Abstract
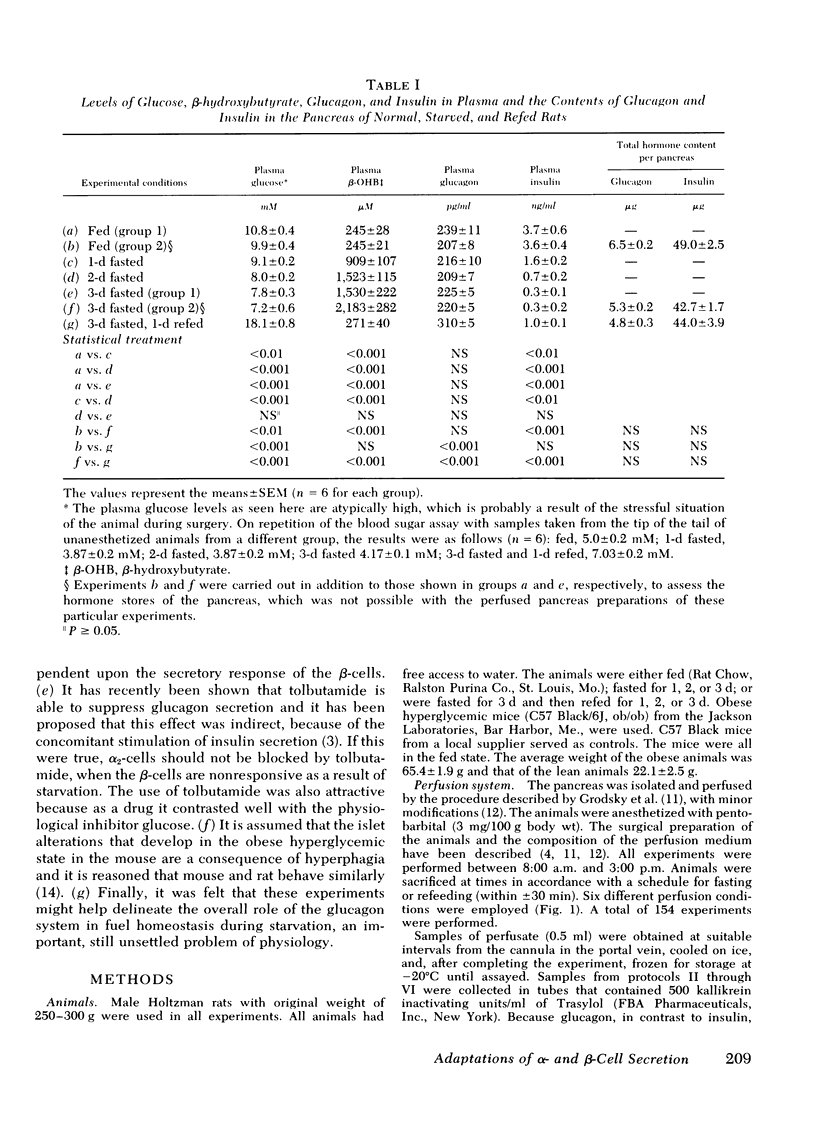
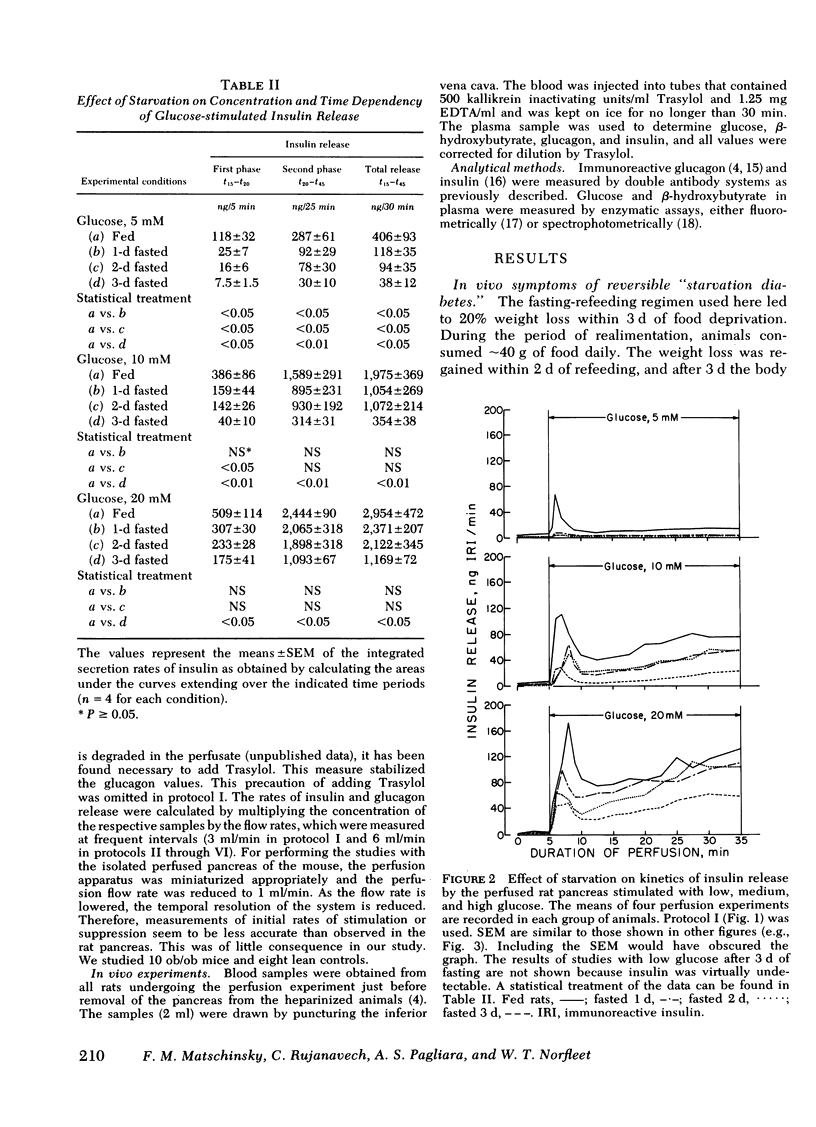
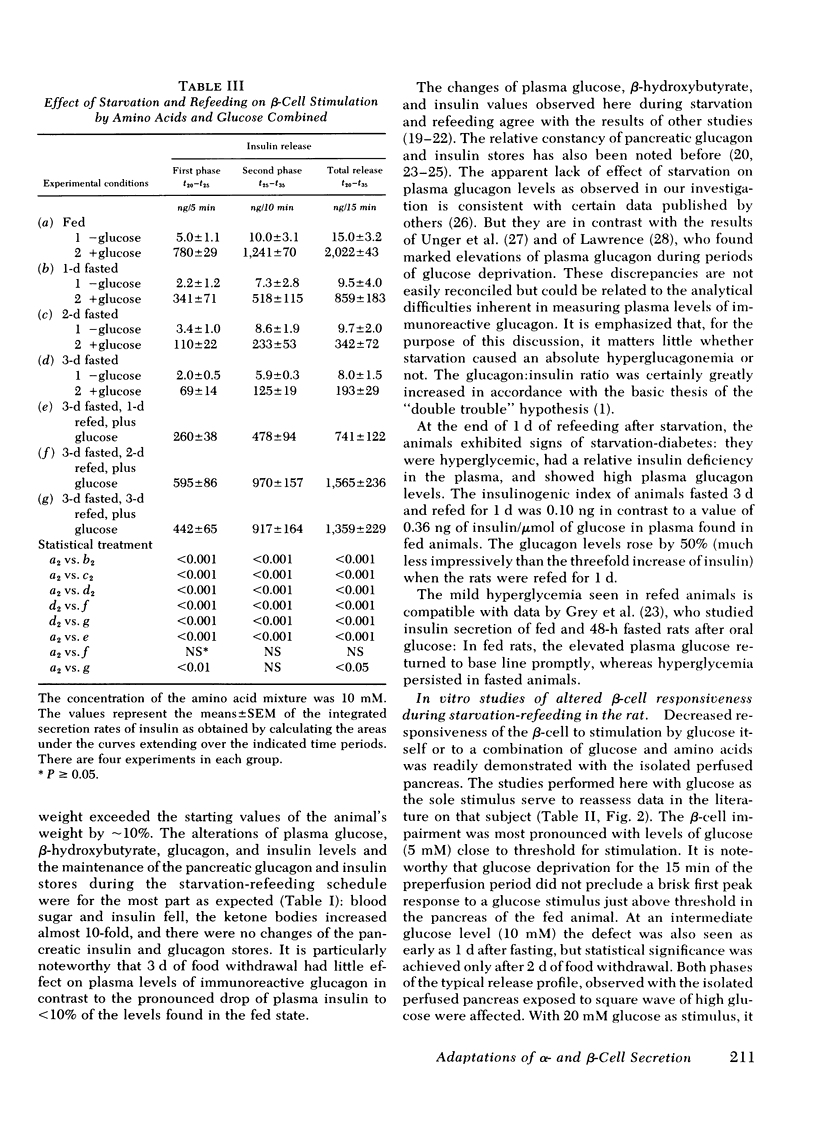
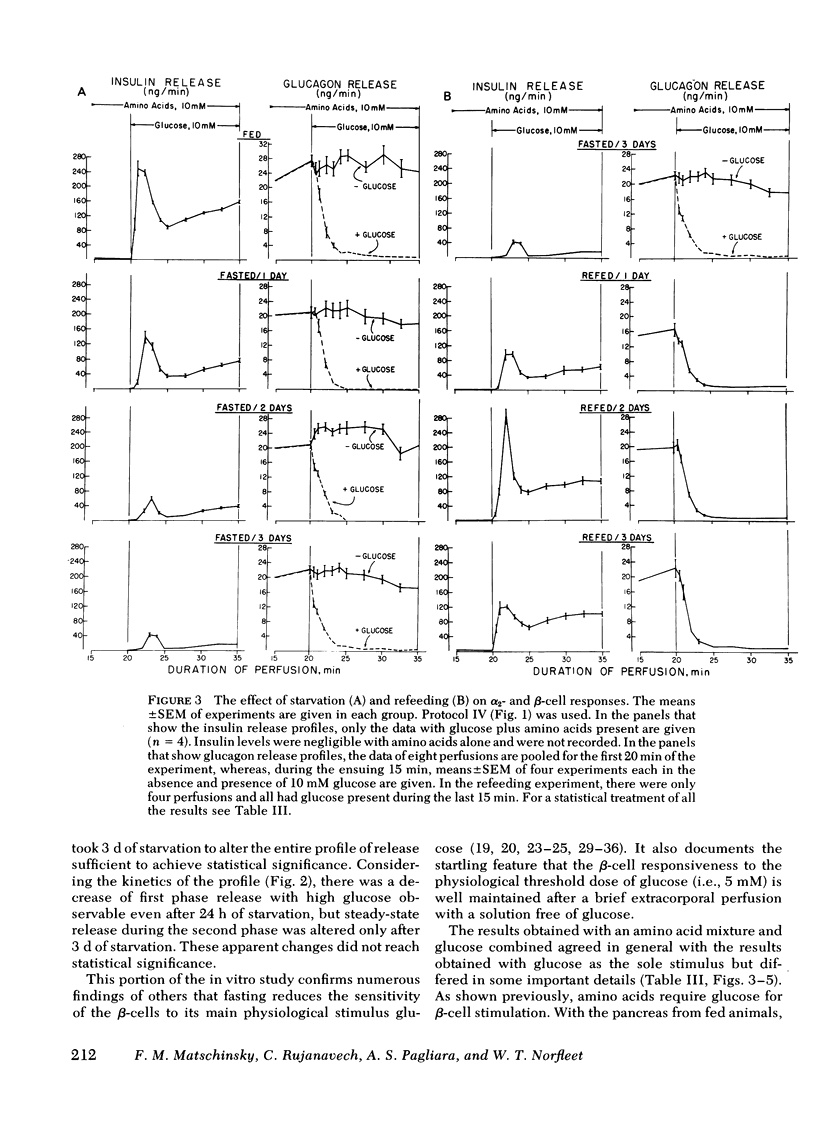
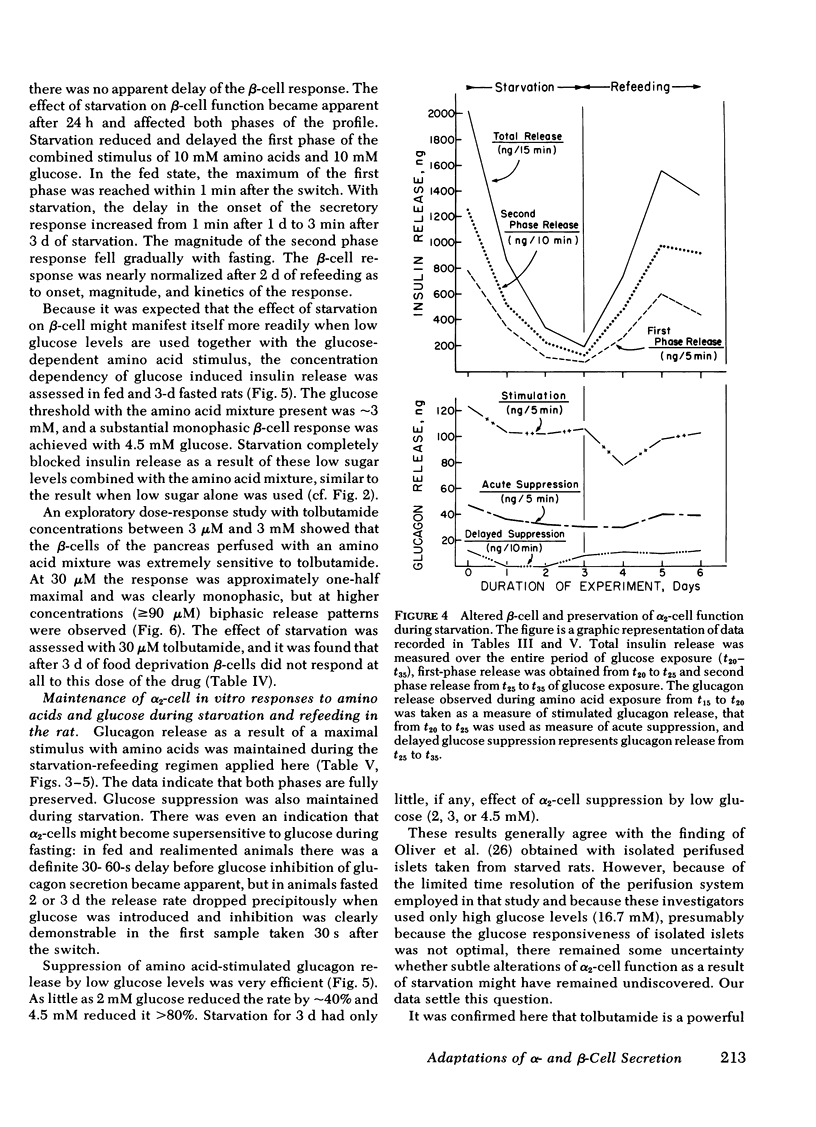
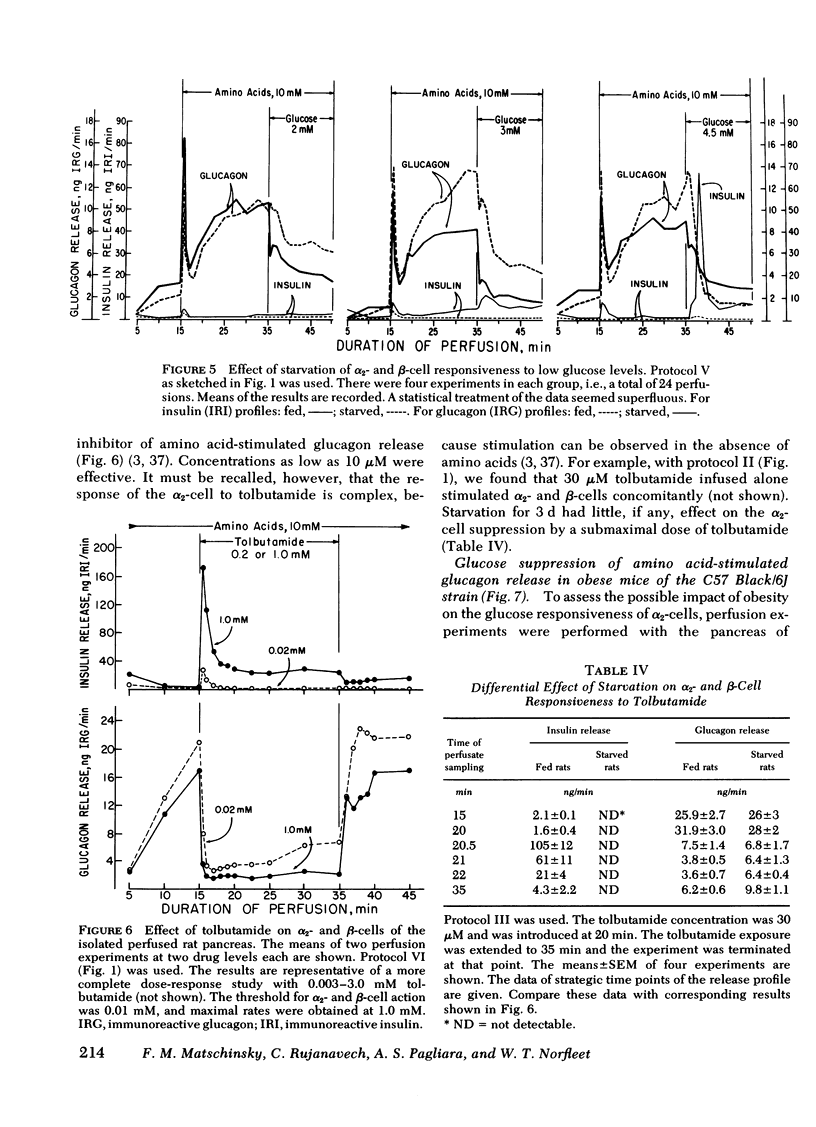
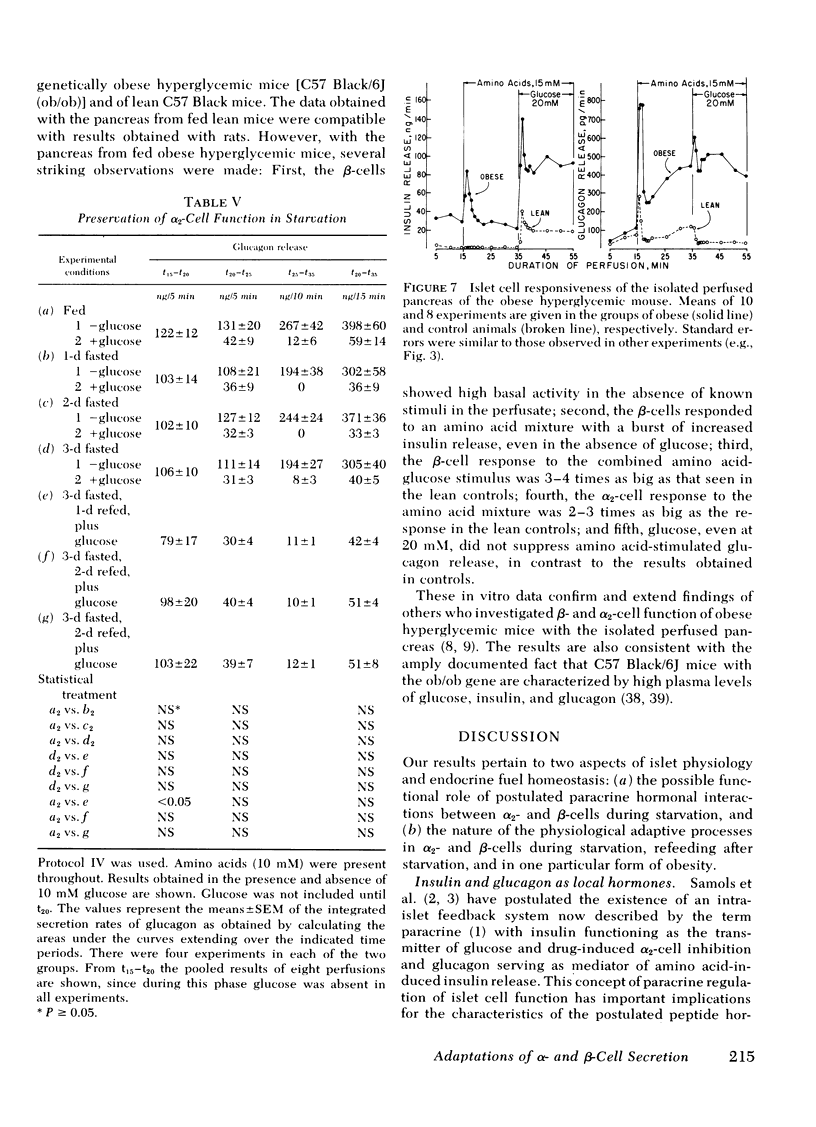
The effects of starvation and refeeding and of obesity on pancreatic alpha2- and beta-cell responses to glucose or tolbutamide were studied with the isolated rat or mouse pancreas perfused with an amino acid mixture in the presence and absence of glucose. It was observed that the physiological adaptation to a regimen of fasting and realimentation and to obesity differed greatly in the two types of endocrine cells. Whereas beta-cells of rats showed a dramatic reduction of glucose- and tolbutamide-stimulated insulin release during starvation that was reversed by refeeding, alpha2-cells preserved their response to stimulators and inhibitors during this experimental manipulation. Amino acid stimulation of glucagon release occurred equally well with the pancreas from fed and starved rats and was suppressed efficiently by glucose and tolbutamide in both nutritional states. Surprisingly, the rate of onset of glucose suppression of alpha2-cells was significantly higher in the fasted than in the fed state. This glucose hypersensitivity was apparent 2 d after after food deprivation and had disappeared again on the 2nd d of refeeding. In the pancreas from animals starved for 3 d, glucose and tolbutamide suppression of alpha2-cells took place in the absence of demonstrable changes of insulin release. In the isolated perfused pancreas taken from the hyperphagic obese hyperglycemic mouse (C57 Black/6J; ob/ob), the observed rate of insulin secretion as a result of a combined stimulus of amino acids and glucose and of glucagon release stimulated by amino acids was about four times higher than achieved by the pancreas of lean controls. However, glucose was unable to suppress the alpha2-cells in the pancreas of obese animals, in spite of the hypersection of the beta-cells, again in contrast to the alpha2-cells of controls that were readily inhibited by glucose. These data imply that the acute suppression of alpha2-cells by glucose is largely independent of a concomitant surge of extracellular insulin levels and that the adaptation of the islet organ to starvation leads to decreased glucose sensitivity of beta-cells, which contrasts with an improved glucose responsiveness of alpha2-cells. However, hyperphagia, which is assumed to be the primary abnormality in the ob/ob mouse, leads to overproduction of insulin and glucagon by the pancreas while greatly reducing the alpha2-cell sensitivity to glucose. An attempt is made to incorporate these data on starvation, refeeding, and obesity, as well as previous results with experimental diabetes, in a comprehensive picture describing a regulative principle underlying the glucose responsivness of alpha2-cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECK P., KOUMANS J. H., WINTERLING C. A., STEIN M. F., DAUGHADAY W. H., KIPNIS D. M. STUDIES OF INSULIN AND GROWTH HORMONE SECRETION IN HUMAN OBESITY. J Lab Clin Med. 1964 Oct;64:654–667. [PubMed] [Google Scholar]
- Bar R. S., Harrison L. C., Muggeo M., Gorden P., Kahn C. R., Roth J. Regulation of insulin receptors in normal and abnormal physiology in humans. Adv Intern Med. 1979;24:23–52. [PubMed] [Google Scholar]
- Bosboom R. S., Zweens J., Bouman P. R. Effects of feeding and fasting on the insulin secretory response to glucose and sulfonylureas in intact rats and isolated perfused rat pancreas. Diabetologia. 1973 Aug;9(4):243–250. doi: 10.1007/BF01221849. [DOI] [PubMed] [Google Scholar]
- Buchanan K. D., Vance J. E., Williams R. H. Effect of starvation on insulin and glucagon release from isolated islets of Langerhans of the rat. Metabolism. 1969 Feb;18(2):155–162. doi: 10.1016/0026-0495(69)90110-3. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuendet G. S., Loten E. G., Cameron D. P., Renold A. E., Marliss E. B. Hormone-substrate responses to total fasting in lean and obese mice. Am J Physiol. 1975 Jan;228(1):276–283. doi: 10.1152/ajplegacy.1975.228.1.276. [DOI] [PubMed] [Google Scholar]
- Dubuc P. U., Mobley P. W., Mahler R. J., Ensinck J. W. Immunoreactive glucagon levels in obese-hyperglycemic (ob/ob) mice. Diabetes. 1977 Sep;26(9):841–846. doi: 10.2337/diab.26.9.841. [DOI] [PubMed] [Google Scholar]
- Fink G., Gutman R. A., Cresto J. C., Selawry H., Lavine R., Recant L. Glucose-induced insulin release patterns: effect of starvation. Diabetologia. 1974 Oct;10(5):421–425. doi: 10.1007/BF01221632. [DOI] [PubMed] [Google Scholar]
- Frankel B. J., Gerich J. E., Hagura R., Fanska R. E., Gerritsen G. C., Grodsky G. M. Abnormal secretion of insulin and glucagon by the in vitro perfused pancreas of the genetically diabetic Chinese hamster. J Clin Invest. 1974 Jun;53(6):1637–1646. doi: 10.1172/JCI107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRODSKY G. M., BATTS A. A., BENNETT L. L., VCELLA C., MCWILLIAMS N. B., SMITH D. F. EFFECTS OF CARBOHYDRATES ON SECRETION OF INSULIN FROM ISOLATED RAT PANCREAS. Am J Physiol. 1963 Oct;205:638–644. doi: 10.1152/ajplegacy.1963.205.4.638. [DOI] [PubMed] [Google Scholar]
- Genuth S. M. Effects of prolonged fasting on insulin secretion. Diabetes. 1966 Nov;15(11):798–806. doi: 10.2337/diab.15.11.798. [DOI] [PubMed] [Google Scholar]
- Grey N. J., Goldring S., Kipnis D. M. The effect of fasting, diet, and actinomycin D on insulin secretion in the rat. J Clin Invest. 1970 May;49(5):881–889. doi: 10.1172/JCI106307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodsky G. M., Epstein G. H., Fanska R., Karam J. H. Pancreatic action of the sulfonylureas. Fed Proc. 1977 Dec;36(13):2714–2719. [PubMed] [Google Scholar]
- Idahl L. A. Dynamics of pancreatic beta-cell responses to glucose. Diabetologia. 1973 Oct;9(5):403–412. doi: 10.1007/BF01239437. [DOI] [PubMed] [Google Scholar]
- Landgraf R., Kotler-Brajtburg J., Matschinsky F. M. Kinetics of insulin release from the perfused rat pancreas caused by glucose, glucosamine, and galactose. Proc Natl Acad Sci U S A. 1971 Mar;68(3):536–540. doi: 10.1073/pnas.68.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube H., Fussgänger R. D., Maier V., Pfeiffer E. F. Hyperglucagonemia of the isolated perfused pancreas of diabetic mice (db-db). Diabetologia. 1973 Oct;9(5):400–402. doi: 10.1007/BF01239436. [DOI] [PubMed] [Google Scholar]
- Laube H., Fussgänger R. D., Pfeiffer E. F. Paradoxical glucagon release in obese hyperglycemic mice. Horm Metab Res. 1974 Sep;6(5):426–426. doi: 10.1055/s-0028-1095724. [DOI] [PubMed] [Google Scholar]
- Lawrence A. M. Radioimmunoassayable glucagon levels in man: effects of starvation, hypoglycemia, and glucose administration. Proc Natl Acad Sci U S A. 1966 Feb;55(2):316–320. doi: 10.1073/pnas.55.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichter S. B., Pagliara A. S., Grieder M. H., Pohl S., Rosai J., Kipnis D. M. Uncontrolled diabetes mellitus and hyperglucagonemia associated with an islet cell carcinoma. Am J Med. 1975 Feb;58(2):285–293. doi: 10.1016/0002-9343(75)90579-3. [DOI] [PubMed] [Google Scholar]
- Lopez-Quijada C., Gomez-Acebo J., Blazquez E. [Effect of starvation upon insulin secretion in the rabbit]. Acta Diabetol Lat. 1969 Oct-Dec;6(4):820–835. doi: 10.1007/BF01548088. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Wright P. H. Effect of fasting upon insulin secretion in the rat. Am J Physiol. 1967 Oct;213(4):843–848. doi: 10.1152/ajplegacy.1967.213.4.843. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M., Ellerman J. E., Krzanowski J., Kotler-Brajtburg J., Landgraf R., Fertel R. The dual function of glucose in islets of Langerhans. J Biol Chem. 1971 Feb 25;246(4):1007–1011. [PubMed] [Google Scholar]
- Matschinsky F. M., Pagliara A. S., Hover B. A., Pace C. S., Ferrendelli J. A., Williams A. Hormone secretion and glucose metabolism in islets of Langerhans of the isolated perfused pancreas from normal and streptozotocin diabetic rats. J Biol Chem. 1976 Oct 10;251(19):6053–6061. [PubMed] [Google Scholar]
- Matschinsky F. M., Pagliara A. S., Stillings S. N., Hover B. A. Glucose and ATP levels in pancreatic islet tissue of normal and diabetic rats. J Clin Invest. 1976 Nov;58(5):1193–1200. doi: 10.1172/JCI108572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. R., Williams V., Wright P. H. Effects of fasting on insulin and glucagon secretion by isolated rat islets of Langerhans. Proc Soc Exp Biol Med. 1977 Feb;154(2):210–214. doi: 10.3181/00379727-154-39639. [DOI] [PubMed] [Google Scholar]
- Pagliara A. S., Stillings S. N., Haymond M. W., Hover B. A., Matschinsky F. M. Insulin and glucose as modulators of the amino acid-induced glucagon release in the isolated pancreas of alloxan and streptozotocin diabetic rats. J Clin Invest. 1975 Feb;55(2):244–255. doi: 10.1172/JCI107928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliara A. S., Stillings S. N., Hover B., Martin D. M., Matschinsky F. M. Glucose modulation of amino acid-induced glucagon and insulin release in the isolated perfused rat pancreas. J Clin Invest. 1974 Oct;54(4):819–832. doi: 10.1172/JCI107822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliara A. S., Stillings S. N., Zawalich W. S., Williams A. D., Matchinsky F. M. Glucose and 3-O-methylglucose protection against alloxan poisoning of pancreatic alpha and beta cells. Diabetes. 1977 Oct;26(10):973–979. doi: 10.2337/diab.26.10.973. [DOI] [PubMed] [Google Scholar]
- Samols E., Harrison J. Intraislet negative insulin-glucagon feedback. Metabolism. 1976 Nov;25(11 Suppl 1):1443–1447. doi: 10.1016/s0026-0495(76)80161-8. [DOI] [PubMed] [Google Scholar]
- Solomon S. S., Ensinck J. W., Williams R. H. Effect of starvation on plasma immunoreactive insulin and non-suppressible insulin-like activity in normal and obese humans. Metabolism. 1968 Jun;17(6):528–534. doi: 10.1016/0026-0495(68)90045-0. [DOI] [PubMed] [Google Scholar]
- Stauffacher W., Orci L., Cameron D. P., Burr I. M., Renold A. E. Spontaneous hyperglycemia and-or obesity in laboratory rodents: an example of the possible usefulness of animal disease models with both genetic and environmental components. Recent Prog Horm Res. 1971;27:41–95. doi: 10.1016/b978-0-12-571127-2.50026-2. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Orci L. The role of glucagon in the endogenous hyperglycemia of diabetes mellitus. Annu Rev Med. 1977;28:119–130. doi: 10.1146/annurev.me.28.020177.001003. [DOI] [PubMed] [Google Scholar]
- Unger R. H. The Banting Memorial Lecture 1975. Diabetes and the alpha cell. Diabetes. 1976 Feb;25(2):136–151. doi: 10.2337/diab.25.2.136. [DOI] [PubMed] [Google Scholar]
- Vance J. E., Buchanan K. D., Williams R. H. Effect of starvation and refeeding on serum immunoreactive glucagon and insulin levels. J Lab Clin Med. 1968 Aug;72(2):290–297. [PubMed] [Google Scholar]
- Voyles N., Gutman R. A., Selawry H., Fink G., Penhos J. C., Recant L. Interaction of various stimulators and inhibitors on insulin secretion in vitro. Horm Res. 1973;4(2):65–73. doi: 10.1159/000178291. [DOI] [PubMed] [Google Scholar]
- Weir G. C., Knowlton S. D., Atkins R. F., McKennan K. X., Martin D. B. Glucagon secretion from the perfused pancreas of streptozotocin-treated rats. Diabetes. 1976 Apr;25(4):275–282. doi: 10.2337/diab.25.4.275. [DOI] [PubMed] [Google Scholar]
- Weir G. C., Samols E., Loo S., Patel Y. C., Gabbay K. H. Somatostatin and pancreatic polypeptide secretion: effects of glucagon, insulin, and arginine. Diabetes. 1979 Jan;28(1):35–40. [PubMed] [Google Scholar]


