Summary
ADAMTS13, a plasma reprolysin-like metalloprotease, cleaves von Willebrand factor (VWF). Severe deficiency of plasma ADAMTS13 activity results in thrombotic thrombocytopenic purpura (TTP), while mild to moderate deficiencies of plasma ADAMTS13 activity are emerging risk factors for developing myocardial and cerebral infarction, preeclampsia, and malignant malaria. Moreover, Adamts13−/− mice develop more severe inflammatory responses, leading to increased ischaemia/perfusion injury and formation of atherosclerosis. Structure-function studies demonstrate that the N-terminal portion of ADAMTS13 (MDTCS) is necessary and sufficient for proteolytic cleavage of VWF under various conditions and attenuation of arterial/venous thrombosis after oxidative injury. The more distal portion of ADAMTS13 (TSP1 2–8 repeats and CUB domains) may function as a disulphide bond reductase to prevent an elongation of ultra large VWF strings on activated endothelial cells and inhibit platelet adhesion/aggregation on collagen surface under flow. Remarkably, the proteolytic cleavage of VWF by ADAMTS13 is accelerated by FVIII and platelets under fluid shear stress. A disruption of the interactions between FVIII (or platelet glycoprotein 1bα) and VWF dramatically impairs ADAMTS13-dependent proteolysis of VWF in vitro and in vivo. These results suggest that FVIII and platelets may be physiological cofactors regulating VWF proteolysis. Finally, the structure-function and autoantibody mapping studies allow us to identify an ADAMTS13 variant with increased specific activity but reduced inhibition by autoantibodies in patients with acquired TTP. Together, these findings provide novel insight into the mechanism of VWF proteolysis and tools for the therapy of acquired TTP and perhaps other arterial thrombotic disorders.
ADAMTS13 and potential human diseases
ADAMTS13, first identified and cloned in 2001, is a member of the ADAMTS (A Disintegrin And Metalloprotease with ThromboSpondin type 1 repeats) family (1,2). It cleaves a large polymeric adhesion protein von Willebrand factor (VWF). VWF is synthesised in vascular endothelial cells and megakaryocytes (3,4). The newly synthesised VWF is stored in intracellular organelles: Weibel-Palade bodies in endothelial cells and α-granules in megakaryocytes and platelets (3,4). VWF is released upon physiological or pathological stimulation and forms an ultra-long or ultra-large (UL) “string-like” structure on the endothelial surface (5–7). These UL-VWF “string-like” structures are hyperactive in recruiting circulating platelets to the site of endothelial activation and/or injury. Plasma ADAMTS13, which is primarily synthesised and released from hepatic stellate cells (8–10) and endothelial cells (11,12), binds and cleaves cell bound UL-VWF strings at the Tyr1605-Met1606 bond, thereby eliminating the UL-VWF from the endothelial surface and resulting in fragmentation of the VWF strings (5–7). In addition, ADAMTS13 cleaves UL or large VWF in solution after being exposed to high fluid shear stress as seen in microcirculation or at the site of narrow vessels and thrombus formation after injury (13–15) (Fig. 1). Arterial shear stress induces conformational changes in soluble multimeric VWF (16–19) so that it becomes accessible by ADAMTS13 for cleavage. The conformational changes can also be induced in vitro by an addition of a denaturant such as urea (20,21) or guanidine-HCl (22,23), which is the molecular basis of various biochemical assays for plasma ADAMTS13 activity.
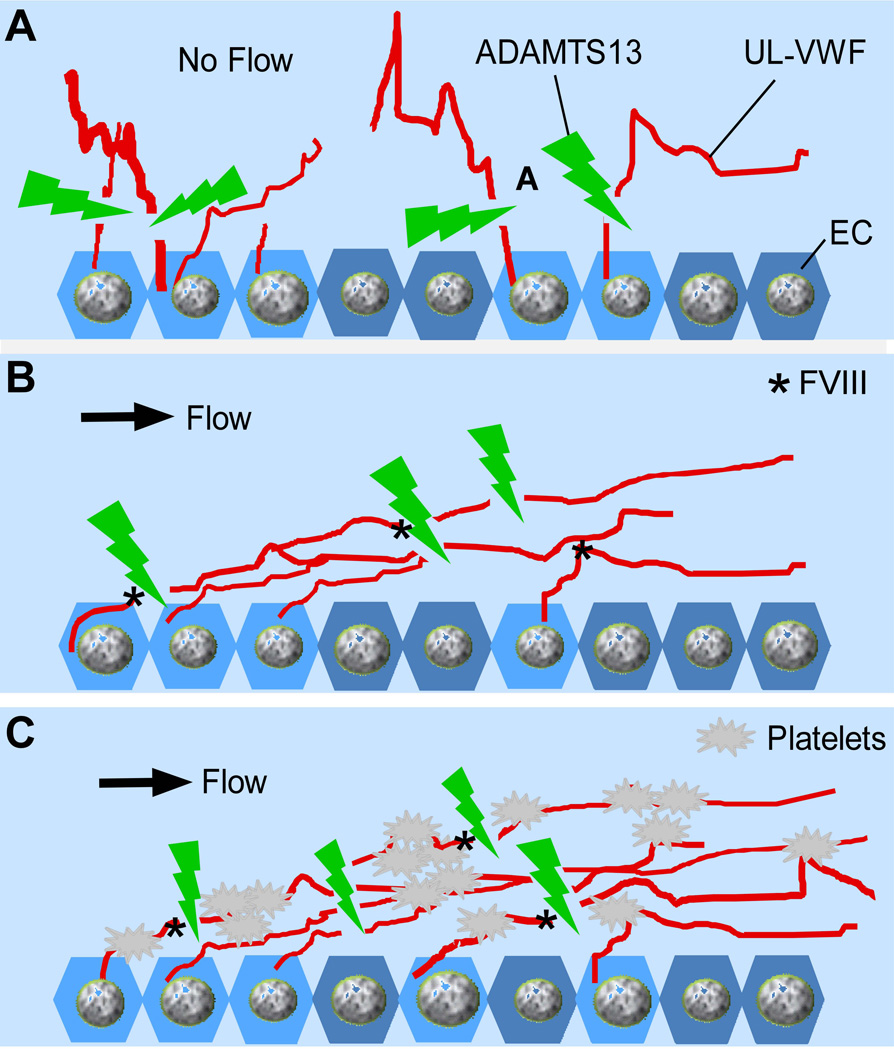
Fig. 1. ADAMTS13 cleaves UL-VWF under various conditions.
A. ADAMTS13 rapidly cleaves newly released UL-VWF or large VWF strings/bundles in the absence (A) and in the presence (B) of flow; ADAMTS13 also cleaves platelet-decorated UL-VWF or large VWF strings/bundles anchored on the endothelial cell surface, in solution, and within growing thrombi under fluid shear stress (C).
An inability to cleave cell-bound and soluble UL or large VWF in circulation because of severe deficiency of plasma ADAMTS13 activity (less than 5% of normal) results in the persistence of hyperactive UL-VWF on endothelial cells (5,6,24,25) and in circulating blood (26). This leads to excessive platelet aggregation and disseminated VWF/platelet-rich thrombus formation (27–29), the characteristic feature of thrombotic thrombocytopenic purpura (TTP). Patients with TTP manifest by marked thrombocytopenia (platelet count, usually less than 20×109/L) and microangiopathic haemolytic anaemia (with low haematocrit, elevated lactate dehydrogenase, and fragmentation of red blood cells) (30–33). Some patients may exhibit neurological signs and symptoms or renal abnormalities (30–33). Idiopathic TTP in most adult patients is caused by an acquired deficiency of plasma ADAMTS13 activity resulting from the formation of inhibitory autoantibodies against ADAMTS13 (23,34,35). Rarely, TTP is caused by one or several germ line mutations in the ADAMTS13 gene (1,34,36–38), resulting in severe deficiency of plasma ADAMTS13 activity at birth. Several animal models have been established for testing novel therapies. Adamts13−/− mice develop a “TTP-like” syndrome only after a trigger such as shigatoxin (39,40) or recombinant VWF challenge (41). However, in baboon, TTP syndrome occurs subsequently after complete inhibition of plasma ADAMTS13 activity by an intravenous administration of anti-ADAMTS13 monoclonal antibodies against the metalloprotease domain without additional triggers (42).
The importance of the discovery of ADAMTS13 extends beyond its association with the potentially fatal TTP syndrome (Table 1). Studies have demonstrated that reduced plasma ADAMTS13 activity and increased plasma VWF (the only known substrate for ADAMTS13) are risk factors for the development of myocardial infarction (43–45), ischaemic stroke (46–48), preeclampsia (49), and malignant (or cerebral) malaria (50–54). Moreover, Adamts13−/− mice demonstrate an increased (~2–5 fold) area of atherosclerotic lesion en face and increased macrophage infiltration as compared with those in WT mice in an ApoE−/− background (55,56). More recently, studies have demonstrated that Adamts13−/− mice exhibit an increase of infarct sizes in the myocardium (57–59) and brain (47,60) after ischaemic/perfusion injury. An infusion of recombinant human ADAMTS13 into Adamts13−/− mice significantly attenuates infarct sizes (47,60). These findings indicate that ADAMTS13 offers systemic protection against ischaemic myocardial and cerebral infarctions. Therefore, an investigation of the biosynthesis, structure-function relationship, and cofactor-dependent regulation of ADAMTS13 protease will provide novel tools for diagnosis and treatment of many potentially fatal human diseases.
Table 1.
ADAMTS13 deficiency and potential human diseases
| Diseases associated | Species | ADAMTS13 status | References cited |
|---|---|---|---|
| TTP | H | <5% | 1, 20, 22, 33 |
| M | Null | 39, 40 | |
| B | <5% | 42 | |
| Ischemic stroke | H | Reduced | 43, 48 |
| M | Null | 46, 47, 60 | |
| Myocardial infarction | H | Reduced | 43–45 |
| M | Null | 56, 58 | |
| Atherosclerosis | H | Reduced | 48 |
| M | Null | 55, 56 | |
| Cerebral malaria | H | Reduced | 50–54 |
| Preeclampsia | H | Reduced | 49 |
TTP, thrombotic thrombocytopenic purpura
H, B, M and R are human, baboon, mouse, and rat, respectively.
Biosynthesis and secretion of ADAMTS13
ADAMTS13 is primarily synthesised in the liver of humans, mice, and rats (1,2,8–10). The mRNA encoding the full-length ADAMTS13 (~4.3 kb) is detected only in the liver by Northern blotting analysis (1,2). However, a truncated form of ADAMTS13 mRNA (~2.4 kb) is found in other tissues such as placenta and skeletal muscle by the same method (2). Using reverse polymerase chain reaction (PCR), fragments of ADAMTS13 mRNA are amplified in many tissues including the kidneys, pancreas, spleen, thymus, prostate, testis, ovary, small intestine, colon, and peripheral blood leukocytes (1,61). Whether it is synthesised in endothelial cells or parenchymal cells remains to be determined.
In the liver, ADAMTS13 is localised to the hepatic stellate cells (HSCs) residing in the interstitial area of human (9), mouse (10), and rat (8) livers. ADAMTS13 mRNA and protein expression in the rat HSCs are dramatically up-regulated upon activation by mechanics and inflammatory cytokines such as transforming growth factor-β (TGF-β) in vitro and in vivo (8), suggesting that ADAMTS13 may play a role in tissue remodelling after injury. In addition, ADAMTS13 protein produced in the HSCs may diffuse into capillaries and enters into the blood stream, thereby regulating plasma levels of ADAMTS13 activity. The evidence supporting this hypothesis include: a) reduced plasma ADAMTS13 activity after a partial hepatectomy in humans (62) or after treatment with dimethylnitrosamine, which damages stellate cells in rats (63); and b) increased expression of ADAMTS13 in HSCs and elevated plasma ADAMTS13 activity upon activation in rat models of cholestasis and steatohepatitis (64).
ADAMTS13 mRNA and protein have also been detected in vascular endothelial cells in vitro and in vivo (11,12,65). It is estimated that unstimulated human umbilical vessel endothelial cells (HUVECs) in culture produce approximately 1 ng ADAMTS13 per millilitre of conditioned medium every 60 minutes. The amount of ADAMTS13 is ~100-fold less than VWF (100 ng/ml) produced by HUVECs under the same conditions (65). Immunohistochemistry demonstrates that ADAMTS13 is not co-localised with VWF in Weibel-Palade bodies (11,12,65), suggesting that ADAMTS13 is constitutively secreted from cells.
The function of ADAMTS13 synthesised in the endothelium is not fully understood. While endothelial cells produce trace amounts of ADAMTS13 in culture, their massive surface coverage suggests a potentially substantial contribution of the endothelium-derived ADAMTS13 to plasma ADAMTS13 activity. In addition, ADAMTS13 released from endothelial cells may cleave newly formed UL-VWF strings on the cell surface, providing an additional mechanism to maintain a VWF-free surface (65–68). Moreover, Lee et al. have demonstrated that ADAMTS13 has either pro-angiogenic or anti-angiogenic effects depending on the cellular environments (69). On one hand, treatment of HUVECs with recombinant ADAMTS13 results in dramatically increased tube formation and cell migration, suggesting enhanced angiogenesis. On the other hand, when vascular endothelial growth factor (VEGF) is present in the culture medium ADAMTS13 inhibits VEGF-induced angiogenic activity. This anti-angiogenic effect is reversed by pre-incubation of ADAMTS13 with a polyclonal antibody against the C-terminal TSP1 5–7 repeats of ADAMTS13 (69), suggesting a role of TSP1 repeats in mediating the pro- and anti-angiogenic effects.
A small quantity of ADAMTS13 mRNA and protein is detectable in human megakaryocytes and platelets (70,71). One study shows that platelets contain less than 160 ng ADAMTS13 per 1×108 platelets (70). The amount of ADAMTS13 produced in platelets may be overestimated. The biological function of platelet-derived ADAMTS13 remains unknown. Preliminary study from our laboratory, presented in the 54th Annual Meeting of the American Society of Hematology, demonstrated that transgenically overexpressed ADAMTS13 in the platelets of Adamts13−/− mice is releasable upon activation by thrombin and collagen, as well as during the thrombus formation after injury with 10% ferric chloride (72). The secreted human ADAMTS13 was able to dramatically inhibit thrombus growth in mesenteric arterioles after oxidative injury and protects Adamts13−/− mice from VWF- and shigatoxin-induced “TTP-like” syndrome (72). These results suggest that platelet-derived ADAMTS13 may be biologically important. The release of ADAMTS13 at the site of thrombus formation may offer a novel treatment for acquired TTP with inhibitors. A similar strategy has been reported for the treatment of haemophiliacs with inhibitors with success in murine models (73–75).
Structure-function relationship of ADAMTS13
ADAMTS13 shares similar domain structure as compared with other ADAMTS family proteases, comprising a signal peptide, a propeptide, a metalloprotease, a disintegrin-like domain, first thrombospondin type 1 repeat (TSP1), Cys-rich and spacer domains. The more distal C-terminus contains seven additional TSP1 repeats and two CUB domains (Fig. 2). The function of each domain of ADAMTS13 in its biosynthesis, secretion, and proteolytic activity has been extensively studied in recent years.
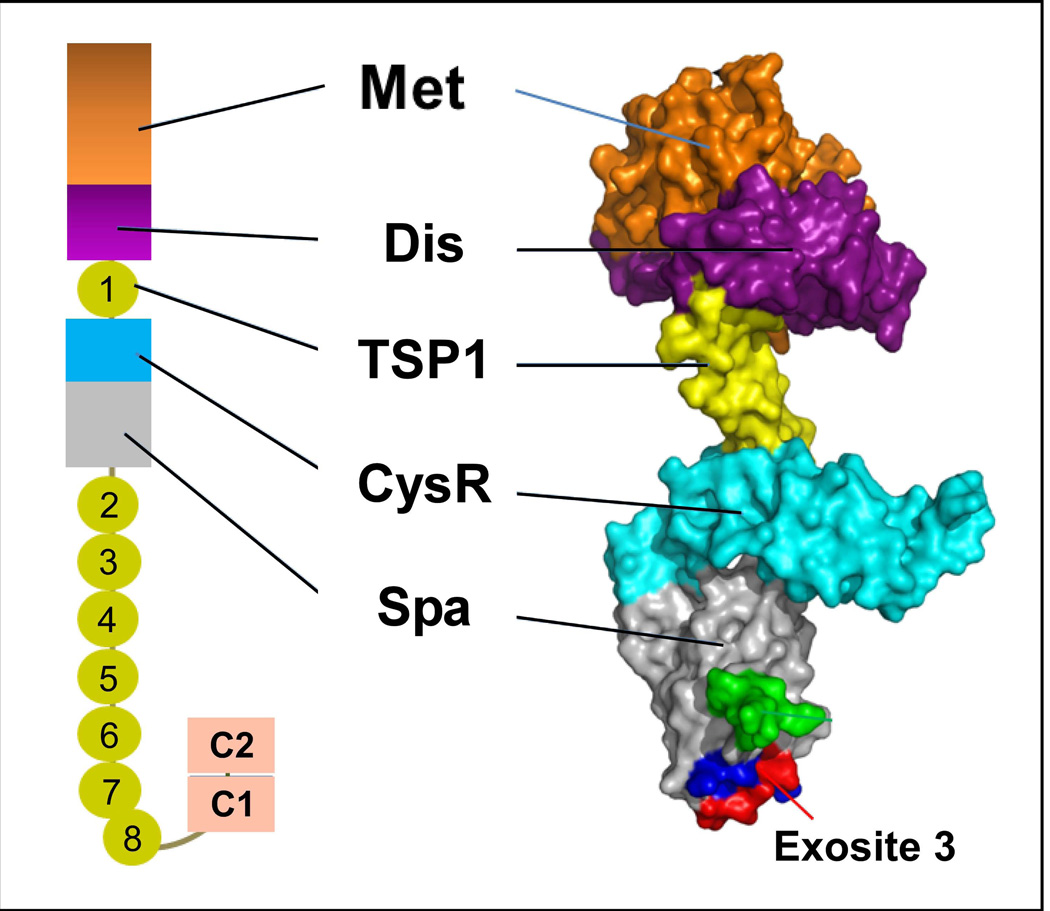
Fig. 2. Domain organisation and partial crystal structure of ADAMTS13.
On the left, the domain organization of human mature ADAMTS13 is shown, which consists of a metalloprotease domain (M), a disintegrin-like domain (D), the first TSP1 repeat, a Cys-rich domain (C), and a spacer domain (S). In addition, the C-terminus contains 7 more TSP1 repeats (2–8) and two CUB domains (C1 and C2). On the right, the surface and carton presentation of the crystal structure of ADAMTS13 disintegrin-like domain (Dis), first TSP1 repeat (TSP1), Cys-rich (Cys) and spacer domain (Spa) in addition to a modelled metalloprotease domain (based on the metalloprotease domains of ADAMTS4 and ADAMTS5).
Unlike the propeptides of other ADAMTS or ADAM family proteases (typically ~200 amino acid residues) (76,77), the ADAMTS13 propeptide is exceptionally short (only ~41 residues). While the propeptides of other ADAMTS proteases function as molecular chaperones to assist protein folding and maintain the latency of the protease by a mechanism of “cysteine-switch”, the ADAMTS13 propeptide is not required for its secretion and activation. Recombinant human ADAMTS13 expressed in cells with or without a propeptide secrets normally and is able to cleave VWF substrates with similar efficacy (78).
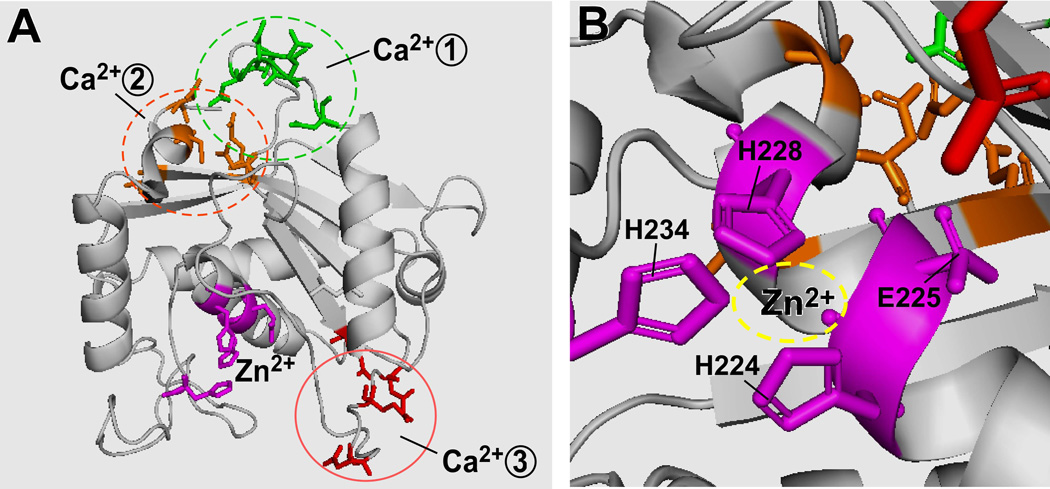
The metalloprotease domain of ADAMTS13 has the expected hallmarks of the reprolysin or adamalysin family of metalloproteases, which include three histidine residues that coordinate the essential Zn2+ ion in the sequence HEXXHXXGXXHD (Fig. 3) (2). In addition, three putative Ca2+-binding sites have been postulated based on modelling the metalloprotease domain of ADAMTS4 and ADAMTS5 (79) (Fig. 3). The first putative Ca2+ binding site comprises amino acid residues Glu83, Asp173, Cys281, and Asp284 that are broadly conserved among ADAMTS and other metalloproteases and appear to mediate low affinity Ca2+ binding. The second putative Ca2+ binding site consists of residues Glu164 and Asp166 in conjunction with one or more of residues Asn162, Asp165, and Asp168. Mutations at the second putative site have no effect on the Ca2+-dependent ADAMTS13 activity. The third site is predicted to include residues Asp187 and Glu212 in conjunction with Asp182 or Glu184 (Fig. 3). Mutations at this site dramatically reduce Ca2+-induced ADAMTS13 activity, suggesting that the residues at the third site play an important role in high-affinity Ca2+ binding and proteolytic activity (79).
Fig. 3. Zinc and calcium binding sites in the metalloprotease domain of ADAMTS13.
A. The ADAMTS13 metalloprotease domain with an active site and three putative calcium-binding sites; B. Three histidine residues and one glutamic acid coordinate the zinc binding.
Like many clotting factors, exosite interaction appears to govern enzymatic activity and substrate specificity. The ADAMTS13 metalloprotease domain alone has no or little proteolytic activity toward VWF substrates. The proteolytic activity increases as more and more non-catalytic domains are sequentially added to the metalloprotease domain (80–83). As shown in the model of MDTCS fragments based on the crystal structure of ADAMTS13-DTCS (84) and the metalloprotease domains of ADAMTS1 (85), ADAMTS4, and ADAMTS5 (86) (Fig. 2), the metalloprotease and disintegrin-like domain (MD) appear to be an inseparable functional unit (80,81,87,88). Experimental data also show that the addition of the disintegrin-like domain to the metalloprotease domain significantly increases cleavage efficiency and specificity (80,87). ADAMTS13 variants lacking the disintegrin-like domain (80,81,87) or carrying point mutations in the variable regions of disintegrin-like domain (Arg349Ala and Leu350Gly) (87) have dramatically reduced proteolytic activity toward both peptide substrates and multimeric VWF, suggesting the importance of the disintegrin-like domain in substrate recognition. Further studies demonstrate that residues Arg349 and Leu350 of the ADAMTS13 disintegrin domain may interact with residues Asp1614 and Ala1612 in the central A2 domain of VWF. Such interactions appear to assist in positioning the Tyr1605-Met1606 bond into the active-site cleft, thereby markedly affecting the rate constant (Km) and catalytic efficiency (kcat) for substrate proteolysis (89,90). More recently, Xiang et al demonstrated that VWF Leu1603 (P3) Tyr1605, and Asp1614 appear to make direct contact with Leu198, Val195, and Arg349 in ADAMTS13, respectively (90). Therefore, the disintegrin domain, working in concert with other non-catalytic domains, ensures that the scissile bond is brought into position over the active centre for cleavage to occur.
Much of our attention has been focused on the role of the Cys-rich and spacer domains of ADAMTS13 in substrate recognition (80–83,88,91). ADAMTS13 variants lacking both the Cys-rich and spacer domains or the spacer domain alone exhibit only minimal activity toward peptidyl substrates but have nearly no activity toward cell bound UL-VWF (66,92) and soluble VWF under various conditions (18,80,82,83). We and others have shown that proteolytic cleavage of a peptidyl substrate increases as function of each of the non-catalytic domains (DTCS) is added to the metalloprotease domain (80,81,83). However, further addition of the TSP1 2–8 and CUB domains does not increase proteolytic activity. These results suggest that the exosite interaction between the ADAMTS13-DTCS domains and VWF-A2 domain is critical for proteolysis of VWF. These non-catalytic (DTCS) domains of ADAMTS13 are shown to directly interact in a linear fashion with various segments in the central VWF-A2 between residues Asp1614 and Arg1668 (81,89,93).
A replacement of the TCS domains of ADAMTS5, a closely related member of ADAMTS proteases, with those of ADAMTS13 alters ADAMTS5 substrate specificity (93). ADAMTS5 is not known to cleave VWF, but a chimeric variant that consists of the metalloprotease and disintegrin domains-like of ADAMTS5 (MD5) and three non-catalytic TCS domains of ADAMTS13 (TCS13) (MD5/TCS13) is able to cleave the Glu1615-Ile1616 bond of VWF domain A2 in peptide substrates or VWF multimers that has been sheared (93). However, this cleavage site is no longer at the Tyr1605-Met1606 bond (93), further confirming the critical role of each of non-catalytic domains of ADAMTS13 in substrate specificity.
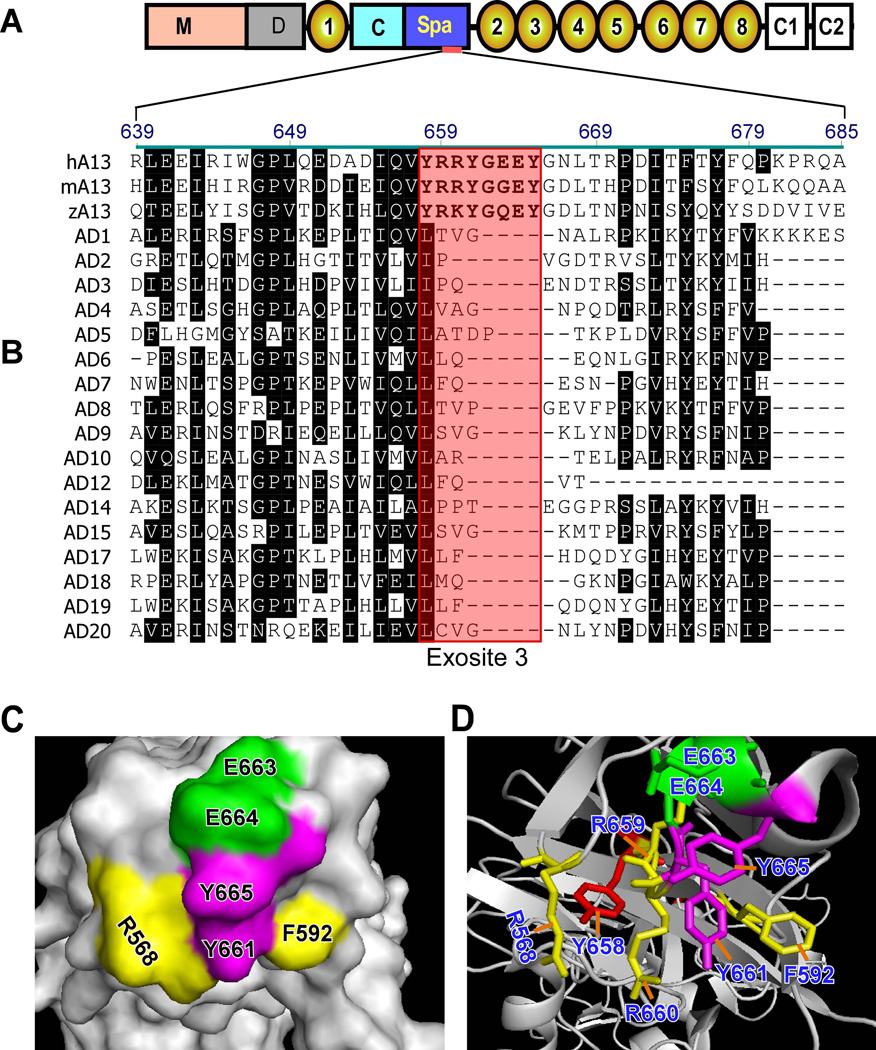
Further sequence analysis of the spacer domains from ADAMTS13 and other ADAMTS family proteases has allowed us to identify several potential exosites that are highly conserved in human, murine, and zebrafish ADAMTS13 but absent in the other members of the ADAMTS family (Fig. 4B) (94). One of these exosites, also described as exosite 3, comprises amino acid residues Tyr658, Arg659, Arg660, Tyr661, and Tyr665 (in conjunction with two surrounding residues Arg568 and Phe592) (Fig. 4C and 4D) (84,94). A deletion of residues Arg659-Glu664 (Δ6aa) or a substitution of residues Arg659, Arg660, and Tyr661 with alanine dramatically reduces proteolytic cleavage of various VWF substrates (94,95).
Fig. 4. Sequence alignment of the spacer domains of ADAMTS13 and other ADAMTS proteases.
A. Domain organization of ADAMTS13; B. Sequence alignment of a partial spacer domain (Arg629-Lys681) from human (h), murine (m), and zebrafish (z) ADAMTS13 (A13), as well as a partial spacer domain of various ADAMTS family proteases (AD1–AD20). Boxed area in pink presents a region (exosite 3) that is highly conserved in the ADAMTS13-spacer domain but absent in the spacer domain in other ADAMTS proteases. C and D are the surface and ribbon representation of exosite 3, respectively.
Additional evidence to support the physiological role of exosite 3 comes from our mouse models of thrombosis. The ADAMTS13-Δ6aa variant exhibits dramatically reduced anti-thrombotic activity compared with WT-ADAMTS13 in the mesenteric arteriolar occlusion assay in Adamts13−/− mice (18). Together, these data suggest the critical role of exosite 3 in the spacer domain in substrate recognition and proteolysis of VWF in vitro and in vivo.
ADAMTS proteases have a variable number of TSP1 repeats that may play a role in cellular localisation and substrate recognition (76,96,97). All TSP1 repeats contain the sequence WXXW, which is often modified by attachment of an α-mannosyl group to the C2- atom of the first Trp (98). Seven of the eight TSP1 repeats also contain the conserved sequence CSX(S/T)CG, in which the hydroxyamino acid at position 4 usually is modified by the disaccharide Glc-Fuc-O-Ser/Thr (98). It is not clear whether these posttranslational modifications play a role in ADAMTS13 function in vivo.
The TSP1 repeats in other ADAMTS proteases participate in substrate recognition and cell surface binding. The first TSP1 repeat of ADAMTS13 binds directly to VWF73 with a Kd of ~136 nM (80). The TSP1 5–8 repeats of ADAMTS13 appear to bind native VWF through the D4 domain (99). Moreover, the C-terminal TSP1 repeats of ADAMTS13 are shown to interact with the endothelial cell surface receptor CD36 (100,101), and such an interaction may enhance proteolytic cleavage of UL-VWF under flow conditions (102). However, human and murine ADAMTS13 variants lacking the C-terminal 2–8 TSP1 repeats and CUB domains (MDTCS) cleave cell bound UL-VWF (66,92,103) and soluble VWF (18) with similar efficacy as does the full-length ADAMTS13. These different conclusions may be attributed to assay sensitivity and the source of recombinant ADAMTS13 variants. Further studies are necessary to elucidate the biological function of the TSP1 repeats.
More recently, the TSP1 repeats of ADAMTS13 are shown to contain free thiols that may react with the free thiols on the surface of UL-VWF or plasma VWF exposed under shear stress (104,105). Such an interaction may prevent disulphide bond exchange and formation between two apposed VWF multimers under flow, thereby attenuating VWF-mediated platelet adhesion and aggregation (104,105).
The CUB domains are unique to ADAMTS13 and are not found in other ADAMTS and ADAM proteases (76,77,97). The role of the ADAMTS13-CUB domains is not fully understood. Recombinant CUB-1 and CUB-1+2 domains or synthetic peptides derived from the CUB-1 domain of ADAMTS13 partially block proteolytic cleavage of endothelial cell bound UL-VWF by full-length ADAMTS13 under flow (106), suggesting that the CUB domains of ADAMTS13 may interact with UL-VWF on the endothelial cell surface. Consistent with this hypothesis, a murine ADAMTS13 variant lacking the CUB domains (delCUB) appears to be defective in cleaving platelets-decorated UL-VWF strings in the mesenteric arterioles of Adamts13−/− mice after ferric chloride injury (92). However, this hypothesis poses some challenges. A human ADAMTS13 variant lacking the CUB domains normally cleaves the newly released UL-VWF strings in the absence of flow (66) or platelet-decorated UL-VWF strings on cultured HUVECs under flow (103). Moreover, a similar human ADAMTS13 variant is significantly able to attenuate the rate of thrombus growth in the mesenteric arteriolar occlusion assay (18), suggesting that the CUB domains are dispensable under these conditions. These conflicting results point to the complexity of assessing ADAMTS13 function under more physiological conditions.
Regulation of ADAMTS13 function
Cofactor-dependent regulation of coagulation enzymes has been well recognised and increases the rate of an enzymatic reaction by several orders of magnitude (107,108). Unlike other clotting factors that are synthesised as inactive zymogens, ADAMTS13 is secreted as a constitutively active protease (10,11,65,83). There has been no inhibitor identified to date. Plasma α2-macroglobulin inhibits many other matrix metalloproteases including ADAMTS-4, 5, 7, and 12 (109–112) but does not seem to bind and affect ADAMTS13 activity toward VWF (83). Therefore, ADAMTS13 function must be regulated at the substrate level.
Newly released UL-VWF anchored on the endothelial cell membrane can be rapidly cleaved by ADAMTS13 in the presence (5–7) or absence of flow (66,67), suggesting that cell bound UL-VWF is in its “open” conformation. The mechanism underlying the “open” conformation is not known. Once released into solution, the UL-VWF rapidly adopts a “closed” conformation that becomes highly resistant to proteolysis by ADAMTS13 in the absence of shear stress or denaturants. The soluble UL-VWF regains its sensitivity to ADAMTS13 when exposed to high fluid shear stress (~20–100 dynes/cm2) that presumably unfolds the central A2 domain of VWF (16,17,113). Such high shear conditions can be found in vivo in narrowed or branching vessels, small arterioles, and microcirculation. The increased VWF proteolysis by ADAMTS13 and a reduction of the plasma VWF activity to antigen ratio (114) correlates with the severity of aortic stenosis (15,115). Surgical correction normalises plasma VWF multimer distribution (15,116).
Arterial shear stress can be simulated in vitro using a cone plate viscometer (117,118), a bench-top mini-vortex (99,113,119), and a microfluidic system (120–122) that generates laminar flow. Under mechanically induced shear stress, proteolytic cleavage of multimeric VWF by recombinant ADAMTS13 increases as a function of increasing shear rate (or shear stress), incubation time, and concentrations of ADAMTS13 enzyme (113). The mechanical force-induced cleavage of an A1A2A3 tri-domain molecule (19) or the A2 domain of VWF (123,124) has also been demonstrated. Together, these findings suggest that fluid shear stress plays a critical role in regulating proteolytic cleavage of soluble VWF by ADAMTS13.
In addition to shear stress, coagulation factor VIII (FVIII), which binds VWF with high affinity (KD, 0.25–0.5 nM), may alter the domain-domain interaction of the neighbouring A1A2A3 of VWF and regulate proteolytic cleavage of the A2 domain by ADAMTS13. To test this hypothesis, recombinant FVIII at various concentrations (0–20 nM) was incubated for 30 minutes with plasma-derived or recombinant multimeric VWF prior to incubation with recombinant ADAMTS13. After 2 minute incubation under constant vortexing (2,500 rpm, ~28.5 dynes/cm2), the proteolytic cleavage product (~350 kDa) increases as a function of increasing concentrations of FVIII (119). In the presence of 20 nM FVIII, the maximal increase in the cleavage product formation approaches ~10–12 fold of that with VWF alone. The rate-enhancing effect of FVIII on VWF proteolysis was detected under fluid shear stress but not under static/denaturing conditions (119), suggesting that the binding of FVIII to VWF may facilitate the unfolding processes of the VWF-A2 domain under these conditions.
Structure-function analysis demonstrates that the B-domain deleted FVIII variant (FVIII-SQ) exhibits a similar rate enhancing effect on proteolysis of VWF by ADAMTS13 as does full-length FVIII (119,125). However, a FVIII variant lacking the acidic (a3) region that contains a major VWF binding site (FVIII-2RKR) has no effect under the same conditions (119,125). Interestingly, a light chain of FVIII (FVIII-LC), despite a 10-fold reduction in its binding affinity to VWF, is sufficient for accelerating the cleavage of VWF to a similar extent as are wild-type FVIII and FVIII-SQ(125), suggesting that binding of FVIII to VWF through its light chain mediates this cofactor activity.
These biochemical findings are further corroborated with those obtained in vivo in a murine model. Hydrodynamic injection is a commonly used method to instantly transfect hepatocytes with plasmids of interest. This manoeuvre also activates endothelial cells, triggering the release of UL-VWF into plasma in mice. When injected with PBS alone, plasma ratios of high to low molecular weight VWF multimers in fVIII−/− mice are higher than those in the fVIII−/− mice reconstituted with a plasmid encoding FVIII-SQ or FVIII-LC (125), suggesting that the expression of a functional VWF-binding FVIII variant eliminates the accumulation of UL-VWF multimers under (patho)physiological conditions.
Additional evidence to support the physiological role of FVIII-dependent regulation of VWF proteolysis by ADAMTS13 comes from our recent findings (presented in part at the ASH meeting, 2009) (126). The result demonstrated that type 2N VWF variants which exhibit a moderate to severe defect for FVIII binding were also compromised in accelerating cleavage of VWF by ADAMTS13 in the presence of FVIII under shear stress (126). Together, our findings support the critical role of FVIII as a physiological cofactor regulating proteolytic cleavage of VWF by ADAMTS13. Such cofactor activity is dependent on the reaction between the light chain of FVIII and the D’D3 domains of VWF.
Platelet glycoprotein 1bα (GP1bα) also binds VWF with high affinity. Studies have demonstrated that an addition of formalin-fixed, lyophilised or fresh platelets, and soluble GP1bα to multimeric VWF increases proteolytic cleavage by ADAMTS13 under static (117,127) or fluid shear conditions (118). Ristocetin, an antibiotic that binds the A1 domain of VWF close to the site that GP1bα binds, also enhances cleavage of multimeric VWF by ADAMTS13 (118,128). These results suggest that the interaction between platelet GP1bα (or ristocetin) and the A1 domain affects the accessibility of the A2 domain by ADAMTS13. Interestingly, ristocetin alleviates the requirement of FVIII to enhance the cleavage of VWF by ADAMTS13 (118), while binding of platelet GP1bα to VWF enhances the effect of FVIII or vice versa as previously demonstrated. In the presence of physiological concentrations of platelets (150×103/µl), the C50 shifts to the left (from 5 nM to 0.5 nM) (118). These results suggest that FVIII and platelet GP1b have synergistic effects that enhance VWF proteolysis by ADAMTS13 under fluid shear stress. It has been postulated that the binding of FVIII to the D’D3 domain of VWF may result in large-scale conformational changes of the VWF multimers, such as pulling away the D’D3 domain from its neighbouring A1 or A2 domain under shear stress. Similarly, binding of platelets or soluble GP1bα to the VWF-A1 domain may expose the A2 domain for cleavage. Two or more platelets bound on each side of the scissile bond may dramatically increase the peak tensile force exerted on the central A2 domain (129), which enhances A2 domain unfolding and proteolysis by ADAMTS13, demonstrated by single molecule experiments (19,123,124).
Mechanism of anti-ADAMTS13 autoantibody inhibition
Idiopathic TTP in adults is caused by a severe deficiency of plasma ADAMTS13 activity due to immunoglobulin G (IgG) type antibodies. Depending on the assays, the inhibitory antibodies are detected in 44% to 100% of acquired idiopathic TTP patients with a severe deficiency of plasma ADAMTS13 activity (130). With more sensitive assays such as an enzyme-linked immunosorbent assay (ELISA) (131,132) or flow cytometry-based technology (133), anti-ADAMTS13 IgGs can be detected in all TTP patients who have a severe deficiency of plasma ADAMTS13 activity (130). Antibody mapping and profiling reveal that anti-ADAMTS13 IgG1 and IgG4 predominate in the plasma of acquired TTP patients (134,135) and nearly all anti-ADAMTS13 IgGs bind the Cys-rich and spacer domains, particularly the spacer domain (35,95,136–140). Other ADAMTS13 domains including the propeptide, metalloprotease domain, disintegrin domain, first TSP1 repeat, more distal TSP1 repeats, and CUB domains are less reactive with autoantibodies (35,136,140). Further analysis demonstrates that the major antigenic epitopes are localised to residues Tyr572-Asn579 (139), Val657-Gly666 (95,139), and Gly662-Val687 (141). A majority of TTP patients (90%) lose reactivity towards ADAMTS13 following the substitution of residues Arg568, Phe592, Arg660, Tyr661, and Tyr665 in exosite 3 of the spacer domain (140). These residues have been demonstrated to play a critical role in substrate recognition and proteolysis of VWF under various conditions (94,95). Therefore, it is conceivable that the binding of anti-ADAMTS13 autoantibodies to this region blocks substrate binding and its proteolytic function.
The mechanism of how patients develop autoantibodies against ADAMTS13 protease is not known. Female predominance (33,35) and autoantibodies observed in identical twin sisters (142) suggest a genetic predisposition. A recent study demonstrates an overrepresentation of the HLA-DRB1*11 allele in acquired TTP patients (143), consistent with the hypothesis. ADAMTS13 is efficiently internalised by immature dendritic cells (antigen-presenting cells) through the surface macrophage mannose receptor (144,145). Interestingly, dendritic cells from HLA-DRB1*11 donors pulsed with higher concentrations of ADAMTS13 in culture present derivatives of a single CUB-2 derived peptide, suggesting that functional presentation of CUB-2 derived peptides on HLA-DRB1*11 may contribute to the onset of acquired TTP by stimulating low affinity self-reactive CD4+ T cells (144,146). Moreover, bacterial or viral infections often precede the acute epitope of initial or recurrent TTP (147–149). This indicates that infections may act as a trigger boasting the production of autoantibody production or substrate release from activated endothelium. However, one cannot exclude the possibility of a “by-stander” or molecular mimic hypothesis in which antibodies against microbes may cross react with ADAMTS13.
Improving on nature, re-engineering ADAMTS13
Others and we have demonstrated that the spacer domain (35,95,136,138–140), particularly exosite 3 (Fig. 2 and Fig. 4), is the major target of anti-ADAMTS13 autoantibodies in patients with acquired TTP (95,140). Autoantibody binding to this region is expected to block substrate binding and inhibit proteolytic activity of ADAMTS13. Replacement of the residues in exosite 3 with alanine nearly completely abolishes anti-ADAMTS13 IgG binding, but also significantly impairs proteolytic activity of ADAMTS13, rendering these mutants useless for therapy. We sought to subtly modify these residues by changing Arg to Lys or vice versa in hopes of eliminating autoantibody binding but retaining ADAMTS13 activity. Through site-directed mutagenesis, we have created and tested a panel of ADAMTS13 mutants for antibody binding and proteolysis. The results show that a substitution of 4–5 residues in exosite 3 generates ADAMTS13 variants (M4: R660K/F592Y/R568K/Y661F and M5: R660K/F592Y/R568K/Y661F/Y665F) with increased specific activity toward FRETS-VWF73 and multimeric VWF by 4- to 5-fold and 10- to12-fold, respectively (Fig. 4) (150). More interestingly, these variants were more resistant than WT and M1-M3 (with one to three residues mutated) to inhibition by anti-ADAMTS13 autoantibodies from acquired TTP patients (150). The reduction M4 and M5 inhibition by autoantibodies is correlated with the impaired binding of anti-ADAMTS13 IgG to the variants (150). Together, our findings indicate that it is possible to re-engineer ADAMTS13 protease to improve specific activity in the presence of autoantibodies, which may offer therapeutic benefits to acquired TTP patients. In summary, tremendous progress has been made in the past decade toward our understandings of the biosynthesis, the structure-function relationship, and the cofactor-dependent regulation of ADAMTS13 function. These advances provide invaluable information concerning the mechanisms of TTP and other atherothrombotic disorders, as well as inflammatory diseases. Therefore, novel diagnostic tools and therapeutics may be developed for managing these potentially fatal diseases.
Acknowledgement
The work presented in the review article was supported by grants from The Children's Hospital of Philadelphia, The University of Pennsylvania, the National Blood Foundation, the American Heart Association (04265532U and 0940100N), and the National Institute of Health (HL-079027 and HL-074124).
Footnotes
Disclosure of Conflicts of Interest: The authors state that they have no conflict of interest.
References
- 1.Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R, Sarode R, Shurin SB, Chandrasekaran V, Stabler SP, Sabio H, Bouhassira EE, Upshaw JD, Jr, Ginsburg D, Tsai HM. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 2.Zheng XL, Chung D, Takayama T, Majerus E, Sadler J, Fujikawa K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276:41059–41063. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 3.Sadler JE. von Willebrand factor. J Biol Chem. 1991;266:22777–22780. [PubMed] [Google Scholar]
- 4.Wagner DD, Marder VJ. Biosynthesis of von Willebrand protein by human endothelial cells: processing steps and their intracellular localization. J Cell Biol. 1984;99:2123–2130. doi: 10.1083/jcb.99.6.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K, Lopez JA. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–4039. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 6.Dong JF, Moake JL, Bernardo A, Fujikawa K, Ball C, Nolasco L, Lopez JA, Cruz MA. ADAMTS-13 metalloprotease interacts with the endothelial cell-derived ultra-large von Willebrand factor. J Biol Chem. 2003;278 doi: 10.1074/jbc.M301385200. 29633-19639. [DOI] [PubMed] [Google Scholar]
- 7.Dong JF. Cleavage of ultra-large von Willebrand factor by ADAMTS-13 under flow conditions. J Thromb Haemost. 2005;3:1710–1716. doi: 10.1111/j.1538-7836.2005.01360.x. [DOI] [PubMed] [Google Scholar]
- 8.Niiya M, Uemura M, Zheng XW, Pollak E, Dockal M, Scheiflinger F, Wells RG, Zheng XL. Increased ADAMTS13 proteolytic activity in rat hepatic stellate cells upon activation in vitro and in vivo. J Thromb Haemost. 2006;4:1063–1070. doi: 10.1111/j.1538-7836.2006.01893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uemura M, Tatsumi K, Matsumoto M, Fujimoto M, Matsuyama T, Ishikawa M, Iwamoto TA, Mori T, Wanaka A, Fukui H, Fujimura Y. Localization of ADAMTS13 to the stellate cells of human liver. Blood. 2005;106:922–924. doi: 10.1182/blood-2005-01-0152. [DOI] [PubMed] [Google Scholar]
- 10.Zhou W, Inada M, Lee TP, Benten D, Lyubsky S, Bouhassira EE, Gupta S, Tsai HM. ADAMTS13 is expressed in hepatic stellate cells. Lab Invest. 2005;85:780–788. doi: 10.1038/labinvest.3700275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shang D, Zheng XW, Niiya M, Zheng XL. Apical sorting of ADAMTS13 in vascular endothelial cells and Madin-Darby canine kidney cells depends on the CUB domains and their association with lipid rafts. Blood. 2006;108:2207–2215. doi: 10.1182/blood-2006-02-002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner N, Nolasco L, Tao Z, Dong JF, Moake J. Human endothelial cells synthesize and release ADAMTS-13. J Thromb Haemost. 2006;4 doi: 10.1111/j.1538-7836.2006.01959.x. 1396-04. [DOI] [PubMed] [Google Scholar]
- 13.Sadler JE. A new name in thrombosis, ADAMTS13. Proc Natl Acad Sci U S A. 2002;99:11552–11554. doi: 10.1073/pnas.192448999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai HM. Shear stress and von Willebrand factor in health and disease. Semin Thromb Hemost. 2003;29:479–488. doi: 10.1055/s-2003-44556. [DOI] [PubMed] [Google Scholar]
- 15.Vincentelli A, Susen S, Le Tourneau T, Six I, Fabre O, Juthier F, Bauters A, Decoene C, Goudemand J, Prat A, Jude B. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med. 2003;349:343–349. doi: 10.1056/NEJMoa022831. [DOI] [PubMed] [Google Scholar]
- 16.Siedlecki CA, Lestini BJ, Kottke-Marchant KK, Eppell SJ, Wilson DL, Marchant RE. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood. 1996;88:2939–2950. [PubMed] [Google Scholar]
- 17.Tsai HM, Sussman I, Nagel RL. Shear stress enhances the proteolysis of von Willebrand factor in normal plasma. Blood. 1994;83:2171–2179. [PubMed] [Google Scholar]
- 18.Xiao J, Jin SY, Xue J, Sorvillo N, Voorberg J, Zheng XL. Essential domains of a disintegrin and metalloprotease with thrombospondin type 1 repeats-13 metalloprotease required for modulation of arterial thrombosis. Arterioscler Thromb Vasc Biol. 2011;31:2261–2269. doi: 10.1161/ATVBAHA.111.229609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu T, Lin J, Cruz MA, Dong JF, Zhu C. Force-induced cleavage of single VWFA1A2A3 tridomains by ADAMTS-13. Blood. 2010;115:370–378. doi: 10.1182/blood-2009-03-210369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furlan M, Robles R, Lammle B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 1996;87:4223–4234. [PubMed] [Google Scholar]
- 21.Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, Brenner B, Krause M, Scharrer I, Aumann V, Mittler U, Solenthaler M, Lammle B. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339:1578–1584. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 22.Tsai HM. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood. 1996;87:4235–4244. [PubMed] [Google Scholar]
- 23.Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339:1585–1594. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chauhan AK, Motto DG, Lamb CB, Bergmeier W, Dockal M, Plaimauer B, Scheiflinger F, Ginsburg D, Wagner DD. Systemic antithrombotic effects of ADAMTS13. J Exp Med. 2006;203:767–776. doi: 10.1084/jem.20051732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong JF. Structural and functional correlation of ADAMTS13. Curr Opin Hematol. 2007;14:270–276. doi: 10.1097/MOH.0b013e3280d35820. [DOI] [PubMed] [Google Scholar]
- 26.Moake JL, Rudy CK, Troll JH, Weinstein MJ, Colannino NM, Azocar J, Seder RH, Hong SL, Deykin D. Unusually large plasma factor VIII:von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med. 1982;307:1432–1435. doi: 10.1056/NEJM198212023072306. [DOI] [PubMed] [Google Scholar]
- 27.Hosler GA, Cusumano AM, Hutchins GM. Thrombotic thrombocytopenic purpura and hemolytic uremic syndrome are distinct pathologic entities. A review of 56 autopsy cases. Arch Pathol Lab Med. 2003;127:834–839. doi: 10.5858/2003-127-834-TTPAHU. [DOI] [PubMed] [Google Scholar]
- 28.Tsai HM. Platelet activation and the formation of the platelet plug: deficiency of ADAMTS13 causes thrombotic thrombocytopenic purpura. Arterioscler Thromb Vasc Biol. 2003;23:388–396. doi: 10.1161/01.ATV.0000058401.34021.D4. [DOI] [PubMed] [Google Scholar]
- 29.Tsai HM. ADAMTS13 and microvascular thrombosis. Expert Rev Cardiovasc Ther. 2006;4:813–825. doi: 10.1586/14779072.4.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell WR, Braine HG, Ness PM, Kickler TS. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med. 1991;325:398–403. doi: 10.1056/NEJM199108083250605. [DOI] [PubMed] [Google Scholar]
- 31.George JN. How I treat patients with thrombotic thrombocytopenic purpura: 2010. Blood. 2010;116:4060–4069. doi: 10.1182/blood-2010-07-271445. [DOI] [PubMed] [Google Scholar]
- 32.Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, Spasoff RA. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med. 1991;325:393–397. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 33.Zheng XL, Richard KM, Goodnough LT, Sadler JE. Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and non-idiopathic thrombotic thrombocytopenic purpura. Blood. 2004;103:4043–4049. doi: 10.1182/blood-2003-11-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng XL, Sadler JE. Pathogenesis of Thrombotic Microangiopathies. Annu Rev Path Mech Dis. 2008;3:249–277. doi: 10.1146/annurev.pathmechdis.3.121806.154311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng XL, Wu HM, Shang D, Falls E, Skipwith CG, Cataland SR, Bennett CL, Kwaan HC. Multiple domains of ADAMTS13 are targeted by autoantibodies against ADAMTS13 in patients with acquired idiopathic thrombotic thrombocytopenic purpura. Haematologica. 2010;95:1555–1562. doi: 10.3324/haematol.2009.019299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kokame K, Matsumoto M, Soejima K, Yagi H, Ishizashi H, Funato M, Tamai H, Konno M, Kamide K, Kawano Y, Miyata T, Fujimura Y. Mutations and common polymorphisms in ADAMTS13 gene responsible for von Willebrand factor-cleaving protease activity. Proc Natl Acad Sci U S A. 2002;99:11902–11907. doi: 10.1073/pnas.172277399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lotta LA, Garagiola I, Palla R, Cairo A, Peyvandi F. ADAMTS13 mutations and polymorphisms in congenital thrombotic thrombocytopenic purpura. Hum Mutat. 2010;31:11–19. doi: 10.1002/humu.21143. [DOI] [PubMed] [Google Scholar]
- 38.Lotta LA, Wu HM, MacKie IJ, Noris M, Veyradier A, Scully MA, Remuzzi G, Coppo P, Liesner R, Donadelli R, Loirat C, Gibbs RA, Horne A, Yang S, Garagiola I, Musallam KM, Peyvandi F. Residual plasmatic activity of ADAMTS13 is correlated with phenotype severity in congenital thrombotic thrombocytopenic purpura. Blood. 2012;120:440–448. doi: 10.1182/blood-2012-01-403113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang J, Motto DG, Bundle DR, Sadler JE. Shiga toxin B subunits induce VWF secretion by human endothelial cells and thrombotic microangiopathy in ADAMTS13-deficient mice. Blood. 2010;116:3653–3659. doi: 10.1182/blood-2010-02-271957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Motto DG, Chauhan AK, Zhu G, Homeister J, Lamb CB, Desch KC, Zhang W, Tsai HM, Wagner DD, Ginsburg D. Shigatoxin triggers thrombotic thrombocytopenic purpura in genetically susceptible ADAMTS13-deficient mice. J Clin Invest. 2005;115:2752–2761. doi: 10.1172/JCI26007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schiviz A, Wuersch K, Piskernik C, Dietrich B, Hoellriegl W, Rottensteiner H, Scheiflinger F, Schwarz HP, Muchitsch EM. A new mouse model mimicking thrombotic thrombocytopenic purpura: correction of symptoms by recombinant human ADAMTS13. Blood. 2012;119:6128–6135. doi: 10.1182/blood-2011-09-380535. [DOI] [PubMed] [Google Scholar]
- 42.Feys HB, Roodt J, Vandeputte N, Pareyn I, Lamprecht S, van Rensburg WJ, Anderson PJ, Budde U, Louw VJ, Badenhorst PN, Deckmyn H, Vanhoorelbeke K. Thrombotic thrombocytopenic purpura directly linked with ADAMTS13 inhibition in the baboon (Papio ursinus) Blood. 2010;116:2005–2010. doi: 10.1182/blood-2010-04-280479. [DOI] [PubMed] [Google Scholar]
- 43.Andersson HM, Siegerink B, Luken BM, Crawley JT, Algra A, Lane DA, Rosendaal FR. High VWF, low ADAMTS13, and oral contraceptives increase the risk of ischemic stroke and myocardial infarction in young women. Blood. 2012;119:1555–1560. doi: 10.1182/blood-2011-09-380618. [DOI] [PubMed] [Google Scholar]
- 44.Matsukawa M, Kaikita K, Soejima K, Fuchigami S, Nakamura Y, Honda T, Tsujita K, Nagayoshi Y, Kojima S, Shimomura H, Sugiyama S, Fujimoto K, Yoshimura M, Nakagaki T, Ogawa H. Serial changes in von Willebrand factor-cleaving protease (ADAMTS13) and prognosis after acute myocardial infarction. Am J Cardiol. 2007;100:758–763. doi: 10.1016/j.amjcard.2007.03.095. [DOI] [PubMed] [Google Scholar]
- 45.Kaikita K, Soejima K, Matsukawa M, Nakagaki T, Ogawa H. Reduced von Willebrand factor-cleaving protease (ADAMTS13) activity in acute myocardial infarction. J Thromb Haemost. 2006;4:2490–2493. doi: 10.1111/j.1538-7836.2006.02161.x. [DOI] [PubMed] [Google Scholar]
- 46.Fujioka M, Nakano T, Hayakawa K, Irie K, Akitake Y, Sakamoto Y, Mishima K, Muroi C, Yonekawa Y, Banno F, Kokame K, Miyata T, Nishio K, Okuchi K, Iwasaki K, Fujiwara M, Siesjo BK. ADAMTS13 gene deletion enhances plasma high-mobility group box1 elevation and neuroinflammation in brain ischemia-reperfusion injury. Neurol Sci. 2012;33:1107–1115. doi: 10.1007/s10072-011-0913-9. [DOI] [PubMed] [Google Scholar]
- 47.Zhao BQ, Chauhan AK, Canault M, Patten IS, Yang JJ, Dockal M, Scheiflinger F, Wagner DD. Von Willebrand factor-cleaving protease ADAMTS13 reduces ischemic brain injury in experimental stroke. Blood. 2009;114:3329–3334. doi: 10.1182/blood-2009-03-213264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bongers TN, De Maat MP, van Goor ML, Bhagwanbali V, van Vliet HH, Gomez Garcia EB, Dippel DW, Leebeek FW. High von Willebrand factor levels increase the risk of first ischemic stroke: influence of ADAMTS13, inflammation, and genetic variability. Stroke. 2006;37:2672–2677. doi: 10.1161/01.STR.0000244767.39962.f7. [DOI] [PubMed] [Google Scholar]
- 49.Stepanian A, Cohen-Moatti M, Sanglier T, Legendre P, Ameziane N, Tsatsaris V, Mandelbrot L, de PD, Veyradier A. Von Willebrand Factor and ADAMTS13: A Candidate Couple for Preeclampsia Pathophysiology. Arterioscler Thromb Vasc Biol. 2011;31:1703–1709. doi: 10.1161/ATVBAHA.111.223610. [DOI] [PubMed] [Google Scholar]
- 50.de Mast Q, Groot E, Asih PB, Syafruddin D, Oosting M, Sebastian S, Ferwerda B, Netea MG, de Groot PG, van dV, Fijnheer R. ADAMTS13 deficiency with elevated levels of ultra-large and active von Willebrand factor in P. falciparum and P. vivax malaria. Am J Trop Med Hyg. 2009;80:492–498. [PubMed] [Google Scholar]
- 51.Larkin D, de LB, Jenkins PV, Bunn J, Craig AG, Terraube V, Preston RJ, Donkor C, Grau GE, Van Mourik JA, O'Donnell JS. Severe Plasmodium falciparum malaria is associated with circulating ultra-large von Willebrand multimers and ADAMTS13 inhibition. PLoS Pathog. 2009;5:e1000349. doi: 10.1371/journal.ppat.1000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bridges DJ, Bunn J, Van Mourik JA, Grau G, Preston RJ, Molyneux M, Combes V, O'Donnell JS, de LB, Craig A. Rapid activation of endothelial cells enables Plasmodium falciparum adhesion to platelet-decorated von Willebrand factor strings. Blood. 2010;115:1472–1474. doi: 10.1182/blood-2009-07-235150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lowenberg EC, Charunwatthana P, Cohen S, van den Born BJ, Meijers JC, Yunus EB, Hassan MU, Hoque G, Maude RJ, Nuchsongsin F, Levi M, Dondorp AM. Severe malaria is associated with a deficiency of von Willebrand factor cleaving protease, ADAMTS13. Thromb Haemost. 2010;103:181–187. doi: 10.1160/TH09-04-0223. [DOI] [PubMed] [Google Scholar]
- 54.Kraisin S, Naka I, Patarapotikul J, Nantakomol D, Nuchnoi P, Hananantachai H, Tsuchiya N, Ohashi J. Association of ADAMTS13 polymorphism with cerebral malaria. Malar J. 2011;10:366. doi: 10.1186/1475-2875-10-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin SY, Tohyama J, Bauer RC, Cao NN, Rader DJ, Zheng XL. Genetic Ablation of Adamts13 Gene Dramatically Accelerates the Formation of Early Atherosclerosis in a Murine Model. Arterioscler Thromb Vasc Biol. 2012;32:1817–1823. doi: 10.1161/ATVBAHA.112.247262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gandhi C, Khan MM, Lentz SR, Chauhan AK. ADAMTS13 reduces vascular inflammation and the development of early atherosclerosis in mice. Blood. 2011;119:2385–2391. doi: 10.1182/blood-2011-09-376202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Meyer SF, Savchenko AS, Haas MS, Schatzberg D, Carroll MC, Schiviz A, Dietrich B, Rottensteiner H, Scheiflinger F, Wagner DD. Protective anti-inflammatory effect of ADAMTS13 on myocardial ischemia/reperfusion injury in mice. Blood. 2012;120:5217–5223. doi: 10.1182/blood-2012-06-439935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doi M, Matsui H, Takeda Y, Saito Y, Takeda M, Matsunari Y, Nishio K, Shima M, Banno F, Akiyama M, Kokame K, Miyata T, Sugimoto M. ADAMTS13 safeguards the myocardium in a mouse model of acute myocardial infarction. Thromb Haemost. 2012;108:1236–1238. doi: 10.1160/TH12-09-0674. [DOI] [PubMed] [Google Scholar]
- 59.Gandhi C, Motto DG, Jensen M, Lentz SR, Chauhan AK. ADAMTS13 deficiency exacerbates VWF-dependent acute myocardial ischemia/reperfusion injury in mice. Blood. 2012;120:5224–5230. doi: 10.1182/blood-2012-06-440255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nishio K, Fujioka M, Hayakawa K, Mishima K, Fujiwara M, Banno F, Kokame K, Miyata T, Shida Y, Sugimoto M, Fukushima H, Okuchi K. ADAMTS13 Gene Deletion Aggravates Ischemic Brain Damage. Blood. 2008;112:102–103. [Google Scholar]
- 61.Plaimauer B, Zimmermann K, Volkel D, Antoine G, Kerschbaumer R, Jenab P, Furlan M, Gerritsen H, Lammle B, Schwarz HP, Scheiflinger F. Cloning, expression, and functional characterization of the von Willebrand factor-cleaving protease (ADAMTS13) Blood. 2002;100:3626–3632. doi: 10.1182/blood-2002-05-1397. [DOI] [PubMed] [Google Scholar]
- 62.Okano E, Ko S, Kanehiro H, Matsumoto M, Fujimura Y, Nakajima Y. ADAMTS13 activity decreases after hepatectomy, reflecting a postoperative liver dysfunction. Hepatogastroenterology. 2010;57:316–320. [PubMed] [Google Scholar]
- 63.Kume Y, Ikeda H, Inoue M, Tejima K, Tomiya T, Nishikawa T, Watanabe N, Ichikawa T, Kaneko M, Okubo S, Yokota H, Omata M, Fujiwara K, Yatomi Y. Hepatic stellate cell damage may lead to decreased plasma ADAMTS13 activity in rats. FEBS Lett. 2007;581:1631–1634. doi: 10.1016/j.febslet.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 64.Watanabe N, Ikeda H, Kume Y, Satoh Y, Kaneko M, Takai D, Tejima K, Nagamine M, Mashima H, Tomiya T, Noiri E, Omata M, Matsumoto M, Fujimura Y, Yatomi Y. Increased production of ADAMTS13 in hepatic stellate cells contributes to enhanced plasma ADAMTS13 activity in rat models of cholestasis and steatohepatitis. Thromb Haemost. 2009;102:389–396. doi: 10.1160/TH08-11-0732. [DOI] [PubMed] [Google Scholar]
- 65.Turner NA, Nolasco L, Ruggeri ZM, Moake JL. Endothelial cell ADAMTS-13 and VWF: production, release, and VWF string cleavage. Blood. 2009;114:5102–5111. doi: 10.1182/blood-2009-07-231597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin SY, Skipwith CG, Shang D, Zheng XL. von Willebrand factor cleaved from endothelial cells by ADAMTS13 remains ultralarge in size. J Thromb Haemost. 2009;7:1749–1752. doi: 10.1111/j.1538-7836.2009.03570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turner N, Nolasco L, Dong JF, Moake J. ADAMTS-13 cleaves long von Willebrand factor multimeric strings anchored to endothelial cells in the absence of flow, platelets or conformation-altering chemicals. J Thromb Haemost. 2009;7:229–232. doi: 10.1111/j.1538-7836.2008.03209.x. [DOI] [PubMed] [Google Scholar]
- 68.Turner N, Nolasco L, Moake J. Generation and breakdown of soluble ultralarge von Willebrand factor multimers. Semin Thromb Hemost. 2012;38:38–46. doi: 10.1055/s-0031-1300950. [DOI] [PubMed] [Google Scholar]
- 69.Lee M, Rodansky ES, Smith JK, Rodgers GM. ADAMTS13 promotes angiogenesis and modulates VEGF-induced angiogenesis. Microvasc Res. 2012;84:109–115. doi: 10.1016/j.mvr.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 70.Liu L, Choi H, Bernardo A, Bergeron AL, Nolasco L, Ruan C, Moake JL, Dong JF. Platelet-derived VWF-cleaving metalloprotease ADAMTS-13. J Thromb Haemost. 2005;3:2536–2544. doi: 10.1111/j.1538-7836.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki M, Murata M, Matsubara Y, Uchida T, Ishihara H, Shibano T, Ashida S, Soejima K, Okada Y, Ikeda Y. Detection of von Willebrand factor-cleaving protease (ADAMTS-13) in human platelets. Biochem Biophys Res Commun. 2004;313:212–216. doi: 10.1016/j.bbrc.2003.11.111. [DOI] [PubMed] [Google Scholar]
- 72.Pickens B, Jin SY, Li D, Zheng XL. Ectopic expression of ADAMTS13 in platelets as a novel therapeutic approach for arterial thrombosis. Blood. 2012;120:491. (abstr.) [Google Scholar]
- 73.Shi Q, Wilcox DA, Fahs SA, Weiler H, Wells CW, Cooley BC, Desai D, Morateck PA, Gorski J, Montgomery RR. Factor VIII ectopically targeted to platelets is therapeutic in hemophilia A with high-titer inhibitory antibodies. J Clin Invest. 2006;116:1974–1982. doi: 10.1172/JCI28416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gewirtz J, Thornton MA, Rauova L, Poncz M. Platelet-delivered factor VIII provides limited resistance to anti-factor VIII inhibitors. J Thromb Haemost. 2008;6:1160–1166. doi: 10.1111/j.1538-7836.2008.02992.x. [DOI] [PubMed] [Google Scholar]
- 75.Zhang G, Shi Q, Fahs SA, Kuether EL, Walsh CE, Montgomery RR. Factor IX ectopically expressed in platelets can be stored in alpha-granules and corrects the phenotype of hemophilia B mice. Blood. 2010;116:1235–1243. doi: 10.1182/blood-2009-11-255612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009;284:31493–31497. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang BL. ADAMTS: a novel family of extracellular matrix proteases. Int J Biochem Cell Biol. 2001;33:33–44. doi: 10.1016/s1357-2725(00)00061-3. [DOI] [PubMed] [Google Scholar]
- 78.Majerus EM, Zheng X, Tuley EA, Sadler JE. Cleavage of the ADAMTS13 propeptide is not required for protease activity. J Biol Chem. 2003;278:46643–46648. doi: 10.1074/jbc.M309872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gardner MD, Chion CK, de Groot, Shah A, Crawley JT, Lane DA. A functional calcium-binding site in the metalloprotease domain of ADAMTS13. Blood. 2009;113:1149–1157. doi: 10.1182/blood-2008-03-144683. [DOI] [PubMed] [Google Scholar]
- 80.Ai J, Smith P, Wang S, Zhang P, Zheng XL. The proximal carboxyl-terminal domains of ADAMTS13 determine substrate specificity and are all required for cleavage of von Willebrand factor. J Biol Chem. 2005;280:29428–29434. doi: 10.1074/jbc.M505513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao W, Anderson PJ, Sadler JE. Extensive contacts between ADAMTS13 exosites and von Willebrand factor domain A2 contribute to substrate specificity. Blood. 2008;112:1713–1719. doi: 10.1182/blood-2008-04-148759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soejima K, Matsumoto M, Kokame K, Yagi H, Ishizashi H, Maeda H, Nozaki C, Miyata T, Fujimura Y, Nakagaki T. ADAMTS-13 cysteine-rich/spacer domains are functionally essential for von Willebrand factor cleavage. Blood. 2003;102:3232–3237. doi: 10.1182/blood-2003-03-0908. [DOI] [PubMed] [Google Scholar]
- 83.Zheng XL, Nishio K, Majerus EM, Sadler JE. Cleavage of von Willebrand factor requires the spacer domain of the metalloprotease ADAMTS13. J Biol Chem. 2003;278:30136–30141. doi: 10.1074/jbc.M305331200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akiyama M, Takeda S, Kokame K, Takagi J, Miyata T. Crystal structures of the noncatalytic domains of ADAMTS13 reveal multiple discontinuous exosites for von Willebrand factor. Proc Natl Acad Sci U S A. 2009;106:19274–19279. doi: 10.1073/pnas.0909755106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gerhardt S, Hassall G, Hawtin P, McCall E, Flavell L, Minshull C, Hargreaves D, Ting A, Pauptit RA, Parker AE, Abbott WM. Crystal structures of human ADAMTS-1 reveal a conserved catalytic domain and a disintegrin-like domain with a fold homologous to cysteine-rich domains. J Mol Biol. 2007;373:891–902. doi: 10.1016/j.jmb.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 86.Takeda S. Three-dimensional domain architecture of the ADAM family proteinases. Semin Cell Dev Biol. 2009;20:146–152. doi: 10.1016/j.semcdb.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 87.de Groot R, Bardhan A, Ramroop N, Lane DA, Crawley JT. Essential role of the disintegrin-like domain in ADAMTS13 function. Blood. 2009;113:5609–5616. doi: 10.1182/blood-2008-11-187914. [DOI] [PubMed] [Google Scholar]
- 88.Gao W, Anderson PJ, Majerus EM, Tuley EA, Sadler JE. Exosite interactions contribute to tension-induced cleavage of von Willebrand factor by the antithrombotic ADAMTS13 metalloprotease. Proc Natl Acad Sci U S A. 2006;103 doi: 10.1073/pnas.0607264104. 19099-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Groot R, Bardhan A, Ramroop N, Lane DA, Crawley JT. Essential role of the disintegrin-like domain in ADAMTS13 function. Blood. 2009;113:5609–5616. doi: 10.1182/blood-2008-11-187914. [DOI] [PubMed] [Google Scholar]
- 90.Xiang Y, de Groot R, Crawley JT, Lane DA. Mechanism of von Willebrand factor scissile bond cleavage by a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) Proc Natl Acad Sci U S A. 2011;108:11602–11607. doi: 10.1073/pnas.1018559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou W, Dong L, Ginsburg D, Bouhassira EE, Tsai HM. Enzymatically active ADAMTS13 variants are not inhibited by anti-ADAMTS13 autoantibodies: a novel therapeutic strategy? J Biol Chem. 2005;280:39934–39941. doi: 10.1074/jbc.M504919200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Maeyer B, De Meyer SF, Feys HB, Pareyn I, Vandeputte N, Deckmyn H, Vanhoorelbeke K. The distal carboxyterminal domains of murine ADAMTS13 influence proteolysis of platelet-decorated VWF strings in vivo. J Thromb Haemost. 2010;8:2305–2312. doi: 10.1111/j.1538-7836.2010.04008.x. [DOI] [PubMed] [Google Scholar]
- 93.Gao W, Zhu J, Westfield LA, Tuley EA, Anderson PJ, Sadler JE. Rearranging exosites in noncatalytic domains can redirect the substrate specificity of ADAMTS proteases. J Biol Chem. 2012;287:26944–26952. doi: 10.1074/jbc.M112.380535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jin SY, Skipwith CG, Zheng XL. Amino acid residues Arg(659), Arg(660), and Tyr(661) in the spacer domain of ADAMTS13 are critical for cleavage of von Willebrand factor. Blood. 2010;115:2300–2310. doi: 10.1182/blood-2009-07-235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pos W, Crawley JT, Fijnheer R, Voorberg J, Lane DA, Luken BM. An autoantibody epitope comprising residues R660, Y661, and Y665 in the ADAMTS13 spacer domain identifies a binding site for the A2 domain of VWF. Blood. 2010;115:1640–1649. doi: 10.1182/blood-2009-06-229203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Turner SL, Blair-Zajdel ME, Bunning RA. ADAMs and ADAMTSs in cancer. Br J Biomed Sci. 2009;66:117–128. doi: 10.1080/09674845.2009.11730257. [DOI] [PubMed] [Google Scholar]
- 97.Jones GC, Riley GP. ADAMTS proteinases: a multi-domain, multi-functional family with roles in extracellular matrix turnover and arthritis. Arthritis Res Ther. 2005;7:160–169. doi: 10.1186/ar1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hofsteenge J, Huwiler KG, Macek B, Hess D, Lawler J, Mosher DF, Peter-Katalinic J. C-mannosylation and O-fucosylation of the thrombospondin type 1 module. J Biol Chem. 2001;276:6485–6498. doi: 10.1074/jbc.M008073200. [DOI] [PubMed] [Google Scholar]
- 99.Zanardelli S, Chion AC, Groot E, Lenting PJ, McKinnon TA, Laffan MA, Tseng M, Lane DA. A novel binding site for ADAMTS13 constitutively exposed on the surface of globular VWF. Blood. 2009;114:2819–2828. doi: 10.1182/blood-2009-05-224915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Asch AS, Tepler J, Silbiger S, Nachman RL. Cellular attachment to thrombospondin. Cooperative interactions between receptor systems. J Biol Chem. 1991;266:1740–1745. [PubMed] [Google Scholar]
- 101.Asch AS, Silbiger S, Heimer E, Nachman RL. Thrombospondin sequence motif (CSVTCG) is responsible for CD36 binding. Biochem Biophys Res Commun. 1992;182:1208–1217. doi: 10.1016/0006-291x(92)91860-s. [DOI] [PubMed] [Google Scholar]
- 102.Vomund AN, Majerus EM. ADAMTS13 bound to endothelial cells exhibits enhanced cleavage of von Willebrand factor. J Biol Chem. 2009;284:30925–30932. doi: 10.1074/jbc.M109.000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tao Z, Wang Y, Choi H, Bernardo A, Nishio K, Sadler JE, Lopez JA, Dong JF. Cleavage of ultralarge multimers of von Willebrand factor by C-terminal-truncated mutants of ADAMTS-13 under flow. Blood. 2005;106:141–143. doi: 10.1182/blood-2004-11-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xiao J, Zheng XL. Carboxyl-Terminal Thrombospondin-Type 1 (TSP-1) Repeats of ADAMTS13 Inhibits Platelet Adhesion to Collagen-Coated Surface Under Shear Stress, Independent of Proteolytic Activity. Blood. 2011;118:195. (abstr.) [Google Scholar]
- 105.Yeh HC, Zhou Z, Choi H, Tekeoglu S, May W, III, Wang C, Turner N, Scheiflinger F, Moake JL, Dong JF. Disulfide bond reduction of von Willebrand factor by ADAMTS-13. J Thromb Haemost. 2010;8:2778–2788. doi: 10.1111/j.1538-7836.2010.04094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tao Z, Peng Y, Nolasco L, Cal S, Lopez-Otin C, Li R, Moake JL, Lopez JA, Dong JF. Role of the CUB-1 domain in docking ADAMTS-13 to unusually large Von Willebrand factor in flowing blood. Blood. 2005;106:4139–4145. doi: 10.1182/blood-2005-05-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rosing J, Tans G, Govers-Riemslag JW, Zwaal RF, Hemker HC. The role of phospholipids and factor Va in the prothrombinase complex. J Biol Chem. 1980;255:274–283. [PubMed] [Google Scholar]
- 108.Tans G, Govers-Riemslag JW, van Rijn JL, Rosing J. Purification and properties of a prothrombin activator from the venom of Notechis scutatus scutatus. J Biol Chem. 1985;260:9366–9372. [PubMed] [Google Scholar]
- 109.Loechel F, Gilpin BJ, Engvall E, Albrechtsen R, Wewer UM. Human ADAM 12 (meltrin alpha) is an active metalloprotease. J Biol Chem. 1998;273:16993–16997. doi: 10.1074/jbc.273.27.16993. [DOI] [PubMed] [Google Scholar]
- 110.Somerville RP, Longpre JM, Apel ED, Lewis RM, Wang LW, Sanes JR, Leduc R, Apte SS. ADAMTS7B, the full-length product of the ADAMTS7 gene, is a chondroitin sulfate proteoglycan containing a mucin domain. J Biol Chem. 2004;279:35159–35175. doi: 10.1074/jbc.M402380200. [DOI] [PubMed] [Google Scholar]
- 111.Tortorella MD, Arner EC, Hills R, Easton A, Korte-Sarfaty J, Fok K, Wittwer AJ, Liu RQ, Malfait AM. Alpha2-macroglobulin is a novel substrate for ADAMTS-4 and ADAMTS-5 and represents an endogenous inhibitor of these enzymes. J Biol Chem. 2004;279:17554–17561. doi: 10.1074/jbc.M313041200. [DOI] [PubMed] [Google Scholar]
- 112.Luan Y, Kong L, Howell DR, Ilalov K, Fajardo M, Bai XH, Di Cesare PE, Goldring MB, Abramson SB, Liu CJ. Inhibition of ADAMTS-7 and ADAMTS-12 degradation of cartilage oligomeric matrix protein by alpha-2-macroglobulin. Osteoarthritis Cartilage. 2008;16:1413–1420. doi: 10.1016/j.joca.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang P, Pan W, Rux AH, Sachais BS, Zheng XL. The cooperative activity between the carboxyl-terminal TSP-1 repeats and the CUB domains of ADAMTS13 is crucial for recognition of von Willebrand factor under flow. Blood. 2007;110:1887–1894. doi: 10.1182/blood-2007-04-083329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pareti FI, Lattuada A, Bressi C, Zanobini M, Sala A, Steffan A, Ruggeri ZM. Proteolysis of von Willebrand factor and shear stress-induced platelet aggregation in patients with aortic valve stenosis. Circulation. 2000;102:1290–1295. doi: 10.1161/01.cir.102.11.1290. [DOI] [PubMed] [Google Scholar]
- 115.Blackshear JL, Wysokinska EM, Safford RE, Thomas CS, Stark ME, Shapiro BP, Ung S, Johns GS, Chen D. Indexes of von Willebrand Factor as Biomarkers of Aortic Stenosis Severity (from the Biomarkers of Aortic Stenosis Severity [BASS] Study) Am J Cardiol. 2013;111:374–381. doi: 10.1016/j.amjcard.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yoshida K, Tobe S, Kawata M. Acquired von Willebrand disease type IIA in patients with aortic valve stenosis. Ann Thorac Surg. 2006;81:1114–1116. doi: 10.1016/j.athoracsur.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 117.Shim K, Anderson PJ, Tuley EA, Wiswall E, Sadler JE. Platelet-VWF complexes are preferred substrates of ADAMTS13 under fluid shear stress. Blood. 2007;111:651–657. doi: 10.1182/blood-2007-05-093021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Skipwith CG, Cao W, Zheng XL. Factor VIII and platelets synergistically accelerate cleavage of von Willebrand factor by ADAMTS13 under fluid shear stress. J Biol Chem. 2010;285:28596–28603. doi: 10.1074/jbc.M110.131227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cao WJ, Krishnaswamy S, Camire RM, Lenting PJ, Zheng XL. Factor VIII accelerates proteolytic cleavage of von Willebrand factor by ADAMTS13. Proc Natl Acad Sci U S A. 2008;105:7416–7421. doi: 10.1073/pnas.0801735105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li M, Ku DN, Forest CR. Microfluidic system for simultaneous optical measurement of platelet aggregation at multiple shear rates in whole blood. Lab Chip. 2012;12:1355–1362. doi: 10.1039/c2lc21145a. [DOI] [PubMed] [Google Scholar]
- 121.Colace T, Falls E, Zheng XL, Diamond SL. Analysis of morphology of platelet aggregates formed on collagen under laminar blood flow. Ann Biomed Eng. 2011;39:922–929. doi: 10.1007/s10439-010-0182-4. [DOI] [PubMed] [Google Scholar]
- 122.Gutierrez E, Petrich BG, Shattil SJ, Ginsberg MH, Groisman A, Kasirer-Friede A. Microfluidic devices for studies of shear-dependent platelet adhesion. Lab Chip. 2008;8:1486–1495. doi: 10.1039/b804795b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang Q, Zhou YF, Zhang CZ, Zhang X, Lu C, Springer TA. Structural specializations of A2, a force-sensing domain in the ultralarge vascular protein von Willebrand factor. Proc Natl Acad Sci U S A. 2009;106:9226–9231. doi: 10.1073/pnas.0903679106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang X, Halvorsen K, Zhang CZ, Wong WP, Springer TA. Mechanoenzymatic cleavage of the ultralarge vascular protein von Willebrand factor. Science. 2009;324:1330–1334. doi: 10.1126/science.1170905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cao W, Sabatino DE, Altynova E, Lange AM, Casina VC, Camire RM, Zheng XL. Light Chain of Factor VIII Is Sufficient for Accelerating Cleavage of von Willebrand Factor by ADAMTS13 Metalloprotease. J Biol Chem. 2012;287:32459–32466. doi: 10.1074/jbc.M112.390690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Skipwith C, Haberichter S, Cao WJ, Gehrand AL, Zheng XL. compromised proteolytic cleavage of von Willebrand factor type 2N variants by ADAMTS13 in the presence of factor VIII (and platelets) under shear stress. Blood. 2009;114:28. (abstr.) [Google Scholar]
- 127.Nishio K, Anderson PJ, Zheng XL, Sadler JE. Binding of platelet glycoprotein Ibalpha to von Willebrand factor domain A1 stimulates the cleavage of the adjacent domain A2 by ADAMTS13. Proc Natl Acad Sci U S A. 2004;101:10578–10583. doi: 10.1073/pnas.0402041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen J, Ling M, Fu X, Lopez JA, Chung DW. Simultaneous exposure of sites in von Willebrand factor for glycoprotein Ib binding and ADAMTS13 cleavage: studies with ristocetin. Arterioscler Thromb Vasc Biol. 2012;32:2625–2630. doi: 10.1161/ATVBAHA.112.254144. [DOI] [PubMed] [Google Scholar]
- 129.Shankaran H, Neelamegham S. Hydrodynamic forces applied on intercellular bonds, soluble molecules, and cell-surface receptors. Biophys J. 2004;86:576–588. doi: 10.1016/S0006-3495(04)74136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tsai HM, Raoufi M, Zhou W, Guinto E, Grafos N, Ranzurmal S, Greenfield RS, Rand JH. ADAMTS13-binding IgG are present in patients with thrombotic thrombocytopenic purpura. Thromb Haemost. 2006;95:886–892. [PMC free article] [PubMed] [Google Scholar]
- 131.Rieger M, Mannucci PM, Kremer Hovinga JA, Herzog A, Gerstenbauer G, Konetschny C, Zimmermann K, Scharrer I, Peyvandi F, Galbusera M, Remuzzi G, Bohm M, Plaimauer B, Lammle B, Scheiflinger F. ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood. 2005;106:1262–1267. doi: 10.1182/blood-2004-11-4490. [DOI] [PubMed] [Google Scholar]
- 132.Tsai HM, Raoufi M, Zhou W, Guinto E, Grafos N, Ranzurmal S, Greenfield RS, Rand JH. ADAMTS13-binding IgG are present in patients with thrombotic thrombocytopenic purpura. Thromb Haemost. 2006;95:886–892. [PMC free article] [PubMed] [Google Scholar]
- 133.Li D, Xiao J, Paessler M, Zheng XL. Novel recombinant glycosylphosphatidylinositol (GPI)-anchored ADAMTS13 and variants for assessment of anti-ADAMTS13 autoantibodies in patients with thrombotic thrombocytopenic purpura. Thromb Haemost. 2011;106:947–958. doi: 10.1160/TH11-05-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ferrari S, Mudde GC, Rieger M, Veyradier A, Kremer Hovinga JA, Scheiflinger F. IgG subclass distribution of anti-ADAMTS13 antibodies in patients with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2009;7:1703–1710. doi: 10.1111/j.1538-7836.2009.03568.x. [DOI] [PubMed] [Google Scholar]
- 135.Ferrari S, Scheiflinger F, Rieger M, Mudde G, Wolf M, Coppo P, Girma JP, Azoulay E, Brun-Buisson C, Fakhouri F, Mira JP, Oksenhendler E, Poullin P, Rondeau E, Schleinitz N, Schlemmer B, Teboul JL, Vanhille P, Vernant JP, Meyer D, Veyradier A. Prognostic value of anti-ADAMTS 13 antibody features (Ig isotype, titer, and inhibitory effect) in a cohort of 35 adult French patients undergoing a first episode of thrombotic microangiopathy with undetectable ADAMTS 13 activity. Blood. 2007;109:2815–2822. doi: 10.1182/blood-2006-02-006064. [DOI] [PubMed] [Google Scholar]
- 136.Klaus C, Plaimauer B, Studt JD, Dorner F, Lammle B, Mannucci PM, Scheiflinger F. Epitope mapping of ADAMTS13 autoantibodies in acquired thrombotic thrombocytopenic purpura. Blood. 2004;103:4514–4519. doi: 10.1182/blood-2003-12-4165. [DOI] [PubMed] [Google Scholar]
- 137.Luken BM, Turenhout EA, Hulstein JJ, Van Mourik JA, Fijnheer R, Voorberg J. The spacer domain of ADAMTS13 contains a major binding site for antibodies in patients with thrombotic thrombocytopenic purpura. Thromb Haemost. 2005;93:267–274. doi: 10.1160/TH04-05-0301. [DOI] [PubMed] [Google Scholar]
- 138.Luken BM, Kaijen PH, Turenhout EA, Kremer Hovinga JA, Van Mourik JA, Fijnheer R, Voorberg J. Multiple B-cell clones producing antibodies directed to the spacer and disintegrin/thrombospondin type-1 repeat 1 (TSP1) of ADAMTS13 in a patient with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2006;4:2355–2364. doi: 10.1111/j.1538-7836.2006.02164.x. [DOI] [PubMed] [Google Scholar]
- 139.Luken BM, Turenhout EA, Kaijen PH, Greuter MJ, Pos W, Van Mourik JA, Fijnheer R, Voorberg J. Amino acid regions 572–579 and 657–666 of the spacer domain of ADAMTS13 provide a common antigenic core required for binding of antibodies in patients with acquired TTP. Thromb Haemost. 2006;96:295–301. doi: 10.1160/TH06-03-0135. [DOI] [PubMed] [Google Scholar]
- 140.Pos W, Sorvillo N, Fijnheer R, Feys HB, Kaijen PH, Vidarsson G, Voorberg J. Residues Arg568 and Phe592 contribute to an antigenic surface for anti-ADAMTS13 antibodies in the spacer domain. Haematologica. 2011;96:1670–1677. doi: 10.3324/haematol.2010.036327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yamaguchi Y, Moriki T, Igari A, Nakagawa T, Wada H, Matsumoto M, Fujimura Y, Murata M. Epitope analysis of autoantibodies to ADAMTS13 in patients with acquired thrombotic thrombocytopenic purpura. Thromb Res. 2011;128:169–173. doi: 10.1016/j.thromres.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 142.Studt JD, Kremer Hovinga JA, Radonic R, Gasparovic V, Ivanovic D, Merkler M, Wirthmueller U, Dahinden C, Furlan M, Lammle B. Familial acquired thrombotic thrombocytopenic purpura: ADAMTS-13 inhibitory autoantibodies in identical twins. Blood. 2004;103:4195–4197. doi: 10.1182/blood-2003-11-3888. [DOI] [PubMed] [Google Scholar]
- 143.Scully M, Brown J, Patel R, McDonald V, Brown CJ, Machin S. Human leukocyte antigen association in idiopathic thrombotic thrombocytopenic purpura: evidence for an immunogenetic link. J Thromb Haemost. 2010;8:257–262. doi: 10.1111/j.1538-7836.2009.03692.x. [DOI] [PubMed] [Google Scholar]
- 144.Sorvillo N, Pos W, van den Berg LM, Fijnheer R, Martinez-Pomares L, Geijtenbeek TB, Herczenik E, Voorberg J. The macrophage mannose receptor promotes uptake of ADAMTS13 by dendritic cells. Blood. 2012;119:3828–3835. doi: 10.1182/blood-2011-09-377754. [DOI] [PubMed] [Google Scholar]
- 145.Zheng XL. ADAMTS13 meets MR, then what? Blood. 2012;119:3652–3654. doi: 10.1182/blood-2012-02-410449. [DOI] [PubMed] [Google Scholar]
- 146.Sorvillo N, van Haren SD, Kaijen PH, Ten BA, Fijnheer R, Meijer AB, Voorberg J. Preferential HLA-DRB1*11 dependent presentation of CUB2 derived peptides by ADAMTS13 pulsed dendritic cells. Blood. 2013 doi: 10.1182/blood-2012-09-456780. [DOI] [PubMed] [Google Scholar]
- 147.Fujimura Y, Matsumoto M. Registry of 919 patients with thrombotic microangiopathies across Japan: database of Nara Medical University during 1998–2008. Intern Med. 2010;49:7–15. doi: 10.2169/internalmedicine.49.2706. [DOI] [PubMed] [Google Scholar]
- 148.Furlan M. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and hemolytic-uremic syndrome. Adv Nephrol Necker Hosp. 2000;30:71–81. [PubMed] [Google Scholar]
- 149.Pos W, Luken BM, Sorvillo N, Hovinga JA, Voorberg J. Humoral immune response to ADAMTS13 in acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2011;9:1285–1291. doi: 10.1111/j.1538-7836.2011.04307.x. [DOI] [PubMed] [Google Scholar]
- 150.Jian C, Xiao J, Gong L, Skipwith CG, Jin SY, Kwaan HC, Zheng XL. Gain-of-function ADAMTS13 variants that are resistant to autoantibodies against ADAMTS13 in patients with acquired thrombotic thrombocytopenic purpura. Blood. 2012;119:3836–3843. doi: 10.1182/blood-2011-12-399501. [DOI] [PMC free article] [PubMed] [Google Scholar]