Abstract
Human embryonic stem cells are derived from the inner cell mass of preimplantation embryo at the blastocyst stage and their differentiation occurs through an intermediate step involving the formation of embryoid bodies (EBs), which are aggregates of embryonic stem cells. The EBs seem to be a powerful tool for investigating the development of embryos, as they can mimic the initial stages of embryonic development. In this study, we aimed to investigate the effect of estrogen compounds on the proliferation and differentiation of short-term and long-term cultured EBs in vitro. For this study, 10-day-old (short-term cultured) and 30-day-old (long-term cultured) EBs were subjected to estradiol (E2), estriol (E3), selective estrogen receptor modulator (raloxifene [RLX]), bisphenol A, and 1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole for 7 days. To confirm the effects of estrogen treatment, ICI-182780 was added to the respective EBs for additional 7 days following estrogen treatment. Quantitative reverse transcription–polymerase chain reaction was performed to analyze the relative expression of differentiation marker genes representing the 3 germ layers. The expression of 7 marker genes, which included α-fetoprotein, hepatocyte nuclear factor (HNF)-3β, HNF-4α (endoderm), brachyury, cardiac actin ([cACT]; mesoderm), nestin (ectoderm), and Oct-4 (undifferentiated), was measured. Significantly, lower expression of HNF-4α in both short-term and long-term cultured EBs was observed after treatment of estrogen compounds compared to control. The expression of HNF-3β in short-term cultured EBs has been positively affected by E2, E3, and RLX. Regarding cACT, higher expression was observed after treatment of E2 (10−7 mol/L) and E3 (10−9 mol/L) in short-term cultured EBs, but opposite effects were demonstrated in long-term cultured EBs. The lower expressions of HNF-4α by E2 and RLX were negated by ICI-182780 treatment, although these findings were not statistically significant in E3-treated group. These findings suggest that estrogen compounds have effects on endodermal and mesodermal differentiation of human EBs.
Keywords: estradiol, estriol, selective estrogen receptor modulator, embryoid body
Introduction
Human embryonic stem cells (hESCs) are derived from the inner cell mass of preimplantation embryo at the blastocyst stage and can serve as a putative source of various cell types in vitro.1–3 Since hESCs have the capacities to self-renew indefinitely, hESCs have the potential to serve as a major resource for cell therapy as well as provide a new experimental model for pharmaceutical drug testing and studies of human development.4
Generally, the initiation of embryonic stem (ES) cell differentiation occurs through an intermediate step involving the formation of embryoid bodies (EBs), which are complex 3-dimensional aggregates of ES cells.5,6 The EBs are a powerful tool for investigating the development of embryos, as they mimic the initial stages of embryonic development7 and such early stage cells are difficult to isolate from developing embryos.8 Moreover, EBs provides a 3-dimensional structure that enhances cell–cell interactions that may be important for developmental model.9
Estrogen, the sex hormone closely associated with reproductive functions, also offers significant effects on diverse stem/progenitor cell populations such as neural stem cells, osteogenic progenitor cells, endothelial progenitor cells, and mesenchymal stem cells.10,11 Furthermore, a few studies recently demonstrated that estradiol (E2) affects the undifferentiation state of EB in mouse stem cells,12 and the addition of E2 to murine EB results in the production of a high amount of osteoblast-like cells.13 However, the studies of ESCs in other species seem to apply to the human disease models due to the interspecies variation,14 and hESCs are likely to be clinically more relevant in studies of human development or diseases.15,16
Estrogen is a group of compounds composed of estrone, E2, and estriol (E3). Although E2 is the major estrogen compound in nonpregnant females, E3 is the primary estrogen expressed during pregnancy. Interestingly, the effect of E3 on hESCs and EBs has not been reported yet. In this study, we aimed to investigate the effect of various estrogen compounds on the proliferation and differentiation of short-term and long-term cultured EBs generated from hESCs.
Materials and Methods
The hESC Culture and EB Formation
The human embryonic stem cell line, SNUhES3,17 was cultured as previously reported.18 Briefly, undifferentiated hESCs were cultured on a mitotically inactivated STO (CRL-1503; ATCC, Manassas, Virginia) feeder layer by mechanical dissociation. The hESC culture medium consisted of DMEM/F12 (Invitrogen, Grand Island, New York), 20% knockout serum replacement (Invitrogen), 1% nonessential amino acids (Invitrogen), 50 U/mL penicillin (Invitrogen), 50 µg/mL streptomycin (Invitrogen), 0.1 mmol/L β-mercaptoethanol (Sigma-Aldrich, St Louis, Missouri), and 4 ng/mL basic fibroblast growth factor (Invitrogen).
For EB formation, undifferentiated hESCs were cultured for 5 days and treated with 2 mg/mL of collagenase type IV (Invitrogen) for 30 minutes at 37°C. Detached hES colonies were centrifuged and washed with phosphate-buffered saline (PBS). Washed hES colonies were transferred and cultured in suspension for several days. For this study, 10-day-old and 30-day-old EBs were used and their medium was changed every other day.
Measurement of EB Growth
To assess the growth of short-term and long-term cultured EBs, growth of EBs was observed and measured under phase-contrast microscope. The diameter of each EBs was measured and calculated using i-solution program (InnerView, KyungGi-do, Korea). To evaluate the proliferation of cultured EBs, the expression of cyclin D1 and Cdk4 was assessed using quantitative reverse transcription–polymerase chain reaction (qRT-PCR).
Treatment With Estrogen Compounds
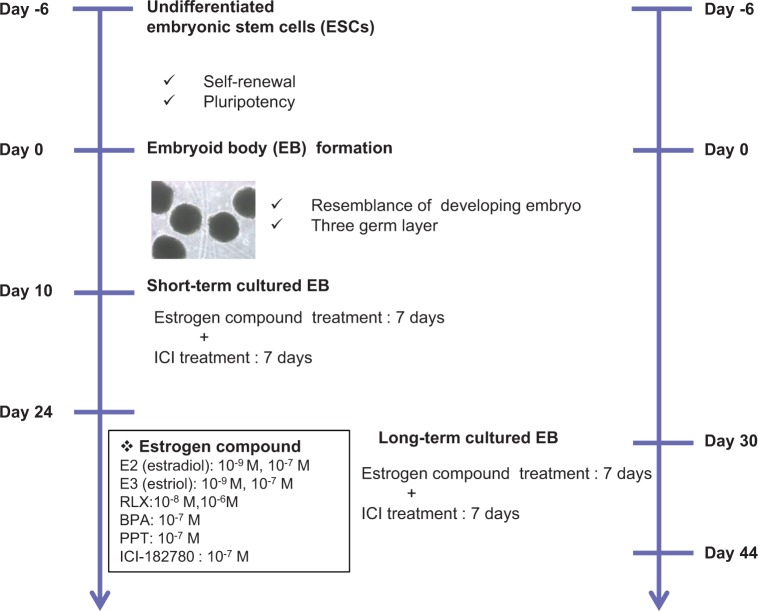
As briefly demonstrated in Figure 1, to investigate the effects of estrogen compounds on the proliferation and differentiation of EB, 10−9 and 10−7 mol/L of E2 (Sigma-Aldrich) and E3 (Sigma-Aldrich), 10−8 and 10−6 mol/L of raloxifene (RLX; Sigma-Aldrich) which is a selective estrogen receptor modulator (SERM), and 10−7 mol/L of bisphenol A (BPA; Sigma-Aldrich) and 1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole (PPT; Tocris Bioscience, Bristol, United Kingdom) were added to the culture media every other day for 7 days. To confirm the effect of estrogen treatment, 10−7 mol/L of ICI-182780 (Tocris Crookson, Minneapolis, Minnesota) was added to respective EBs every other day for 7 days after treatment with estrogen.
Figure 1.
The schematic presentation of experiment. Undifferentiated hESCs formed into hEBs by enzymatic dissociation and cultured for 10 days (short-term cultured EB) and 30 days (long-term cultured EB). Each estrogen compound and the inhibitor including physiological level were added and the expression of representative germ layer markers was evaluated. EB indicates embryoid body; hEBs, human EBs; hESCs, human embryonic stem cells.
Immunostaining
The samples were fixed with 4% paraformaldehyde (Sigma-Aldrich) for 15 minutes at room temperature. And then, the samples were washed twice with PBS and incubated with 3% bovine serum albumin blocking solution to inhibit nonspecific reaction. Primary antibodies, mouse anti-estrogen receptor (ER)-α (Abcam, Cambridge, Massachusetts), and mouse anti-ER-β (GeneTex, Irvine, California) were added and incubated for 60 minutes at 37°C and washed twice with PBS containing tween 20 (PBST). Secondary antibodies, Alexa Fluor 488 goat anti-mouse and Alexa Fluor 594 goat anti-mouse, were added and incubated for 60 minutes at 37°C and washed twice with PBST. After treatment with anti-fade solution containing 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen), samples were observed by confocal scanning laser microscope (BioRad, Hercules, California).
Quantitative RT-PCR
Total RNA was extracted from RNeasy mini kit (Qiagen, Valencia, California), according to the manufacturer’s protocol. Complementary DNA (DNA) was synthesized from 1 µg of total RNA using Accute RT-premix (Bioneer, Daejeon, Korea). Specific primers used for PCR are shown in Table 1. Quantitative PCR was performed in RotorGene 3000 (Corbett Life Science, Sydney, Australia) using QuantiTect SYBR green PCR kit (Qiagen). The amplification program included an initial step at 95°C for 15 minutes, followed by 45 cycles of denaturation at 95°C for 15 seconds, annealing at 58°C for 20 seconds, and extension at 72°C for 30 seconds. All reactions were run in triplicate and relative gene expression was normalized against the corresponding glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression.
Table 1.
The Sequences of Primers Used for qRT-PCR
| Gene | Forward | Reverse |
|---|---|---|
| GAPDH | GGCGTTCTCTTTGGAAAGGTGTTC | GTACTCAGCGGCCAGCATCG |
| αFP | AGAACCTGTCACAAGCTGTG | GACAGCAAGCTGAGGATGTC |
| HNF-3β | CTACGCCAACATGAACTCCA | GAGGTCCATGATCCACTGGT |
| HNF-4α | TGTCCCGACAGATCACCTC | CACTCAACGAGAACCAGCAG |
| Brachyury | TAAGGTGGATCTTCAGGTAGC | CATCTCATTGGTGAGCTCCCT |
| cACT | GGAGTTATGGTGGGTATGGGTC | AGTGGTGACAAAGGAGTAGCCA |
| Oct4 | GAGAACAATGAGAACCTTCAGGA | CTCGAACCACATCCTTCTCT |
| Nestin | CAGCTGGCGCACCTCAAGATG | AGGGAAGTTGGGCTCAGGACTGG |
| Cyclin D1 | GATCAAGTGTGACCCGGACT | TCCTCCTCCTCTTCCTCCTC |
| CDK4 | GAAACTCTGAAGCCGACCAG | ACATCTCGAGGCCAGTCATC |
Abbreviation: qRT-PCR, quantitative reverse transcription–polymerase chain reaction.
Statistical Analysis
Statistical analysis was performed using SPSS version 17.0 (SPSS Inc, Chicago, Illinois). Data are expressed as the medians ± interquartile range and were analyzed by Kruskal-Wallis test as a nonparametric method, followed by least significant difference test for post hoc analysis. P values less than .05 were considered to be statistically significant.
Results
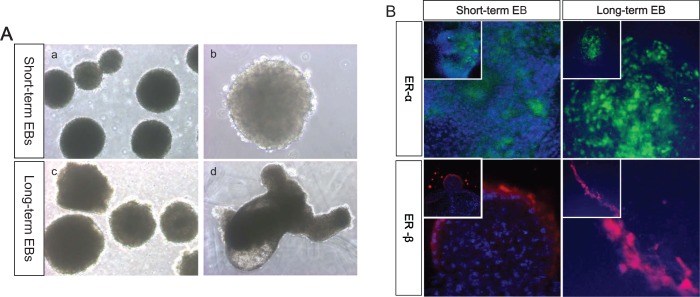
The scheme of whole experiment is represented as Figure 1. The hESCs-derived EBs were cultured in suspension and classified according to culture period. Human EBs formed spherical mass that has typical morphology and some of EBs showed cystic region in long-term cultured EBs (Figure 2A). For determination of proper time point for treatment with estrogen compounds, we evaluated the expression of ER-α and ER-β at days 3, 6, 9, and 12 in cultured EBs. The expression of ER-α and ER-β was first observed in day 6 EBs; however, the expression level was very low. The expression levels of ER reached their peak in EBs at day 9 and the levels were similar to those of EBs at day 12. Therefore, we added the estrogen compounds at day 10 (short-term cultured EB; Figure 2B). Similarly, we also assessed ER-α and ER-β expression levels in further cultured EBs and the expression of ER-α and ER-β was continued. Therefore, we defined 30-day-old EBs as long-term culture EB and estrogen compounds were added.
Figure 2.
The growth of EBs and expression of estrogen receptors. The growth of each group of EBs was observed and their diameters used as indication of growth. A, The morphological appearance of hEBs in suspension. Upper panel: short-term cultured EBs (day 10); lower panel: long-term cultured EBs (day 30), magnification: a, ×40; b, ×100; c, ×40; and d, ×100. B, Immunostaining of ER receptors in differentiating EBs. Upper panel: expression of ER-α in short-term (day 10) and long-term cultured EB (day 30), lower panel: expression of ER-β in short-term (day 10) and long-term cultured EB (day 30), magnification: inner box, ×200; outer box, ×400. EB indicates embryoid body; ER, estrogen receptor; hEBs, human EBs.
To detect the effect of estrogen compounds on the differentiation of short-term and long-term cultured EBs, the expression of 7 marker genes was measured as follows: α-fetoprotein (AFP), hepatocyte nuclear factor (HNF)-3β, HNF-4α (endoderm), brachyury, cardiac actin ([cACT] mesoderm), nestin (ectoderm), and Oct-4 (undifferentiated).
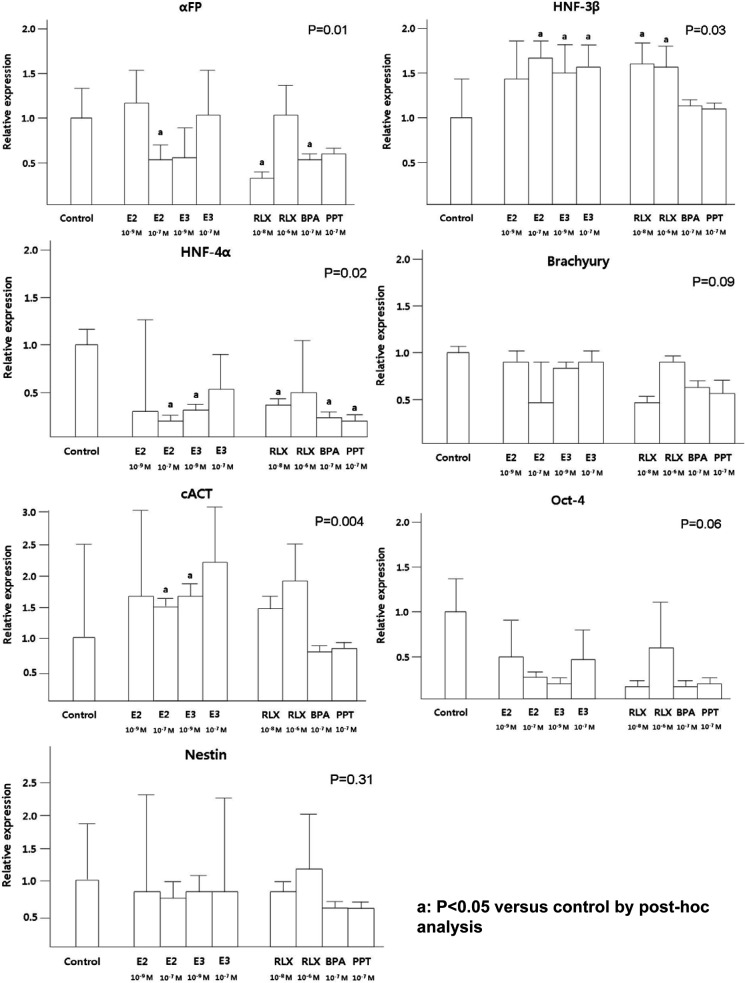
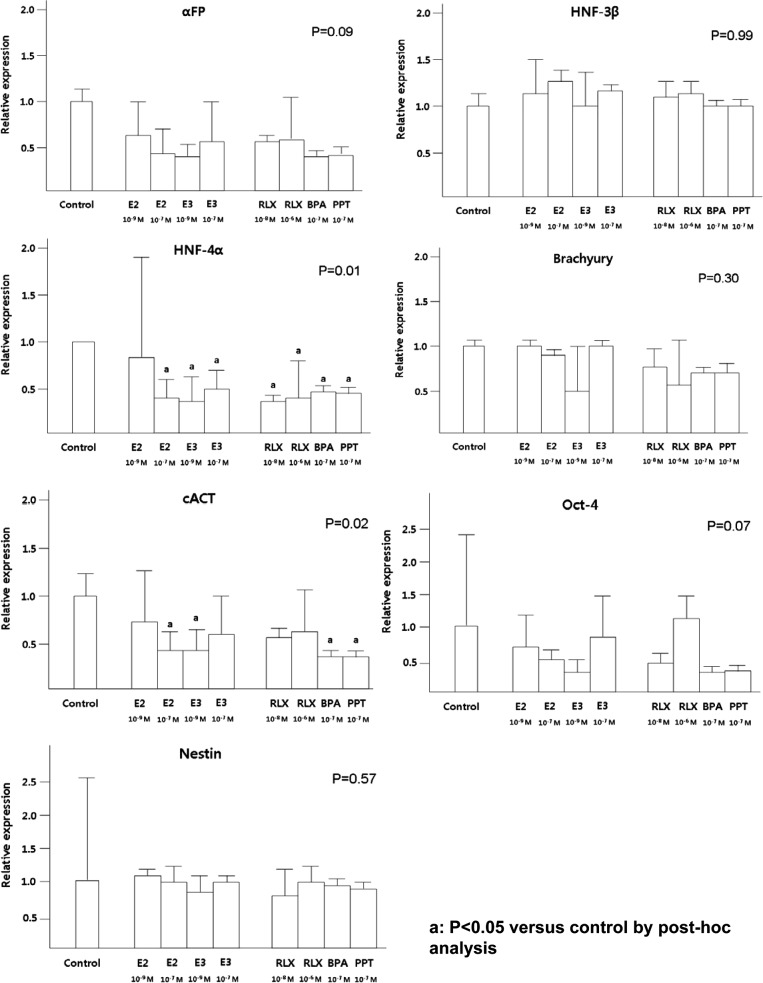
In short-term cultured EBs, E2 (10−7 mol/L), RLX (10−8 mol/L) and BPA inhibited AFP and HNF-4α expression (Figure 3). Moreover, low concentration of E3 (10−9 mol/L) and PPT also suppressed HNF-4α expression. Similarly, significantly lower expression of HNF-4α in long-term cultured EBs was observed after treatment with all estrogen compounds except low level of E2 (10−9 mol/L), compared to the control (P = .01; Figure 4).
Figure 3.
The effects of estrogen compounds on the expression of short-term cultured EBs. The expression of 3 germ layer markers was evaluated using qRT-PCR in short-term cultured EBs. Data are combined from 3 separate experiments and expression of differentiation marker gene was normalized to GAPDH expression as an internal control. Percentage of expression in the control group was set at 1. P values (by Kruskal-Wallis test) less than .05 are considered significant. a P < .05 compared to control by post hoc analysis. BPA indicates bisphenol-A; E2, estradiol; E3, estriol; EBs, embryoid bodies; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PPT, 1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole; qRT-PCR, quantitative reverse transcription polymerase chain reaction; RLX, raloxifene.
Figure 4.
The effects of estrogen compounds on the expression of long-term cultured EBs. The expression of 3 germ layer markers was evaluated using qRT-PCR in long-term cultured EBs. Data are combined from 3 separate experiments and expression of differentiation marker gene was normalized to GAPDH expression as an internal control. The percentage of expression in the control group was set at 1. P value (by Kruskal-Wallis test) less than .05 are considered significant. a P < .05 compared to control by post hoc analysis. BPA indicates bisphenol-A; E2, estradiol; E3, estriol; EBs, embryoid bodies; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PPT, 1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole; qRT-PCR, quantitative reverse transcription–polymerase chain reaction; RLX, raloxifene.
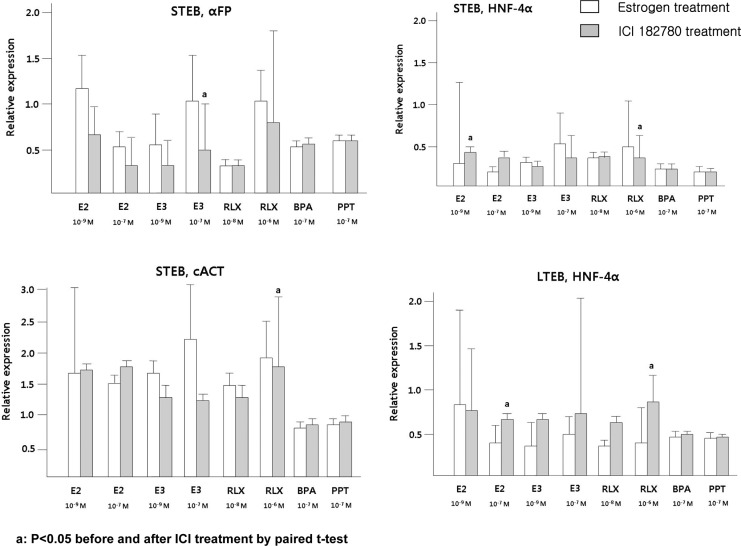
The expression of HNF-3β in short-term cultured EBs has been positively affected by E2, E3, and RLX, however, these findings were not observed in long-term cultured EBs. Higher expression of cACT was observed after treatment with E2 (10−7 mol/L) and E3 (10−9 mol/L) in short-term cultured EBs, but the opposite effects were demonstrated in long-term cultured EBs. The lower expressions of HNF-4α by E2 and RLX were negated by ICI-182780 treatment, although these findings were not statistically significant in E3-treated group (Figure 5). Additionally, proliferation assessed by cyclin D1 and Cdk4 expression did not demonstrate significant difference according to the treatment of diverse estrogen compounds (data not shown).
Figure 5.
After serial treatment of estrogen compound and ICI-182780, the expression of 3 germ layer markers was evaluated using qRT-PCR and the percentage of expression in the control group was set at 1. a P < .05 before versus after ICI-182780 treatment by paired t test. BPA indicates bisphenol-A; E2, estradiol; E3, estriol; EBs, embryoid bodies; HNF, hepatocyte nuclear factor; PPT, 1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole; qRT-PCR, quantitative reverse transcription–polymerase chain reaction; RLX, raloxifene.
Discussion
Estrogen is an indispensible sex steroid hormone and is known to be involved in cell proliferation, differentiation, and survival.19 Estrogen is also shown to be implicated in the development or progression of numerous diseases, including various types of cancer, cardiovascular disease, and lupus erythematosus.20 The progression of autoimmune diseases such as lupus or rheumatoid arthritis is associated with estrogen level, especially increased level during pregnancy.21 Additionally, adverse effects during development have been reported, which may be associated with alterations to the endocrine milieu.22,23 Recently, several researchers have reported the effect of E2 on the differentiation of ESCs and EBs. Murashov et al24 demonstrated that the addition of E2 to mouse ESCs differentiating into neurons increased neurite branching, and Tielens et al13 reported that treatment of E2 on the osteogenic differentiation of mouse EBs resulted in the production of a high amount of osteoblast-like cells. Since mouse ESCs differ from hESCs not merely in expression of cell surface markers but also in cell cycle regulation, control of apoptosis, and cytokine expression,25 the results of these studies in murine ESCs may not easily apply to hESCs.
Hong et al26 confirmed the presence of ER in undifferentiated hESCs and EBs and demonstrated the upregulated expression of endodermal markers such as GATA-4 and αFP in the 10−5 mol/L E2-treated group. However, in their study, a concentration of 10−5 mol/L E2 is about 10 000-fold higher than the physiologic concentration of E2 (10−9-10−10 mol/L) and the concentration of 10−5 mol/L seems to be impossible in a normal developmental environment. On the contrary, we found downregulation of AFP in short-term and long-term cultured EBs after E2 treatment. These contradictory findings of expression pattern seem to be due to different hES cell lines, different culture period of EBs, and different concentration of E2. In our study, physiologic concentrations of estrogen compounds were used as we aimed to mimic an environment of human development in vitro. In addition, differentiation potential among hESC cell lines may be different according to the origin of cell lines and culture environment.
Although E2 is a major estrogen component in nonpregnant women, E3 is the predominant estrogen during pregnancy.27 Therefore, we investigated the effect of E3 on the differentiation of EBs, in addition to E2 in this study. E3 is measured in maternal serum and used for mid-trimester prenatal screening test.28 Abnormally low levels of E3 in a pregnant women suggest chromosomal aberrations including Down syndrome (trisomy 21) or Edward syndrome (trisomy 18),28 but pregnancy outcomes have not been reported in case of elevated E3 concentration yet.
Estrogen receptor is of 2 types, ER-α and ER-β. The ER-α is mainly distributed in endometrium, breast, and ovarian tissue,20,29 whereas ER-β has been detected in bone, heart, and brain.30 Estradiol binds equally well to the ER-α and ER-β,31 however, E3 preferentially binds to ER-β.32 Raloxifene, SERM, binds with high affinity to both ER-α and ER-β and induces subtle changes in the conformation of the RLX-receptor complex which are likely the basis for the pharmacological differences from E2.33,34 In the present study, HNF-4α expression decreased significantly in most estrogen-treated EBs, and it was also observed in PPT-treated EBs, which is a pure ER-α agonist. Therefore, it can be hypothesized that decreased HNF-4α expression in short-term and long-term cultured EB is induced via both ER-α and ER-β though the exact mechanism for this phenomenon will need to be investigated in future studies.
HNF-4α is a protein enriched in liver, kidney, and pancreas35 and plays an important role in the maintenance of hepatocyte differentiation and is a major in vivo regulator of genes involved in the control of lipid homeostasis.36 Interestingly, it has been demonstrated that ER interacts commonly with nuclear receptors including HNF-4, and this interaction has been suggested to mediate signaling pathway.37 Moreover, HNF-4 has been reported to antagonize ER-α-mediated induction of human coagulation factor gene.38 These findings may partially explain low HNF-4α expression after treatment of estrogen compounds in this study, and it seems to be through ER-α- signaling pathway.
HNF-3β is another member of HNF subfamily, which is shown to be involved in cellular differentiation, and Berger et al39 have previously reported high level of HNF-3β messenger RNA expression by estrogen treatment. Similarly, in our study, higher HNF-3β expression was observed in estrogen-treated short-term cultured EBs and it may be conducted through non-ER-α-mediated signaling pathway. Moreover, there was no significant change in HNF-3β expression in long-term cultured EBs, and it seems that the effect of estrogen on HNF-3β may be different according to the culture duration of EBs. Regarding cACT expression, estrogen including E2 and E3 can affect cardiac differentiation and the magnitude of effect seems to be different according to the concentration of estrogen and the stage of EBs, which is similar to timing hypothesis of estrogen with heart disease.40
In the present study, there are a few limitations as follows: first, the expression of marker genes was determined with estrogen concentrations that are known to be physiologic level. Therefore, it remains possible that the level of gene expression might be different in other concentrations. For example, significant change in HNF-4α expression was observed after treatment of physiologic concentration (10−7 mol/L) of E2 with pregnancy compared to control but not with nonpregnant physiologic level (10−9 mol/L). Second, we hypothesize that the developmental potential differs according to the culture duration of EBs; however, it is not reported to which period of embryonic development long-term cultured EBs correspond to. Therefore, the further investigation regarding the temporal relation between EB culture and their corresponding developmental period should be considered in later studies.
In conclusion, our findings suggest that estrogen compounds have effects on endodermal and mesodermal differentiation of human EBs. Further large-scale, in-depth investigation is needed to confirm these results.
Footnotes
Authors’ Note: The authors Hoon Kim and Yoon Young Kim contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A111539) and from Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012-006959).
References
- 1. Itskovitz-Eldor J, Schuldiner M, Karsenti D, et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6(2):88–95. [PMC free article] [PubMed] [Google Scholar]
- 2. Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18(4):399–404. [DOI] [PubMed] [Google Scholar]
- 3. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. [DOI] [PubMed] [Google Scholar]
- 4. Pera MF, Trounson AO. Human embryonic stem cells: prospects for development. Development. 2004;131(22):5515–5525. [DOI] [PubMed] [Google Scholar]
- 5. Dang SM, Kyba M, Perlingeiro R, Daley GQ, Zandstra PW. Efficiency of embryoid body formation and hematopoietic development from embryonic stem cells in different culture systems. Biotechnol Bioeng. 2002;78(4):442–453. [DOI] [PubMed] [Google Scholar]
- 6. Khoo ML, McQuade LR, Smith MS, Lees JG, Sidhu KS, Tuch BE. Growth and differentiation of embryoid bodies derived from human embryonic stem cells: effect of glucose and basic fibroblast growth factor. Biol Reprod. 2005;73(6):1147–1156. [DOI] [PubMed] [Google Scholar]
- 7. Meshorer E, Misteli T. Chromatin in pluripotent embryonic stem cells and differentiation. Nat Rev Mol Cell Biol. 2006;7(7):540–546. [DOI] [PubMed] [Google Scholar]
- 8. Desbaillets I, Ziegler U, Groscurth P, Gassmann M. Embryoid bodies: an in vitro model of mouse embryogenesis. Exp Physiol. 2000;85(6):645–651. [PubMed] [Google Scholar]
- 9. Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19(10):1129–1155. [DOI] [PubMed] [Google Scholar]
- 10. Ray R, Novotny NM, Crisostomo PR, Lahm T, Abarbanell A, Meldrum DR. Sex steroids and stem cell function. Mol Med. 2008;14(7-8):493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun H, Wang H, Hu S. Effects of estrogen on diverse stem cells and relevant intracellular mechanisms. Sci China Life Sci. 2010;53(5):542–547. [DOI] [PubMed] [Google Scholar]
- 12. Jung EM, Choi KC, Yu FH, Jeung EB. Effects of 17beta-estradiol and xenoestrogens on mouse embryonic stem cells. Toxicol In Vitro. 2010;24(6):1538–1545. [DOI] [PubMed] [Google Scholar]
- 13. Tielens S, Wymeersch F, Declercq H, Cornelissen M. Effect of 17beta-estradiol on the in vitro differentiation of murine embryonic stem cells into the osteogenic lineage. In Vitro Cell Dev Biol Anim. 2008;44(8-9):368–378. [DOI] [PubMed] [Google Scholar]
- 14. Hurtt ME, Cappon GD, Browning A. Proposal for a tiered approach to developmental toxicity testing for veterinary pharmaceutical products for food-producing animals. Food Chem Toxicol. 2003;41(5):611–619. [DOI] [PubMed] [Google Scholar]
- 15. McNeish J. Embryonic stem cells in drug discovery. Nat Rev Drug Discov. 2004;3(1):70–80. [DOI] [PubMed] [Google Scholar]
- 16. Pal R, Mamidi MK, Das AK, Bhonde R. Human embryonic stem cell proliferation and differentiation as parameters to evaluate developmental toxicity. J Cell Physiol. 2011;226(6):1583–1595. [DOI] [PubMed] [Google Scholar]
- 17. Oh SK, Kim HS, Ahn HJ, et al. Derivation and characterization of new human embryonic stem cell lines: SNUhES1, SNUhES2, and SNUhES3. Stem Cells. 2005;23(2):211–219. [DOI] [PubMed] [Google Scholar]
- 18. Oh SK, Kim HS, Park YB, et al. Methods for expansion of human embryonic stem cells. Stem Cells. 2005;23(5):605–609. [DOI] [PubMed] [Google Scholar]
- 19. McLachlan JA. Environmental signaling: what embryos and evolution teach us about endocrine disrupting chemicals. Endocr Rev. 2001;22(3):319–341. [DOI] [PubMed] [Google Scholar]
- 20. Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116(3):561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lockshin MD. Sex differences in autoimmune disease. Lupus. 2006;15(11):753–756. [DOI] [PubMed] [Google Scholar]
- 22. Guerrero-Bosagna C, Sabat P, Valladares L. Environmental signaling and evolutionary change: can exposure of pregnant mammals to environmental estrogens lead to epigenetically induced evolutionary changes in embryos? Evol Dev. 2005;7(4):341–350. [DOI] [PubMed] [Google Scholar]
- 23. Wang H, Zhou C, Chen W, Li T, Huang J, Zhuang G. Supraphysiological estrogen levels adversely impact proliferation and histone modification in human embryonic stem cells: possible implications for controlled ovarian hyperstimulation assisted pregnancy. Eur J Obstet Gynecol Reprod Biol. 2011;155(1):58–64. [DOI] [PubMed] [Google Scholar]
- 24. Murashov AK, Pak ES, Hendricks WA, Tatko LM. 17beta-Estradiol enhances neuronal differentiation of mouse embryonic stem cells. FEBS Lett. 2004;569(1-3):165–168. [DOI] [PubMed] [Google Scholar]
- 25. Ginis I, Luo Y, Miura T, et al. Differences between human and mouse embryonic stem cells. Dev Biol. 2004;269(2):360–380. [DOI] [PubMed] [Google Scholar]
- 26. Hong SH, Nah HY, Lee YJ, et al. Expression of estrogen receptor-alpha and -beta, glucocorticoid receptor, and progesterone receptor genes in human embryonic stem cells and embryoid bodies. Mol Cells. 2004;18(3):320–325. [PubMed] [Google Scholar]
- 27. Zhou J, Seidel ER. Estrogens induce visfatin expression in 3T3-L1 cells. Peptides. 2010;31(12):271–274. [DOI] [PubMed] [Google Scholar]
- 28. Graves JC, Miller KE, Sellers AD. Maternal serum triple analyte screening in pregnancy. Am Fam Physician. 2002;65(5):915–920. [PubMed] [Google Scholar]
- 29. Walker VR, Korach KS. Estrogen receptor knockout mice as a model for endocrine research. ILAR J. 2004;45(4):455–461. [DOI] [PubMed] [Google Scholar]
- 30. Saunders PT. Oestrogen receptor beta (ER beta). Rev Reprod. 1998;3(3):164–171. [DOI] [PubMed] [Google Scholar]
- 31. Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. 8th ed Philadelphia, PA: Wolters Kluwer; 2011. [Google Scholar]
- 32. Zhu BT, Han GZ, Shim JY, Wen Y, Jiang XR. Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: Insights into the structural determinants favoring a differential subtype binding. Endocrinology. 2006;147(9):4132–4150. [DOI] [PubMed] [Google Scholar]
- 33. Bryant HU, Glasebrook AL, Yang NN, Sato M. An estrogen receptor basis for raloxifene action in bone. J Steroid Biochem Mol Biol. 1999;69(1-6):37–44. [DOI] [PubMed] [Google Scholar]
- 34. Rich RL, Hoth LR, Geoghegan KF, et al. Kinetic analysis of estrogen receptor/ligand interactions. Proc Natl Acad Sci U S A. 2002;99(13):8562–8567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sladek FM, Zhong WM, Lai E, Darnell JE., Jr Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 1990;4(12B):2353–2365. [DOI] [PubMed] [Google Scholar]
- 36. Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol. 2001;21(4):1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee SK, Choi HS, Song MR, Lee MO, Lee JW. Estrogen receptor, a common interaction partner for a subset of nuclear receptors. Mol Endocrinol. 1998;12(8):1184–1192. [DOI] [PubMed] [Google Scholar]
- 38. Farsetti A, Moretti F, Narducci M, et al. Orphan receptor hepatocyte nuclear factor-4 antagonizes estrogen receptor alpha-mediated induction of human coagulation factor XII gene. Endocrinology. 1998;139(11):4581–4589. [DOI] [PubMed] [Google Scholar]
- 39. Berger RR, Sanders MM. Estrogen modulates HNF-3beta mRNA levels in the developing chick oviduct. DNA Cell Biology. 2000;19(2):103–112. [DOI] [PubMed] [Google Scholar]
- 40. Barrett-Connor E. Hormones and heart disease in women: the timing hypothesis. Am J Epidemiol. 2007;166(5):506–510. [DOI] [PubMed] [Google Scholar]