickle infection boosted gerbils show both IL-4 and IFN-γ responses.
Acknowledgement
We would like to thank NIAID/NIH Filariasis Research Reagent Resource Center (FR3) at the University of Georgia, Athens, GA, for providing us with parasite materials for this study. This study was supported by NIH grant AI064745.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Global programme to eliminate lymphatic filariasis: progress report, 2011. Releve epidemiologique hebdomadaire / Section d'hygiene du Secretariat de la Societe des Nations -Weekly epidemiological record / Health Section of the Secretariat of the League of Nations. 2012;87(37):346–356. Epub 2012/09/18. [PubMed] [Google Scholar]
- 2.Day KP. The endemic normal in lymphatic filariasis: A static concept. Parasitol Today. 1991;7(12):341–343. doi: 10.1016/0169-4758(91)90215-a. Epub 1991/01/01. [DOI] [PubMed] [Google Scholar]
- 3.Gnanasekar M, Rao KV, He YX, Mishra PK, Nutman TB, Kaliraj P, et al. Novel phage display-based subtractive screening to identify vaccine candidates of Brugia malayi . Infection and immunity. 2004;72(8):4707–4715. doi: 10.1128/IAI.72.8.4707-4715.2004. Epub 2004/07/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramachandran S, Kumar MP, Rami RM, Chinnaiah HB, Nutman T, Kaliraj P, et al. The larval specific lymphatic filarial ALT-2: induction of protection using protein or DNA vaccination. Microbiology and immunology. 2004;48(12):945–955. doi: 10.1111/j.1348-0421.2004.tb03624.x. Epub 2004/12/22. [DOI] [PubMed] [Google Scholar]
- 5.Anand SB, Gnanasekar M, Thangadurai M, Prabhu PR, Kaliraj P, Ramaswamy K. Immune response studies with Wuchereria bancrofti vespid allergen homologue (WbVAH) in human lymphatic filariasis. Parasitology research. 2007;101(4):981–988. doi: 10.1007/s00436-007-0571-2. Epub 2007/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anand SB, Murugan V, Prabhu PR, Anandharaman V, Reddy MV, Kaliraj P. Comparison of immunogenicity, protective efficacy of single and cocktail DNA vaccine of Brugia malayi abundant larval transcript (ALT-2) and thioredoxin peroxidase (TPX) in mice. Acta tropica. 2008;107(2):106–112. doi: 10.1016/j.actatropica.2008.04.018. Epub 2008/06/13. [DOI] [PubMed] [Google Scholar]
- 7.Dakshinamoorthy G, Samykutty AK, Munirathinam G, Shinde GB, Nutman T, Reddy MV, et al. Biochemical characterization and evaluation of a Brugia malayi small heat shock protein as a vaccine against lymphatic filariasis. PloS one. 2012;7(4):e34077. doi: 10.1371/journal.pone.0034077. Epub 2012/04/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veerapathran A, Dakshinamoorthy G, Gnanasekar M, Reddy MV, Kalyanasundaram R. Evaluation of Wuchereria bancrofti GST as a vaccine candidate for lymphatic filariasis. PLoS neglected tropical diseases. 2009;3(6):e457. doi: 10.1371/journal.pntd.0000457. Epub 2009/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devaney E, Bancroft A, Egan A. The effect of irradiation on the third stage larvae of Brugia pahangi . Parasite immunology. 1993;15(7):423–427. doi: 10.1111/j.1365-3024.1993.tb00627.x. Epub 1993/07/01. [DOI] [PubMed] [Google Scholar]
- 10.Dixit S, Gaur RL, Sahoo MK, Joseph SK, Murthy PS, Murthy PK. Protection against L3 induced Brugia malayi infection in Mastomys coucha pre-immunized with BmAFII fraction of the filarial adult worm. Vaccine. 2006;24(31–32):5824–5831. doi: 10.1016/j.vaccine.2006.05.003. Epub 2006/06/08. [DOI] [PubMed] [Google Scholar]
- 11.Sharmila S, Christiana I, Kiran P, Reddy MV, Kaliraj P. The adjuvant-free immunoprotection of recombinant filarial protein Abundant Larval Transcript-2 (ALT-2) in Mastomys coucha and the immunoprophylactic importance of its putative signal sequence. Experimental parasitology. 2011;129(3):247–253. doi: 10.1016/j.exppara.2011.08.005. Epub 2011/08/26. [DOI] [PubMed] [Google Scholar]
- 12.Joseph SK, Sambanthamoorthy S, Dakshinamoorthy G, Munirathinam G, Ramaswamy K. Protective immune responses to biolistic DNA vaccination of Brugia malayi abundant larval transcript-2. Vaccine. 2012;30(45):6477–6482. doi: 10.1016/j.vaccine.2012.07.084. Epub 2012/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalyanasundaram R, Balumuri P. Multivalent vaccine formulation with BmVAL-1 and BmALT-2 confer significant protection against challenge infections with Brugia malayi in mice and jirds. Research and reports in tropical medicine. 2011;2011(2):45–56. doi: 10.2147/RRTM.S13679. Epub 2011/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abhilash S, Gajalakshmi D, Ramaswamy K. Multivalent Vaccine for Lymphatic Filariasis. Procedia in Vaccinology. 2010;Volume 3:12–18. doi: 10.1016/j.provac.2010.11.003. DNA Vaccines 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dakshinamoorthy G, Samykutty AK, Munirathinam G, Reddy MV, Kalyanasundaram R. Multivalent fusion protein vaccine for lymphatic filariasis. Vaccine. 2013;31(12):1616–1622. doi: 10.1016/j.vaccine.2012.09.055. Epub 2012/10/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thirugnanam S, Pandiaraja P, Ramaswamy K, Murugan V, Gnanasekar M, Nandakumar K, et al. Brugia malayi: comparison of protective immune responses induced by Bm-alt-2 DNA, recombinant Bm-ALT-2 protein and prime-boost vaccine regimens in a jird model. Experimental parasitology. 2007;116(4):483–491. doi: 10.1016/j.exppara.2007.02.017. Epub 2007/04/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gnanasekar M, Anand SB, Ramaswamy K. Identification and cloning of a novel tetraspanin (TSP) homologue from Brugia malayi . DNA sequence : the journal of DNA sequencing and mapping. 2008;19(2):151–156. doi: 10.1080/10425170701517614. Epub 2007/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barthold E, Wenk P. Dose-dependent recovery of adult Acanthocheilonema viteae (Nematoda: Filarioidea) after single and trickle inoculations in jirds. Parasitology research. 1992;78(3):229–234. doi: 10.1007/BF00931731. Epub 1992/01/01. [DOI] [PubMed] [Google Scholar]
- 19.Ash LR, Riley JM. Development of subperiodic Brugia malayi in the jird, Meriones unguiculatus, with notes on infections in other rodents. The Journal of parasitology. 1970;56(5):969–973. Epub 1970/10/01. [PubMed] [Google Scholar]
- 20.Snow LC, Michael E. Transmission dynamics of lymphatic filariasis: density-dependence in the uptake of Wuchereria bancrofti microfilariae by vector mosquitoes. Med Vet Entomol. 2002 Dec;16(4):409–423. doi: 10.1046/j.1365-2915.2002.00396.x. [DOI] [PubMed] [Google Scholar]
- 21.Subramanian S, Krishnamoorthy K, Ramaiah KD, Habbema JD, Das PK, Plaisier AP. The relationship between microfilarial load in the human host and uptake and development of Wuchereria bancrofti microfilariae by Culex quinquefasciatus: a study under natural conditions. Parasitology. 1998 Mar;116(Pt 3):243–255. doi: 10.1017/s0031182097002254. [DOI] [PubMed] [Google Scholar]
- 22.Joseph SK, Verma SK, Sahoo MK, Dixit S, Verma AK, Kushwaha V, et al. Sensitization with anti-inflammatory BmAFI of Brugia malayi allows L3 development in the hostile peritoneal cavity of Mastomys coucha . Acta tropica. 2011;120(3):191–205. doi: 10.1016/j.actatropica.2011.08.005. Epub 2011/08/31. [DOI] [PubMed] [Google Scholar]
- 23.Chandrashekar R, Rao UR, Subrahmanyam D. Antibody-mediated cytotoxic effects in vitro and in vivo of rat cells on infective larvae of Brugia malayi . International journal for parasitology. 1990;20(6):725–730. doi: 10.1016/0020-7519(90)90005-8. Epub 1990/10/01. [DOI] [PubMed] [Google Scholar]
- 24.Ughasi J, Bekard HE, Coulibaly M, Adabie-Gomez D, Gyapong J, Appawu M, Wilson MD, Boakye DA. Mansonia africana and Mansonia uniformis are vectors in the transmission of Wuchereria bancrofti lymphatic filariasis in Ghana. Parasit Vectors. 2012;5:89. doi: 10.1186/1756-3305-5-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenbeiss WF, Apfel H, Meyer TF. Protective immunity linked with a distinct developmental stage of a filarial parasite. J Immunol. 1994;152(2):735–742. Epub 1994/01/15. [PubMed] [Google Scholar]
- 26.Gregory WF, Atmadja AK, Allen JE, Maizels RM. The abundant larval transcript-1 and −2 genes of Brugia malayi encode stage-specific candidate vaccine antigens for filariasis. Infection and immunity. 2000;68(7):4174–4179. doi: 10.1128/iai.68.7.4174-4179.2000. Epub 2000/06/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang W, Li J, Duke M, Jones MK, Kuang L, Zhang J, Blair D, Li Y, McManus DP. Inconsistent protective efficacy and marked polymorphism limits the value of Schistosoma japonicum tetraspanin-2 as a vaccine target. PLoS Negl Trop Dis. 2011;5(5):e1166. doi: 10.1371/journal.pntd.0001166. Epub 2011 May 31. PubMed PMID: 21655308; PubMed Central PMCID: PMC3104969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cupit PM, Steinauer ML, Tonnessen BW, Eric Agola L, Kinuthia JM, Mwangi IN, Mutuku MW, Mkoji GM, Loker ES, Cunningham C. Polymorphism associated with the Schistosoma mansoni tetraspanin-2 gene. Int J Parasitol. 2011 Oct;41(12):1249–1252. doi: 10.1016/j.ijpara.2011.07.007. Epub 2011 Aug 22. PubMed PMID: 21889508; PubMed Central PMCID: PMC3188324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sim BK, Kwa BH, Mak JW. Immune responses in human Brugia malayi infections: serum dependent cell-mediated destruction of infective larvae in vitro. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1982;76(3):362–370. doi: 10.1016/0035-9203(82)90191-2. Epub 1982/01/01. [DOI] [PubMed] [Google Scholar]
- 30.Babayan SA, Attout T, Vuong PN, Le Goff L, Gantier JC, Bain O. The subcutaneous movements of filarial infective larvae are impaired in vaccinated hosts in comparison to primary infected hosts. Filaria journal. 2005;4:3. doi: 10.1186/1475-2883-4-3. Epub 2005/05/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Goff L, Martin C, Oswald IP, Vuong PN, Petit G, Ungeheuer MN, et al. Parasitology and immunology of mice vaccinated with irradiated Litomosoides sigmodontis larvae. Parasitology. 2000;120(Pt 3):271–280. doi: 10.1017/s0031182099005533. Epub 2000/04/12. [DOI] [PubMed] [Google Scholar]
- 32.Sahoo MK, Sisodia BS, Dixit S, Joseph SK, Gaur RL, Verma SK, et al. Immunization with inflammatory proteome of Brugia malayi adult worm induces a Th1/Th2-immune response and confers protection against the filarial infection. Vaccine. 2009;27(32):4263–4271. doi: 10.1016/j.vaccine.2009.05.015. Epub 2009/05/20. [DOI] [PubMed] [Google Scholar]
- 33.Martin C, Saeftel M, Vuong PN, Babayan S, Fischer K, Bain O, et al. B-cell deficiency suppresses vaccine-induced protection against murine filariasis but does not increase the recovery rate for primary infection. Infection and immunity. 2001;69(11):7067–7073. doi: 10.1128/IAI.69.11.7067-7073.2001. Epub 2001/10/13. [DOI] [PMC free article] [PubMed] [Google Scholar]