Abstract
BACKGROUND:
Polycystic ovary syndrome (PCOS) is the commonest endocrinopathy in women that is associated with reproductive and metabolic disorders.
OBJECTIVES:
We compared the ovulation and conception rates after the treatment with clomiphene citrate (CC) alone and in combination with metformin in infertile patients presented with polycystic ovarian syndrome (PCOS).
MATERIALS AND METHODS:
This randomized controlled trial of independent cases and controls was conducted at the Department of Obstetrics and Gynecology, Hera General Hospital, Makkah, Saudi Arabia from February 01 to December 31, 2008. The 42 subjects diagnosed as PCOS were divided into group A and B (21 subjects in each) for management with CC + metformin and CC alone, respectively. Group A received 500 mg three times a day of metformin continuously from the first cycle for 6 months or till pregnancy was confirmed. In both groups CC was started at a dose of 50 mg from day-2 till day-6 of the menstrual cycle. The dose of CC was increased to 100 mg in second and 150 mg in third cycle, and then remained 150 mg for the remaining three cycles. With ovulation the dose of CC was unaltered in both groups. Data were analyzed using Statistical Package for the Social Sciences (SPSS) version 16.
RESULTS:
More than 50% females in both groups were had body mass index > 25. Group A achieved high rate of regular cycles, ovulation success, and conception than group B (71.4% vs. 38.1%; P = 0.03), (76.2% vs. 38.1%; P = 0.021), and (66.6% vs. 28.6%; P = 0.01), respectively.
CONCLUSION:
Management with metformin + CC increased the ovulation and conception rates.
KEY WORDS: Anovulation, clomiphene citrate, polycystic ovaries, metformin
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a heterogeneous disorder with an incidence of (4-7%) among reproductive aged women. It is characterized by chronic anovulation, hyperandrogenism, and is the most frequent endocrinopathy in women.[1] It is commonly linked with insulin resistance and compensatory hyperinsulinemia.[2,3] Ovarian hyperandrogenism and chronic anovulation are highly linked with hyperinsulinemia. However, weight loss in obese women with PCOS leads to decrease in insulin and androgen levels with an obvious improved fertility outcome.[4] Clomiphene citrate (CC) was the first-line therapy for ovulation induction in these patients but it has still high failure rate. However, CC usage for more than six cycles had high risk of ovarian cancer in these patients.[5,6]
According to latest recommendations, insulin-sensitizing agents (ISA), such as metformin and thiazolidinediones, have been preferred for PCOS treatment. These agents have both metabolic and endocrine/ovarian favorable effects. In addition to the favorable metabolic actions, i.e., improvement in glucose, lipid, and proinflammatory profiles, these agents also put forth beneficial endocrine and ovarian effects, e.g., amelioration of reproductive abnormalities, restoration of ovulation and menstrual cycles, reduction of androgen production, and increase in pregnancy rates. For that reason, the use of insulin sensitizers along with lifestyle measures and/or other agents, in women with PCOS, particularly in the situations of insulin- or clomifene-resistance, have been emphasized.[7,8]
To the best of our knowledge and understanding, the studies done on this topic are scarce in Saudi Arabia so we set out the objectives of this study to compare the effects of CC alone and in combination with metformin in females presented with PCOS.
MATERIALS AND METHODS
This randomized controlled trial of independent cases and controls with one control per case was conducted at the Department of Obstetrics and Gynecology, Hera General Hospital, Makkah, Saudi Arabia from February 01 to December 31, 2008.
The sample size was determined according to Jones et al., criteria[9] as 42 subjects because Vandermolen et al., indicated the ovulation failure rate of (P1 = 73%) in the group treated with CC alone and (P2 = 25%) for experimental group treated with both CC and metformin.[10] The standardized difference was calculated as 0.96 by following formula. This sample size was calculated to reject the null hypothesis that the failure rates for experimental and control subjects were equal with probability (power) 90%. The Type I error probability (alpha) associated with this test of this null hypothesis is 0.05. A line was drawn in the nomogram from 0.96 to the power axis at 0.9, and found 42 subjects from the intersect with the central axis, at 0.05 a level in the study.
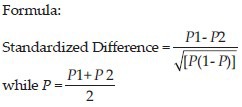
The first 42 diagnosed patients of PCOS were enrolled. The diagnosis was made on the bases of the presence of two of the three following criteria:
Polycystic ovaries (either 12 or more peripheral follicles or increased ovarian volume (greater than 10 cm3).
Oligo- or anovulation (irregular cycles, amenorrhea).
Clinical and/or biochemical signs of hyperandrogenism (Acne, hirsutism, voice changes, and clitoromegaly).
PCOS was only diagnosed after excluding other etiologies like thyroid dysfunction, congenital adrenal hyperplasia, hyperprolactinemia, and androgen-secreting tumors. The baseline screening tests were done, i.e., thyroid function tests, a serum prolactin, and free androgen index (total testosterone divided by sex hormone binding globulin (SHBG) ×100 to give a calculated free testosterone level). In cases with clinical evidence of hyperandrogenism and total testosterone > 5 nmol/l, 17-hydroxyprogesterone was advised to exclude androgen-secreting tumors. Male factor was kept normal in both groups by making semen analysis to rule out male infertility. Females with recent history of pelvic inflammatory disease were also not selected. Hysterosalpingogram was done to exclude tubal factor.
The females were divided into group A (cases) and group B (controls) (21 subjects in each) by randomization for management with CC along with metformin and CC alone, respectively. The randomization was done by opening opaque envelopes containing cards bearing the management protocols.
The group A received 500 mg three times a day of metformin continuously (the same dose) from the first cycle for 6 months or till pregnancy was confirmed. In both groups, CC was started at a dose of 50 mg from 2nd day of the menstrual cycle till 6th day for a total of 5 days.
All females were followed for six cycles for evidence of ovulation as detected by follicle tracking (ovarian volume, follicular size in millimeter and number of follicles) on ultrasonography by professionals and regulation of menstrual cycles. Ovulation was confirmed by dominant follicle (18-20 millimeter) on 12th day with absent follicles on 16th day. After ovulation Human Chorionic Gonadotropin 10,000 IU intramuscularly was given to patients followed by advice of intercourse after 36 hours. With ovulation the dose of CC was unaltered in both groups. The dose of CC was increased, if the size of dominant follicle was remained <18 mm, i.e., 100 mg during the second cycle and 150 mg in the 3rd cycle and kept the same dose of 150 mg for the remaining three cycles till ovulation occurred.
Confirmation of conception was done by positive urine pregnancy test in those women who did not menstruate. In females, who became pregnant, metformin was continued up to 08 weeks of pregnancy. Clinical pregnancy was confirmed by gestational sac detection on ultrasonography.
Data were analyzed using Statistical Package for the Social Sciences (SPSS) version 16 (SPSS Inc., Chicago, IL, USA) and subjected to descriptive analysis, i.e., number, percentage. Chi-square test (2 × 2 contingency Table) was applied to categorical variables.
ETHICAL ISSUES
The Hospital granted us permission to conduct this study and we declared that we have no financial or personal relationship(s) which may have inappropriately influenced us in writing this paper.
A written formal informed consent from all study participants was taken after they had been made aware of study procedure.
RESULTS
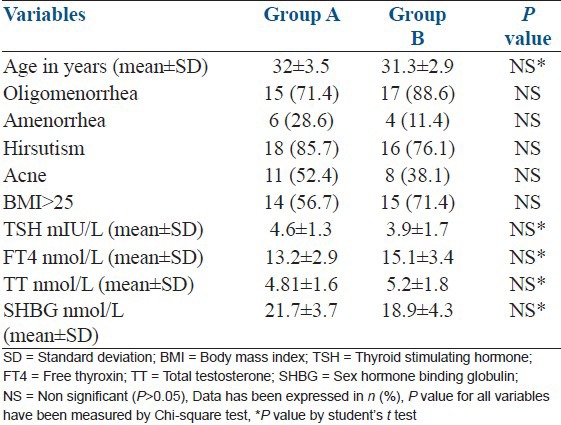
The subjects’ mean ± SD age (years) of group A was insignificantly high than group B, i.e., 32 ± 3.5 vs. 31.3 ± 2.9; P > 0.05. Amenorrhea was common in group A 6 (28.6) while group B females were more frequently suffered from oligomenorrhea, i.e., 17 (88.6). More than 50% females in both groups were had body mass index >25 [Table 1].
Table 1.
Base line characteristics of subjects
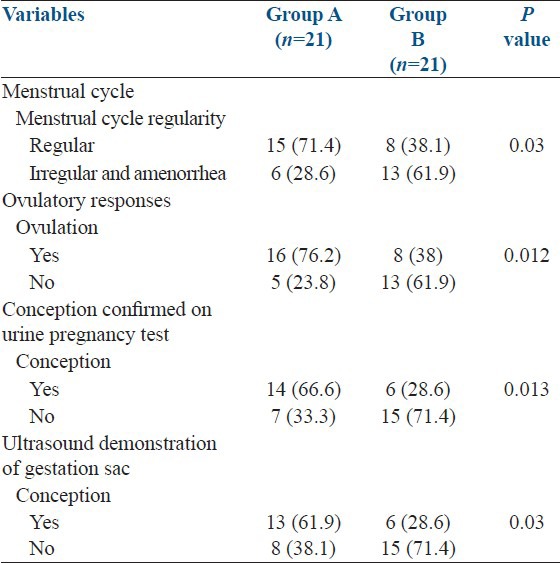
Group A achieved high rate of regular cycles than group B (71.4% vs. 38.1%; P = 0.03) while ovulatory response was also found higher in group A than group B (76.2% vs. 38.1%; P = 0.021). Similarly more females were found to be conceived confirmed by urine pregnancy test in group A than group B (66.6% vs. 28.6%; P = 0.01) as well as group A had high conception rate confirmed on presence of gestational sac by ultrasound (61.9% vs. 28.6%; P = 0.03) [Table 2].
Table 2.
Subjects’ outcome
In group A, 60% females had complained of loss of appetite, 18% had nausea and vomiting, but none of them discontinued therapy. There were no cases of hyperstimulation either in the group A or B. No teratogenic effects were observed in patients who conceived after treatment with metformin for ovulation induction. In both groups, no twin pregnancy was found.
DISCUSSION
PCOS were commonly treated by CC and gonadotrophins, but this mechanism has given a new direction towards its management with insulin sensitizing drugs that gave promising results and higher success rates of ovulation as well as pregnancy as compared with CC alone.[11]
A major development in clinical endocrinology was happened with the discovery that metformin is effective in ovulation induction in women with PCOS.[10] Placebo-controlled studies as mentioned by Cochrane review obviously indicated that metformin is superior to placebo for inducing ovulation in infertile women with PCOS with an odds ratio of 3.9 (confidence interval 2.3-6.7).[12]
Our study is comparable to a number of studies done internationally. A study was carried out at Jordan showing the effects of metformin along with CC compared with placebo-CC, on the ovulation and pregnancy rates in CC-resistant women with PCOS. It was found increased rates of ovulation (68.6% versus 25%, P < 0.05) and pregnancy (56.3% versus 16.6%, P < 0.05) in the metformin-CC group vs. placebo-CC controls. However, ovarian hyperstimulation in placebo-CC group was observed.[13] Overall ovulation rate in CC group of our study was comparable to certain studies, i.e., Batakan found 28.2% with 100 mg of CC, Boostanfar et al., found 45% with CC dose of 150 mg, Memon et al. found 23% with 100 mg of CC, and Lobo et al., found 32% with 200 mg of CC.[5,14,15,16]
In our study, regularity of menstrual cycle was found nearly double in the group A, that highlighted the fact that metformin helps in regulating the menses as determined by Katsiki et al., Zain et al., and Pasquali and Gambineri.[7,17,18]
A controlled trial in India found that the metformin + CC resulted in higher rate of ovulation than CC alone group (P = 0.0016). The pregnancy rate was 8% with CC and 24% with metformin + CC group. Loss of appetite was found in 80% females of the study group while 24% had nausea and vomiting. Moreover, no case of hyperstimulation was seen in either group. Finally, it was concluded that this combination should be initiated early in the course of treatment.[19]
Vandermolen et al. tried to determine whether metformin treatment increased the ovulation and pregnancy rates in addition to CC in women with PCOS who were resistant to CC alone through a randomized, double-blind, and placebo-controlled trial. Higher rates of ovulation as well as conception in metformin + CC vs. placebo + CC group were found, i.e., (75% vs. 27%) and (55% vs. 7%), respectively.[11]
Study of Zain et al., compared metformin alone, CC alone, and metformin + CC in three groups of subjects suffering from PCOS and found highest success rate of ovulation 68.7% and conception (21.1%) in metformin + CC group. However, they concluded that CC should be used as first line therapy in these females.[17]
Contrary to the study of Zain et al., Neveu et al., found nearly similar ovulation rates among PCOS females treated with metformin alone and metformin + CC group. Furthermore, conception rates were also not different in the three groups, i.e., metformin alone, CC alone, and combination group. It was suggested that metformin can be used first for ovulation induction in females with PCOS despite of their weight and insulin levels because of its efficacy and known safety profile.[20]
Decision-analytic model by Jungheim and Odibo comparing the same three treatment strategies using probability estimates derived after reviewing literature and sensitivity analyses performed on the baseline assumptions. Contrary to studies of Neveu et al., and Zain et al., it was finalized that combination therapy should be the first line therapy.[18,21]
CONCLUSION
A combination of metformin and CC significantly regulated the menstrual cycle and increased the ovulation and conception rates in study patients without complications, so we prefer this combination therapy as first line therapy.
RECOMMENDATIONS
A multicenteric randomized controlled trial has been highly recommended to prove the effects of metformin on menstrual cycle regularity in PCOS subjects.
Footnotes
Source of Support: Hospital itself
Conflict of Interest: None declared
REFERENCES
- 1.Costello MF. Theme: Polycystic ovary syndrome – a management update. Aust Fam Physician. 2005;34:127–33. [PubMed] [Google Scholar]
- 2.Burghen GA, Givens JR, Kitabachi AE. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab. 1980;50:113–6. doi: 10.1210/jcem-50-1-113. [DOI] [PubMed] [Google Scholar]
- 3.Dunaif A. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Semin Reprod Endocrinol. 1994;12:15–20. [Google Scholar]
- 4.Kiddy DS, Hamilton-Fairley D, Bush A, Short F, Anyaoku V, Reed MJ, et al. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 1992;36:105–11. doi: 10.1111/j.1365-2265.1992.tb02909.x. [DOI] [PubMed] [Google Scholar]
- 5.Labo RA, Granger LR, Davajan V, Mishell DR., Jr An extended regimen of clomiphene citrate in women unresponsive to standard therapy. Fertil Steril. 1982;37:462–6. doi: 10.1016/s0015-0282(16)46335-1. [DOI] [PubMed] [Google Scholar]
- 6.Balasch J, Barri PN. Follicular stimulation and ovarian cancer. Hum Reprod. 1993;8:990–6. doi: 10.1093/oxfordjournals.humrep.a138215. [DOI] [PubMed] [Google Scholar]
- 7.Katsiki N, Georgiadou E, Hatzitolios AI. The role of insulin-sensitizing agents in the treatment of polycystic ovary syndrome. Drugs. 2009;69:1417–31. doi: 10.2165/00003495-200969110-00001. [DOI] [PubMed] [Google Scholar]
- 8.Fact sheet from reproductive facts.org. Birmingham, Alabama: American Society For Reproductive Medicine; c1996-2012. [Last cited on 2012 sep 15, Last accessed on 2012 sep 15]. Polycystic ovarian syndrome. Available from: http://www. reproductivefacts.org/Insulin_sensitizing_agents_and_pcos . [Google Scholar]
- 9.Jones SR, Carley S, Harrison M. An introduction to power and sample size estimation. Emerg Med J. 2003;20:453–8. doi: 10.1136/emj.20.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandermolen DT, Ratts VS, Evans WS, Stovall DW, Kauma SW, Nestler JE. Metformin increases the ovulatory rate and pregnancy rate from clomiphene citrate in patients with polycystic ovary syndrome who are resistant to clomiphene citrate alone. Fertil Steril. 2001;75:310–5. doi: 10.1016/s0015-0282(00)01675-7. [DOI] [PubMed] [Google Scholar]
- 11.Maria JI, Nestler JE. Insulin-lowering drugs in polycystic ovary syndrome. Obstet Gynecol Clin North Am. 2001;28:153–64. doi: 10.1016/s0889-8545(05)70191-1. [DOI] [PubMed] [Google Scholar]
- 12.Lord JM, Flight IH, Norman RJ. Metformin in polycystic ovary syndrome: Systematic review and meta-analysis. Br Med J. 2003;327:951–3. doi: 10.1136/bmj.327.7421.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malkawi HY, Qublan HS. The effect of metformin plus clomiphene citrate on ovulation and pregnancy rates in clomiphene-resistant women with polycystic ovary syndrome. Saudi Med J. 2002;23:663–6. [PubMed] [Google Scholar]
- 14.Batukan C, Baysal B. Metformin improves ovulation and pregnancy rates in patients with polycystic ovarian syndrome. Arch Gynecol Obstet. 2001;265:124–7. doi: 10.1007/s004040100176. [DOI] [PubMed] [Google Scholar]
- 15.Boostanfar R, Jain JK, Mishell DR, Jr, Paulson RJ. A prospective randomized trial comparing clomiphene citrate with tamoxifen citrate for ovulation induction. Fertil Steril. 2001;75:1024–6. doi: 10.1016/s0015-0282(01)01749-6. [DOI] [PubMed] [Google Scholar]
- 16.Memon Z, Karim N, Agha F. Effect of metformin versus clomiphene citrate on ovulation and conception rate in polycystic ovarian syndrome patients. J Basic Appl Sci. 2011;7:55–9. [Google Scholar]
- 17.Zain MM, Jamaluddin R, Ibrahim A, Norman RJ. Comparison of clomiphene citrate, metformin, or the combination of both for first-line ovulation induction, achievement of pregnancy, and live birth in Asian women with polycystic ovary syndrome: A randomized controlled trial. Fertil Steril. 2009;91:514–21. doi: 10.1016/j.fertnstert.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Pasquali R, Gambineri A. Insulin-sensitizing agents in polycystic ovary syndrome. Eur J Endocrinol. 2006;154:763–75. doi: 10.1530/eje.1.02156. [DOI] [PubMed] [Google Scholar]
- 19.Dasari P, Pranahita GK. The efficacy of metformin and clomiphene citrate combination compared with clomiphene citrate alone for ovulation induction in infertile patients with PCOS. J Hum Reprod Sci. 2009;2:18–22. doi: 10.4103/0974-1208.51337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neveu N, Granger L, St-Michel P, Lavoie HB. Comparison of clomiphene citrate, metformin, or the combination of both for first-line ovulation induction and achievement of pregnancy in 154 women with polycystic ovary syndrome. Fertil Steril. 2007;87:113–20. doi: 10.1016/j.fertnstert.2006.05.069. [DOI] [PubMed] [Google Scholar]
- 21.Jungheim ES, Odibo AO. Fertility treatment in women with polycystic ovary syndrome: A decision analysis of different oral ovulation induction agents. Fertil Steril. 2010;94:2659–64. doi: 10.1016/j.fertnstert.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]


