Summary
The protein C pathway provides multiple important functions to maintain a regulated balance between haemostasis and host defence systems in response to vascular and inflammatory injury. The anticoagulant protein C pathway is designed to regulate coagulation, maintain the fluidity of blood within the vasculature, and prevent thrombosis, whereas the cytoprotective protein C pathway prevents vascular damage and stress. The cytoprotective activities of activated protein C (APC) include anti-apoptotic activity, anti-inflammatory activity, beneficial alterations of gene expression profiles, and endothelial barrier stabilisation. These cytoprotective activities of APC, which require the endothelial protein C receptor (EPCR) and protease-activated receptor-1 (PAR1), have been a major research focus. Recent insights, such as non-canonical activation of PAR1 at Arg46 by APC and biased PAR1 signalling, provided better understanding of the molecular mechanisms by which APC elicits cytoprotective signalling through cleavage of PAR1. The discovery and development of anticoagulant-selective and cytoprotective-selective APC mutants provided unique opportunities for preclinical research that has been and may continue to be translated to clinical research. New mechanisms for the regulation of EPCR functionality, such as modulation of EPCR-bound lipids that affect APC’s cytoprotective activities, may provide new research directions to improve the efficacy of APC to convey its cytoprotective activities to cells. Moreover, emerging novel functions for EPCR expand the roles of EPCR beyond mediating protein C activation and APC-induced PAR1 cleavage. These discoveries increasingly develop our understanding of the protein C pathway, which will conceivably expand its physiological implications to many areas in the future.
Keywords: Protein C, Endothelial Protein C Receptor, Protease-Activated Receptor 1, Vascular Endothelium, Blood Coagulation Factors
The protein C pathway
The protein C pathway provides multiple important functions that regulate both haemostasis and host defence systems in response to vascular and inflammatory injury, respectively. The anticoagulant protein C pathway is designed to regulate coagulation, maintain the fluidity of blood within the vasculature, and prevent thrombosis, whereas the cytoprotective protein C pathway provides anti-inflammatory and cytoprotective activities to prevent vascular damage and stress [1-5]. Through its different biologic activities, activated protein C (APC) and the protein C pathway components have important implications for translational research and potential treatment strategies in medical disorders such as thrombosis, inflammation, ischaemic stroke and neurodegenerative disease [6-8]. Regulation of APC’s biologic activities involves interactions with cofactors that facilitate the assembly of APC in macromolecular complexes for efficient proteolysis of different substrates that produce APC’s numerous physiologic effects. Studies of APC’s structure-function relationships have provided insights into the components, molecular mechanisms and presumed assembly of these macromolecular complexes [1, 3, 5, 9]. From these and other studies, the endothelial protein C receptor (EPCR) emerges as a key receptor that regulates APC’s diverse activities. In addition to the discussion of the latest insights into the molecular mechanisms of the anticoagulant and cytoprotective protein C pathway, this review will also focus on the emerging novel functions of EPCR and the regulation of its biological function.
The protein C pathway at the crossroads of coagulation and inflammation
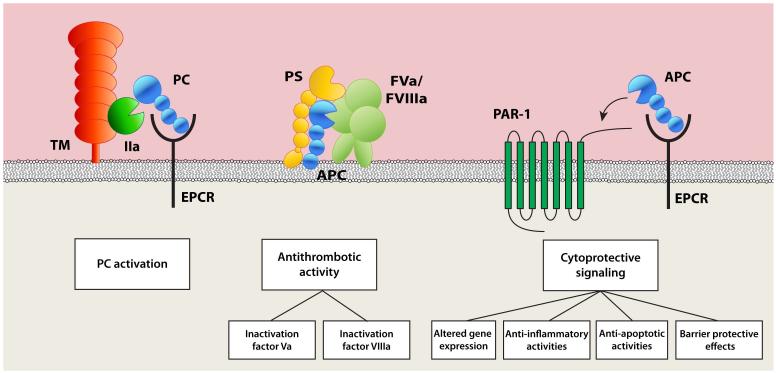
Physiological proteolytic activation of protein C by thrombin occurs on the surface of endothelial cells and involves two membrane receptors, thrombomodulin, and EPCR [10, 11]. Binding of thrombin to thrombomodulin shields thrombin’s procoagulant exosite I and promotes activation of protein C. This reaction is augmented by localisation of protein C on the endothelial surface by binding to EPCR (Fig 1) [12]. As an anticoagulant enzyme, APC proteolytically inactivates factors Va (FVa) and VIIIa (FVIIIa) with enhancements from various lipid and protein cofactors (Fig 1). In addition to facilitating activation of protein C by the thrombin-thrombomodulin complex, EPCR also mediates effects of APC directly on cells. APC associated with EPCR conveys cytoprotective signalling events through cleavage of protease-activated receptor-1 (PAR1) (Fig 1) [13, 14]. EPCR-dependent cytoprotective effects of APC include anti-inflammatory and anti-apoptotic activities, alterations of gene expression profiles, and protection of the endothelial barrier function (Fig 1) [1]. Thus, EPCR operates at the crossroads of the protein C anticoagulant and APC cytoprotective pathways. The importance of the protein C pathway is best illustrated by the pro-thrombotic and pro-inflammatory complications associated with protein C deficiency and has been extensively demonstrated in animal models for ischaemic stroke, inflammatory disease, atherosclerosis, and vascular disease [6-8]. While APC is best known for its anticoagulant activity, the potential therapeutic application of APC in these diseases mostly relies on its cytoprotective properties mediated via EPCR.
Figure 1. The components and reactions of the protein C pathway.
The three major reactions of the protein C pathway, depicted from left to right, are protein C activation, the anticoagulant protein C pathway, and the cytoprotective protein C pathway. (left) Protein C activation. Physiological activation of protein C (PC) by the thrombin (IIa)-thrombomodulin (TM) complex on the surface of endothelial cells is facilitated by EPCR. (middle) The anticoagulant protein C pathway. APC exerts its anticoagulant activities by proteolytic inactivation of FVa and FVIIIa aided by PS on negatively charged phospholipid membranes. (right) The cytoprotective protein C pathway. APC associated with EPCR cleaves PAR1 to initiate cell signalling with cytoprotective effects that may include anti-inflammatory and anti-apoptotic activities, altered gene expression profiles, and barrier protective effects.
The anticoagulant protein C pathway
The APC-mediated inactivation of FVa and FVIIIa on negatively charged phospholipid membranes is assisted by cofactors protein S (PS) and FV [15, 16]. Since FVa enhances prothrombinase ~10,000-fold, inactivation of FVa by APC effectively shuts down thrombin formation. Although cleavage of FVa at Arg306 by APC results in a complete loss of function, cleavage at Arg506 is kinetically favoured but results only in partial inactivation of FVa [15-17]. Molecular mechanisms for enhanced APC-mediated cleavage of FVa by PS have been partially elucidated and involve a PS-induced change in the geometry of APC relative to the membrane [18]. Limited structural information is available and thus the putative spatial orientation of the APC-PS-FVa complex remains highly speculative. Nevertheless, complex assembly involves interactions of an extended basic exosite on the protease domain of APC with FVa and multiple reciprocal interactions of APC with PS, and PS with FVa [1, 3, 5].
Homologous to FV, FVIIIa is an essential cofactor for the tenase complex and enhances factor Xa (FXa) formation approximately 200,000-fold [19]. However, the physiological relevance of FVIIIa inactivation by APC was questioned due to the rapid spontaneous dissociation of the A2-domain, resulting in a FVIIIa half-life of only 2 min [20]. Nevertheless, several observations, including stabilisation of FVIIIa by FIXa in the tenase complex, support a role for APC in the inactivation of FVIIIa [20-22]. Homologous to inactivation of FVa, inactivation of FVIIIa by APC occurs upon cleavage at Arg336 and Arg562. In contrast to FVa, cleavage of FVIIIa at either site results in a complete loss of cofactor activity. Both PS and FV but not FVa provide additional enhancement of APC-mediated inactivation of FVIIIa [21-23].
The cytoprotective protein C pathway
Since the initial conceptualisation of this pathway a decade ago, when APC was shown to inhibit apoptosis and activate PAR1 in the presence of EPCR, the basic paradigm, although refined, has remained much the same [13, 24]. APC-mediated cytoprotective signalling requires co-localisation of PAR1 and EPCR in caveolin-1 enriched lipid rafts or caveolae, possibly induced by occupancy of EPCR, and is initiated when APC bound to EPCR activates PAR1 [1, 25, 26]. APC’s cytoprotective activities generally counteract cellular injury, promote cell survival and, dependent on the cell type and injury, may include anti-apoptotic and anti-inflammatory activities, alteration of gene expression profiles and protection of endothelial barrier function. In addition to the many studies showing that PAR1 and EPCR are required for APC’s protective effects on cells, other receptors such as sphingosine-1-phosphate receptor 1 (S1P1), apolipoprotein E receptor 2 (ApoER2), glycoprotein Ib, CD11b/CD18 (αMβ2; Mac-1; CR3), PAR-3, and Tie2 may independently or cooperatively contribute to APC-initiated signalling on endothelial and other cells [27-29]. Thus, different cells may employ different receptor ensembles to convey the various aspects of APC’s cytoprotective cellular effects. APC’s cytoprotective effects involving proteolytic inactivation of extracellular histones also protect against sepsis in mice but presumably are independent from APC’s cell signalling effects [30].
Since the anticoagulant and cytoprotective activities of APC differ both in substrates (FVa and FVIIIa vs. PAR1) and cofactors (phospholipids/PS vs. EPCR), engineering approaches of APC exploiting these differences have provided anticoagulant-selective and cytoprotective-selective APC mutants. These activity-selective APC mutants have provided valuable tools that have provided important insights into the relative contributions of APC’s distinct functions towards the beneficial effects of APC in various murine injury and disease models. To date, cytoprotective-selective APC mutations have targeted the interaction with PS (e.g. L38D) or with FVa (e.g. R229A/R230A, K191A/K192A/K193A (a.k.a. 3K3A-APC or combined a.k.a. 5A-APC) and R222C/D237C stabilising the 70-80 loop) [1, 31-34]. Vice versa, anticoagulant-selective APC mutations have targeted EPCR binding (e.g. L8V) and an exosite on APC (e.g. E330A and E333A) that is required for interactions with PAR1 [35, 36]. In addition, an enigmatic anticoagulant-selective E149A-APC, which lacks cytoprotective activities but cleaves PAR1 like wild-type APC, provides a challenging test of our current understanding of the cytoprotective protein C pathway as the molecular basis for its defective cytoprotective activities remains unsolved to this date [37]. Generally consistent with the concept that APC’s cytoprotective activities protect cells and APC’s anticoagulant activities prevent occlusive thrombosis, cytoprotective-selective but not anticoagulant-selective APC mutants provide beneficial effects in models of inflammation, sepsis, and ischaemic stroke, whereas anticoagulant-selective but not cytoprotective-selective APC mutants prevent thrombosis [7, 31, 37]. Strikingly however, in a model of death due to total body radiation anticoagulant-selective E149A-APC reduced death but the cytoprotective-selective 5A-APC did not [38].
In 2001, the PROWESS trial reported that recombinant APC (Xigris) reduced 28-day all-cause mortality in adult severe sepsis patients [39]. However, 10 years later, the repeat PROWESS-SHOCK trial did not reproduce the beneficial effects of Xigris, resulting in its market withdrawal in late 2011 [40]. These conflicting results raise many questions about the individual trials, their comparison, and improvements in standards of care for sepsis patients. It is important to realise that the PROWESS and PROWESS-SHOCK clinical trials employed a 96-h long, low dose infusion regimen, which was based on the assumption that APC’s anticoagulant activity was responsible for its beneficial effects. From multiple in vivo studies, it now appears that the cytoprotective activities of APC are most important for mortality reduction in sepsis and neuroprotection for stroke [7, 31, 37]. Furthermore, the 96-h low dose infusion regimen was associated with serious bleeding risk [39, 40]. In the future, cytoprotective-selective APC mutants with greatly reduced risk of bleeding should permit trials with altered dosing regimens, consistent with APC’s ability to alter cell signalling and provide multiple cytoprotective activities.
The PAR1 paradox
One of the reasons that PAR1-mediated cytoprotective signalling by APC initially drew considerable scepticism was based on the major question of how APC could induce relevant PAR1-mediated signalling when thrombin, the archetypal PAR1 activator, activates PAR1 so much more efficiently. Not only does thrombin induce PAR1-dependent proinflammatory and endothelial barrier disruptive effects that contrast APC’s cytoprotective and endothelial barrier protective effects, the requirement for thrombin to activate protein C seemed incompatible with a role for PAR1-mediated cytoprotective effects of endogenously generated APC [14, 41-43]. Finding satisfactory answers for this paradox turned out to be quite a challenge and numerous different mechanisms have been suggested over the past few years. Some insights came from demonstrations that membrane localisation of PAR1 with EPCR in caveolae, or caveolin-1 rich microdomains, was required for selective PAR1 signalling by APC [44, 45].
Other suggested explanations for selective PAR1 signalling by APC involved PAR1-dependent transactivation of S1P1, and Tie2 [42, 43, 46]. Phenotypic resemblance of the barrier protective aspects of APC and sphingosine 1-phosphate (S1P)-mediated signalling led to investigations of the role of S1P1 in the cytoprotective signalling pathway of APC [42, 43]. Indeed, APC induces an interaction between EPCR and S1P1, and S1P1 is required for APC-mediated protection against thrombin-induced endothelial permeability. Activation of sphingosine kinase-1 (SK1) induced by APC, increases the intracellular levels of S1P, and provides the ligand for S1P1 activation [43]. Collectively, these observations are suggestive of transactivation of S1P1 by the APC-EPCR-PAR1 pathway that may involve S1P1-mediated activation of PI3 kinase and Akt signalling to mediate APC’s barrier-protective effects [42, 43]. In contrast, PAR1 activation by thrombin does not result in S1P1 activation [42]. APC was also found to modulate the angiopoietin (Ang)/Tie2 axis with potential implications for S1P1-induced signalling by APC. Activation of the endothelial Tie2 receptor is regulated by a dynamic balance between dummy ligand Ang2 that keeps the receptor quiescent and agonist ligand Ang1. The Ang/Tie2 axis is critical for remodelling, maturation, and stability of developing vasculature [47] and stimulation of Tie2 by Ang1 results in barrier protection [46]. APC enhances Tie2 expression and shifts the Ang1/Ang2 balance by stimulating Ang1 while inhibiting Ang2 expression in an EPCR- and PAR1-dependent manner [46, 48]. Consistent with APC’s effects on SK1 activation, Ang1 induces barrier protection by activation of SK1, suggesting that APC’s effects on the Ang/Tie2 axis and S1P1 may be more intimately linked [49]. Contributions of other PAR receptors and modulation of PAR1 signalling via formation of heterodimers provides additional selectivity between APC and thrombin mediated PAR1 signalling. Thus far, PAR1 and three other family members, PAR2, PAR3 and PAR4 have been identified and are activated on many different cell types by various proteases with a broad spectrum of biologic activities [14, 50]. APC can cleave PAR2 on cytokine-stimulated endothelial cells. However, no PAR2-dependent effects of APC on endothelial cells have been reported, although PAR2 was implicated in mediating APC effects on other cell types such as epithelial cells [51]. Cleavage of PAR2 by APC fails to induce specific gene expression responses and does not alter the PAR1-dependency of APC signalling [52]. Furthermore, contrary to PAR1 activation by thrombin, activation by APC does not lead to PAR2 transactivation [52]. Although thrombin cannot cleave PAR2, thrombin-cleaved PAR1 supports formation of PAR1-PAR2 heterodimer resulting in transactivation of PAR2 and explaining why some thrombin responses require both PAR1 and PAR2 [53, 54]. The intriguing effects of PAR2 transactivation by PAR1 were shown in a study where early PAR1 activation had deleterious effects but late PAR1 signalling dependent on PAR2 was beneficial and culminated in barrier stabilisation [55].
PAR3 also contributes to APC’s cytoprotective effects in the brain, particularly on neurons, in the kidney, and on podocytes [56-58]. PAR3 is considered a non-signalling receptor, and is therefore the least studied member of the PAR family. Little is known about PAR3 functions in the APC cytoprotective pathway but neuroprotection by APC in murine brain injury models required PAR1, EPCR and PAR3 [56]. Heterodimerisation of PAR3 with PAR1 in murine podocytes or PAR2 in human podocytes that lack PAR1 seems required for APC’s cytoprotective effects [57]. These observations suggest modulation of APC-mediated signalling by PAR3, consistent with modulation of thrombin-mediated PAR1 signalling by PAR3 due to PAR1-PAR3 heterodimer formation that enhances barrier disruptive effects of thrombin [59].
Biased signalling of PAR1, a G protein-coupled receptor (GPCR)
The potential contributions of S1P1, the Ang/Tie2 axis and PAR2/PAR3 transactivation to APC’s cytoprotective effect fail to explain why PAR1 activation by APC is fundamentally different from activation by thrombin. The selective and opposite nature of thrombin and APC for PAR1-dependent effects on endothelial barrier function is striking. Thrombin induces activation of Ras homolog gene family member A (RhoA) that promotes endothelial barrier leakage, whereas APC induces activation of Ras-related C3 botulinum toxin substrate 1 (Rac1) that promotes barrier stabilisation [41, 42, 60]. A major piece of the puzzle to explain the PAR1 paradox was found by Trejo and colleagues, who demonstrated that RhoA activation by thrombin requires G protein dependent signalling, whereas Rac1 activation by APC requires β-arrestin 2-dependent signalling [61]. Thus, not only are the functional effects of PAR1 signalling by APC and thrombin different but also the PAR1 signalling itself is qualitatively different. Insights gleaned from mechanisms regulating GPCR signalling suggest that β-arrestin 2-dependent signalling is characteristic of biased signalling, thus implying that PAR1 can induce biased signalling. According to the emerging biased signalling paradigm, GPCRs exist in dynamic conformational ensembles with each conformational subset linked to highly selective signalling outcomes (e.g., coupling to particular G proteins to initiate G protein dependent signalling or initiating β-arrestin 2-dependent signalling) [62-64]. Since biased signalling is a property of the ligand-receptor complex, both the ligand and/or the receptor can be biased. This provides a conceptual framework to comprehend the complexities associated with biased agonism due to different agonists and allosteric modulation [62, 65-68]. Structural and pharmacologic information supports the notion that biased agonists can selectively and differentially stabilise one or more conformational subsets of the receptor at the expense of others. Thus, providing an explanation how different activators such as APC and thrombin (or different PAR1 agonist, see also next section non-canonical PAR1 activation by APC) can induce qualitative different signalling outcomes. Particular conformational subsets of the receptor induced by one activator (APC) versus another activator (thrombin) are linked to highly selective signalling outcomes and functional consequences, such as Rac1 versus RhoA activation. Alternatively, receptor bias can be induced by allosteric ligands or modulators. Accordingly, allosteric ligands or modulators stabilise one or more conformational subsets of the receptor linked to particular signalling outcomes and functional consequences regardless of the activating ligand. Although hypothetical, the phenomenon of “receptor occupancy of EPCR,” switching thrombin signalling from barrier disruptive to barrier protective, may be consistent with binding of an allosteric ligand to EPCR. Allosteric modulation of PAR1 signalling by EPCR and/or caveolin-1 may explain why cytoprotective APC signalling is dependent on caveolae. Thus, because PAR1 was shown to be a biased receptor, application of the biased signalling paradigm to PAR1 activation by APC and thrombin provides a comprehensive, conceptual explanation why phenomena such as receptor occupancy of EPCR and localisation of PAR1 and EPCR in caveolae result in such remarkable differences in functional selectivity when PAR1 is activated by two different proteases [25, 61, 69, 70].
Non-canonical PAR1 activation by APC
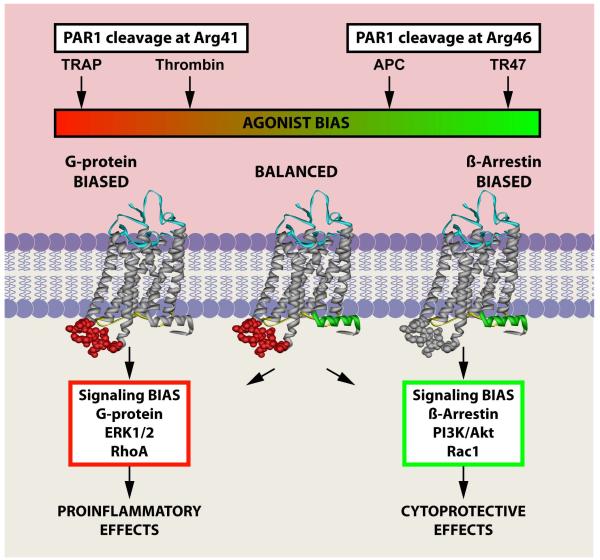
Another important piece of the PAR1 paradox puzzle was found by recent reports of non-canonical PAR1 activation by APC, providing strong support for the application of the biased signalling paradigm to PAR1 activation by APC and thrombin. Thrombin activates PAR1 at the canonical Arg41 site, which generates the agonist N-terminal tethered-ligand that begins with residue Ser42, but APC cleaves PAR1 predominantly at Arg46 in the presence of EPCR [14, 71-73]. APC-mediated cleavage at Arg46 generates a novel N-terminal tethered-ligand that begins with residue Asn47. A synthetic peptide comprising PAR1 residues 47-66 (TR47) mimics APC-like protective signalling in endothelial cells as reflected by Akt and GSK3β phosphorylation that is dependent on PAR1. TR47 stabilises endothelial barriers in vitro and provides vascular barrier protective effects in vivo [73]. Furthermore, TR47 induces Rac1 activation, consistent with inducing a biased agonist response in PAR1 that employs β-arrestin 2-dependent signalling to activate Rac1. In contrast to PAR1 agonist peptides starting at Ser42 (TRAP), TR47 does not induce platelet activation [74] and does not induce phosphorylation of ERK1/2, which is typical of TRAP-induced PAR1 signalling [73]. These data indicate that the novel PAR1 N-terminus beginning at residue Asn47, generated by APC-mediated PAR1 cleavage at Arg46, induces APC-like cytoprotective signalling that involves Rac1 activation. In contrast, the PAR1 N-terminus beginning at residue Ser42, generated by thrombin (or APC-mediated cleavage at Arg41), induces G protein-dependent activation of RhoA. Thus, TRAP and TR47 represent two different PAR1 agonists that induce differential biased PAR1 signalling. Derived from the biased signalling paradigm, proteolysis at Arg41 versus Arg46 presumably alters the spectrum of conformations of PAR1 due to differences in the tethered-ligand sequences implied by cleavage at either Arg41 or Arg46. Consequently, cleavage of PAR1 at Arg46 by APC induces a subset of PAR1 conformations with functional consequences that differ from that induced by PAR1 activation at Arg41 (Fig 2). Thus, activation of PAR1 at different sites by thrombin and APC, generating different tethered-ligand agonists and resulting in the activation of different signalling pathways, can understandably mediate the often-opposite effects of thrombin and APC.
Figure 2. Biased agonism due to canonical and non-canonical activation of PAR1.
Because thrombin cleaves PAR1 at Arg41 and APC cleaves PAR1 at Arg46, the tethered-ligand sequences generated by these proteases differ and accordingly result in activation of different signalling pathways, which has been labelled “biased agonism.” The agonist bias is thus directly related to the cleavage sites of the tethered-ligand and the new N-terminal sequence as represented by the TRAP peptide that exists after cleavage at Arg41 or the TR47 peptide that exists after cleavage at Arg46 [73]. The conformer subsets of PAR1 that are stabilised by different agonist can be different and have distinctly different properties because one conformer subset can prefer binding of G proteins, whereas another conformer subset can prefer signalling mediated by β-arrestin-2. TRAP and thrombin promote signalling via different G proteins while the dynamic conformational ensembles of PAR1 stabilised by APC’s action, and presumably TR47, preferentially promote signalling mediated by β-arrestin-2. This figure was originally published in Blood [73]. L.O. Mosnier, R.K. Sinha, L. Burnier, E.A. Bouwens, J.H. Griffin. Biased agonism of protease-activated receptor 1 by activated protein C caused by noncanonical cleavage at Arg46. Blood 2012;120:5237-46. © the American Society of Hematology.
Another pending PAR1 paradox question is how a kinetically inefficient activation of PAR1 by APC can have physiologic effects compared to efficient PAR1 cleavage by thrombin. Thrombin-cleaved PAR1 is rapidly internalised to provide regulation of PAR1-initiated signalling, but APC-activated PAR1 tends to remain on the cell surface [45, 75]. Although reasons for the lack of PAR1 internalisation after activation by APC remain to be determined, it is conceivable that PAR1 cleavage by APC at Arg46 might be a contributing factor. Such a model would imply accumulation of Arg46-cleaved PAR1 on the membrane until a critical mass required to induce prolonged cytoprotective signalling is reached, consistent with the observations that APC’s functional effects on cells generally require prolonged incubation with APC and that in murine models high-dose APC bolus generally achieves better outcomes than low-dose infusion [27].
Regulation of EPCR function
The presence of functional EPCR on the cell surface is essential for APC’s cytoprotective effects on endothelial cells and many other cells. Binding of APC to EPCR for cytoprotective effects provides a mechanistic distinction compared to APC’s anticoagulant activities that require binding of the APC Gla-domain to negatively charged phospholipid membranes. Thus, expression of APC’s anticoagulant activities is incompatible with binding of the Gla-domain to EPCR [76-78]. In vivo studies using genetically modified mice with low or high EPCR expression clearly demonstrate the importance of EPCR for the efficacy of cytoprotective effects by APC [31, 79], thus prompting the question what mechanisms regulate EPCR expression and function on cells.
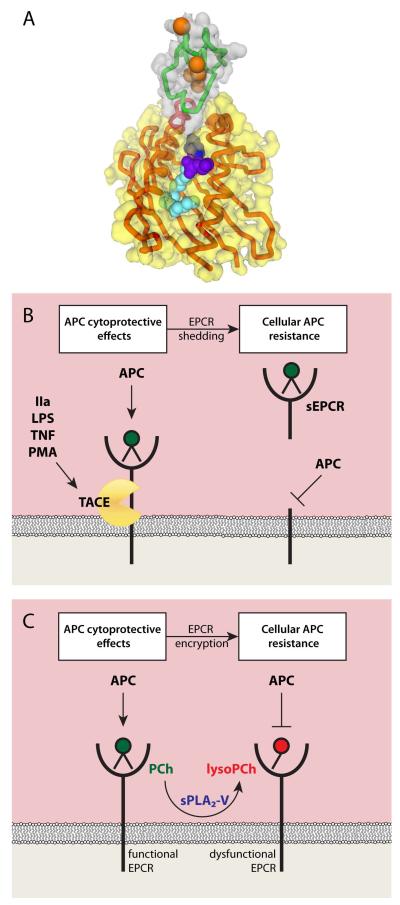
EPCR is homologous to CD1/major histocompatibility complex (MHC) molecules that consist of three extracellular domains, α-1, α-2, and α-3, although EPCR lacks the α-3-domain and, consequently, does not associate with β2-microglobulin [76]. The α-1 and α-2 domains are located along opposing edges of a planar platform. This forms a hydrophobic binding groove that contains a phospholipid, most likely phosphatidylcholine (PCh), important for correct spacing of the α-1 and α-2 domains to support (A)PC binding (Fig 3A) [76, 80]. EPCR’s short cytoplasmic tail suggests that palmitoylation of the C-terminal Cys residue may help localise EPCR to certain lipid rafts or caveolae to facilitate modulation of cell signalling pathways but also implies that induction of direct cell signalling by EPCR is unlikely [45, 81]. Several mechanisms have been described for modification of EPCR function, including EPCR shedding and more recently lipid editing by phospholipase A2 (PLA2) [80, 82]. EPCR is shed from the endothelial cell surface by the metalloproteinase tumour necrosis factor (TNF)-α converting enzyme (TACE), and shedding releases the soluble extracellular EPCR domain (sEPCR) [83] (Fig 3B). Shedding can be stimulated by both inflammatory mediators such as interleukin 1β, phorbol esters, and TNFα, and activated coagulation factors such as thrombin [84, 85]. Accordingly, higher levels of sEPCR have been reported in patients with systemic inflammatory diseases and conditions associated with enhanced thrombin generation [86].
Figure 3. EPCR and mechanisms for regulation of EPCR functionality and induction of cellular APC resistance.
(A) Structure of the N-terminal part of the APC GLA-domain (green) and Ca2+ ions (orange) in complex with EPCR (red) based on 1LQV [76]. The phosphatidylcholine (PCh) embedded in the hydrophobic groove of EPCR is indicated with the lipid head group in purple and the fatty acid chains in blue. Cleavage of the lipid by sPLA2 at the sn-2 position to generate lyso-PCh results in loss of the fatty acid chain indicated in light blue. (B) Inflammatory mediators and activated coagulation factors such as thrombin (IIa), lipopolysaccharide (LPS), TNFα and phorbol myristate acetate (PMA) can up regulate the metalloproteinase TACE. TACE cleaves EPCR at the cell membrane, which results in shedding of soluble EPCR (sEPCR) in the circulation. As a result APC, which normally binds to EPCR on the cell surface, is unable to bind to the cell. This abrogates APC’s direct cytoprotective effects on cells thereby inducing cellular APC resistance with potential implications for the susceptibility to thrombosis and inflammation. (C) Functional EPCR loaded with a PCh (green) in its hydrophobic groove promotes the generation of APC by the thrombin-thrombomodulin complex and supports APC’s direct cytoprotective effects on cells. Secreted phospholipase A2 group V (sPLA2-V) changes the PCh in EPCR for a lysoPCh [80]. This lysoPCh-loaded EPCR (red), or encrypted EPCR, is dysfunctional with regard to protein C and APC binding. Thus, sPLA2-V modified EPCR fails to promote APC generation by the thrombin-thrombomodulin complex and leads to cellular APC resistance as APC can no longer exert its cytoprotective effects directly on the cell.
Recently, a mechanism was described by which an endothelial-derived phospholipase can modify or “encrypt” EPCR to lose its ability to bind APC, thereby rendering EPCR unable to mediate APC’s direct cytoprotective activities on cells (Fig 3C) [80]. These findings conceptualise a novel phenomenon of “cellular APC resistance” similar to the well-known “anticoagulant APC resistance” associated with an increased risk for venous thrombosis [1, 87]. When PCh metabolites, such as lysoPCh and platelet-activating factor, occupy EPCR’s hydrophobic groove, the affinity for APC is reduced [80]. This raises immediate questions of whether other lipids can occupy EPCR and what will be the consequences of incorporation of such lipids on EPCR function. As a branch of the large phospholipase family, PLA2s hydrolyse phospholipids at the sn-2 position producing free fatty acids and lysophospholipids. Most PLA2s are intracellular, but some secreted PLA2s circulate in plasma and are best known for their pro-atherogenic properties and participation in inflammation via the generation of bioactive lipid mediators [88]. Secreted PLA2 group V can modify the EPCR lipid, as incubation of endothelial cells or purified EPCR with secreted PLA2-V results in diminished APC binding and inhibition of EPCR-dependent anti-apoptotic effects of APC on cells [80]. Cellular APC resistance may have important implications for thrombotic and inflammatory vascular disease because EPCR inactivation in vivo, either genetically or induced by blocking antibodies, increases susceptibility to thrombotic and inflammatory disease [89]. Although it is recognised that the type of lipid molecule embedded in the hydrophobic groove of EPCR is essential for binding of APC to EPCR and that sPLA2 can modify this lipid, the precise role of sPLA2 in regulating the function of EPCR has not been established [76, 80]. In addition, whether cells can employ shedding of encrypted EPCR as a possible counter measure to restore EPCR’s cytoprotective functions after induction of cellular APC remains to be determined.
APC-independent actions of EPCR
EPCR also functions independent of APC as a receptor for factor VII (FVII) and FVIIa. Binding of FVII(a) to EPCR occurs in similar manner compared to APC and seems to have implications for its circulation half-life, tissue factor-dependent procoagulant activity and possibly PAR-dependent signalling. Recent studies showed that FVIIa associates with the endothelium when administered intravenously to mice, and that this affects its circulation half-life [90, 91]. FVIIa rapidly binds to EPCR on the endothelial surface where EPCR-mediated transcytosis of FVIIa across the endothelial barrier promotes the perivascular build-up of FVIIa. Intriguingly, functionally active FVIIa remains in tissues for periods of time far exceeding its circulatory half-life and one wonders whether this phenomenon contributes to tissue thrombosis or is designed to protect against bleeding [90, 91]. Similar to these observations, EPCR-dependent transcytosis of APC across the blood-brain barrier was suggested to contribute to APC’s ability to provide neuroprotective activities in the brain [92]. Also similar to APC, FVIIa binding to EPCR seems to induce a shift from FVIIa’s activity in coagulation to activities on cells. Binding of FVIIa to EPCR inhibited the procoagulant activity of the tissue factor (TF)/FVIIa complex [93]. Some studies suggested that FVIIa does not activate PAR1, that FVIIa binding to EPCR does not facilitate PAR1 cleavage, and that FVIIa fails to prevent thrombin-induced endothelial cell barrier permeability [81, 94]. However, other studies indicate that EPCR-bound FVIIa can activate PAR1 on primary endothelial cells and induce downstream protective signalling similar to APC [95, 96]. Moreover, the ternary (FX-TF-FVIIa) complex is known to initiate PAR1 and PAR2 signalling and EPCR has been described to modulate PAR activation in this setting [97].
Although FXa has also been reported to require EPCR to initiate PAR-dependent signalling [98], this remains a controversial subject. The interaction between FXa and EPCR is one of low affinity in purified systems [99, 100], and FX fails to compete with APC or FVII binding in various cell-based assays [10, 99, 100]. Moreover, binding of FXa to endothelial cells seems only partly EPCR-dependent as a portion of FXa remains bound to the endothelial cells in the presence of large amounts of APC or a blocking anti-EPCR antibody [98]. A more elaborate review on this subject has been published recently [99].
A new molecular partner for EPCR came from its identification as a ligand for the T cell receptor (TCR) on a subpopulation of T cells [101]. Based on structural similarity of EPCR with CD1/MHC molecules and the fact that EPCR binds lipids analogously to CD1d, a role for lipid antigen presentation by EPCR was anticipated. However, mutagenesis of the lipid-binding surface of EPCR showed no involvement in binding to the TCR, and recognition of EPCR by the TCR did not involve discrimination of lipid antigens. Instead, structural moieties in the β-sheet of EPCR were identified to mediate the EPCR-TCR interaction, unlike the recognition of CD1d by TCRs. EPCR is proposed to contribute to the regulation of the T cells in response to infection or malignancy in concert with other secondary signals. Possibly, T cells may use EPCR as a molecular beacon to identify a target cell as endothelial, with additional factors distinguishing infected cells from uninfected cells [101].
Emerging insight on the role of the protein C pathway in cancer biology
Malignant tumour cells make use of cytoprotective pathways for survival and mitogenic pathways for growth. Although the underlying triggers are different, some of the signalling pathways involved in malignant cell growth intersect with PAR1-dependent signalling pathways involved in the cytoprotective effects of APC and the mitogenic effects of thrombin [102, 103]. This raises several questions regarding the potential role of the cytoprotective protein C pathway and its receptors in tumour growth and metastasis. Can tumour cells adopt APC’s cytoprotective signalling mechanisms to promote survival or metastatic potential? Or vice versa, can inhibition of APC’s cytoprotective signalling mechanisms on tumour cells inhibit survival and reduce metastasis? Is there good and bad PAR1-mediated signalling and how does biased PAR1 signalling affect tumour growth, survival and metastasis? Alternatively, can the cytoprotective protein C pathway be employed to protect the surrounding healthy tissues during anti-tumour therapies aimed at inflicting maximal damage to the tumour cells?
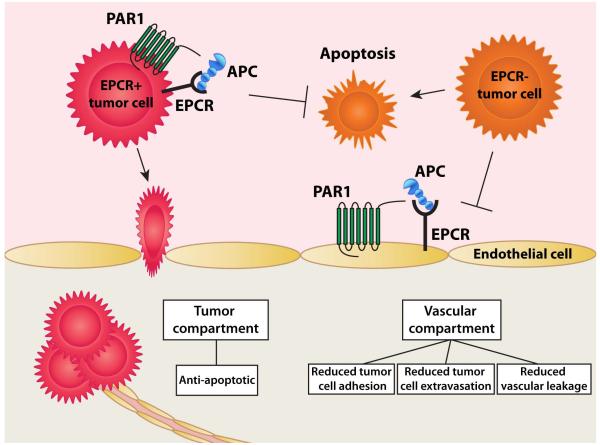
Studies addressing these questions suggest divergent effects of APC and its receptors on tumour cell migration, invasion, and metastasis. EPCR is expressed in a variety of human tumours cells and some studies found that APC induced EPCR- and PAR1-dependent anti-apoptotic signalling pathways in certain tumour cells that enhanced survival and increased the potential of these cells to form metastatic foci [104-108]. However, other studies have found anti-metastatic effects of APC consisting of down-regulation of vascular adhesion molecules that are involved in tumour cell adhesion and improvement of vascular barrier functions thereby limiting the extravasation of potentially metastatic tumour cells [109, 110]. These observations imply a two compartment model, that of the tumour and that of the vasculature (Fig 4). Different effects of APC are to be anticipated in the vasculature compared to the tumour depending on the expression of the ensemble of protein C pathway receptors in each compartment.
Figure 4. Multiple effects of APC on tumour cell migration, invasion, and metastasis.
Emerging studies on the effects of the protein C pathway for tumour cell migration, invasion, and metastasis suggest a two-compartment model, the tumour and the vasculature. Different effects of APC are to be anticipated in each compartment depending on the expression of the ensemble of protein C pathway receptors. Initial reports suggests that on tumour cells that express EPCR (EPCR+ cells (red)) anti-apoptotic APC effects can convey survival benefits that cooperate the metastatic potential of these cells [104-106]. In contrast, APC does not convey a survival advantage on EPCR deficient tumour cells (EPCR-cells (orange)). In the vasculature, APC and the protein C pathway provide anti-metastatic effects by down-regulating vascular adhesion molecules that are involved in tumour cell adhesion and enhancing vascular barrier function thereby limiting the extravasation of potentially metastatic tumour cells [109, 110]. Thus, important considerations for the interpretation of the multiple different effects of APC on tumour cell migration, invasion, and metastasis include: 1) the compartment(s) targeted by APC, and; 2) the ensemble of protein C pathway receptors on the tumour cell and on the surrounding vasculature.
Modulation of EPCR expression on tumour cells versus the vasculature reveals striking different outcomes. Vascular over-expression of EPCR diminished metastasis in a melanoma model in which endogenous APC limited cancer cell extravasation and protected against metastasis by enhancing vascular barrier functions [109, 110]. However, studies targeting EPCR expression on the tumour cell revealed different, opposite effects of EPCR expression. In a model of lung metastasis, over-expression of EPCR on lung adenocarcinoma cells led to prometastatic effects, while silencing of EPCR expression reduced metastasis [106]. These apparent contrasting effects of EPCR may be attributed to differences in targeting EPCR expression in the vasculature (melanoma model) versus in the tumour cells (lung metastasis model) and to the lack of EPCR expression on the melanoma cells compared to the lung carcinoma cells that express abundant EPCR. Although these observations are fascinating, the phenotypic and biochemical characteristics of tumour cells are notorious heterogeneous and inherently dependent on the experimental model used. Thus, further studies are needed to better understand the ramifications of the protein C pathway in cancer biology.
Concluding remarks
The protein C pathway has come from an antithrombotic pathway to a full-fledge vascular protective pathway. Developments in the last decade fostered a new branch in the protein C pathway, i.e. the direct effects of APC on cells that result in cytoprotective signalling. Recent insights into the APC cytoprotective pathway provide molecular mechanisms that involve EPCR and PAR1. Moreover, a major paradox of how PAR1 can mediate opposing effects by APC and thrombin was solved by showing that these proteases activate PAR1 at different sites, resulting in different tethered-ligands with different signalling characteristics. Efforts by many have resulted in new possibilities for basic and preclinical studies that may be translated to clinical applications. Notably, a cytoprotective-selective APC (3K3A-APC) has recently entered phase1 clinical trial for ischaemic stroke. The emerging new insights into the molecular mechanisms of the protein C pathway provide additional exciting avenues for mimetics with APC-like activities in translational research with potential clinical applications, such as the treatment of ischaemic stroke, inflammatory disease, and vascular disease. In line with biased agonism and allosteric modulation of PAR1 by thrombin and APC, drug discovery efforts to identify compounds that antagonise PAR1’s G protein-dependent signalling but not β-arrestin 2-dependent signalling may prove fruitful for treating a variety of diseases where thrombosis, inflammation and barrier disruption play a key role. Similarly, APC-mimetic compounds that promote cytoprotective PAR1-dependent signalling while avoiding thrombin-mimetic signalling would encompass therapeutically desirable biased PAR1 agonists. In addition, emerging studies on the regulation of EPCR functionality might provide therapeutically relevant avenues to improve efficacy of endogenous or pharmacologic cytoprotective activities of APC. To name but one example, sPLA2 inhibitors developed to treat atherosclerosis [111] might be used as a therapeutic strategy to prevent EPCR encryption and boost the endogenous protein C anticoagulant and cytoprotective pathways. Extending studies of the protein C pathway in new fields such as cancer research may uncover novel molecular mechanisms and potential targets for therapy. In summary, recent discoveries have expanded the real potential of the cytoprotective protein C pathway and have opened up exciting new horizons for translational research.
Acknowledgements
We apologise to our colleagues whose work could not be cited due to space limitations. We like to thank Dr. John H. Griffin for many stimulating discussions. This work was supported by an American Heart Association Western States Affiliate postdoctoral fellowship (E.A.B.) and National Institutes of Health (NHLBI) grant HL104165 (L.O.M.).
Footnotes
Conflict of Interest disclosure L.O. Mosnier is a co-inventor for intellectual property owned by The Scripps Research Institute that is related APC mutants and non-canonical PAR1 activation by APC discussed here. The other authors have nothing to disclose.
References
- 1.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–72. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 2.Rosing J, Tans G. Coagulation factor V: an old star shines again. Thromb Haemost. 1997;78:427–33. [PubMed] [Google Scholar]
- 3.Dahlback B, Villoutreix BO. Regulation of blood coagulation by the protein C anticoagulant pathway: novel insights into structure-function relationships and molecular recognition. Arterioscler Thromb Vasc Biol. 2005;25:1311–20. doi: 10.1161/01.ATV.0000168421.13467.82. [DOI] [PubMed] [Google Scholar]
- 4.Weiler H. Regulation of inflammation by the protein C system. Crit Care Med. 2010;38:S18–25. doi: 10.1097/CCM.0b013e3181c9cbb5. [DOI] [PubMed] [Google Scholar]
- 5.Rezaie AR. Regulation of the protein C anticoagulant and antiinflammatory pathways. Curr Med Chem. 2010;17:2059–69. doi: 10.2174/092986710791233706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danese S, Vetrano S, Zhang L, Poplis VA, Castellino FJ. The protein C pathway in tissue inflammation and injury: pathogenic role and therapeutic implications. Blood. 2010;115:1121–30. doi: 10.1182/blood-2009-09-201616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zlokovic BV, Griffin JH. Cytoprotective protein C pathways and implications for stroke and neurological disorders. Trends Neurosci. 2011;34:198–209. doi: 10.1016/j.tins.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esmon CT. Protein C anticoagulant system-anti-inflammatory effects. Semin Immunopathol. 2012;34:127–32. doi: 10.1007/s00281-011-0284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wildhagen KC, Lutgens E, Loubele ST, Ten Cate H, Nicolaes GA. The structure-function relationship of activated protein C. Lessons from natural and engineered mutations. Thromb Haemost. 2011;106:1034–45. doi: 10.1160/TH11-08-0522. [DOI] [PubMed] [Google Scholar]
- 10.Fukudome K, Esmon CT. Identification, cloning, and regulation of a novel endothelial cell protein C/activated protein C receptor. J Biol Chem. 1994;269:26486–91. [PubMed] [Google Scholar]
- 11.Esmon CT, Owen WG. The discovery of thrombomodulin. J Thromb Haemost. 2004;2:209–13. doi: 10.1046/j.1538-7933.2003.00537.x. [DOI] [PubMed] [Google Scholar]
- 12.Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci U S A. 1996;93:10212–6. doi: 10.1073/pnas.93.19.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–2. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 14.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–14. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 15.Kalafatis M, Rand MD, Mann KG. The mechanism of inactivation of human factor V and human factor Va by activated protein C. J Biol Chem. 1994;269:31869–80. [PubMed] [Google Scholar]
- 16.Nicolaes GA, Tans G, Thomassen MC, Hemker HC, Pabinger I, Varadi K, Schwarz HP, Rosing J. Peptide bond cleavages and loss of functional activity during inactivation of factor Va and factor VaR506Q by activated protein C. J Biol Chem. 1995;270:21158–66. doi: 10.1074/jbc.270.36.21158. [DOI] [PubMed] [Google Scholar]
- 17.Nesheim ME, Taswell JB, Mann KG. The contribution of bovine Factor V and Factor Va to the activity of prothrombinase. J Biol Chem. 1979;254:10952–62. [PubMed] [Google Scholar]
- 18.Yegneswaran S, Smirnov MD, Safa O, Esmon NL, Esmon CT, Johnson AE. Relocating the active site of activated protein C eliminates the need for its protein S cofactor. A fluorescence resonance energy transfer study. J Biol Chem. 1999;274:5462–8. doi: 10.1074/jbc.274.9.5462. [DOI] [PubMed] [Google Scholar]
- 19.van Dieijen G, Tans G, Rosing J, Hemker HC. The role of phospholipid and factor VIIIa in the activation of bovine factor X. J Biol Chem. 1981;256:3433–42. [PubMed] [Google Scholar]
- 20.Lenting PJ, van Mourik JA, Mertens K. The life cycle of coagulation factor VIII in view of its structure and function. Blood. 1998;92:3983–96. [PubMed] [Google Scholar]
- 21.O’Brien LM, Mastri M, Fay PJ. Regulation of factor VIIIa by human activated protein C and protein S: inactivation of cofactor in the intrinsic factor Xase. Blood. 2000;95:1714–20. [PubMed] [Google Scholar]
- 22.Gale AJ, Cramer TJ, Rozenshteyn D, Cruz JR. Detailed mechanisms of the inactivation of factor VIIIa by activated protein C in the presence of its cofactors, protein S and factor V. J Biol Chem. 2008;283:16355–62. doi: 10.1074/jbc.M708985200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cramer TJ, Gale AJ. The anticoagulant function of coagulation factor V. Thromb Haemost. 2012;107:15–21. doi: 10.1160/TH11-06-0431. [DOI] [PubMed] [Google Scholar]
- 24.Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene Expression Profile of Antithrombotic Protein C Defines New Mechanisms Modulating Inflammation and Apoptosis. J Biol Chem. 2001;276:11199–203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- 25.Rezaie AR. The occupancy of endothelial protein C receptor by its ligand modulates the par-1 dependent signaling specificity of coagulation proteases. IUBMB Life. 2011;63:390–6. doi: 10.1002/iub.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russo A, Soh UJ, Trejo J. Proteases display biased agonism at protease-activated receptors: location matters. Mol Interv. 2009;9:87–96. doi: 10.1124/mi.9.2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiler H. Multiple receptor-mediated functions of activated protein C. Hamostaseologie. 2011;31:185–95. doi: 10.5482/ha-1166. [DOI] [PubMed] [Google Scholar]
- 28.Cao C, Gao Y, Li Y, Antalis TM, Castellino FJ, Zhang L. The efficacy of activated protein C in murine endotoxemia is dependent on integrin CD11b. J Clin Invest. 2010;120:1971–80. doi: 10.1172/JCI40380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang XV, Banerjee Y, Fernandez JA, Deguchi H, Xu X, Mosnier LO, Urbanus RT, de Groot PG, White-Adams TC, McCarty OJ, Griffin JH. Activated protein C ligation of ApoER2 (LRP8) causes Dab1-dependent signaling in U937 cells. Proc Natl Acad Sci U S A. 2009;106:274–9. doi: 10.1073/pnas.0807594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–21. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerschen EJ, Fernandez JA, Cooley BC, Yang XV, Sood R, Mosnier LO, Castellino FJ, Mackman N, Griffin JH, Weiler H. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J Exp Med. 2007;204:2439–48. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bae JS, Yang L, Manithody C, Rezaie AR. Engineering a disulfide bond to stabilize the calcium-binding loop of activated protein C eliminates its anticoagulant but not its protective signaling properties. J Biol Chem. 2007;282:9251–9. doi: 10.1074/jbc.M610547200. [DOI] [PubMed] [Google Scholar]
- 33.Mosnier LO, Gale AJ, Yegneswaran S, Griffin JH. Activated protein C variants with normal cytoprotective but reduced anticoagulant activity. Blood. 2004;104:1740–4. doi: 10.1182/blood-2004-01-0110. [DOI] [PubMed] [Google Scholar]
- 34.Harmon S, Preston RJ, Ni Ainle F, Johnson JA, Cunningham MS, Smith OP, White B, O’Donnell JS. Dissociation of activated protein C functions by elimination of protein S cofactor enhancement. J Biol Chem. 2008;283:30531–9. doi: 10.1074/jbc.M802338200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L, Bae JS, Manithody C, Rezaie AR. Identification of a specific exosite on activated protein C for interaction with protease-activated receptor 1. J Biol Chem. 2007;282:25493–500. doi: 10.1074/jbc.M702131200. [DOI] [PubMed] [Google Scholar]
- 36.Preston RJ, Villegas-Mendez A, Sun YH, Hermida J, Simioni P, Philippou H, Dahlback B, Lane DA. Selective modulation of protein C affinity for EPCR and phospholipids by Gla domain mutation. FEBS J. 2005;272:97–108. doi: 10.1111/j.1432-1033.2004.04401.x. [DOI] [PubMed] [Google Scholar]
- 37.Mosnier LO, Zampolli A, Kerschen EJ, Schuepbach RA, Banerjee Y, Fernández JA, Yang XV, Riewald M, Weiler H, Ruggeri ZM, Griffin JH. Hyperantithrombotic, noncytoprotective Glu149Ala-activated protein C mutant. Blood. 2009;113:5970–8. doi: 10.1182/blood-2008-10-183327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geiger H, Pawar SA, Kerschen EJ, Nattamai KJ, Hernandez I, Liang HP, Fernandez JA, Cancelas JA, Ryan MA, Kustikova O, Schambach A, Fu Q, Wang J, Fink LM, Petersen KU, Zhou D, Griffin JH, Baum C, Weiler H, Hauer-Jensen M. Pharmacological targeting of the thrombomodulin-activated protein C pathway mitigates radiation toxicity. Nat Med. 2012;18:1123–9. doi: 10.1038/nm.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ., Jr. Recombinant human protein CWEiSSsg. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 40.Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gardlund B, Marshall JC, Rhodes A, Artigas A, Payen D, Tenhunen J, Al-Khalidi HR, Thompson V, Janes J, Macias WL, Vangerow B, Williams MD, Group P-SS Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–64. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 41.Komarova YA, Mehta D, Malik AB. Dual regulation of endothelial junctional permeability. Sci STKE. 2007;2007:re8. doi: 10.1126/stke.4122007re8. [DOI] [PubMed] [Google Scholar]
- 42.Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem. 2005;280:17286–93. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 43.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–84. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 44.Bae JS, Yang L, Rezaie AR. Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells. Proc Natl Acad Sci U S A. 2007;104:2867–72. doi: 10.1073/pnas.0611493104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russo A, Soh UJ, Paing MM, Arora P, Trejo J. Caveolae are required for protease-selective signaling by protease-activated receptor-1. Proc Natl Acad Sci U S A. 2009;106:6393–7. doi: 10.1073/pnas.0810687106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minhas N, Xue M, Fukudome K, Jackson CJ. Activated protein C utilizes the angiopoietin/Tie2 axis to promote endothelial barrier function. FASEB J. 2010;24:873–81. doi: 10.1096/fj.09-134445. [DOI] [PubMed] [Google Scholar]
- 47.Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, Yancopoulos GD, Isner JM. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233–40. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- 48.Bae JS, Rezaie AR. Thrombin upregulates the angiopoietin-Tie2 Axis: endothelial protein C receptor occupancy prevents the thrombin mobilization of angiopoietin 2 and P-selectin from Weibel-Palade bodies. J Thromb Haemost. 2010;8:1107–15. doi: 10.1111/j.1538-7836.2010.03812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Stankovic M, Bonder CS, Hahn CN, Parsons M, Pitson SM, Xia P, Proia RL, Vadas MA, Gamble JR. Basal and angiopoietin-1-mediated endothelial permeability is regulated by sphingosine kinase-1. Blood. 2008;111:3489–97. doi: 10.1182/blood-2007-05-092148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramachandran R, Noorbakhsh F, Defea K, Hollenberg MD. Targeting proteinase-activated receptors: therapeutic potential and challenges. Nat Rev Drug Discov. 2012;11:69–86. doi: 10.1038/nrd3615. [DOI] [PubMed] [Google Scholar]
- 51.Julovi SM, Xue M, Dervish S, Sambrook PN, March L, Jackson CJ. Protease activated receptor-2 mediates activated protein C-induced cutaneous wound healing via inhibition of p38. Am J Pathol. 2011;179:2233–42. doi: 10.1016/j.ajpath.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riewald M, Ruf W. Protease-activated receptor-1 signaling by activated protein C in cytokine-perturbed endothelial cells is distinct from thrombin signaling. J Biol Chem. 2005;280:19808–14. doi: 10.1074/jbc.M500747200. [DOI] [PubMed] [Google Scholar]
- 53.O’Brien PJ, Prevost N, Molino M, Hollinger MK, Woolkalis MJ, Woulfe DS, Brass LF. Thrombin responses in human endothelial cells. Contributions from receptors other than PAR1 include the transactivation of PAR2 by thrombin-cleaved PAR1. J Biol Chem. 2000;275:13502–9. doi: 10.1074/jbc.275.18.13502. [DOI] [PubMed] [Google Scholar]
- 54.Shi X, Gangadharan B, Brass LF, Ruf W, Mueller BM. Protease-activated receptors (PAR1 and PAR2) contribute to tumor cell motility and metastasis. Mol Cancer Res. 2004;2:395–402. [PubMed] [Google Scholar]
- 55.Kaneider NC, Leger AJ, Agarwal A, Nguyen N, Perides G, Derian C, Covic L, Kuliopulos A. ‘Role reversal’ for the receptor PAR1 in sepsis-induced vascular damage. Nat Immunol. 2007;8:1303–12. doi: 10.1038/ni1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo H, Liu D, Gelbard H, Cheng T, Insalaco R, Fernandez JA, Griffin JH, Zlokovic BV. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron. 2004;41:563–72. doi: 10.1016/s0896-6273(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 57.Madhusudhan T, Wang H, Straub BK, Grone E, Zhou Q, Shahzad K, Muller-Krebs S, Schwenger V, Gerlitz B, Grinnell BW, Griffin JH, Reiser J, Grone HJ, Esmon CT, Nawroth PP, Isermann B. Cytoprotective signaling by activated protein C requires protease-activated receptor-3 in podocytes. Blood. 2012;119:874–83. doi: 10.1182/blood-2011-07-365973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, Corat MA, Zeier M, Blessing E, Oh J, Gerlitz B, Berg DT, Grinnell BW, Chavakis T, Esmon CT, Weiler H, Bierhaus A, Nawroth PP. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007;13:1349–58. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- 59.McLaughlin JN, Patterson MM, Malik AB. Protease-activated receptor-3 (PAR3) regulates PAR1 signaling by receptor dimerization. Proc Natl Acad Sci U S A. 2007;104:5662–7. doi: 10.1073/pnas.0700763104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bir N, Lafargue M, Howard M, Goolaerts A, Roux J, Carles M, Cohen MJ, Iles KE, Fernandez JA, Griffin JH, Pittet JF. Cytoprotective-selective activated protein C attenuates Pseudomonas aeruginosa-induced lung injury in mice. Am J Respir Cell Mol Biol. 2011;45:632–41. doi: 10.1165/rcmb.2010-0397OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soh UJ, Trejo J. Activated protein C promotes protease-activated receptor-1 cytoprotective signaling through beta-arrestin and dishevelled-2 scaffolds. Proc Natl Acad Sci U S A. 2011;108:E1372–80. doi: 10.1073/pnas.1112482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol. 2012;52:179–97. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DeWire SM, Violin JD. Biased ligands for better cardiovascular drugs: dissecting G-protein-coupled receptor pharmacology. Circ Res. 2011;109:205–16. doi: 10.1161/CIRCRESAHA.110.231308. [DOI] [PubMed] [Google Scholar]
- 64.Valant C, Robert Lane J, Sexton PM, Christopoulos A. The best of both worlds? Bitopic orthosteric/allosteric ligands of g protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2012;52:153–78. doi: 10.1146/annurev-pharmtox-010611-134514. [DOI] [PubMed] [Google Scholar]
- 65.Katritch V, Cherezov V, Stevens RC. Diversity and modularity of G protein-coupled receptor structures. Trends Pharmacol Sci. 2012;33:17–27. doi: 10.1016/j.tips.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Granier S, Kobilka B. A new era of GPCR structural and chemical biology. Nat Chem Biol. 2012;8:670–3. doi: 10.1038/nchembio.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu JJ, Horst R, Katritch V, Stevens RC, Wuthrich K. Biased signaling pathways in beta2-adrenergic receptor characterized by 19F-NMR. Science. 2012;335:1106–10. doi: 10.1126/science.1215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boehr DD, Nussinov R, Wright PE. The role of dynamic conformational ensembles in biomolecular recognition. Nat Chem Biol. 2009;5:789–96. doi: 10.1038/nchembio.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bae JS, Yang L, Rezaie AR. Lipid raft localization regulates the cleavage specificity of protease activated receptor 1 in endothelial cells. J Thromb Haemost. 2008;6:954–61. doi: 10.1111/j.1538-7836.2008.02924.x. [DOI] [PubMed] [Google Scholar]
- 70.Russo A, Soh UJK, Paing MM, Arora P, Trejo J. Caveolae are required for protease-selective signaling by protease-activated receptor–1. Proc Natl Acad Sci U S A. 2009;106:6393–7. doi: 10.1073/pnas.0810687106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–68. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 72.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–64. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 73.Mosnier LO, Sinha RK, Burnier L, Bouwens EA, Griffin JH. Biased agonism of protease-activated receptor 1 by activated protein C caused by non-canonical cleavage at Arg46. Blood. 2012 doi: 10.1182/blood-2012-08-452169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vassallo RR, Jr., Kieber-Emmons T, Cichowski K, Brass LF. Structure-function relationships in the activation of platelet thrombin receptors by receptor-derived peptides. J Biol Chem. 1992;267:6081–5. [PubMed] [Google Scholar]
- 75.Schuepbach RA, Feistritzer C, Brass LF, Riewald M. Activated protein C-cleaved protease activated receptor-1 is retained on the endothelial cell surface even in the presence of thrombin. Blood. 2008;111:2667–73. doi: 10.1182/blood-2007-09-113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oganesyan V, Oganesyan N, Terzyan S, Qu D, Dauter Z, Esmon NL, Esmon CT. The crystal structure of the endothelial protein C receptor and a bound phospholipid. J Biol Chem. 2002;277:24851–4. doi: 10.1074/jbc.C200163200. [DOI] [PubMed] [Google Scholar]
- 77.Liaw PC, Neuenschwander PF, Smirnov MD, Esmon CT. Mechanisms by which soluble endothelial cell protein C receptor modulates protein C and activated protein C function. J Biol Chem. 2000;275:5447–52. doi: 10.1074/jbc.275.8.5447. [DOI] [PubMed] [Google Scholar]
- 78.Regan LM, Stearns-Kurosawa DJ, Kurosawa S, Mollica J, Fukudome K, Esmon CT. The endothelial cell protein C receptor. Inhibition of activated protein C anticoagulant function without modulation of reaction with proteinase inhibitors. J Biol Chem. 1996;271:17499–503. doi: 10.1074/jbc.271.29.17499. [DOI] [PubMed] [Google Scholar]
- 79.Li W, Zheng X, Gu J, Hunter J, Ferrell GL, Lupu F, Esmon NL, Esmon CT. Overexpressing endothelial cell protein C receptor alters the hemostatic balance and protects mice from endotoxin. J Thromb Haemost. 2005;3:1351–9. doi: 10.1111/j.1538-7836.2005.01385.x. [DOI] [PubMed] [Google Scholar]
- 80.Lopez-Sagaseta J, Puy C, Tamayo I, Allende M, Cervero J, Velasco SE, Esmon CT, Montes R, Hermida J. sPLA2-V inhibits EPCR anticoagulant and antiapoptotic properties by accommodating lysophosphatidylcholine or PAF in the hydrophobic groove. Blood. 2012;119:2914–21. doi: 10.1182/blood-2011-05-353409. [DOI] [PubMed] [Google Scholar]
- 81.Bae JS, Yang L, Manithody C, Rezaie AR. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor 1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007;110:3909–16. doi: 10.1182/blood-2007-06-096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu J, Qu D, Esmon NL, Esmon CT. Metalloproteolytic release of endothelial cell protein C receptor. J Biol Chem. 2000;275:6038–44. doi: 10.1074/jbc.275.8.6038. [DOI] [PubMed] [Google Scholar]
- 83.Qu D, Wang Y, Esmon NL, Esmon CT. Regulated endothelial protein C receptor shedding is mediated by tumor necrosis factor-alpha converting enzyme/ADAM17. J Thromb Haemost. 2007;5:395–402. doi: 10.1111/j.1538-7836.2007.02347.x. [DOI] [PubMed] [Google Scholar]
- 84.Menschikowski M, Hagelgans A, Eisenhofer G, Siegert G. Regulation of endothelial protein C receptor shedding by cytokines is mediated through differential activation of MAP kinase signaling pathways. Exp Cell Res. 2009;315:2673–82. doi: 10.1016/j.yexcr.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 85.Gu JM, Katsuura Y, Ferrell GL, Grammas P, Esmon CT. Endotoxin and thrombin elevate rodent endothelial cell protein C receptor mRNA levels and increase receptor shedding in vivo. Blood. 2000;95:1687–93. [PubMed] [Google Scholar]
- 86.Kurosawa S, Stearns-Kurosawa DJ, Carson CW, D’Angelo A, Della Valle P, Esmon CT. Plasma levels of endothelial cell protein C receptor are elevated in patients with sepsis and systemic lupus erythematosus: lack of correlation with thrombomodulin suggests involvement of different pathological processes. Blood. 1998;91:725–7. [PubMed] [Google Scholar]
- 87.Castoldi E, Rosing J. APC resistance: biological basis and acquired influences. J Thromb Haemost. 2010;8:445–53. doi: 10.1111/j.1538-7836.2009.03711.x. [DOI] [PubMed] [Google Scholar]
- 88.Mallat Z, Lambeau G, Tedgui A. Lipoprotein-associated and secreted phospholipases A(2) in cardiovascular disease: roles as biological effectors and biomarkers. Circulation. 2010;122:2183–200. doi: 10.1161/CIRCULATIONAHA.110.936393. [DOI] [PubMed] [Google Scholar]
- 89.Esmon CT. The endothelial cell protein C receptor. Thromb Haemost. 2000;83:639–43. [PubMed] [Google Scholar]
- 90.Nayak RC, Sen P, Ghosh S, Gopalakrishnan R, Esmon CT, Pendurthi UR, Rao LV. Endothelial cell protein C receptor cellular localization and trafficking: potential functional implications. Blood. 2009;114:1974–86. doi: 10.1182/blood-2009-03-208900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gopalakrishnan R, Hedner U, Ghosh S, Nayak RC, Allen TC, Pendurthi UR, Rao LV. Bio-distribution of pharmacologically administered recombinant factor VIIa (rFVIIa) J Thromb Haemost. 2010;8:301–10. doi: 10.1111/j.1538-7836.2009.03696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deane R, LaRue B, Sagare AP, Castellino FJ, Zhong Z, Zlokovic BV. Endothelial protein C receptor-assisted transport of activated protein C across the mouse blood-brain barrier. J Cereb Blood Flow Metab. 2009;29:25–33. doi: 10.1038/jcbfm.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lopez-Sagaseta J, Montes R, Puy C, Diez N, Fukudome K, Hermida J. Binding of factor VIIa to the endothelial cell protein C receptor reduces its coagulant activity. J Thromb Haemost. 2007;5:1817–24. doi: 10.1111/j.1538-7836.2007.02648.x. [DOI] [PubMed] [Google Scholar]
- 94.Petersen LC, Thastrup O, Hagel G, Sorensen BB, Freskgard PO, Rao LV, Ezban M. Exclusion of known protease-activated receptors in factor VIIa-induced signal transduction. Thromb Haemost. 2000;83:571–6. [PubMed] [Google Scholar]
- 95.Sen P, Gopalakrishnan R, Kothari H, Keshava S, Clark CA, Esmon CT, Pendurthi UR, Rao LV. Factor VIIa bound to endothelial cell protein C receptor activates protease activated receptor-1 and mediates cell signaling and barrier protection. Blood. 2011;117:3199–208. doi: 10.1182/blood-2010-09-310706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ghosh S, Pendurthi UR, Steinoe A, Esmon CT, Rao LV. Endothelial cell protein C receptor acts as a cellular receptor for factor VIIa on endothelium. J Biol Chem. 2007;282:11849–57. doi: 10.1074/jbc.M609283200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Disse J, Petersen HH, Larsen KS, Persson E, Esmon N, Esmon CT, Teyton L, Petersen LC, Ruf W. The endothelial protein C receptor supports tissue factor ternary coagulation initiation complex signaling through protease-activated receptors. J Biol Chem. 2011;286:5756–67. doi: 10.1074/jbc.M110.201228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schuepbach RA, Riewald M. Coagulation factor Xa cleaves protease-activated receptor-1 and mediates signaling dependent on binding to the endothelial protein C receptor. J Thromb Haemost. 2010;8:379–88. doi: 10.1111/j.1538-7836.2009.03682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Montes R, Puy C, Molina E, Hermida J. Is EPCR a multi-ligand receptor? Pros and cons. Thromb Haemost. 2012;107 doi: 10.1160/TH11-11-0766. [DOI] [PubMed] [Google Scholar]
- 100.Sen P, Nayak R, Clark CA, Gopalakrishnan R, Esmon CT, Pendurthi UR, Rao LV. Factor X binding to endothelial cell protein C receptor: comparison with factor VIIa and activated protein C. Blood. 2011;118:2635–6. doi: 10.1182/blood-2011-05-354571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Willcox CR, Pitard V, Netzer S, Couzi L, Salim M, Silberzahn T, Moreau JF, Hayday AC, Willcox BE, Dechanet-Merville J. Cytomegalovirus and tumor stress surveillance by binding of a human gammadelta T cell antigen receptor to endothelial protein C receptor. Nat Immunol. 2012;13:872–9. doi: 10.1038/ni.2394. [DOI] [PubMed] [Google Scholar]
- 102.Yang E, Boire A, Agarwal A, Nguyen N, O’Callaghan K, Tu P, Kuliopulos A, Covic L. Blockade of PAR1 signaling with cell-penetrating pepducins inhibits Akt survival pathways in breast cancer cells and suppresses tumor survival and metastasis. Cancer Res. 2009;69:6223–31. doi: 10.1158/0008-5472.CAN-09-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spek CA, Arruda VR. The protein C pathway in cancer metastasis. Thromb Res. 2012;129(Suppl 1):S80–4. doi: 10.1016/S0049-3848(12)70022-1. [DOI] [PubMed] [Google Scholar]
- 104.Beaulieu LM, Church FC. Activated protein C promotes breast cancer cell migration through interactions with EPCR and PAR-1. Exp Cell Res. 2007;313:677–87. doi: 10.1016/j.yexcr.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suzuki K, Hayashi T. Protein C and its inhibitor in malignancy. Semin Thromb Hemost. 2007;33:667–72. doi: 10.1055/s-2007-991534. [DOI] [PubMed] [Google Scholar]
- 106.Anton I, Molina E, Luis-Ravelo D, Zandueta C, Valencia K, Ormazabal C, Martinez-Canarias S, Perurena N, Pajares MJ, Agorreta J, Montuenga LM, Segura V, Wistuba II, De Las Rivas J, Hermida J, Lecanda F. Receptor of activated protein C promotes metastasis and correlates with clinical outcome in lung adenocarcinoma. Am J Respir Crit Care Med. 2012;186:96–105. doi: 10.1164/rccm.201110-1826OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scheffer GL, Flens MJ, Hageman S, Izquierdo MA, Shoemaker RH, Scheper RJ. Expression of the vascular endothelial cell protein C receptor in epithelial tumour cells. Eur J Cancer. 2002;38:1535–42. doi: 10.1016/s0959-8049(02)00108-9. [DOI] [PubMed] [Google Scholar]
- 108.Tsuneyoshi N, Fukudome K, Horiguchi S, Ye X, Matsuzaki M, Toi M, Suzuki K, Kimoto M. Expression and anticoagulant function of the endothelial cell protein C receptor (EPCR) in cancer cell lines. Thromb Haemost. 2001;85:356–61. [PubMed] [Google Scholar]
- 109.Bezuhly M, Cullen R, Esmon CT, Morris SF, West KA, Johnston B, Liwski RS. Role of activated protein C and its receptor in inhibition of tumor metastasis. Blood. 2009;113:3371–4. doi: 10.1182/blood-2008-05-159434. [DOI] [PubMed] [Google Scholar]
- 110.Van Sluis GL, Niers TM, Esmon CT, Tigchelaar W, Richel DJ, Buller HR, Van Noorden CJ, Spek CA. Endogenous activated protein C limits cancer cell extravasation through sphingosine-1-phosphate receptor 1-mediated vascular endothelial barrier enhancement. Blood. 2009;114:1968–73. doi: 10.1182/blood-2009-04-217679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Suckling K. Phospholipase A2s: developing drug targets for atherosclerosis. Atherosclerosis. 2010;212:357–66. doi: 10.1016/j.atherosclerosis.2010.03.011. [DOI] [PubMed] [Google Scholar]