Abstract
Background
Observational studies and meta-analyses of trials suggest daily aspirin use may affect cancer risk, particularly for colorectal cancer, but evidence regarding alternate-day use is scant.
Objective
To examine the association between long-term use of alternate-day low-dose aspirin and cancer incidence in healthy women.
Design
Observational follow-up of a randomized controlled trial.
Setting
U.S. female health professionals.
Participants
39,876 women aged 45 and over in the Women’s Health Study, 33,682 of whom continued observational follow-up.
Intervention
100 mg of aspirin or placebo administered every other day until March 2004, with a median 10-year follow-up. Post-trial observational follow-up continued through March 2012.
Measurements
Incidence of cancer.
Results
5,071 cancers were confirmed throughout follow-up, including 2,070 breast, 451 colorectal, 431 lung cancers, and 1,391 cancer deaths. Over the entire follow-up there was no overall effect of aspirin on total (hazard ratio (HR) = 0.97, 95% confidence interval (CI) = 0.92-1.03, p=0.31), breast (HR=0.98, 95% CI = 0.90-1.07 p=0.65) or lung (HR=1.04, 95% CI = 0.86-1.26, p=0.67) cancer. Incidence of colorectal cancer was lower in the aspirin group (HR=0.80, 95% CI = 0.67-0.97, p=0.021), primarily due to a reduction in proximal colon cancer (HR=0.73, 95% CI = 0.55-0.95, p=0.022), with the effect emerging after 10 years. The post-trial reduction in colorectal cancer was 42% (HR=0.58, 95% CI = 0.42-0.80, p<0.001). There was no extended effect on cancer deaths or colorectal polyps. There were more reported gastrointestinal bleeds (HR=1.14, 95% CI=1.06-1.22, p<0.001) and peptic ulcers (HR=1.17, 95% CI=1.09-1.27, p<0.001) in the aspirin group.
Limitations
Data were available only for women. Not all women received extended follow-up, and the possibility of ascertainment bias post-trial cannot be ruled out. Gastrointestinal bleeding, peptic ulcer, and polyp information was obtained only from self-report during extended follow-up.
Conclusions
Long-term use of alternate-day, low-dose aspirin may reduce risk for colorectal cancer in healthy women.
Evidence has recently emerged that aspirin may provide prophylaxis for cancer, primarily colorectal cancer (1), but also other gastric cancers, and breast, ovarian, prostate, and lung cancers (2, 3). In a meta-analysis of trials of colorectal adenoma or polyp recurrence Cole et al (4) found a reduction in colorectal adenoma, particularly in advanced tumors. More recently, Rothwell et al (5, 6) pooled data from trials of aspirin and cardiovascular disease (CVD) over 20 years of follow-up, finding sizeable reductions in colorectal cancer incidence and mortality. Furthermore, the effect emerged only after 5-10 years, with sizeable delayed effects (5, 7). Benefits increased with duration of use, with an earlier effect in trials using a higher dose (8). Stronger effects were seen for adenocarcinoma, particularly metastatic adenocarcinoma (9).
These meta-analyses were limited to trials of daily aspirin use. The two largest randomized trials of aspirin, the Women’s Health Study (WHS) and the Physicians’ Health Study (PHS), used an alternate-day dose and were not included. Previous analyses from the WHS found no effect of alternate-day aspirin on cancer, including total or colorectal cancer, over a mean 10-year follow-up (10). Extended 12-year follow-up in the PHS also found no association of alternate-day aspirin on colorectal cancer (11). While it is plausible that aspirin timing and dose may play a role in carcinogenesis, that is unproven (12).
Since the end of the active intervention in 2004, the WHS has conducted follow-up for both cardiovascular and cancer outcomes. A primary aim of the extension was to examine long-term effects of aspirin on cancer, particularly colorectal cancer. We report here results for aspirin and incident cancer through a median follow-up of up to 18 years. Key questions include whether alternate-day, low-dose aspirin is associated with reduced risk of colorectal cancer, and, if so, whether this could be explained by increased endoscopy with polyp detection and removal.
METHODS
Design Overview
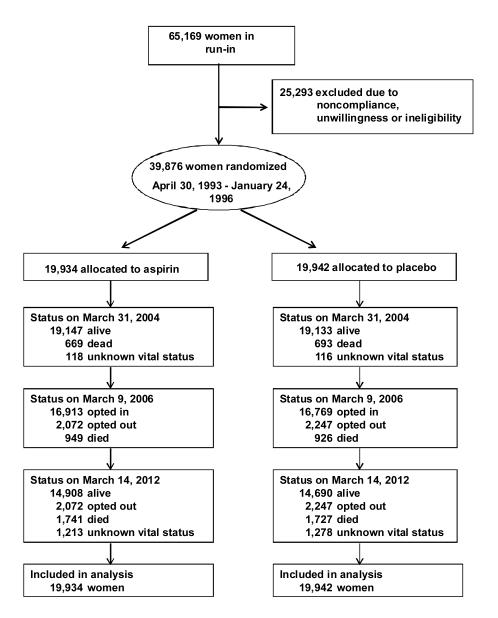
The WHS was a randomized factorial trial of aspirin (100 mg every other day, supplied by Bayer HealthCare) and vitamin E in the primary prevention of cancer and cardiovascular disease conducted entirely by mail. Detailed methods have been described (13, 14). Female health professionals were eligible if they were aged 45 years or older without previous history of cancer (except non-melanoma skin cancer), cardiovascular disease, or other major chronic illness, and willing to forego outside use of study medications. A total of 39,876 women were willing, eligible, and compliant during a three-month placebo run-in (Figure 1). Randomization took place from April 30, 1993, through January 24, 1996, using blocks of size 16 within 5-year age groups. Written informed consent was obtained from all participants. The trial was approved by the Institutional Review Board of Brigham and Women’s Hospital and monitored by an external Data and Safety Monitoring Board.
Figure 1.
Flow of participants through the Women’s Health Study including extended follow-up.
Trial Follow-up
Participants were sent annual supplies of monthly calendar packs containing active agents or placebo. At six and twelve months, and then yearly, participants were mailed questionnaires seeking information on study drug compliance, side effects, non-study aspirin use, clinical endpoints, and risk factors. Study medications and endpoint ascertainment were continued in blinded fashion through the scheduled end of the trial in March, 2004.
Post-trial Follow-up
Following the end of the active intervention on March 31, 2004, participants were invited for further annual follow-up. Women could opt-out of future mailings at the first follow-up questionnaire collected in 2005-06. Opt-out status is reported as of March 9, 2006, when this mailing was complete. This analysis includes endpoints accrued and confirmed through March 14, 2012.
During the post-trial period women were sent annual questionnaires in the same form as those used during the intervention period. However, monthly calendar packs were no longer mailed. Questions regarding the outside use of non-study aspirin were identical to those used during the trial period, as were questions regarding side effects, clinical endpoints, and various risk factors.
Study Outcomes
When a cancer or cardiovascular event was reported during either the trial or post-trial period, written consent for medical record review was requested from the participant, or next of kin if deceased, and medical records were obtained from hospitals or treating physicians. All relevant information was reviewed by an Endpoints Committee composed of physicians blinded to treatment assignment. Only confirmed endpoints were included.
The primary cancer endpoint for the WHS was any invasive cancer, excluding nonmelanoma skin cancer. Incidence of breast, colorectal, and lung cancer were secondary endpoints. Reports of cancer were confirmed by pathology or cytology reports, or, rarely, based on strong clinical and radiologic or laboratory marker evidence (e.g., elevated CA-125). Cardiovascular endpoints were confirmed as previously described (15), and a National Death Index (NDI) search was conducted when cause of death was unconfirmed. Endpoint review is complete for 95% of reported cancers, 95% of myocardial infarctions (MIs), and 94% of strokes. The confirmation rate among those with records is 82% for cancer, 61% for MI, and 68% for stroke. Of all deaths, 60% have cause confirmed by medical records, 78% are confirmed or have death certificates, and 85% are confirmed, have death certificates, or NDI reports.
Women were asked whether they were diagnosed with a colon polyp, peptic ulcer, or gastrointestinal bleeding on annual questionnaires. During the active intervention, medical records were reviewed from a small, random subset (n=558) of women reporting incident colon polyps; of these, 295 (53%; 55% in the aspirin and 51% in the placebo groups) were confirmed to be adenomatous polyps (10). Self-reported information on colonoscopy or sigmoidoscopy was collected at 1 year, 9 years, end of trial, and in post-trial years 1, 2, 3, and 5, with the questions at 9 years and end of trial asking about procedures since randomization. Incident gastrointestinal bleeding or peptic ulcers were confirmed by supplementary questionnaire during the active intervention period only. Reports of gastrointestinal bleeding or peptic ulcers were collected intermittently during post-trial follow-up and were not confirmed. Use of outside non-study aspirin was assessed using the same questions as used during the trial period.
Statistical Analysis
Primary analyses were intent-to-treat. Kaplan-Meier survival curves were used to estimate incidence over time, and the log-rank test compared curves. Cox proportional hazards models (16) were used to estimate the hazard ratio (HR) and 95% confidence interval (CI) comparing women randomized to active aspirin vs. aspirin placebo over the entire follow-up, adjusting for age and vitamin E assignment. Tests for proportionality included an interaction of aspirin with the logarithm of time. Effects during the trial (active intervention) versus post-trial (extended follow-up) periods were estimated separately using the counting process style input to the Cox regression model (17).
Analyses were conducted for total, breast, colorectal, and lung, as well as other site-specific cancers. For comparison with published meta-analyses (8, 9), we also grouped sites into broad categories (8), including gastrointestinal tract (esophagus, stomach, pancreas, biliary tract, liver, small bowel, colon and rectum); urinary tract (kidney, ureter, bladder, or urethra); respiratory tract (lung, pleura, larynx, pharynx or nasopharynx); reproductive (breast, endometrium, ovary, cervix and vagina); and hematological (lymphoma, leukemia and myeloma), and examined metastatic cancer (9) (defined as distant blood-born metastases) and adenocarcinoma, separately and combined. For completeness, we also examined cardiovascular endpoints and total deaths.
To determine whether aspirin assignment affected screening and detection of colon polyps, we examined proportions screened and all self-reports of colon polyps over time by aspirin group. We also examined self-reported gastrointestinal bleeds and peptic ulcers over time by aspirin assignment.
Because a subset opted out of extended post-trial follow-up, we examined baseline characteristics and intervening trial events such as gastrointestinal symptoms, cancer screening, and study pill compliance by randomized aspirin group among those who opted in. To adjust for differences in opt-out response by intervention, in a sensitivity analysis we used inverse probability weighting to adjust for any imbalances (17). We constructed a propensity score for opting-in to further follow-up including the characteristics above as well as aspirin assignment and its interaction with compliance, which served as the denominator for the weights. Stabilized weights were formed by including as the numerator a model with aspirin assignment only (18).
In addition, we examined cancer outcomes following the trial by post-trial aspirin use as well as randomized aspirin intervention. We defined post-trial aspirin use as more than 3 days per month collected on the first (or second if missing) observational questionnaire. We again used inverse probability weighting, which has been found to have low bias and minimum error for confounder adjustment (19). All analyses were conducted in SAS version 9.2. All p-values are two-sided.
The funding sources had no role in the design, conduct, and analysis of our study or in the decision to submit the manuscript for publication.
RESULTS
At the first post-trial questionnaire in 2005-06, 1,875 women (4.7%) had died since randomization, with 949 (4.8%) vs. 926 (4.6%) in the aspirin and placebo groups, respectively (p=0.58) (Figure 1). Of survivors, 4,319 women (10.8%) opted out of further follow-up, and 33,682 women (88.6% of survivors) agreed to continue participation. Slightly more women in the aspirin arm compared with the placebo arm opted in for extended follow-up (16,913; 89.1% vs. 16,769, 88.2%; p=0.006). The median follow-up among survivors during the active intervention was 10.3 years (range 8.2-10.9), and for those who opted in for further follow-up was 17.5 years (range 10.4-18.8). Participants were mean age 55 years at randomization; 54% were postmenopausal, and 13% were current smokers. There were no baseline differences by randomized aspirin assignment (Table 1). Among those opting in for continued follow-up, there were no differences in baseline characteristics between randomized groups.
Table 1.
Baseline characteristics by randomized intervention among all participants and among survivors who participated in post-trial follow-up.
| Randomized |
Post-trial Follow-up |
|||||||
|---|---|---|---|---|---|---|---|---|
| Aspirin (N=19,934) |
Placebo (N=19,942) |
Aspirin (N=16,913) |
Placebo (N=16,769) |
|||||
|
|
||||||||
| N | % | N | % | N | % | N | % | |
| Age | ||||||||
| 45-54 | 12010 | 60.2 | 12015 | 60.2 | 10392 | 61.4 | 10369 | 61.8 |
| 55-64 | 5876 | 29.5 | 5878 | 29.5 | 5021 | 29.7 | 4930 | 29.4 |
| 65+ | 2048 | 10.3 | 2049 | 10.3 | 1500 | 8.9 | 1470 | 8.8 |
| Smoking | ||||||||
| Current | 2580 | 12.9 | 2655 | 13.3 | 1917 | 11.3 | 1975 | 11.8 |
| Past | 7167 | 36.0 | 7098 | 35.6 | 6139 | 36.3 | 6017 | 35.9 |
| Never | 10171 | 51.1 | 10169 | 51.0 | 8845 | 52.3 | 8765 | 52.3 |
| BMI (kg/m2) | ||||||||
| <25 | 10069 | 50.6 | 10094 | 50.7 | 8678 | 51.3 | 8587 | 51.2 |
| 25-<30 | 6158 | 30.9 | 6193 | 31.1 | 5201 | 30.8 | 5202 | 31.0 |
| 30+ | 3674 | 18.5 | 3633 | 18.2 | 3024 | 17.9 | 2973 | 17.7 |
| Alcohol use | ||||||||
| < 1 drink/wk | 11627 | 58.3 | 11599 | 58.2 | 9688 | 57.3 | 9572 | 57.1 |
| 1+ drink/wk | 8302 | 41.7 | 8338 | 41.8 | 7220 | 42.7 | 7192 | 42.9 |
| Physical activity | ||||||||
| <1000 kcal/wk | 12923 | 65.7 | 13071 | 66.3 | 10911 | 65.3 | 10933 | 66.0 |
| ≥ 1000 kcal/wk | 6751 | 34.3 | 6632 | 33.7 | 5792 | 34.7 | 5631 | 34.0 |
| Menopausal status | ||||||||
| Premenopausal | 5478 | 27.6 | 5495 | 27.6 | 4835 | 28.6 | 4797 | 28.7 |
| Perimenopausal | 3521 | 17.7 | 3628 | 18.2 | 2988 | 17.7 | 3090 | 18.5 |
| Postmenopausal, on HT |
6037 | 30.4 | 5911 | 29.7 | 5223 | 31.0 | 5050 | 30.2 |
| Postmenopausal, not on HT |
4850 | 24.4 | 4854 | 24.4 | 3832 | 22.7 | 3791 | 22.7 |
| Family history of cancer |
||||||||
| Yes | 3529 | 17.7 | 3517 | 17.6 | 2993 | 17.7 | 2930 | 17.5 |
| No | 16405 | 82.3 | 16425 | 82.4 | 13920 | 82.3 | 13839 | 82.5 |
| Randomized | ||||||||
| Vitamin E | ||||||||
| Active | 9966 | 50.0 | 9971 | 50.0 | 8464 | 50.0 | 8437 | 50.3 |
| Placebo | 9968 | 50.0 | 9971 | 50.0 | 8449 | 50.0 | 8332 | 49.7 |
Information on study aspirin use was reported by 99% of surviving women at year 2, 96% at year 4 and 95% at year 8. During the trial, use of white study pills (active aspirin or aspirin placebo) was consistently lower by about 1% in the active aspirin group though follow-up (Appendix Figure 1) (10). In the final year of active intervention, use of at least two-thirds of the study medication was 64% in the aspirin group and 65% in the placebo group. Women in both groups took the white pills a median 9 years (25th, 75th percentiles = 4, 11 years.) Information on outside non-study aspirin use during the trial was reported by 98% of surviving women at year 2, 96% at year 4 and 92% at year 8. Non-study aspirin use for more than three days per month was similar between groups, with a median of three years among those who took it.
Among those who opted in to post-trial follow-up, 96%, 95%, and 94% of surviving women provided information on aspirin use on the first, third, and fifth observational questionnaires, respectively. Use of non-study aspirin at least 3 days/month was higher in the randomized aspirin group, reaching 46% in the aspirin group and 43% in the placebo group at year 15. Those who used post-trial aspirin took it for a median three years (25th, 75th percentiles = 2, 5 years.) Non-aspirin NSAIDs were used by 20% of women at the end of the trial, and 28% at the end of post-trial follow-up, similarly between groups.
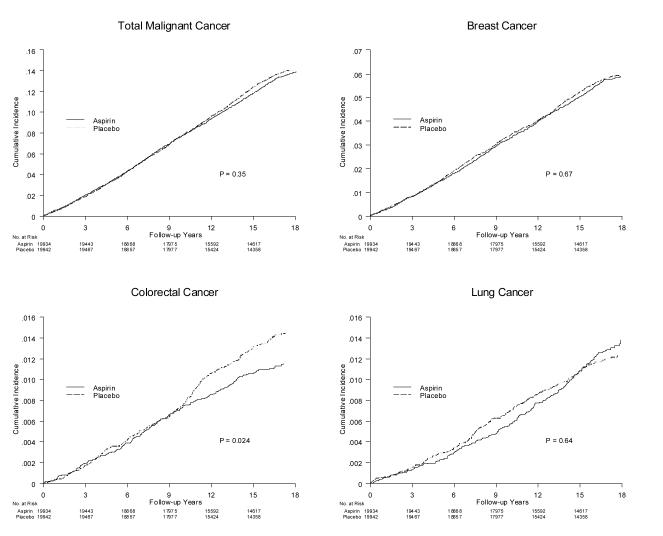
Throughout the entire 18-year follow-up, 5,071 incident malignant cancers were confirmed, including 2,070 breast, 451 colorectal, and 431 lung cancers (Table 2). Total cancer remained balanced by randomized group overall (0.127 vs. 0.132 16-year incidence for aspirin vs. placebo, HR = 0.97, 95% CI = 0.92-1.03, p=0.31) (Figure 2A), as was breast cancer (0.054 vs. 0.056 16-year incidence, HR=0.98, 95% CI = 0.90-1.07, p=0.65) (Figure 2B). There was an overall imbalance in colorectal cancers (0.011 vs. 0.014 16-year incidence, HR=0.80, 95% CI = 0.67-0.97, p=0.021) (Figure 2C). This was primarily due to a reduction in colon cancer, particularly proximal colon cancer (HR=0.73, 95% CI = 0.55-0.95, p=0.022) (Appendix Table 1). Survival curves suggested that the difference emerged after 10 years (figure 2C). Although the interaction with log time did not reach statistical significance (p=0.066), the difference in effect in the trial and post-trial periods was statistically significant (p=0.012), (post-trial HR=0.58, 95% CI = 0.42-0.80, p<0.001). There were no apparent interactions with baseline characteristics (Appendix Table 2). There were no overall differences in lung cancers (Figure 2D); the slight reduction seen during the trial balanced out over post-trial follow-up.
Table 2.
Trial and post-trial cancer incidence by randomized aspirin assignment in the Women’s Health Study.
| Cases (%) |
||||||
|---|---|---|---|---|---|---|
| Cancer Type | Aspirin (N=19,934) |
Placebo (N=19,942) |
Hazard Ratio |
95% CI | P Value |
P trial vs. post-trial |
| OVERALL | ||||||
| Total cancer | 2509 (12.6) | 2562 (12.8) | 0.97 | 0.92-1.03 | 0.31 | 0.23 |
| Breast Cancer | 1028 (5.2) | 1042 (5.2) | 0.98 | 0.90-1.07 | 0.65 | 0.48 |
| Colorectal Cancer | 202 (1.0) | 249 (1.2) | 0.80 | 0.67-0.97 | 0.021 | 0.012 |
| Lung Cancer | 221 (1.1) | 210 (1.1) | 1.04 | 0.86-1.26 | 0.67 | 0.028 |
| TRIAL | ||||||
| Total cancer | 1568 (7.9) | 1568 (7.9) | 1.00 | 0.93-1.07 | 0.96 | |
| Breast Cancer | 643 (3.2) | 671 (3.4) | 0.96 | 0.86-1.07 | 0.43 | |
| Colorectal Cancer | 144 (0.7) | 150 (0.8) | 0.96 | 0.76-1.20 | 0.70 | |
| Lung Cancer | 117 (0.6) | 134 (0.7) | 0.87 | 0.68-1.12 | 0.27 | |
| POST-TRIAL | ||||||
| Total cancer | 941 (5.4) | 995 (5.8) | 0.93 | 0.85-1.02 | 0.113 | |
| Breast Cancer | 385 (2.2) | 371 (2.2) | 1.02 | 0.89-1.18 | 0.77 | |
| Colorectal Cancer | 58 (0.3) | 99 (0.6) | 0.58 | 0.42-0.80 | <0.001 | |
| Lung Cancer | 104 (0.6) | 76 (0.4) | 1.34 | 1.00-1.80 | 0.052 | |
Figure 2.
Cumulative incidence of A) total, B) breast, C) colorectal, and D) lung cancer from time of randomization by randomized aspirin assignment, with p-value from log-rank test.
There were no differences in incidence of other cancer types, or in cancer or overall deaths (Appendix Table 1). When cancer types were grouped into broader categories(8) (Appendix Table 3), there was a difference in all gastrointestinal cancers (0.016 vs. 0.019 16-year incidence, HR=0.83, 95% CI = 0.72-0.97, p=0.021). There was an interaction with time (p=0.015) (Appendix Figure 2), with a post-trial HR of 0.64 (95% CI = 0.49-0.82, p<0.001).
There was an overall reduction in metastatic cancer that did not reach statistical significance (HR=0.88, 95% CI=0.77-1.00, p=0.055) (Appendix Table 4). While there was no significant deviation from proportionality, effects in the post-trial period were larger for metastatic cancer (HR=0.81, 95% CI=0.65-1.01, p=0.063), as well as metastatic adenocarcinoma (HR=0.73, 95% CI=0.56-0.96, p=0.025) but not for adenocarcinoma (Appendix Figures 3-5).
For CVD the reduction in stroke seen in the trial period was reduced (Appendix Table 5), and there was no difference in major CVD or CVD death.
There were more self-reported GI bleeds in the aspirin group during overall follow-up (0.085 vs. 0.075 16-year incidence, HR=1.14, 95% CI=1.06-1.22, p<0.001) (Table 3). The difference by randomized groups was restricted to the trial period with no further difference post-trial (p=0.79) (Appendix Figure 6). There were only 6 deaths overall with a confirmed cause listed as GI hemorrhage, 3 in the active and 3 in the placebo group. Overall rates of self-reported peptic ulcer were also higher (0.077 vs. 0.065 16-year incidence, HR=1.17, 95% CI=1.09-1.27, p<0.001). The difference was again restricted to the trial period, with no further differences post-trial (p=0.26), though cumulative incidence remained higher for both GI bleeds and peptic ulcer in the aspirin group at the end of follow-up (Appendix Figures 6 and 7).
Table 3.
Gastrointestinal (GI) side effects and polyps by randomized aspirin assignment after extended follow-up in the Women’s Health Study.
| Cases (%) |
||||||
|---|---|---|---|---|---|---|
| Aspirin (N=19,934) |
Placebo (N=19,942) |
Hazard Ratio |
95% CI | P Value | P trial vs. post- trial |
|
| OVERALL | ||||||
| Reported GI bleed | 1645 (8.3) | 1452(7.3) | 1.14 | 1.06-1.22 | <0.001 | 0.37 |
| Reported peptic ulcer | 1456 (7.3) | 1242 (6.2) | 1.17 | 1.09-1.27 | <0.001 | 0.29 |
| Reported colon polyp | 5187 (26.0) | 5151 (25.8) | 1.00 | 0.96-1.04 | 0.94 | 0.145 |
| TRIAL | ||||||
| Reported GI bleed | 1489 (7.5) | 1301 (6.5) | 1.15 | 1.07-1.24 | <0.001 | |
| Reported peptic ulcer | 1115 (5.6) | 931 (4.7) | 1.20 | 1.10-1.31 | <0.001 | |
| Reported colon polyp | 2901 (14.6) | 2967 (14.9) | 0.97 | 0.92-1.02 | 0.31 | |
| POST-TRIAL | ||||||
| Reported GI bleed | 156 (0.9) | 151 (0.9) | 1.03 | 0.82-1.29 | 0.79 | |
| Reported peptic ulcer | 341 (2.0) | 311 (1.8) | 1.09 | 0.94-1.28 | 0.26 | |
| Reported colon polyp | 2286 (14.6) | 2184 (14.0) | 1.03 | 0.97-1.09 | 0.29 | |
Colon polyps were reported by 10,332 women, with no differences between groups overall (HR=1.00, 95% CI = 0.96-1.04, p=0.94), or during the trial (p=0.31) or post-trial (p=0.27) (Table 3, Appendix Figure 8). There were few differences in reported endoscopies between groups either during the trial or post-trial (Appendix Table 6). When analyses were restricted to those undergoing endoscopy at least once during overall follow-up, there was no substantial change in effect for either polyps or colorectal cancer.
There were several differences in events or conditions occurring during the trial by randomized group, including GI symptoms and compliance to study medication. Appendix Table 7 presents the propensity score for opting-in for further follow-up. Women who opted in were more likely to be good compliers during the trial, to have experienced GI bleeds, and to have screening endoscopies. They were also younger and less likely to be black, current smokers, nondrinkers, or past hormone therapy users. Inverse-probability weighting led to little change in the estimated effects, however, either overall or post-trial (Appendix Table 8). The estimated hazard ratio for aspirin and colorectal cancer remained 0.80 (95% CI = 0.66-0.97, p=0.021) over all follow-up and 0.58 (95% CI = 0.41-0.81, p=0.002) post-trial.
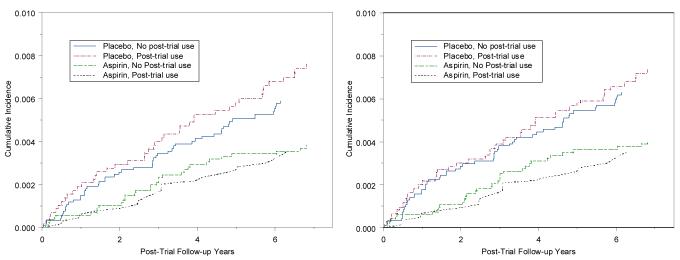
In further analyses we examined the effects of aspirin according to post-trial use as well as randomized assignment. Compared to those in the placebo group who did not start regular aspirin use, those in the placebo group who started aspirin had a 21% higher rate of colorectal cancer post-trial (HR=1.21, 95% CI = 0.81-1.80) (Appendix Table 9). Among those in the aspirin group, those who did not use aspirin post-trial had a 33% lower rate (HR=0.67, 95%CI = 0.43-1.02) (Figure 3A), while those who continued to use aspirin post-trial had a 43% lower rate of colorectal cancer (HR=0.57, 95%CI = 0.35-0.93), although differences between the latter two groups were not statistically significant. Post-trial observational use, however, was positively associated with many risk factors for cancer (Appendix Table 10), including age, body mass index, and alcohol use. Women who underwent more screening procedures during the trial were also more likely to use aspirin post-trial, as were women in the active aspirin, but not placebo, group who were compliant to study pill-taking. Using inverse probability weighting adjusting for these factors, the estimates remained consistent with reduction in colorectal cancer with longer-term aspirin use (Figure 3B, Appendix Table 9). The increase among those who started aspirin in the placebo group was somewhat attenuated (HR=1.17, 95% CI = 0.77-1.77), while the estimated effects in the other two groups were largely unchanged.
Figure 3.
Post-trial incidence of colorectal cancer by randomized aspirin and post-trial aspirin use A) unweighted and B) weighted by inverse propensity of aspirin use post-trial.
DISCUSSION
At the end of the 10-year active intervention in the WHS, there was difference in overall, colorectal, or other site-specific cancer by randomized aspirin group (10). The WHS was specifically designed to study effects on both cardiovascular and cancer outcomes, and had the same detailed collection of cancer outcomes, side effects, and screening procedures in extended follow-up. After a median 18-year follow-up, a difference in colorectal cancer by aspirin group has emerged.
Recent literature supports a delayed effect of aspirin on cancer. Rothwell and colleagues (5-9) conducted meta-analyses of long-term effects of aspirin on cancer outcomes using trials of aspirin and CVD. In the British Doctors’ and UK-TIA trials (7), allocation to 300 mg daily aspirin reduced colorectal cancer after a latency of 10 years, with greater differences for proximal colon cancer (5). These beneficial findings extended to mortality at various cancer sites (6), to incident cancer at various sites (8), and to metastatic cancers and adenocarcinoma (9). The strongest difference was for gastrointestinal cancer in these and other data. A recent trial of aspirin among patients with Lynch syndrome, or hereditary non-polyposis colon cancer, found no difference during the initial mean 29 month intervention period, but a longer-term 37% reduction in colon cancer over an average 56 months (20). Previous meta-analyses were limited to trials of daily aspirin, thus excluding the WHS, the largest trial of aspirin to date. We found similar long-term differences in cancer incidence, particularly colorectal cancer, with a hazard ratio of 0.80 compared to 0.76 in the meta-analysis (5).
The effects of aspirin on cardiovascular disease and on bleeds appear to be more immediate, with attenuation of the randomized effect once aspirin is stopped. This is consistent with effects on the aggregation of platelets, which have a high turnover (21). Aspirin may affect carcinogenesis through the COX-2 pathway (22), which may require larger doses and a shorter dosing interval due to the rapid resynthesis of the enzyme in nucleated cells(21). The WHS data, however, suggest that even low doses on alternate days could play a role in carcinogenesis, at least in the colon. A potential platelet-mediated mechanism for low-dose and even alternate-day aspirin has been hypothesized (23). Permanent inactivation of platelet COX-1 could inhibit COX-2 upregulation in adjacent cell types in the intestinal mucosa at sites of mucosal injury, where platelets are likely to be recruited and activated. The induction of COX-2 and its downstream signalling could lead to reduced apoptosis and increased cell proliferation and angiogenesis. Other mechanisms including non-COX-dependent pathways have been hypothesized (24), although exact mechanisms remain to be determined. A recent study raised the intriguing possibility that aspirin therapy may increase survival primarily among patients with mutated-PIK3CA colorectal cancer (25). This suggests that the PIK3CA mutation in colorectal cancer may serve as a predictive biomarker, and that PI3K-related pathways may play a role in aspirin’s apparent effect on cancer.
The delayed differences in cancer outcomes by aspirin assignment may indicate an effect in the early stages of carcinogenesis, requiring a long latent period especially at low doses. Alternative explanations are that increased bleeding leads to more endoscopy and early polyp removal. Rates of colonoscopy or sigmoidoscopy were similar by intervention in the WHS, however, and we found no difference in reported colon polyp occurrence over time. Furthermore, the greatest effect was seen in the proximal colon, less affected by sigmoidoscopy. Even if reduced cancer is caused by increased endoscopy and polyp removal due to symptoms, the net clinical effect appears to be a reduction in colorectal cancers.
A limitation of the current analysis is that post-trial information on GI bleeds, peptic ulcer, and polyps was from self-report only. Not all polyp reports were reviewed, and none in the observational follow-up. The confirmation rate for those reviewed was only 53% (10). Endoscopy was irregular and could be influenced by increased bleeding or GI symptoms. Since trials of polyp recurrence using a regular screening protocol have suggested lower rates with aspirin use (4), it is possible that lower polyp incidence is balanced by increased polyp detection in the WHS. This question, however, cannot be answered definitively except by a long-term trial including regular screening colonoscopy, which would be difficult and expensive.
Other limitations include the opt-out of post-trial follow-up. Women who remained in post-trial follow-up were more likely to have had endoscopy procedures, which may have led to the slight uptick in rates of colorectal cancer seen in the placebo group post-trial (Figure 2C), which could be due to ascertainment bias. Since there was little difference between randomized groups, however, the randomized comparisons continue to be valid, and are similar in adjusted and unadjusted analyses. There was also imperfect compliance to aspirin assignment during the trial. Cross-over following the intervention likely diluted the post-trial effect as in the other trials examined. In analyses of post-trial use, however, those who continued to take aspirin in the post-trial period continued to exhibit lower rates of colorectal cancer. Post-trial observational aspirin use was influenced by many factors related to cancer incidence and diagnosis, and these latter analyses could still be subject to unmeasured confounding even after inverse probability weighting.
What are the implications of these findings regarding recommendations for aspirin use? While aspirin is clearly protective in the secondary prevention of CVD, its effects are more limited in primary prevention (26). The U.S. Preventive Services Task Force (27) recommends aspirin in women aged 55-79 years only when potential benefit outweighs potential harm. With more evidence for long-term effects in carcinogenesis, recommendations might be reconsidered. Over the 18-year study, there were 53 net fewer cancers (47 colorectal, 56 gastrointestinal) in the aspirin group, compared to 48 fewer cases of CVD (47 strokes), which may extrapolate to 84 cases (66 strokes) if aspirin use had continued. Aspirin’s adverse effects, however, cannot be forgotten. Even the WHS’s low dose led to a net increase of 193 GI bleeds and 214 peptic ulcers reported, though some were minor. Relative risks for confirmed bleeds were higher during the intervention period (15). A recent study examining the net benefit of aspirin use concluded that aspirin could not be recommended for primary prevention in any subgroup of the WHS, including older women (28). Whether new results regarding long-term benefits for cancer will tip the balance in favor of aspirin remains to be determined.
Supplementary Material
ACKNOWLEDGEMENTS
Funding/Support: Supported by grants (HL043851, HL080467, HL099355, and CA407988) from the National Heart, Lung, and Blood Institute and the National Cancer Institute, Bethesda, Maryland.
Aspirin and aspirin placebo were provided by Bayer Healthcare. Vitamin E and vitamin E placebo were provided by the Natural Source Vitamin E Association.
Primary Funding Source National Institutes of Health.
Footnotes
Authors Contributions: All authors have made substantial contributions to the intellectual content for the paper, and have given final approval for the submitted manuscript. Dr. Cook conducted the statistical analysis and had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest: None
ClinicalTrials.gov Identifier NCT00000479
REFERENCES
- 1.Chan AT, Arber N, Burn J, et al. Aspirin in the chemoprevention of colorectal neoplasia: an overview. Cancer Prev Res (Phila) 2012;5(2):164–78. doi: 10.1158/1940-6207.CAPR-11-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuzick J, Otto F, Baron JA, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10(5):501–7. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 3.Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol. 2012;23(6):1403–15. doi: 10.1093/annonc/mds113. [DOI] [PubMed] [Google Scholar]
- 4.Cole BF, Logan RF, Halabi S, et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst. 2009;101(4):256–66. doi: 10.1093/jnci/djn485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741–50. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 6.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 7.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369(9573):1603–13. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 8.Rothwell PM, Price JP, Fowkes GR, et al. Short-term effects of daily aspirin on cancer incidence, mortality and non-vascular death: analysis of the time-course of risks and benefits in randomised controlled trials. Lancet. 2012;379:1602–12. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 9.Rothwell PM, Wilson M, Price JF, Belch JFF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 10.Cook NR, Lee IM, Gaziano MJ, et al. Low-dose aspirin in the primary prevention of cancer. The Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 11.Sturmer T, Glynn RJ, Lee IM, Manson JAE, Buring JE, Hennekens CH. Aspirin use and colorectal cancer: post-trial follow-up from the Physicians’ Health Study. Ann Intern Med. 1998;128:713–20. doi: 10.7326/0003-4819-128-9-199805010-00003. [DOI] [PubMed] [Google Scholar]
- 12.Chan AT, Cook NR. Are we ready to recommend aspirin for cancer prevention? Lancet. 2012;379:1570–1. doi: 10.1016/S0140-6736(11)61654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buring JE, Hennekens CH, for the Women’s Health Study Research Group The Women’s Health Study: Summary of the study design. J Myocardiol Ischemia. 1992;4:27–9. [Google Scholar]
- 14.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women’s Health Study. J Womens Health Gend Based Med. 2000;9(1):19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 16.Cox DR. Regression models and life tables (with discussion) J Roy Stat Soc B. 1972;34:187–220. [Google Scholar]
- 17.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Springer; New York, NY: 2000. [Google Scholar]
- 18.Hernán M, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiol. 2000;11(5):561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013 doi: 10.1002/sim.5705. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burn J, Gerdes A-M, Macrae F, on behalf of the CAPP2 Investigators Long-term eff ect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081–7. doi: 10.1016/S0140-6736(11)61049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patrono C, Coller B, Dalen J, et al. Platelet-active drugs: the relationship among dose, effectiveness, and side effects. Chest. 2001;119:39S–63S. doi: 10.1378/chest.119.1_suppl.39s. [DOI] [PubMed] [Google Scholar]
- 22.Thun MJ. Beyond willow bark: aspirin in the prevention of chronic disease. Epidemiol. 2000;11:371–14. doi: 10.1097/00001648-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9(5):259–67. doi: 10.1038/nrclinonc.2011.199. [DOI] [PubMed] [Google Scholar]
- 24.Langley RE, Burdett S, Tierney JF, Cafferty F, Parmar MKB, Venning G. Aspirin and cancer: has aspirin been overlooked as an adjuvant therapy? Brit J Canc. 2011;105:1107–13. doi: 10.1038/bjc.2011.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao X, Lochhead P, Nishihara R, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367(17):1596–606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seshasai SRK, Wijesuriya S, Sivakumaran R, et al. Effect of aspirin on vascular and nonvascular outcomes: meta-analysis of randomized controlled trials. Arch Int Med. 2012;172(3):209–216. doi: 10.1001/archinternmed.2011.628. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Preventive Services Task Force Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2009;150:396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]
- 28.Dorresteijn JA, Visseren FL, Ridker PM, et al. Aspirin for primary prevention of vascular events in women: individualized prediction of treatment effects. Eur Heart J. 2011;32(23):2962–9. doi: 10.1093/eurheartj/ehr423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.