Abstract
Objective:
To elucidate the regulation of the nitric oxide (NO) and carbon monoxide (CO) pathways in preeclampsia and to evaluate the ratio of asymmetric dimethylarginine (ADMA) to symmetric dimethylarginine (SDMA) as a marker for preeclampsia.
Methods:
Maternal plasma and placental samples were obtained from 20 participants with preeclampsia and 23 controls. Enzyme-linked immunosorbent assay was used to measure plasma NO, ADMA, and SDMA as well as placental NO and hemeoxygnase 1 (HO-1). Western blot was used to measure placental dimethylarginine dimethylaminotransferases (DDAH-I and DDAH-II).
Results:
Placental DDAH-I, placental DDAH-II, placental NO, and placental HO-1 were significantly decreased in participants with preeclampsia. While ADMA and SDMA levels were decreased in preeclampsia, the ADMA-SDMA ratio was not significantly different.
Conclusions:
Decreased DDAH and HO with preeclampsia suggest that they are important points in the regulatory pathways of NO and CO production that are altered in preeclampsia. The ADMA-SDMA ratio is not a useful test for preeclampsia.
Keywords: asymmetric dimethylarginine, carbon monoxide, hemeoxygenase, nitric oxide, preeclampsia
Introduction
Hypertensive disorders of pregnancy complicate 6% to 8% of pregnancies in the United States and are a major cause of both maternal and neonatal morbidity worldwide.1 Improved understanding of the pathophysiology of preeclampsia will result in improved screening and therapeutic options. Despite extensive research, the underlying cause of preeclampsia remains unclear and many competing theories persist. Endothelial dysfunction and abnormal cytotrophoblast invasion of the maternal spiral arteries are important factors resulting in the development of preeclampsia.2 The nitric oxide (NO) and the carbon monoxide (CO) synthesizing pathways are 2 major pathways known to be important in endothelial-mediated vasodilatation.3,4
Nitric oxide is a potent vasodilator produced by NO synthase (NOS) from the substrate l-arginine and is subsequently released from endothelial cells. It is an important regulator of maternal vascular resistance. Abnormal regulation of NO has been implicated in the development of preeclampsia.3 Production of NO by NOS is downregulated by reversible, competitive inhibition of the enzyme by asymmetric dimethylarginine (ADMA).3 The biologic plausibility of the role of this pathway in preeclampsia is supported by the fact that elevated levels of ADMA have been implicated in the pathology of other microvascular diseases.3 However, while numerous studies have demonstrated elevated plasma levels of ADMA in preeclampsia,3,5–11 2 other studies did not demonstrate any difference.12,13 Fewer studies of ADMA in placental tissue have been conducted but levels do not appear to be altered in preeclampsia.14 It is not clear whether placental levels of ADMA correlate with plasma levels or not.
Asymmetric dimethylarginine is itself metabolized to l-citrulline and dimethylamine by the enzyme dimethylarginine dimethylaminohydrolase (DDAH), which exists in 2 isoforms (DDAH-I and DDAH-II).3 Dimethylarginine dimethylaminohydrolase has not been as extensively studied as ADMA in preeclampsia, likely in part because it is not released into the maternal circulation. Dimethylaminohydrolase-I is primarily found in the liver and kidney and DDAH-II is primary found in the endothelium, but both are present in the placenta and metabolize ADMA. Dimethylaminohydrolase-I has been most strongly correlated with a reduction in ADMA levels,3 and preliminary data suggest that DDAH-I has a higher affinity to ADMA than DDAH-II, but further detailed biochemical studies of DDAH-II are needed.15 Two studies examining placental DDAH in preeclampsia had conflicting results.9,13 The isomer symmetric dimethylarginine (SDMA) is not an active inhibitor of NOS and is not metabolized by DDAH.3If DDAH is implicated in the development of preeclampsia, then elevated ADMA levels in relation to SDMA may be expected as it primarily metabolizes ADMA and not SDMA. However, Braekke et al found the ADMA-SDMA ratio to be 12% lower in pregnancies complicated by preeclampsia in comparison to controls.10
Carbon monoxide is another vasodilator that has been recently implicated with the vascular dysfunction of preeclampsia.4 Hemeoxygenases, particularly the inducible hemeoxygenase 1 (HO-1), produce CO with degradation of heme to biliverdin IX. Hemeoxygenases have been identified in the placenta, and it appears that CO plays an essential role with both trophoblast invasion and maintaining placental vascular tone throughout pregnancy.4 However, once again, studies measuring HO-1 expression in placentas from pregnancies complicated by preeclampsia have yielded conflicting results.4,16,17
We conducted a prospective study designed to simultaneously evaluate, at the maternal plasma and placental level, both pathways in pregnancies complicated by preeclampsia compared to healthy controls. We specifically had an interest in the relatively understudied and potentially crucial endothelial cell enzyme DDAH and the potential of using the ADMA-SDMA ratio as a marker in preeclampsia.
Materials and Methods
Study Population
After obtaining institutional review board (IRB) approval, women diagnosed with mild or severe preeclampsia between 34 and 42 weeks of gestation were considered for inclusion. Preeclampsia was defined according to the guidelines of the International Society for the Study of Hypertension in Pregnancy.18 Briefly, preeclampsia was defined as the presence of systolic blood pressure (BP) of ≥140 mm Hg or diastolic BP of ≥ 90 mm Hg on 2 occasions at least 6 hours apart, proteinuria ≥1+ on 2 random dipstick or a 24-hour urine collection of 300 mg or greater. Women with a history of chronic hypertension, preexisting renal disease, tobacco use, or recreational drug use were excluded from participation. The control group consisted of normotensive healthy participants without a history of tobacco or recreational drug use. Eligible participants were enrolled upon consenting to participate in the study.
Specimen Collection
Within 30 minutes of delivery, maternal blood was drawn. The blood was then separated into 1 heparinized tube and 1 tube containing EDTA. The samples were centrifuged at 2000g for 10 minutes and the supernatant transferred to separate tubes and stored at −80 C. Placental biopsies (approximately 1 g) were obtained from the placental bed (maternal–fetal interface). Placental tissue was homogenized in sterile phosphate-buffered saline containing EDTA, centrifuged at 10 000 rpm for 20 minutes and the supernatant transferred to a clean tube prior to freezing at −80 C.
Analysis of Samples
Plasma ADMA and SDMA
Plasma ADMA and SDMA levels were quantified using an enzyme-linked immunosorbent assay (ELISA; Enzo Life Sciences, Plymouth Meeting, Pennsylvania). Standard and testing samples, 20 µL, were pipetted into the respective wells on the plate and subsequently 25 µL of acylation buffer was added to each well. Then 200 μg of equalizing reagent was added to the wells and mixed for 10 seconds. The plate was incubated for 20 minutes on an orbital shaker. In all, 50 µL of the standard, control, and samples were then placed in the respective wells of the coated microtiter strips, with duplicates. Antiserum, 50 µL, was then added and the trays covered and incubated over night. The contents of the wells were then discarded. The wells were washed with buffer 4 times; 100 µL of enzyme conjugate was then placed in the wells followed by 60 minutes of incubation and 4 more washings. The stop solution was then pipetted into each well and the optical density was read at 450 nm in a microplate photometer. The ADMA and SDMA concentrations were then determined using standard curves. The sensitivity was 0.05 µmol/L.
The DDAH-I and DDAH-II
Expression of DDAH-I and DDAH-II was measured from homogenized placental tissue using Western blot analysis. Protein extracts were prepared from homogenates of placental villous tissues in a nondenaturing buffer as described previously.19 Initially the amount of solubalized protein was determined with the detergent compatible protein assay kit II (Bio-Rad Laboratories, Hercules, California). Equal amounts of protein extracts (100-150 μg/lane) were separated on 12% gel sodium dodecyl sulfate–polyacrylamide gel electrophoresis and electrically transferred to polyvinylidene fluoride membranes. For DDAH-I, goat anti-DDAH-I antibody (1:100 dilution, overnight at 4°C; Santa Cruz SC-26068, Santa Cruz, California) was followed by donkey anti-goat immunoglobulin G (IgG; 1:2000 dilution, room temperature for 1 hour; Santa Cruz SC-2020) and for DDAH-II rabbit anti-DDAH-II antibody (1:100 dilution, overnight at 4°C; Santa Cruz SC-32859) was followed by goat anti-rabbit IgG (1:2000 dilution, room temperature for 1 hour; Cell Signaling SC-7074, Danvers, Massachusetts). Detection of bound antibodies was performed with the use of ECL Western blotting detection system (GE Healthcare, Piscataway, New Jersey). Digital images were captured and semiquantitative analysis was performed with NIH Image J software. Data presented are normalized to β-actin and expressed as fold change.
Hemeoxygenase 1
Hemeoxygenase 1 was measured in placental tissue samples using colorimetric immunometric enzyme immunoassay (Stressgen, Ann Arbor, Michigan). Hemeoxygenase 1 standards and samples were added in duplicate to the 96-well anti HO-1 immunoassay plate and then incubated and washed. Diluted anti-HO-1 was added and the specimens were incubated and washed again. Diluted anti-Rabbit IgG-HRP conjugate was then added and the specimens incubated and then washed. A dilution factor of 25 was used. The specimens were developed with tetramethylbenzidine (TMB) substrate and the stop solution added. Absorbance was measured at 450 nm and the concentrations determined by measuring against the HO-1 standard curve. The sensitivity of the test was 0.78 ng/mL.
Total NO (NOx) production
Nitric oxide levels were estimated using the NO quantitation kit (Actif Motif, Carlsbad, California). The kit was used according to the manufacturer’s instructions and ELISA was used to measure nitrate and nitrite (NOx) levels. The detection limit of the test is <1 μmol/L of nitrite/nitrate, and it provides results from 2 to 55 μmol/L. Absorbances were detected with a spectrophotometer using a 540-nm filter. The plasma specimens were appropriately diluted with the assay buffer and filtered through a 10 000-Da micropore filter prior to the assay. The placental samples were homogenized in ice-cold hydrochloric acid (20% wt/vol). The sample was then filtered through a 10 000-Da micropore filter prior to the assay.
Statistical Analysis
Data were managed and analyzed with SPSS statistical software (version 11.5; SPSS, Chicago, Illinois) and JMP (version 9.0; SAS Institute Inc, Cary, North Carolina). A 2-sided Student t test was used for the comparison of continuous variables between the groups and the χ2 test was used for the comparison of categorical variables unless an individual cell had less than 6 observations in which case Fisher exact test was used. A P value of .05 or less was considered to be statistically significant.
Results
A total of 43 patients were enrolled in the study between January 2005 and August 2007 at an academically affiliated community hospital. Of the 20 participants included in the preeclampsia group, 10 had severe preeclampsia. At enrollment, 2 women had a platelet count of below 100 000/mL, 2 women had a serum glutamic pyruvic transaminase (SGPT) and serum glutamic oxaloacetic transaminase (SGOT) of greater than 72 U/L, and 2 had creatinine levels above 1.0 mg/dL. No women developed eclampsia and all women with preeclampsia received magnesium sulfate for seizure prophylaxis.
The preeclampsia specimens were collected between January 2005 and July 2006, and the control specimens were collected from March 2006 to August 2007. The patient characteristics are presented in Table 1. There were no significant differences between the 2 groups in regard to race or maternal age. Gestational age at delivery and infant birth weight were lower in the preeclampsia group and the women in this group were more likely to be nulliparous and obese. Due to technical problems in sampling and in running the assays, there were some samples and assays that were not collected and performed for each participant. This is indicated in the appropriate figures.
Table 1.
Patient Characteristics.a
| Characteristic | Preeclampsia (n = 20) | Control (n = 23)b | P Value |
|---|---|---|---|
| Maternal age (years) | 29.8 ± 9.1 | 28.2 ± 6.7 | .54 |
| Race/ethnicity | .66 | ||
| White | 5 (25) | 4 (18.2) | |
| Black | 4 (20) | 5 (22.7) | |
| Hispanic | 9 (45) | 11 (55) | |
| Other | 2 (10) | 2 (9.1) | |
| Nulliparous | 15 (75) | 7 (31.8) | < .01 |
| Gestational age at delivery (week gestation) | 37.6 ± 2.2 | 39.6 ± 1.1 | < .01 |
| Maximum systolic blood pressure (mm Hg) | 164.2 ± 17.3 | 121.6 ± 13.5 | < .01 |
| Body mass index (kg/m2) | 40.0 ± 12.5 | 32.1 ± 7.7 | .03 |
| Birth weight (g) | 2852 ± 972 | 3481 ± 438 | .01 |
| Cesarean delivery | 13 (65.0) | 7 (31.8) | .06 |
a Data presented as n (%) or mean ± SD.
b Data unavailable for 1 participant.
Maternal Plasma
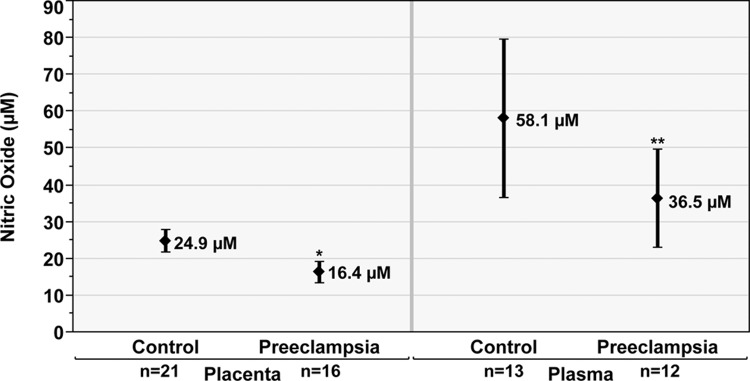
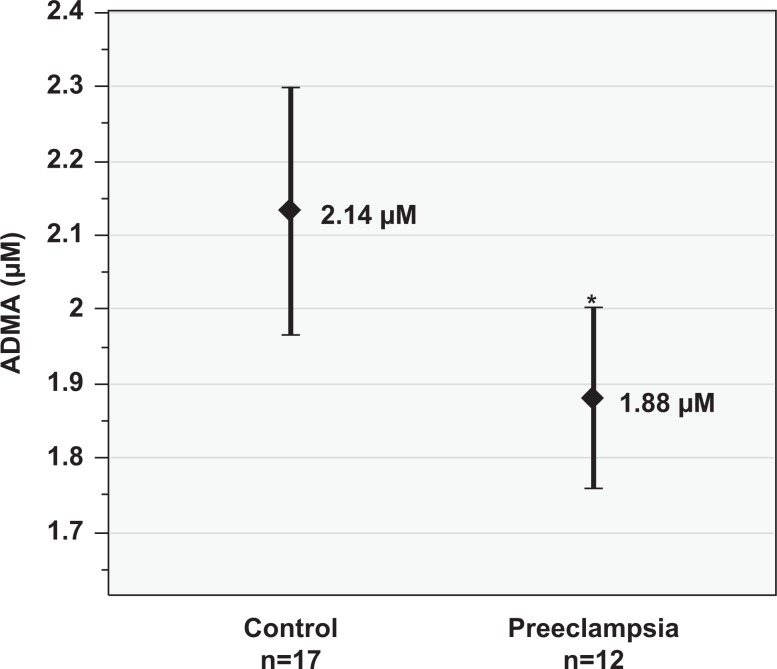
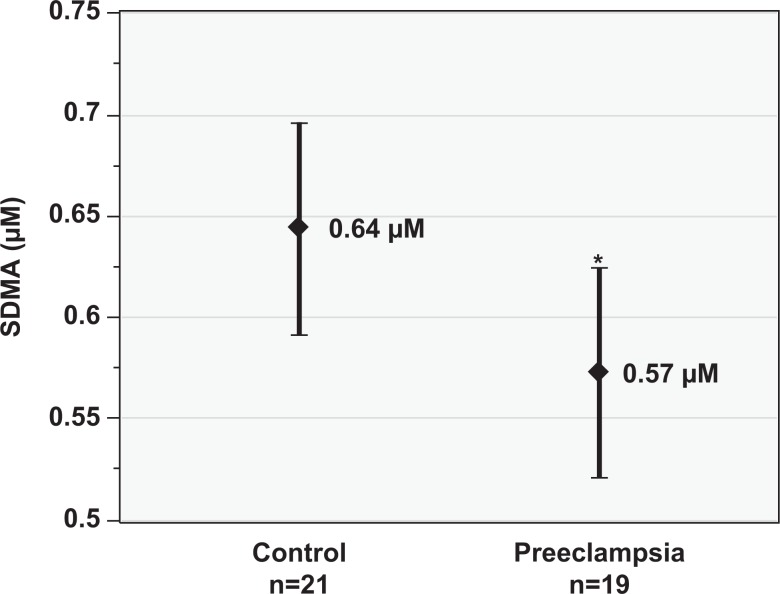
Total plasma NOx levels (mean ± standard deviation [SD], µmol/L) were lower in the preeclampsia group (36.5 ± 20.9) compared to the control group (58.1 ± 40.3); however, this did not reach statistical significance (P = .08, Figure 1). Maternal plasma ADMA (mean ± SD, µmol/L) was lower in the preeclampsia group (1.88 ± 0.19) than the control group (2.14 ± 0.33; P < .01, Figure 2). Similarly, SDMA (mean ± SD, µmol/L) levels were lower in the preeclampsia group (0.57 ± 0.11) than the control group (0.64 ± 0.12; P = .03, Figure 3). The ADMA-SDMA ratio was 9% higher in the preeclampsia group compared to the control group; however, this was not statistically significant (P = .89).
Figure 1.
Placental and plasma nitric oxide (NOx, µmol/L) in the preeclampsia compared to control groups. Data are reported as mean and 95% confidence interval. *P < .01 versus control group. **P = .08 versus control group.
Figure 2.
Plasma asymmetric dimethylarginine (ADMA, µmol/L) in the preeclampsia versus control groups. Data are reported as mean and 95% confidence interval. *P < .01 versus control group.
Figure 3.
Plasma asymmetric dimethylarginine (ADMA, µmol/L) versus control groups. Data are reported as mean and 95% confidence interval. *P < .03 versus control group.
Placenta
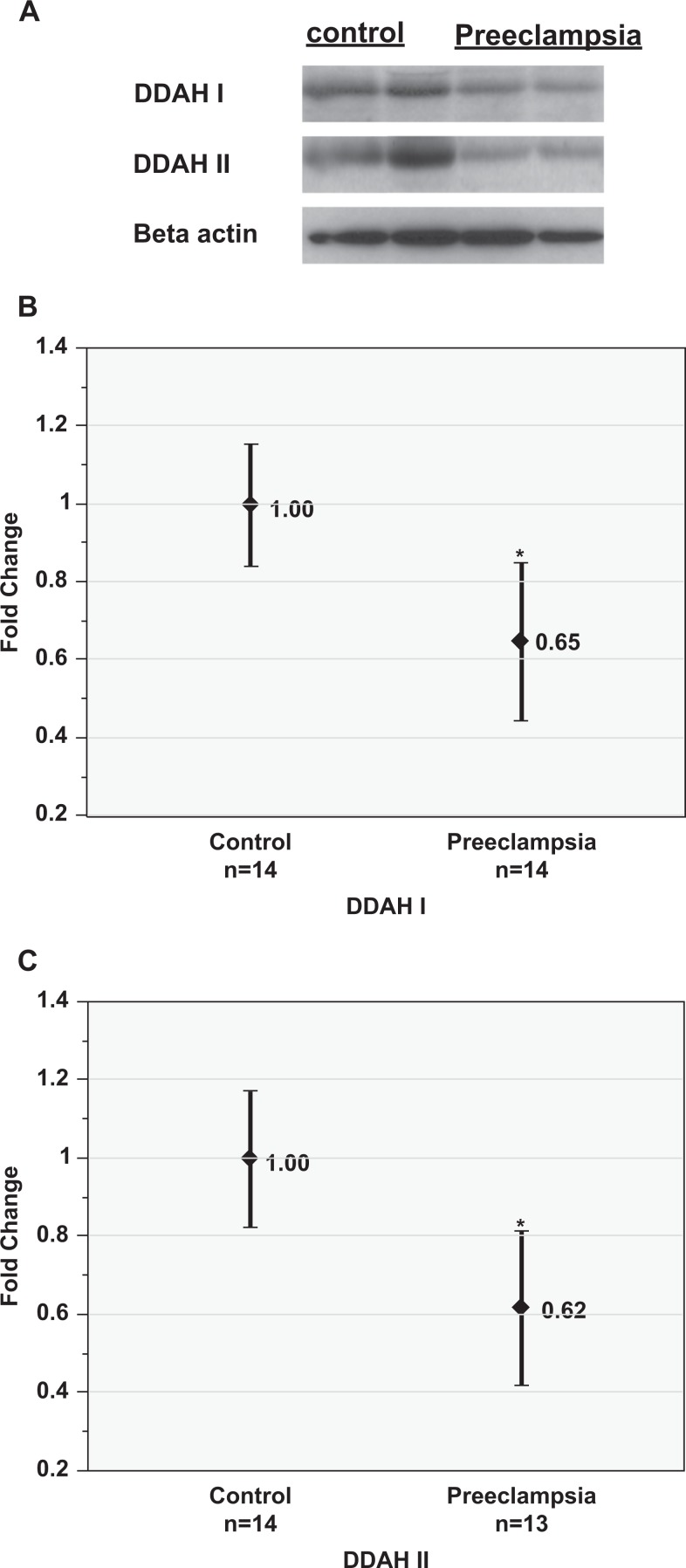
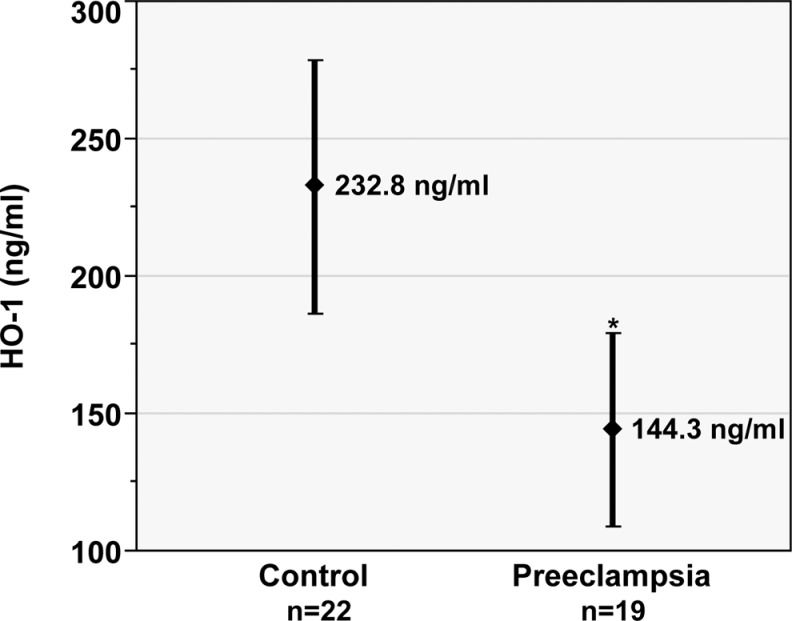
In the placental specimens, total NOx levels (mean ± SD, µmol/L) were lower in the preeclampsia group (16.4 ± 5.5) than the control group (24.9 ± 6.9; P < .01, Figure 1). Western blot studies demonstrated significantly reduced protein expression of both DDAH-I (0.65 ± 0.34) and DDAH-II (0.62 ± 0.33), measured as fold change (mean ± SD) in the preeclampsia group compared to that in controls (P < .01, Figure 4). Placental HO-1 concentrations (mean ±SD, ng/mL) were also lower in the preeclampsia group (144.3 ±72.6) than that in the control group (232.8 ± 103.6; P < .01, Figure 5).
Figure 4.
Placental expression of dimethylaminohydrolase (DDAH) I and II proteins in preeclampsia versus control groups. A, Representative Western blots showing DDAH-I and DDAH-II proteins in the control and preeclampsia groups. β-actin served as an equal protein loading control. Quantification of DDAH-I (B) and DDAH-II (C) proteins in preeclampsia compared to controls. Data are reported as mean and 95% confidence interval. *P < .01 versus control group.
Figure 5.
Placental expression of hemeoxygenase 1 (HO-1) protein in preeclampsia compared to controls. Data are reported as mean and 95% confidence interval. *P < .01 versus control group.
Discussion
Our findings support the theories that there are abnormalities in the regulation of both NO and CO synthesizing pathways in the setting of preeclampsia. All placental tissue concentrations of NO, HO-1, DDAH-I, and DDAH-II that we hypothesized would be lower in preeclampsia were found to be decreased in our study population. Specifically, our data support the theory that reduced DDAH-I and DDAH-II activity may be important in the pathogenesis of preeclampsia, leading to reduced production of NO and maternal vasodilatation.
The lower placental tissue concentrations of DDAH-I and DDAH-II support the findings of Ellis et al that this may be an integral factor in the development of preeclampsia.9However, this decrease in DDAH concentration was expected to be associated with elevated levels of maternal plasma ADMA in the preeclampsia group, while our data actually showed lower ADMA levels. In attempting to explain these apparently contradictory results, it is important to note that all assays were performed shortly after specimen collection, with the exception of the ADMA and SDMA assays that were delayed due to logistical and technical limitations. The importance of time in the possible degradation of the enzymes of interest in the frozen specimens is unclear. This, and the fact that the ADMA and SDMA findings are not consistent with most previous studies,3,5–11 lead us to believe that the ADMA and SDMA results should be viewed with caution and that the findings regarding DDAH-I and DDAH-II are valid and important. This is further supported by the finding of decreased placental NO concentrations in the preeclampsia group.
While decreased DDAH expression in the preeclampsia group was expected to correlate with elevated ADMA levels, SDMA levels were expected to remain unchanged; thus we expected to find a higher ADMA-SDMA ratio in the setting of preeclampsia. However, the SDMA maternal plasma concentrations were also significantly higher in controls, leading to no significant difference in the ADMA-SDMA ratio between the 2 groups. Neither our study nor Braekke et al demonstrated an increase in the ADMA-SDMA ratio as hypothesized. Symmetric dimethylarginine is renally cleared; potentially, its clearance is compromised in preeclampsia and therefore its levels are not proportionally different than those of ADMA.10
Our data support the theory that abnormal CO production by HO-1 at the level of the placenta plays a role in the development of preeclampsia. We recognize that data from previous studies of this pathway have been particularly contradictory, which may be in part due to the variability in patient population, laboratory methods, or severity of disease.4,16,17 Similar to our findings, Ahmed et al demonstrated lower placental HO-1 levels in pregnancies complicated by preeclampsia.16 McLaughlin et al actually showed an increase in HO-1; however, their study included only 4 participants in each group20; Barber et al failed to detect HO-1 in the placenta.17 Based on our findings, the studies discussed above, and biologic plausibility, a compelling argument can be made that abnormalities of HO-1 expression and CO production play an integral role in the development of preeclampsia.
The major strength of our study lies in its inclusion of both the major endothelial cell pathways regulating maternal vascular tone and the finding that the functions of both pathways were significantly depressed in the preeclampsia group. A second strength was our inclusion of measurements from both the placenta and the maternal plasma. In the future, this approach may allow differentiation between dysfunction at the level of the placental interface versus the maternal periphery and evaluation for compensating responses.
The small sample size is a limitation of this study that is unfortunately shared by many similar studies. This limited our ability to perform subgroup analyses, evaluate the interaction between the 2 pathways, or adjust for important potential confounders, such as obesity or parity. While the difference in gestational age was statistically significant, it is of questionable clinical significance.
Our data, in combination with the existing literature, suggest that the clinical syndrome of preeclampsia results from a complex interaction between multiple pathways and responses that are influenced by a variety of environmental and genetic factors. Thus, the ideal future study would prospectively evaluate a large number of patients for multiple markers, starting in the first trimester of pregnancy. Additionally, genomics may prove to play a pivotal role in the future. For example, Akbar et al reported on 132 women with preeclampsia and 112 controls and uncovered 8 single-nucleotide polymorphisms forming 4 common haplotypes in the DDAH gene, 2 of which were associated with preeclampsia.21 It may be that different women respond best to different treatments or preventative measures and that genomics can assist in determining the appropriate management for each individual.
The ultimate purpose of understanding these pathways is to design interventions that target specific deficiencies or weaknesses and either prevent or reduce the severity of the disease. For example, therapies that either upregulate CO or NO production or would offer similar biologic effects may be helpful. A recent trial conducted in Mexico demonstrated that administration of l-arginine in combination with vitamins to high-risk women during pregnancy reduced the risk of preeclampsia.22 Some small trials evaluating supplementation of l-arginine in patients with preeclampsia have suggested that such treatment may achieve a reduction in blood pressure, but these studies were not sufficiently powered to assess safety or for clinically important outcomes.23 Our data suggest that DDAH activators may be a novel treatment or preventative option. The most effective therapies may be those that target first or second trimester, preclinical abnormalities.
Therapies for the prevention and treatment of preeclampsia carry the potential to dramatically reduce maternal and neonatal morbidity worldwide. The NO and CO synthesizing pathways regulating maternal endothelial cell function have each been inconsistently, and yet convincingly, implicated in the pathogenesis of this disease. This study provides further evidence that both pathways are important and dysfunctional in preeclampsia. Taken in context of the existing literature, we believe that further large, prospective trials anticipating heterogeneity of underlying etiologies, as well as studies to further investigate the role of genomics in the development and prevention of preeclampsia are crucial.
Footnotes
Authors’ Note: This study was presented at the 31st Society of Maternal–Fetal Medicine Annual Meeting, San Francisco, California, February 12, 2011.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial Support for this project was provided by the MemorialCare Foundation (MHS 801001-R142030).
References
- 1.Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102(1):181–192. [DOI] [PubMed] [Google Scholar]
- 2.Merviel P, Carbillon L, Challier JC, Rabreau M, Beaufils M, Uzan S. Pathophysiology of preeclampsia: links with implantation disorders. Eur J Obstet Gynecol Reprod Biol. 2004;115(2):134–147. [DOI] [PubMed] [Google Scholar]
- 3.Boger RH, Diemert A, Schwedhelm E, Luneburg N, Maas R, Hecher K. The role of nitric oxide synthase inhibition by asymmetric dimethylarginine in the pathophysiology of preeclampsia. Gynecol Obstet Invest. 2010;69(1):1–13. [DOI] [PubMed] [Google Scholar]
- 4.Bainbridge SA, Smith GN. HO in pregnancy. Free Radic Biol Med. 2005;38(8):979–988. [DOI] [PubMed] [Google Scholar]
- 5.Fickling SA, Williams D, Vallance P, Nussey SS, Whitley GS. Plasma concentrations of endogenous inhibitor of nitric oxide synthesis in normal pregnancy and pre-eclampsia. Lancet. 1993;342(8865):242–243. [DOI] [PubMed] [Google Scholar]
- 6.Holden DP, Fickling SA, Whitley GS, Nussey SS. Plasma concentrations of asymmetric dimethylarginine, a natural inhibitor of nitric oxide synthase, in normal pregnancy and preeclampsia. Am J Obstet Gynecol. 1998;178(3):551–556. [DOI] [PubMed] [Google Scholar]
- 7.Speer PD, Powers RW, Frank MP, Harger G, Markovic N, Roberts JM. Elevated asymmetric dimethylarginine concentrations precede clinical preeclampsia, but not pregnancies with small-for-gestational-age infants. Am J Obstet Gynecol. 2008;198(1):112.e1–112.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pettersson A, Hedner T, Milsom I. Increased circulating concentrations of asymmetric dimethyl arginine (ADMA), an endogenous inhibitor of nitric oxide synthesis, in preeclampsia. Acta Obstet Gynecol Scand. 1998;77(8):808–813. [PubMed] [Google Scholar]
- 9.Ellis J, Wennerholm UB, Bengtsson A, et al. Levels of dimethylarginines and cytokines in mild and severe preeclampsia. Acta Obstet Gynecol Scand. 2001;80(7):602–608. [PubMed] [Google Scholar]
- 10.Braekke K, Ueland PM, Harsem NK, Staff AC. Asymmetric dimethylarginine in the maternal and fetal circulation in preeclampsia. Pediatr Res 2009;66(4):411–415. [DOI] [PubMed] [Google Scholar]
- 11.Mao D, Che J, Li K, et al. Association of homocysteine, asymmetric dimethylarginine, and nitric oxide with preeclampsia. Arch Gynecol Obstet. 2010;282(4):371–375. [DOI] [PubMed] [Google Scholar]
- 12.Maas R, Boger RH, Schwedhelm E, et al. Plasma concentrations of asymmetric dimethylarginine (ADMA) in Colombian women with pre-eclampsia. JAMA. 2004;291(7):823–824. [DOI] [PubMed] [Google Scholar]
- 13.Siroen MP, Teerlink T, Bolte AC, et al. No compensatory upregulation of placental dimethylarginine dimethylaminohydrolase activity in preeclampsia. Gynecol Obstet Invest 2006;62(1):7–13. [DOI] [PubMed] [Google Scholar]
- 14.King RG, Di Iulio JL, Gude NM, Brennecke SP. Effect of asymmetric dimethyl arginine on nitric oxide synthase activity in normal and pre-eclamptic placentae. Reprod Fertil Dev 1995;7(6):1581–1584. [DOI] [PubMed] [Google Scholar]
- 15.Pope AJ, Karuppiah K, Cardounel AJ. Role of the PRMT-DDAH-ADMA axis in the regulation of endothelial nitric oxide production. Pharmacol Res. 2009;60(6):461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed A, Rahman M, Zhang X, et al. Induction of placental heme oxygenase-1 is protective against TNFalpha-induced cytotoxicity and promotes vessel relaxation. Mol Med. 2000;6(5):391–409. [PMC free article] [PubMed] [Google Scholar]
- 17.Barber A, Robson SC, Myatt L, Bulmer JN, Lyall F. Heme oxygenase expression in human placenta and placental bed: reduced expression of placenta endothelial HO-2 in preeclampsia and fetal growth restriction. FASEB J. 2001;15(7):1158–1168. [DOI] [PubMed] [Google Scholar]
- 18.Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy. 2001;20(1): IX–XIV. [DOI] [PubMed] [Google Scholar]
- 19.Liao WX, Magness RR, Chen DB. Expression of estrogen receptors-alpha and -beta in the pregnant ovine uterine artery endothelial cells in vivo and in vitro. Biol Reprod. 2005;72(3):530–537. [DOI] [PubMed] [Google Scholar]
- 20.McLaughlin BE, Lash GE, Smith GN, et al. Heme Oxygenase Expression in Selected Regions of Term Human Placenta. Exp Biol Med. 2003;228(5):564–567. [DOI] [PubMed] [Google Scholar]
- 21.Akbar F, Heinonen S, Pirskanen M, Uimari P, Tuomainen TP, Salonen JT. Haplotypic association of DDAH1 with susceptibility to pre-eclampsia. Mol Hum Reprod. 2005;11(1):73–77. [DOI] [PubMed] [Google Scholar]
- 22.Vadillo-Ortega F, Perichart-Perera O, Espino S, et al. Effect of supplementation during pregnancy with L-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: randomised controlled trial. BMJ. 2011;342: d2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rytlewski K, Olszanecki R, Korbut R, Zdebski Z. Effects of prolonged oral supplementation with l-arginine on blood pressure and nitric oxide synthesis in preeclampsia. Eur J Clin Invest. 2005;35(1):32–37. [DOI] [PubMed] [Google Scholar]