Abstract
Steroidogenesis in testicular cells depends upon the availability of cholesterol within testicular mitochondria besides the activities of 3β-hydroxysteroid dehydrogenase (3β-HSD, 17β-hydroxysteroid dehydrogenase [17b-HSD]), and the tissue levels of steroidogenic acute regulatory protein (StAR), androgen-binding protein (ABP), and testosterone (T). Cellular cholesterol biosynthesis is regulated by endogenous oxycholesterols acting through nuclear hormone receptors. Plant oxysterols, such as 28-homobrassinolide (28-HB), available to human through diet, was shown to exhibit antihyperglycemic effect in diabetic male rat. Its role in rat testicular steroidogenesis and lipid peroxidation (LPO) was therefore assessed using normal and streptozotocin-induced diabetic male rats. Administration of 28-HB (333 µg/kg body weight) by oral gavage for 15 consecutive days to experimental rats diminished LPO, increased antioxidant enzyme, 3β-HSD and 17β-HSD activities, and elevated StAR and ABP expression and T level in rat testis. We report that 28-HB induced steroidogenesis in normal and diabetic rat testis.
Keywords: 28-homobrassinolide, phyto-oxysterol, testis, lipid peroxidation, steroidogenesis
Introduction
Cellular-signaling processes are mediated through cytoplasmic- and/or membrane (plasma or nuclear)-localized protein receptors in animal cells. Steroids and peptide growth factors functioned as ligands to such receptors to induce cell signaling. Oxygenated steroids remain as an additional group of signal-transducing ligands. Cytochrome P450 catalyses the oxygenation of endogenous cholesterol and yield oxycholesterol (oxysterol) compounds. These oxycholesterols signaled through nuclear membrane–localized receptors to autoregulate cholesterol biosynthesis in animal cells.1 Some oxycholesterols have now been considered as possible ligands to the nuclear orphan receptors.2 Oxysterols are available through food due to their ubiquitous presence in plant and animal kingdom. Brassinostreroids are plant oxysterols that are present at 10 to 100 µg/kg wet plant tissue. Dietary consumption of these compounds by herbivores and omnivores allow for their assimilation in the animal cells with a potential to affect cell function and growth in the host in a temporal manner. In recent years, phytohormones have been considered biologically significant in animal health and in agriculture. Hormones, in general, work with the immune and nervous systems to regulate growth, immunity, metabolism, reproduction, cognition, and behavior. Altering hormonal signals can therefore affect tissue functions leading to health disorders. Phytohormones can have substantial effect on human health when consumed through fortified foods and herbal medications.3 Information on their in vivo influences on cellular ultrastructure, metabolism, reproduction, and genetics in higher animals however remains very limited.
The biosynthesis of all hormonal steroids in response to trophic hormone and other steroidogenic stimuli begins with cholesterol to form the first steroid, pregnenolone, synthesized in all steroidogenic tissues.4 Subsequently, the synthesis of testosterone (T) is under the influence of 3β- and 17β-hydroxy steroid dehydrogenases. Steroidogenesis is dependent on the complex cellular process of cholesterol transport to inner mitochondrial membrane, a rate-limiting step in steroid biosynthesis. Steroidogenic acute regulatory protein (StAR) is crucial for the transport of cholesterol to mitochondria where the biosynthesis of steroids is initiated.
Testosterone is primarily produced by the Leydig cells and is critical for developmental and reproductive functions in the male. The androgen-binding protein (ABP) produced by Sertoli cells in the seminiferous tubules of the testis specifically binds T with high affinity and transports them to the epididymis. Leydig cell function is reportedly impaired by a decrease in T production as a consequence of suppressed CYP11A1, CYP17A1, 3β-HSD and 17β-HSD activities.5,6 The intratissue sex steroid concentration is implicated as a significant marker in evaluating the expression of steroid-metabolizing enzymes in a tissue.7 Studies investigating the effects of 28-homobrassinolide (28-HB) on diabetic male rats indicated antihyperglycemic potency in this phytohormone.8 Since hyperglycemia was known to suppress testicular and ovarian steroidogenesis in the rat,9 it provided a basis for evaluating the biopotency of this oxysterol in rat testicular steroidogenesis. The present study was designed to elucidate the effects of 28-HB on testicular steroidogenesis in normal and streptozotocin (STZ)-induced diabetic rats.
Materials and Methods
Chemicals, Reagents, and Antibodies
Streptozotocin was purchased from Sigma-Aldrich (St. Louis, Missouri). 28-Homobrassinolide (technical grade) used in the study was received courtesy of Dr Vyas, Godrej Agrovet, Mumbai, India. Actin (sc-7210) was purchased from Santa Cruz Biotechnology (Santa Cruz, California). Polyclonal antibody against rat ABP was a gift from Dr C. Y. Cheng, The Population Council, New York. Antisera against a synthetic peptide consisting of amino acid sequences 88 to 98 of the mouse StAR protein generated in rabbit was a gift from Dr D. M. Stocco, Texas Tech University Health Science Centre, Lubbock, Texas. Horseradish peroxidase–conjugated goat anti-rabbit immunoglobulin G (IgG) was obtained from Bangalore Genei (Bangalore, India). All other chemicals used for various assays were of analytical grade and were obtained from local commercial source.
Experimental Design
Male Wistar strain rats were obtained from Sri Ragavendra Enterprises, Bangalore, India. The animals were housed in polypropylene cages with autoclaved paddy husk as bedding and were provided with standard laboratory chow and tap water ad libitum. The animals were maintained at 24°C ± 2°C under a well-regulated dark and light (12:12 hours) schedule. The experiments were carried out in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA 2003), Government of India. Normal rats weighing 180 to 250 g were divided into 4 groups as follows. Group I: control, group II: rats treated with 28-HB, group III: diabetic rats, and group IV: diabetic rats treated with 28-HB. Diabetes was induced with STZ by a single dose of 50 mg/kg body weight ([bw] dissolved in 0.1 mol/L of citrate buffer, pH 4.5) administered intraperitoneally. The control rats were administered citrate buffer alone. Diabetes in STZ-treated rats was confirmed by determining the fasting blood glucose content, 72 hours postinjection employing a portable glucose analyzer on rat tail blood samples. Control blood glucose content was measured in the animals prior to tissue collection. Animals whose blood glucose content showed greater than 250 mg/dL were considered diabetic and used for the experiment. Treatment group of rats were administered 50 µg of 28-HB in 50 μL of 50% ethanol (333 μg/kg bw) through oral gavage for 15 consecutive days. Control group of animals were given an equal volume of the vehicle alone. The selection of the doses was based on earlier data published from this laboratory.10 All groups of animals were allowed free access to a standard diet. The weight of each animal was monitored until the end of the treatment period. Twenty-four hours following the last dose, the animals were anesthetized and killed by cervical dislocation.
Preparation of Tissue Homogenate
Rat testes were dissected out, weighed, and the tissue homogenate 10% (w/v) was prepared in cold 0.1 mol/L phosphate buffer, pH 7.4 using a glass Teflon homogenizer (Remi RQ-127A, Remi motors, Mumbai, India). The homogenate was centrifuged at 10 000g in a Sorvall RC-5C refrigerated centrifuge for 30 minutes and the supernatants were collected and used for the biochemical assays described below.
Testicular Steroidogenic Enzyme Activities
The activities of 3β- and 17β-hydrogenases were measured by the method of Bergmeyer et al.11 The reaction mixture in a volume of 2.0 mL contained 100 µmol of sodium pyrophosphate buffer (pH 9.0) and 0.5 µmol cofactor nicotinamide adenine dinucleotide (NAD) for 3β-hydroxysteroid dehydrogenase (3β-HSD) and nicotinamide adenine dinucleotide phosphate (NADPH) for 17β-HSD, 0.08 µmol of substrate (dehydroepiandrosterone for 3β-HSD and androstenedione for 17β-HSD), and 100 µL of coenzyme source. The reactions were carried out in a quartz cuvette of 1.0 cm path length at 23°C. The absorbance at 340 nm was measured at an interval of 20 seconds for 3 minutes in a ultraviolet (UV)-visible spectrophotometer (UV-1700, Shimadzu, Japan). The specific activities of the enzymes were expressed as nmol of NAD converted to NADH/mg protein/min for 3β-HSD or nmol of NADPH converted to NADP/mg protein/min for 17β-HSD.
Immunoblot Analysis of StAR and ABP in Testis
Immunoblotting was used to assay the key steroidogenesis factors StAR and ABP. The protein content of testis homogenate supernatant was determined by Lowry method.12 Protein-matched samples were applied to different lanes of a 10% sodium dodecyl sulfate (SDS)–polyacrylamide gels for electrophoresis (Mini Proteau II system, Biorad, Hercules, California) by the Laemmli method.13 Electrophoresis was performed at 75 V and the resolved proteins were electrophoretically transferred onto a nitrocellulose membrane (NYTRAN, Keene, New Hampshire) using the transfer buffer (0.2 mol/L glycine, 25 mmol/L Tris, and 20% methanol, pH 8.5). Successful transfer was confirmed by staining the blots with Ponceau S. The protein blots were incubated in blocking buffer (phosphate-buffered saline containing 0.1% [v/v] Tween 20 and 5% [w/v] nonfat dry milk powder) for 5 hours at room temperature followed by incubation with StAR antisera (1:1000 dilution) generated in rabbit. The incubation with the primary antibody was carried out overnight at 4°C. The following day, the blots were washed in phosphate-buffered saline and incubated for 1 hour at room temperature with horseradish peroxidase–conjugated anti-rabbit IgG (1 in 1000 dilution). Immunodetection of proteins were revealed using tetramethylbenzidine/H2O2 (Bangalore Genei) as substrate and the resulting immune-specific bands were quantified by densitometry. In the case of ABP, blots were incubated with primary antibody for ABP (1 in 1000) overnight at 4°C and processed for blot analysis and densitometry.
Testosterone Assay
Rat blood plasma T concentration was measured according to the protocol described by enzyme-linked immunosorbent assay using Pathozyme kit, Omega diagnostics, Scotland, UK. The intensity of the color developed was inversely proportional to the concentration of T in the sample and was expressed as ng/mg tissue.
Evaluation of Tissue Antioxidant Enzymes and Protein Content
The thiobarbituric acid reactive substance (TBARS) levels, measured as an index of malondialdehyde (MDA) production and hence lipid peroxidation (LPO), were determined by the method of Ohkawa et al.14 Measurement of superoxide dismutase (SOD) activity was carried out by the method of Marklund and Marklund.15 Catalase activity was assayed by the method of Claiborne16 and reduced glutathione activity by the method of Beutler et al.17 Protein estimation was carried out by the method of Lowry et al.12
Statistical Analysis
Data were expressed as mean ± standard deviation for 6 animals per group. Statistical analyses were performed by 1-way analysis of variance followed by Tukey posttest using SPSS (student version 7.5, SPSS Inc, UK). P < .05 was taken as statistically significant. Images were compiled using Adobe Photoshop (version 7.0, San Jose, California). Densitometric scanning was performed using Gene tool (version 3.05, Synoptics Ltd, Cambridge, UK).
Results
The normal glucose content in Wistar rats range from 50 to 135 mg/100 mL. When the blood glucose content exceeds 200 mg/100 mL, the animals are considered diabetic. In our studies, the STZ-treated rats had a blood glucose content >350 mg/dL. Experimental animals orally administered with 28-HB (50 μg/mL) for 15 consecutive days not only exhibited reduced circulating blood glucose content but also yielded changes in the activities of 3β- and 17β-HSD enzymes and in the testicular content of StAR and ABP. A decrease in the activity of antioxidant enzymes and an increase in LPO in rat testis were noted following STZ administration. 28-Homobrassinolide administration for 15 days decreased the tissue LPO and increased the antioxidant properties in these tissues.
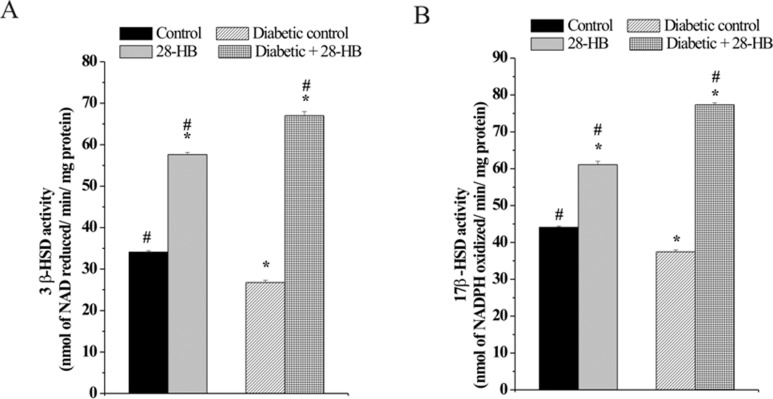
Effect of 28-HB on 3β- and 17β-HSD Enzyme Activities
The activity of the steroidogenic enzymes 3β- and 17β-HSD was lower in diabetic rats compared to the normal rats. The 3β-HSD enzyme activity of normal rat testis elevated 71% and that of diabetic rats to 152% above the respective controls following 28-HB treatment. In diabetic control, 3β-HSD activity was noted 28% below the normal tissue content (Figure 1A). Similarly, the 17β-HSD enzyme activity of normal rat testis increased 39% and that of diabetic rat testis 105% following 28-HB treatment (Figure 1B). The diabetic control was 14% below that of normal control testicular enzyme activity.
Figure 1.
Effect of 28-homobrassinolide on the specific activity of 3β-hydroxysteroid dehydrogenase (A) and 17β-hydroxy steroid dehydrogenase (B) in rat testis. n = 6; P < .05.
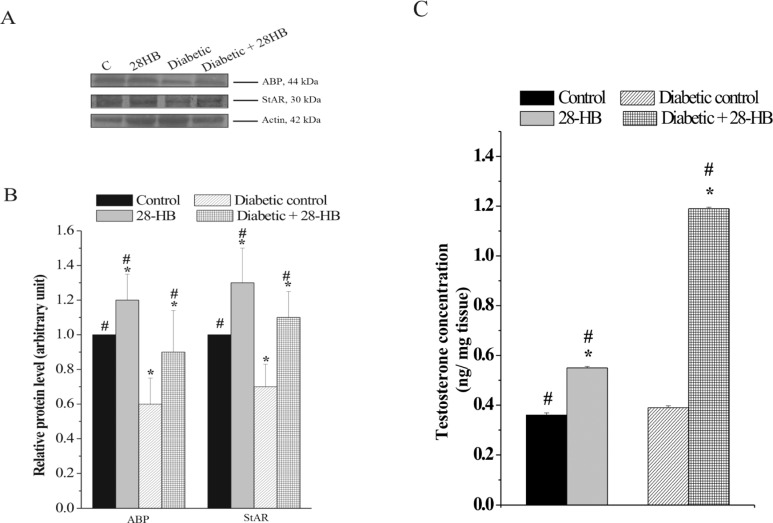
Effect of 28-HB on ABP and StAR Protein Content
A 30% to 37% increase in ABP and StAR protein content was detected as a consequence of 28-HB induction in normal and diabetic rats. However, the concentration of both the proteins decreased in diabetic control when compared to normal control rats (Figure 2A and B).
Figure 2.
Changes in the levels of ABP and StAR protein in the testis of 28-homobrassinolide-treated rats. A, Immunoblots showing significant increase in proteins both in normal and in diabetic rats at the dose of 333 µg/kg body weight of the pytohormone. The blots were probed with actin to show equal protein loading. B, Graph representing densitometrically scanned results corresponding to the blots depicted in A. The control was arbitrarily set at 1. C, Effect of 28-homobrassinolide on testicular testosterone levels. n = 6; P < .05. ABP indicates androgen-binding protein; StAR, steroidogenic acute regulatory protein.
Effect of 28-HB on Testicular T Content
Measurement of tissue T level in rats indicated 57% elevation of this steroid content in the normal tissue and 120% in diabetic tissue following 28-HB administration. Both controls were however closely matched in their values (Figure 2C).
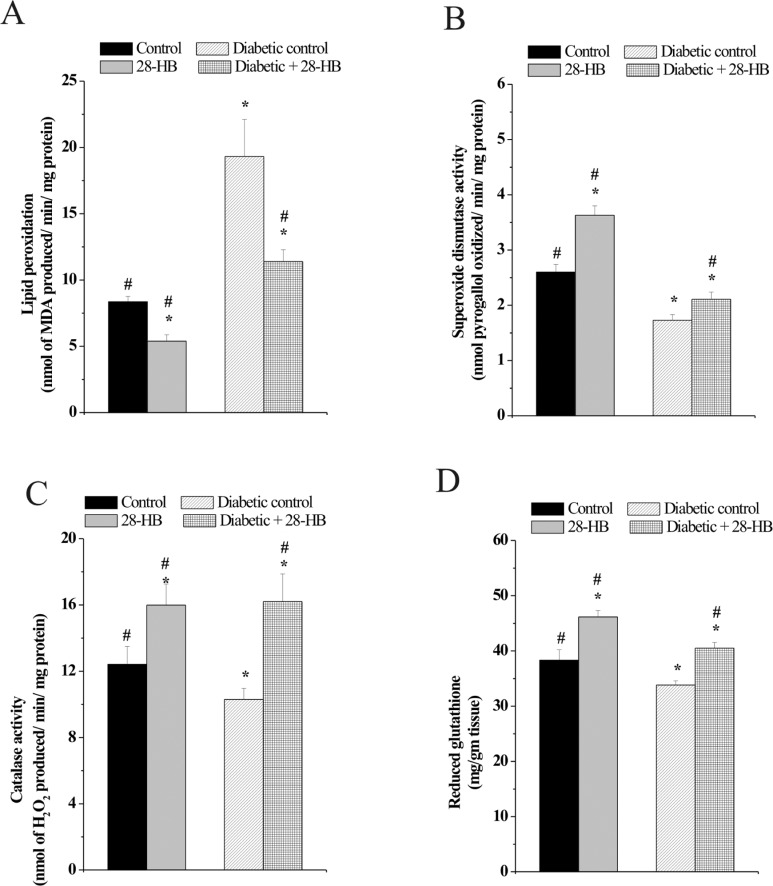
Effect of 28-HB on LPO and Antioxidant Enzymes
In the present study, a decrease in the activities of antioxidant enzymes and an increase in the LPO level in testis following STZ administration were observed. Treatment with 28-HB for 15 days, on the contrary, decreased LPO and increased the activities of antioxidant enzymes. The potent antioxidant property of 28-HB was recognized through the measurement of SOD and catalase activities and tissue glutathione (GSH) content in normal and diabetic-treated rats.
The MDA content was determined to assess the LPO status in rat testicular cells. Peroxidation damage noted in the diabetic control rats was nearly 138% greater than the corresponding control rat testicular cells. Administration of 28-HB diminished the MDA generated by 38% in control rat testicular cells, while the reduction was noted to be 42% in diabetic control rat testicular cells (Figure 3A).
Figure 3.
Effect of 28-homobrassinolide on lipid peroxidation (A), superoxide dismutase activity (B), catalase activity (C), and GSH activity (D) in rat testis. n = 6; P < .05. GSH indicates glutathione.
Antioxidant enzyme, SOD measurement indicates a 40% enhancement in activity in control rats due to 28-HB induction, whereas SOD activity in diabetic control was 35% less than the normal control and increased to about 24% following 28-HB treatment (Figure 3B). Similarly measurement of GSH content in the rat testis indicated a 13% reduction in diabetic control compared to normal and a similar reduction in the 28-HB-treated diabetic and control rat tissues. In either instance however, 28-HB augmented GSH content of normal and diabetic control in a closely related manner (Figure 3D). Increase in catalase activity following 28-HB treatment was noted in normal and diabetic rat testis relative to their respective controls. An 18% decrease in catalase enzyme activity was noted in diabetic rat testis compared to the normal tissue (Figure 3C). The catalase activity of normal rat testis increased 28% following treatment with 28-HB and 57% increase in case of the diabetic rat testis relative to their controls.
Discussion
Steroidogenesis is a multifactorial biological process that occurs under regulation of endogenous hormones. Though androgens and estrogens are known to be involved in reproductive maturation and function,18 endogenous oxysterols seem to occupy a critical role in the management and regulation of steroidogenesis in mammals.19,20 In diabetic rats, it has been reported that testicular and ovarian steroidogenesis remain suppressed due to hyperglycemia.9 Earlier studies by Muthuraman and Srikumar8 employing STZ diabetic male rats suggested that the plant brassinosteroid isoform, 28-HB, renormalized hyperglycemic levels of circulating blood glucose in the experimental rats. Since brassinosteroids as plant oxysterols bear close structural relationship to animal oxycholesterol, the specific effects of the phytooxysterol 28-HB on rat testicular steroidogenesis was therefore investigated using groups of control and experimentally induced diabetic male rats.
Steroidogenesis is dependent upon the availability of cholesterol in the inner mitochondrial membrane. Though other proteins may be involved in the replenishment of mitochondrial cholesterol, there is abundant biochemical, clinical, and genetic evidences to implicate StAR as a labile protein mediator21 for the process. Overexpression of StAR in mouse Leydig cells reportedly increased the basal steroidogenic rate.22 Our studies show that changes in the StAR protein content following 28-HB treatment (Figure 2A and B) augmented steroidogenesis in the experimental rat testis.
It is known that the synthesis of T in animal tissues is under the influence of 3β- and 17β-hydroxy steroid dehydrogenases. Increase in the activities of 3β- and 17β-HSD observed in the present study (Figure 1A and B) was suggestive of the active involvement of these enzymes in rat testicular steriodogenesis. Elevated 17β-HSD activity due to 28-HB was noted in relation to the elevated StAR content in normal rat testicular tissue. However, elevated 17β-HSD activity did not correlate with the StAR content of diabetic rat testis, suggestive of a disproportionate link between HSD activity and StAR content in the diabetic rat testis probably influenced by 28-HB. While the loss of functional StAR reduces steroid production, it does not eliminate all of it, indicating the existence of StAR-independent pathways for steroid generation. It is however unclear what factors contributed to StAR-independent steroidogenesis23 in animal cells. The present study suggests that oxysterols may contribute to such a dichotomy observed in steroidogenesis.
It is reported that androgens reduced T biosynthesis in adult Leydig cells and in Leydig cell lines in an autoregulatory manner through receptor-mediated inhibition of StAR expression under normal physiological conditions.24,25 On the contrary, the increase in StAR protein level along with the relatively high level of T (Figure 2C) detected in the testis of male rats used in this study is due to the specific effect of the phytooxysterol 28-HB. Even though StAR and ABP were positively regulated by administered 28-HB, the observed increase in testicular T content in diabetic rat is to be considered excessive. It is however reported that diabetic rats do not respond to T by inhibition of gonadotropin-releasing hormone.26 Studies have shown that the rat plasma T content remains elevated up to 4 weeks in diabetic rats following STZ induction.27 Subsequent decline in plasma T was noted only beyond 120 to 140 days in diabetic rat.28 The increase in the T content noted in the rat testis in our study is therefore a reflection of the STZ effect in the experimental diabetic rats used for the study. While d-aspartic acid has been shown to be a T booster in male rat,29 the use of 28-HB in the present study was also noted to be a booster for T production in the experimental male rats. It is therefore suggested that 28-HB possibly acted as an additional natural factor for the process. However, correlated changes in the T-ABP levels in the rat testis were not observed. Even though T is the major in vivo regulator of ABP synthesis in diabetic rats the marked increase in T seems to downregulate ABP expression in rat testis.30,31
Lipid peroxidation is a well-known process of oxidative cellular injury in plants and animals and is an indicator of oxidative stress in tissues. Polyunsaturated fatty acid peroxides generate MDA and measurement of MDA content has been used as biomarkers of LPO status in the animal tissues. Diabetogens, such as STZ, enhanced ROS generation and induced LPO and protein carbonyl expression in the animal testis.32 These effects could be attenuated by the administration of antioxidants or herbal extracts.33 In hyperglycemia, the oxidative stress was due to inactivation and glycation of antioxidant enzymes. Elevated antioxidant enzymes, SOD (Figure 3B) and catalase activities (Figure 3C), were measured in experimental rat testis. Increased GSH content (Figure 3D) and changes in the TBARS level were also noted following 28-HB administration to the experimental rats. Our study confirms the antioxidative property of 28-HB reported34 earlier. Increased oxidative stress and decreased antioxidant level was, however, observed in STZ-diabetic rat testis. Recovery of the antioxidant status was achieved through elevation in catalase and SOD activities concomitant with a decrease in oxidative stress (reduced TBARS) as cited earlier. 28-Homobrassinolide thus reduced rat testicular tissue LPO significantly (Figure 3A).
The metabolism of brassinosteroids in animal tissues is not fully known. Close structural similarity to oxycholesterols containing the ganone ring system may presumably subject this organic cyclic molecule to a metabolic fate similar to that of any steroid nucleus, generating intermediates potentially capable of influencing cellular metabolic events,19 steroidogenesis, and histological changes in a host tissue. It is not clear whether 28-HB was assimilated into mammalian cells through simple diffusion process or required specific mechanism for transport of this molecular species across plasma/nuclear membranes. The structural features of 28-HB would allow it to be considered similar to endogenous oxysterols involved in receptor mediated cellular signaling process, eliciting a biological response.35 It is proposed that the induction of biological activity in mammalian cells by phytohormones may be related to their capacity to elicit specific gene expression within target cells. Experimental evidence to such a proposition is made available through (1) the ability to regulate 3-hydroxy-3-methylglutaryl-CoA reductase enzyme activity to influence cholesterol biosynthesis in mammalian cells by oxycholesterols and (2) the ability to induce hexokinase gene expression in the rat tissues following oral administration of 28-HB to a group of male rats.36
Conclusion
The brassinosteroid plant hormone isoform 28-HB is an inducer of T production in normal and diabetic rat testicular cells. This phytooxysterol increased StAR and ABP expression in rat testicular cells, clearly indicative of the steroidogenic potential of 28-HB in the experimental animals. Based on the insight generated by this study, it can be postulated that specific oxysterols of animal cell origin probably played a role in testostserone biosynthesis and StAR protein production in normal testicular cells. The StAR protein level was however noticeably disproportionate to the increase in T content of the tissue following 28-HB use, suggesting that testicular steroidogenesis in adult rat can possibly exist independent of StAR’s regulatory role. The reduced T levels in the testis is a primary reason for reproductive dysfunction in diabetic male rats. Results of our study indicate a possible role for endogenous oxysterols in animal cells in the regulation of male reproductive function, since 28-HB as a plant oxysterol is capable of eliciting the observed biological responses in rat testicular function. It therefore becomes imperative to review Leydig cell dysfunction associated with male infertility, from this point of view. The significant possibility of endogenous oxycholesterols having a physiological role to influence testicular steroidogenesis is highlighted through this study.
Acknowledgments
R. Jubendradass acknowledges Department of Science and Technology, New Delhi, for the Inspire Fellowship. R. Premalatha wish to acknowledge P. Muthuraman for his help with the initial animal handling experiments.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: grants from the University Grants Commission, New Delhi, Department of Science and Technology, Government of India and DST-FIST program.
References
- 1.Lund E, Bjoerkhem I. Role of oxysterols in the regulation of cholesterol homeostasis: a critical evaluation. Acc Chem Res. 1995;28(6):241–249. [Google Scholar]
- 2.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signaling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383(6602):728–731. [DOI] [PubMed] [Google Scholar]
- 3.Verger Ph, Leblanc JC. Concentration of phytohormones in food and feed and their impact on the human exposure. Pure Appl Chem. 2003;75(11):1873–1880. [Google Scholar]
- 4.Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17(3):221–244. [DOI] [PubMed] [Google Scholar]
- 5.Saradha B, Vaithinathan S, Mathur PP. Single exposure to low dose of lindane causes transient decrease in testicular steroidogenesis in adult male Wistar rats. Toxicology. 2008;244(2-3):190–197. [DOI] [PubMed] [Google Scholar]
- 6.D'Cruz SC, Vaithinathan S, Jubendradass R, Mathur PP. Effects of plants and plant products on the testis. Asian J Androl. 2010;12(4):468–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Page ST. Physiologic role and regulation of intratesticular sex steroids. Curr Opin Endocrinol Diabetes Obes. 2011;18(3):217–223. [DOI] [PubMed] [Google Scholar]
- 8.Muthuraman P, Srikumar K. A brassinosteroid as an antihyperglycemic in alloxan induced diabetic rats. J Curr Sci. 2009;10(1):19–28. [Google Scholar]
- 9.Cameron DF, Rountree J, Schultz RE, Repetta D, Murray FT. Sustained hyperglycemia results in testicular dysfunction and reduced fertility potential in BBWOR diabetic rats. Am J Physiol. 1990;259(6 pt 1):E881–E889. [DOI] [PubMed] [Google Scholar]
- 10.Muthuraman P, Ravikumar S, Vikramathithan J, Nirmalkumar G, Srikumar K. Effect of phytohormones on tissue hexokinase and on some blood components in Wistar rats. Int J Drug Delivery. 2010;2:168–172. [Google Scholar]
- 11.Bergmeyer HU, Grassl M, Walter HE. Methods of Enzymatic Analysis. Vol 2 3rd ed Deerfield Beach, FL: Verlag Chemie; 1983. [Google Scholar]
- 12.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 13.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. [DOI] [PubMed] [Google Scholar]
- 14.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. [DOI] [PubMed] [Google Scholar]
- 15.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47(3):469–474. [DOI] [PubMed] [Google Scholar]
- 16.Claiborne A. Catalase activity. In: Greenwald R, ed. CRC Handbook of Methods for Oxygen Radical Research. FL: CRC Press; 1985. [Google Scholar]
- 17.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 18.Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocr Rev. 1987;8(1):1–28. [DOI] [PubMed] [Google Scholar]
- 19.Schroepfer GJ., Jr Oxysterols: modulators of cholesterol metabolism and other processes. Physiol Rev. 2000;80(1):361–554. [DOI] [PubMed] [Google Scholar]
- 20.Xie W, Evans RM. Orphan nuclear receptors: the exotics of xenobiotics. J Biol Chem. 2001;276(41):37739–37742. [DOI] [PubMed] [Google Scholar]
- 21.Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol. 2005;19(11):2647–2659. [DOI] [PubMed] [Google Scholar]
- 22.Svechnikov K, Landreh L, Weisser J, et al. Origin, development and regulation of human Leydig cells. Horm Res Paediatr. 2010;73(2):93–101. [DOI] [PubMed] [Google Scholar]
- 23.Watari H, Arakane F, Moog-Lutz C, et al. MLN64 contains a domain with homology to the steroidogenic acute regulatory protein (StAR) that stimulates steroidogenesis. Proc Natl Acad Sci U S A. 1997;94(16):8462–8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houk CP, Pearson EJ, Martinelle N, Donahoe PK, Teixeira J. Feedback inhibition of steroidogenic acute regulatory protein expression in vitro and in vivo by androgens. Endocrinology. 2004;145(3):1269–1275. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz de Galarreta CM, Fanjul LF, Meidan R, Hsueh AJ. Regulation of 3 beta-hydroxysteroid dehydrogenase activity by human chorionic gonadotropin, androgens, and anti-androgens in cultured testicular cells. J Biol Chem. 1983;258(18):10988–10996. [PubMed] [Google Scholar]
- 26.Jackson FL, Hudson JC. Altered responses to androgen in diabetic male rats. Diabetes. 1984;33(9):819–824. [DOI] [PubMed] [Google Scholar]
- 27.Leaming AB, Mathur RS, Levine JH. Increased plasma testosterone in streptozotocin-diabetic female rats. Endocrinology.1982;111(4):1329–1333. [DOI] [PubMed] [Google Scholar]
- 28.Ho SM. Prostatic androgen receptor and plasma testosterone levels in streptozotocin-induced diabetic rats. J Steroid Biochem Mol Biol. 1991;38(1):67–72. [DOI] [PubMed] [Google Scholar]
- 29.D'Aniello A, Di Cosmo A, Di Cristo C, Annunziato L, Petrucelli L, Fisher G. Involvement of D-aspartic acid in the synthesis of testosterone in rat testes. Life Sci. 1996;59(2):97–104. [DOI] [PubMed] [Google Scholar]
- 30.Tindall DJ, Means AR. Concerning the hormonal regulation of androgen binding protein in rat testis. Endocrinology. 1976;99(3):809–818. [DOI] [PubMed] [Google Scholar]
- 31.Danzo BJ, Pavlou SN, Anthony HL. Hormonal regulation of androgen binding protein in the rat. Endocrinology. 1990;127(6):2829–2838. [DOI] [PubMed] [Google Scholar]
- 32.Matsunami T, Sato Y, Sato T, Yukawa M. Antioxidant status and lipid peroxidation in diabetic rats under hyperbaric oxygen exposure. Physiol Res. 2010;59(1):97–104. [DOI] [PubMed] [Google Scholar]
- 33.Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 2008;1(1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muthuraman P, Srikumar K. A comparative study on the effect of homobrassinolide and gibberellic acid on lipid peroxidation and antioxidant status in normal and diabetic rats. J Enzyme Inhib Med Chem. 2009;24(5):1122–1127. [DOI] [PubMed] [Google Scholar]
- 35.Smith LL. Cholesterol Auto Oxidation. New York, NY: Plenum Press; 1981:1–674. [Google Scholar]
- 36.Muthuraman P, Srikumar K. Induction of hexokinase I expression in normal and diabetic rats by a brassinosteroid isoform. Eur J Pharm Sci. 2010;41(1):1–9. [DOI] [PubMed] [Google Scholar]