Abstract
Primary intraosseous hemangiomas are benign, vascular malformations that account for approximately 1% of all primary bone neoplasms. These tumors are mostly found in vertebral bodies and are rarely seen in the calvarium, where they represent 0.2% of bony neoplasms. When found in the skull, they tend to present with vague symptoms and do not have the typical radiological findings suggestive of hemangiomas. Because of this, these tumors can be missed in many cases or may be misinterpreted as more ominous lesions like multiple myeloma or osteosarcoma. Involvement of the skull base is exceedingly rare, and presentation with cranial nerve unilateral polyneuropathies has not been reported. We report a patient case with review of recent pertinent literature.
Keywords: clivus, hemangioma, endoscopic transnasal surgery
Case Report
History
The patient is an 89-year-old right-handed woman with past medical history significant for rheumatoid arthritis, hypertension, osteoporosis, and hypothyroidism. She presented with 2 days of dysarthric speech, drooling, and difficulty swallowing. She endorsed new constant “pressure” headaches that began 4 weeks prior to presentation that radiated to behind her left eye, ear, and jaw. She denied any previous history of headaches.
Computed tomography (CT) and magnetic resonance imaging (MRI) imaging demonstrated two separate abnormalities: A 3.4 cm × 2.3 cm hypodense soft tissue fullness at the anterior craniocervical junction with erosion of the inferior aspect of the clivus (Fig. 1) and a 2.0 cm × 1.9 cm erosive mass of the upper clivus that preserved the cortical margin (Fig. 2). The former lesion was characteristic for rheumatoid pannus, but the latter lesion was concerning for tumor. A subsequent CT of the chest/abdomen/pelvis was notable for a 2-cm mass of the right kidney suspicious for renal cell carcinoma. A conventional cerebral angiogram was obtained to determine the vascularity of this lesion, given the metastatic renal cell carcinoma concern. This angiogram, however, did not demonstrate any evidence of vascular lesions within the clivus (Fig. 3).
Fig. 1.
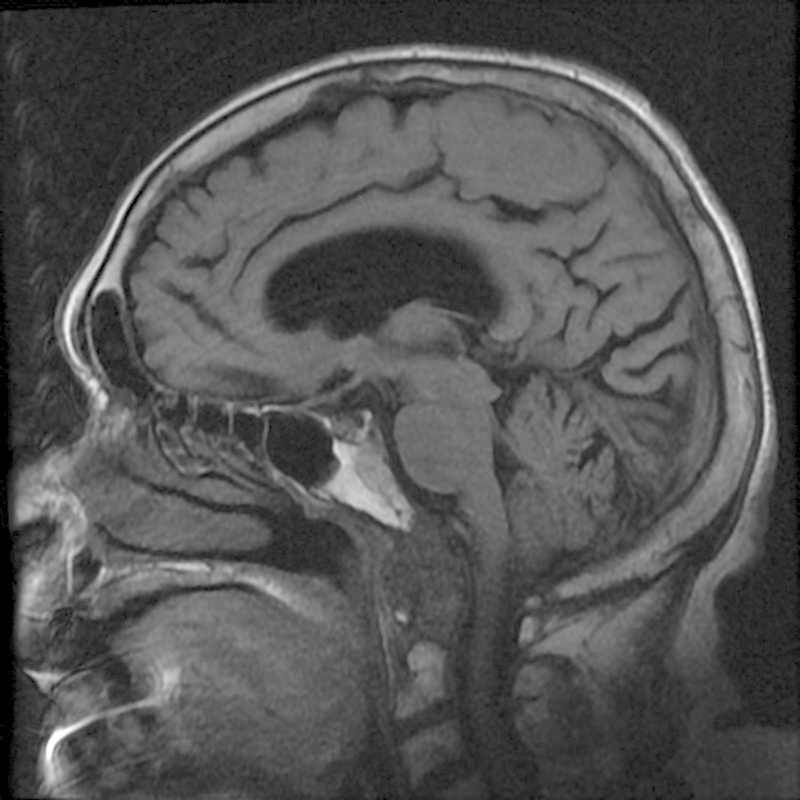
Sagittal T1-weighted magnetic resonance imaging demonstrating erosion of the inferior aspect of the clivus consistent with rheumatoid pannus.
Fig. 2.
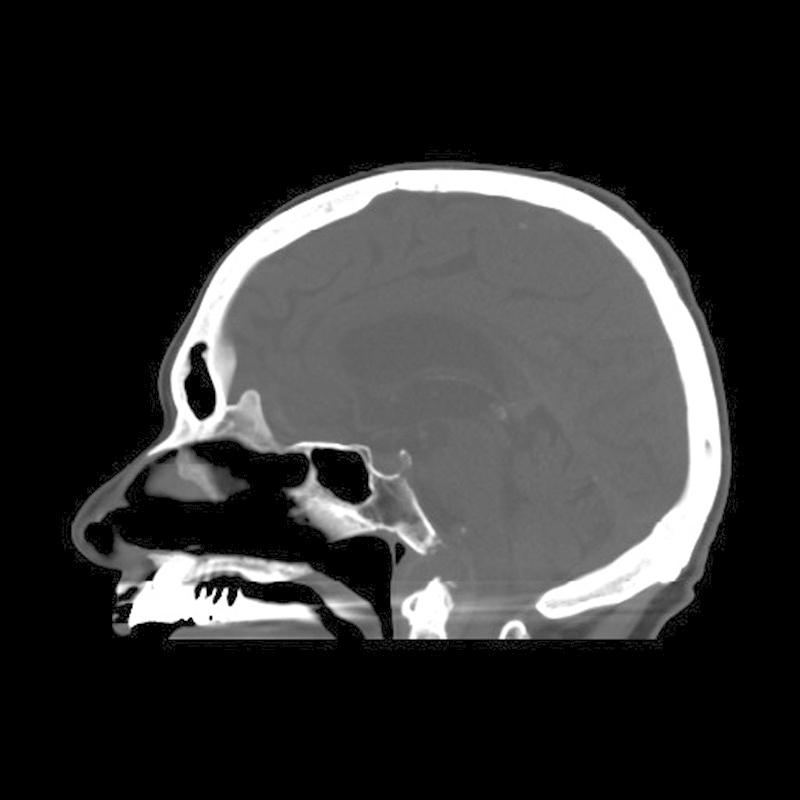
Sagittal computed tomography reformatted image demonstrating an erosive mass of the upper clivus that preserves the cortical margin.
Fig. 3.
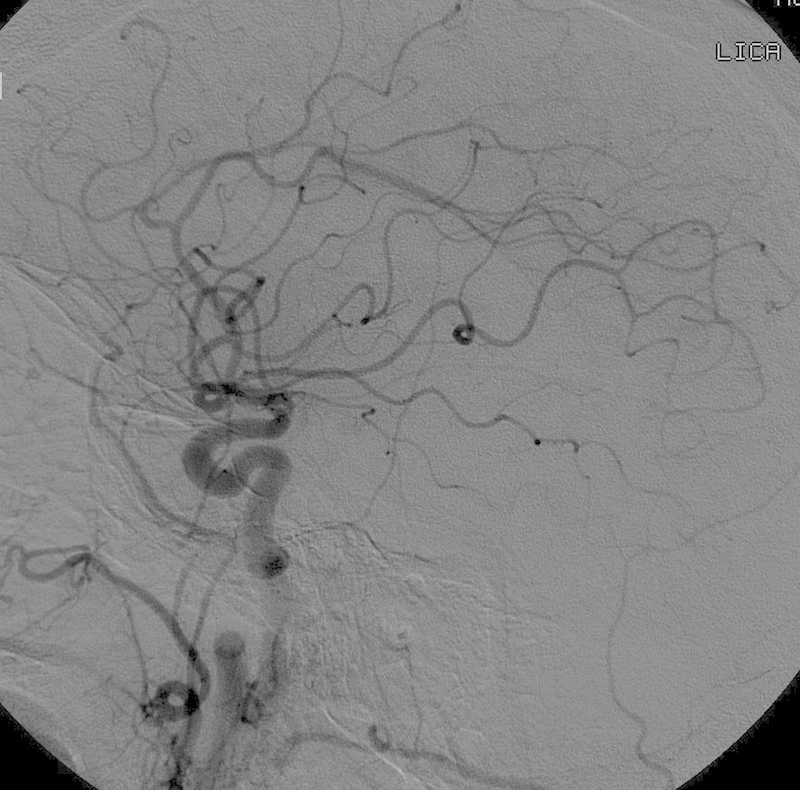
Digital subtraction angiography of the left common carotid artery demonstrating no significant tumor blush.
Neurological Examination
Her neurological exam was notable for multiple cranial neuropathies. She had left facial numbness in the maxillary and mandibular divisions of the trigeminal nerve, left tongue numbness, left facial droop, decreased left gag reflex, and protrusion of her tongue to the left. The remainder of her neurological examination was stable.
Surgical Biopsy
Based on the patient's cranial neuropathies and evolving presentation, the decision was made at interdisciplinary tumor board to proceed with an endoscopic endonasal clival biopsy and partial tissue debulking. The patient was taken to the operating room for endoscopic transnasal biopsy of the clival lesion. To aid with intraoperative localization of the lesion, stereotactic, frameless, computer-assisted surgical navigation was utilized (Fig. 4). Epinephrine-soaked pledgets were utilized to decongest the nasal cavity and allow for adequate vasoconstriction. Preoperative cefuroxime and 10 mg of dexamethasone were administered. A zero-degree endoscope was employed to survey the bilateral nasal cavity and used for the remainder of the case. A mucoperiosteal flap was developed bilaterally using a long needle-tipped cautery spanning the face of the sphenoid and taken onto the posterior septum, which was subsequently reflected inferiorly.
Fig. 4.
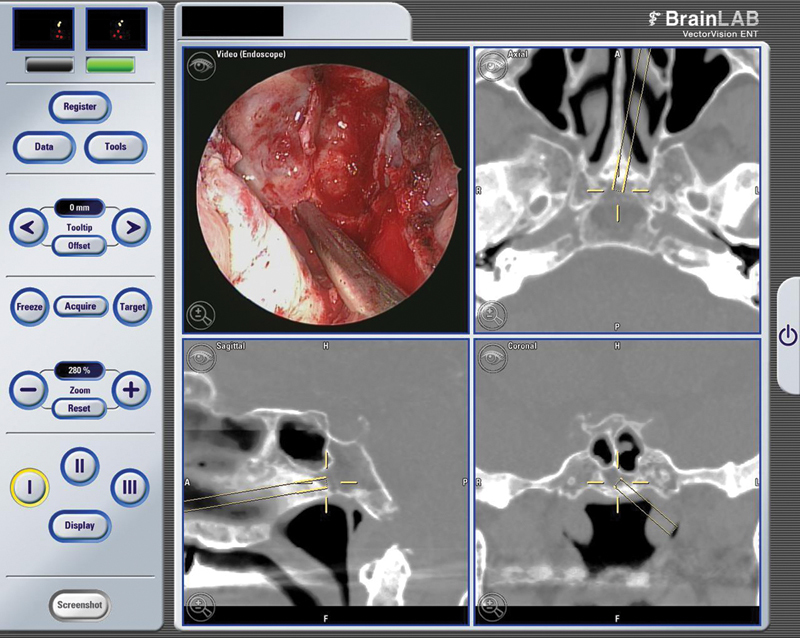
Intraoperative stereotactic snapshot image correlating intraoperative pathology with sagittal computed tomography scan.
The sphenoid ostia were enlarged using Kerrison rongeurs, allowing access to the central rostrum, which was taken down with a high-speed cutting and diamond drill bur. The intersinus sphenoid septum was resected, providing exposure to the central clivus. The anterior portion of the clivus was addressed using the high-speed drill and thinned, allowing the transclival biopsy to occur. The bone quality was abnormally soft and extremely vascular.
Upon confirming midline location with stereotactic navigation, multiple segments of cortical margins were curetted and sent for permanent specimen. Using the #3 round diamond bur, the clival area was outlined and its center was fractured. The bone quality was soft and fractured like an egg shell. We encountered a honeycomb appearance of osteolytic cells with significant blood clot. The anterior core was soft, suggestive of a cystic lesion, and the edges were scraped and sent off for pathology. The posterior clival bony margin was not violated (Fig. 5).
Fig. 5.
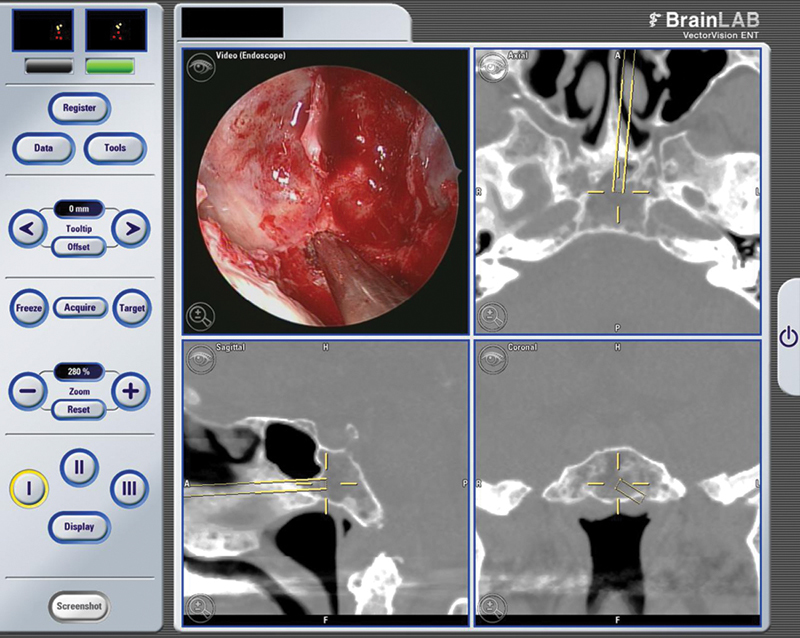
Similar Intraoperative snapshot image of a deeper and posterior limit of the biopsy.
Once the biopsy was completed, the skull base defect was reconstructed with a combination of Surgifoam (Ethicon, Inc.; Somerville, New Jersey, USA) and SurgiSeal (Adhezion Biomedical, LLC; Wyomissing, Pennsylvania, USA). Gelfoam (Pfizer, New York, New York, USA) was used to bolster the reconstruction, in lieu of nasal packing.
Pathological Findings
Grossly the specimen consisted of hemorrhagic bone fragments. On hematoxylin and eosin staining, two of the three bony specimens revealed sclerotic bone trabeculae that were thickened with increased collagen bands and ectatic vascular channels in the intertrabecular spaces. These ectatic vessels had thin walls composed of benign endothelial cells and filled much of the observed marrow spaces. The findings of sclerotic bone and a benign vascular proliferation were most consistent with a diagnosis of intraosseous hemangioma (Fig. 6).
Fig. 6.
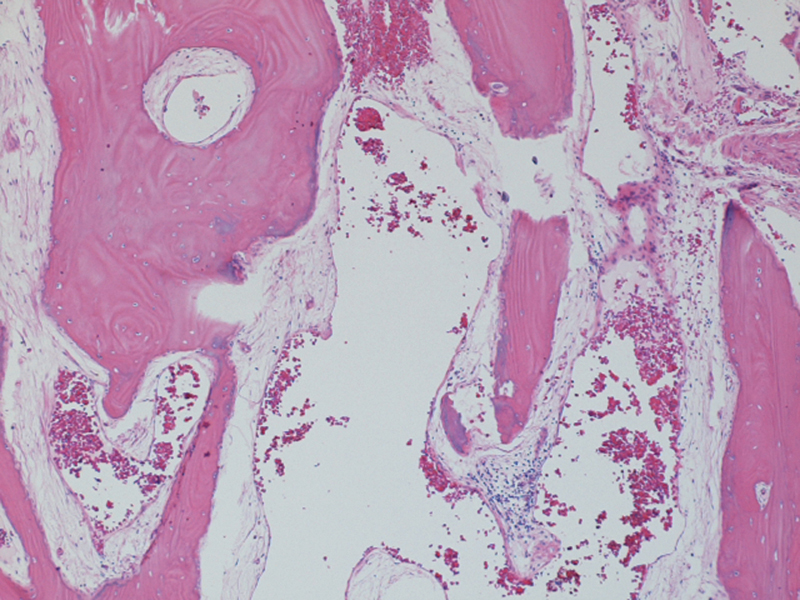
Hematoxylin and eosin staining (5 ×) demonstrating sclerotic bone trabeculae that are thickened with increased collagen bands and ectatic vascular channels in the intertrabecular spaces.
Postoperative Course
The patient was successfully extubated in the operating room and taken to the neurosurgical intensive care unit for overnight observation. She remained at her neurological baseline and was transferred to the regular floor on postoperative day one. She returned home neurologically stable.
At her postoperative visit a week later, she remained at her neurological baseline and continued to have difficulty swallowing and moving her tongue on her right side. She denied any fevers, congestion, or spinal fluid leak. She continued to be seen by the visiting nurse services (VNS), who were in the process of sending her for intensive speech and swallow therapy. She was also seen by otolaryngology for postoperative sinus endoscopy and debridement, which proceeded without incident and was remarkable only for normal postoperative crusting.
Several weeks later, the patient began reporting some easement of her left facial numbness but continued reporting left temporal headaches. In addition, her erythrocyte sedimentation rate (ESR) was found to be 81, and, in consultation with the neurology service, a biopsy was performed to rule out giant cell arteritis. The biopsy, however, was negative for arteritis. The patient is currently 9 months postsurgery with surgical defects well healed and no evidence of cerebrospinal fluid leak or infections. She continues to have stable cranial neuropathies without evidence of malignant dissemination of the suspected renal cell carcinoma. It does appear that all of the patient's cranial neuropathies were secondary to the clival hemangioma.
Discussion
Primary Intraosseous Hemangiomas
Primary intraosseous hemangiomas are benign, malformed vascular lesions that account for approximately 1% of all primary bone neoplasms.1,2 In contrast, cavernous hemangiomas of the brain are characterized by ectatic, mostly thin-walled blood vessels with little intervening brain tissue. They occur most frequently in the spine (30 to 50%) and skull (20%), whereas involvement of other sites (such as the long bones, short tubular bones, and ribs) is rare. Histologically, hemangiomas of the bone are classified as cavernous and capillary subtypes.1,4 The former comprised thin-walled blood vessels that have variable amounts of intervening collagenous fibrosis that may show calcification. Capillary hemangiomas, in contrast, lack fibrous septa, have smaller vessel lumens, and affect the vertebral column.2
Although most hemangiomas of bone are asymptomatic incidental findings, cerebral hemangiomas can present with seizures, headache, focal neurological deficits, and occasionally hemorrhage. Bone hemangiomas can be expansile lesions, producing local swelling when symptomatic, with associated local pain or fractures.2 Cerebral cavernous hemangiomas may be hereditary. Some are autosomal dominant, caused by mutations of the KRITI1 gene on the long arm of chromosome 7.6 This encodes a protein that interacts with the tumor suppressor gene krev-1/rap1a. Occasionally, the mutation gives rise to hyperkeratotic cutaneous venous malformations, as well. Mutations on 7p and 3q are also linked to familial cavernous hemangiomas.7
Skeletal hemangiomatosis is characteristically not hereditary and is associated with visceral involvement 60 to 70% of the time and lymphangiomatosis.8 Visceral involvement is mostly of the spleen, liver, kidney, mesentery, chest, and lymph nodes and confers a poorer prognosis. Gorham disease may represent a more severe form of multifocal cavernous hemangiomatosis. It is extremely rare, occurs in children and young adults, and is characterized by resorption of affected bone; hence the name disappearing or phantom bone disease.8
Calvarial Cavernous Hemangiomas
Calvarial cavernous hemangiomas are typically diagnosed incidentally after a CT or MRI scan is performed for other reasons. Occasionally, they may present as a peripheral, slowly enlarging calvarial lump that may be associated with dull or throbbing headaches. As the hemangioma expands, the headaches may increase in severity. Neurological findings, however, are remarkably rare, as these rarely expand intracranially.1
Calvarial cavernous hemangiomas most frequently affect the frontal and parietal bones, but hemangiomas arising from the craniofacial bones (zygoma, maxilla, vomer, and mandible) have been reported as well.3,4,9,10,11,12,13,14,15 In these locations, they typically manifest as painless, progressive facial swelling. When arising from bones of the orbit, some authors classify them as primary intraosseous orbital hemangiomas.1,16,17 These tend to present as supraorbital swelling or neuralgia or nasolacrimal obstruction. In advanced cases, proptosis, blepharoptosis, diplopia, and visual loss may ensue as tumors expand into the orbital cavity.1,16,17 Cavernous hemangiomas of the skull base are exceptionally rare, and only two have been reported to arise from the clivus.18,19
The radiographic characteristics of intraosseous hemangiomas have been well described. Typically, these lesions appear as well-circumscribed, expansive areas of rarefaction owing to resorption of trabeculae by enlarged vascular channels. This leads to a honeycomb or polka-dotted pattern seen on CT, representing coarse trabeculae seen in axial cuts.1,18,19 This is particularly apparent in vertebral hemangiomas but is not well appreciated in calvarial hemangiomas. Although the cortex may be greatly expanded, a thin bony shell and periosteum is invariably maintained, differentiating it from osteogenic sarcomas, in which the periosteum and surrounding tissues are usually invaded.1
The signal characteristics on MRI are variable, largely depending on the amount of slow-moving venous blood, as well as on the ratio of red marrow to converted fatty marrow present within the lesion. Hence, on T1-weighted sequences, smaller lesions or those with more fatty content tend to have higher signal intensity. On T2-weighted sequences, areas with slow venous flow or blood pooling have increased signal intensity.1,18,19 Generally, though, intraosseous hemangiomas have heterogeneous signal in both T1- and T2-weighted sequences, and CT scan is more useful for operative planning.
On angiography, larger hemangiomas tend to demonstrate a delayed blush with feeding arteries but no draining veins. Preoperative embolization may be very useful in managing these lesions.1,18,20
Small calvarial hemangiomas, especially those in the skull base, may not demonstrate many of the typical radiographic characteristics of intraosseous hemangiomas. In these instances, diagnosis may be made only after pathological examination.5
Skull Base Clival Hemangioma
Our case is unique with regards to the patient's presentation, radiographic findings, and lesion location. Indeed, presentation with headache and severe multiple lower cranial nerve neuropathies has not been described previously in the literature and is more consistent with Garcin syndrome, which is a rare syndrome of unilateral paralysis of many or all cranial nerves seen in tumors of the nasopharynx or skull base.21 Prognosis is generally unfavorable, presumably owing to a late manifestation of an aggressive disease process. Although many etiologies of Garcin syndrome have been reported, including nasopharyngeal carcinoma, skull base sarcoma, metastasis, giant aneurysm, basal meningitis, and carcinomatous meningitis,21 hemangioma has never been identified. Similarly, the typical radiographic characteristics of intraosseous hemangiomas, such as angiographic blush and honeycomb trabeculations, were not apparent in this lesion, possibly owing to the small lesion size and relative lack of weight-bearing stress in the clivus, respectively.
Primary intraosseous hemangiomas grow very slowly but do not spontaneously involute.1 Generally, indications for surgical removal include neurological compromise, unremitting but attributable headache, mass effect, cosmetic deformity, or need for definitive diagnosis. When these criteria are met, en bloc resection is recommended with establishment of normal bony margins.1,2 Of course, this is much more difficult to attain in the skull base, and subtotal resection or biopsy may be the only feasible options. This approach, however, may be complicated by severe hemorrhage from the mass itself. After en bloc resection, prognosis is excellent and recurrence rare.1,2 Our intent was to biopsy the lesion for definitive diagnosis.
The literature supporting radiation therapy for intraosseous hemangiomas of the calvarium is sparse but may support it as an alternative or adjunct to surgical resection.1,2,22 Other authors, however, cite concerns of malignant transformation, secondary malignancy generation, hypopituitarism, or cranial nerve deficit after radiation.1 Expectant management of intraosseous hemangiomas in sensitive locations may be preferable in such instances. The role of radiosurgery in the treatment of intracranial cavernous hemangiomas remains controversial. Currently, it may be supported for deep, eloquent hemangiomas, such as those in the cavernous sinus, especially if they have bled previously, or when risk of direct microsurgical resection is overwhelming.23
In our patient, the consensus among the tumor board was that radiation therapy for a clival hemangioma was not supported by the literature. In the end, tissue diagnosis was instrumental in preventing our patient from receiving unnecessary radiation to this sensitive area. Instead, given her comorbidities and advanced age, expectant observation was agreed upon.
References
- 1.Liu J K, Burger P C, Harnsberger H R, Couldwell W T. Primary intraosseous skull base cavernous hemangioma: case report. Skull Base. 2003;13(4):219–228. doi: 10.1055/s-2004-817698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peterson D L Murk S E Story J L Multifocal cavernous hemangioma of the skull: report of a case and review of the literature Neurosurgery 1992305778–781., discussion 782 [PubMed] [Google Scholar]
- 3.Heckl S, Aschoff A, Kunze S. Cavernomas of the skull: review of the literature 1975-2000. Neurosurg Rev. 2002;25(1-2):56–62, discussion 66-67. doi: 10.1007/s101430100180. [DOI] [PubMed] [Google Scholar]
- 4.Sade B, Lee D K, Prayson R A, Hughes G B, Lee J H. Intraosseous cavernous angioma of the petrous bone. Skull Base. 2009;19(3):237–240. doi: 10.1055/s-0028-1114294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khanam H, Lipper M H, Wolff C L, Lopes M B. Calvarial hemangiomas: report of two cases and review of the literature. Surg Neurol. 2001;55(1):63–67, discussion 67. doi: 10.1016/s0090-3019(00)00268-8. [DOI] [PubMed] [Google Scholar]
- 6.Verlaan D J, Davenport W J, Stefan H, Sure U, Siegel A M, Rouleau G A. Cerebral cavernous malformations: mutations in Krit1. Neurology. 2002;58(6):853–857. doi: 10.1212/wnl.58.6.853. [DOI] [PubMed] [Google Scholar]
- 7.Craig H D, Günel M, Cepeda O. et al. Multilocus linkage identifies two new loci for a mendelian form of stroke, cerebral cavernous malformation, at 7p15-13 and 3q25.2-27. Hum Mol Genet. 1998;7(12):1851–1858. doi: 10.1093/hmg/7.12.1851. [DOI] [PubMed] [Google Scholar]
- 8.Verbeke S L, Bovée J V. Primary vascular tumors of bone: a spectrum of entities? Int J Clin Exp Pathol. 2011;4(6):541–551. [PMC free article] [PubMed] [Google Scholar]
- 9.Clauser L, Meneghini F, Riga M, Rigo L. Haemangioma of the zygoma. Report of two cases with a review of the literature. J Craniomaxillofac Surg. 1991;19(8):353–358. doi: 10.1016/s1010-5182(05)80278-1. [DOI] [PubMed] [Google Scholar]
- 10.Cuesta Gil M, Navarro-Vila C. Intraosseous hemangioma of the zygomatic bone. A case report. Int J Oral Maxillofac Surg. 1992;21(5):287–291. doi: 10.1016/s0901-5027(05)80739-8. [DOI] [PubMed] [Google Scholar]
- 11.Guibert-Tranier F, Piton J, Riche M C, Merland J J, Caille J M. Vascular malformations of the mandible (intraosseous haemangiomas). The importance of preoperative embolization. A study of 9 cases. Eur J Radiol. 1982;2(4):257–272. [PubMed] [Google Scholar]
- 12.Moore S L, Chun J K, Mitre S A, Som P M. Intraosseous hemangioma of the zygoma: CT and MR findings. AJNR Am J Neuroradiol. 2001;22(7):1383–1385. [PMC free article] [PubMed] [Google Scholar]
- 13.Nakahira M, Kishimoto S, Miura T, Saito H. Intraosseous hemangioma of the vomer: a case report. Am J Rhinol. 1997;11(6):473–477. doi: 10.2500/105065897780914956. [DOI] [PubMed] [Google Scholar]
- 14.Ozdemir R, Alagoz S, Uysal A C, Unlu R E, Ortak T, Sensoz O. Intraosseous hemangioma of the mandible: a case report and review of the literature. J Craniofac Surg. 2002;13(1):38–43. doi: 10.1097/00001665-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Stassi J, Rao V M, Lowry L. Hemangioma of bone arising in the maxilla. Skeletal Radiol. 1984;12(3):187–191. doi: 10.1007/BF00361085. [DOI] [PubMed] [Google Scholar]
- 16.Banerji D, Inao S, Sugita K, Kaur A, Chhabra D K. Primary intraosseous orbital hemangioma: a case report and review of the literature. Neurosurgery. 1994;35(6):1131–1134. doi: 10.1227/00006123-199412000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Relf S J Bartley G B Unni K K Primary orbital intraosseous hemangioma Ophthalmology 1991984541–546., discussion 547 [PubMed] [Google Scholar]
- 18.Tashiro T, Inoue Y, Nemoto Y. et al. Cavernous hemangioma of the clivus: case report and review of the literature. AJNR Am J Neuroradiol. 1991;12(6):1193–1194. [PMC free article] [PubMed] [Google Scholar]
- 19.Vanhoenacker F M, De Praeter G, Kools D, Voormolen M, Parizel P M. Unusual lesion of the clivus: diagnosis and discussion. Skeletal Radiol. 2011;40(2):243–244. doi: 10.1007/s00256-010-0981-6. [DOI] [PubMed] [Google Scholar]
- 20.Lobato R D, Lamas E, Amor T, Rivas J J. Primary calvarial hemangioma: angiographic study. Surg Neurol. 1978;10(6):389–394. [PubMed] [Google Scholar]
- 21.Mubaidin S I, Sunna J B, Beiruti M A, Shennak M M, Ayoub M S. Renal cell carcinoma presenting as Garcin's syndrome. J Neurol Neurosurg Psychiatry. 1990;53(7):613–614. doi: 10.1136/jnnp.53.7.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sweet C, Silbergleit R, Mehta B. Primary intraosseous hemangioma of the orbit: CT and MR appearance. AJNR Am J Neuroradiol. 1997;18(2):379–381. [PMC free article] [PubMed] [Google Scholar]
- 23.Chou C W Wu H M Huang C I et al. Gamma knife surgery for cavernous hemangiomas in the cavernous sinus Neurosurgery 2010673611–616., discussion 616 [DOI] [PubMed] [Google Scholar]


